Abstract
BACKGROUND
Calcium channel blocker poisonings account for a substantial number of reported deaths from cardiovascular drugs. While supportive care is the mainstay of treatment, experimental therapies such as high dose insulin-euglycemia and lipid emulsion have been studied in animal models and used in humans. In the most severe cases even aggressive care is inadequate and deaths occur. In both experimental models and clinical cases of vasodilatory shock, methylene blue improves hemodynamic measures. Methylene blue acts as both a nitric oxide scavenger and inhibits guanylate cyclase that is responsible for the production of cGMP. Excessive cGMP production is associated with refractory vasodilatory shock in sepsis and anaphylaxis. The aim of this study was to determine the efficacy of methylene blue in an animal model of amlodipine-induced shock.
METHODS
Sprague-Dawley rats were anesthetized, ventilated and instrumented for continuous blood pressure and heart rate monitoring. The dose of amlodipine that produced death within 60 minutes was 17 mg/kg/hour (LD50). Rats were divided into 2 groups: amlodipine followed by methylene blue or amlodipine followed by normal saline (NS) with 15 rats in each group. Rats received methylene blue at 2 mg/kg over 5 mins or an equivalent amount of NS in three intervals from the start of the protocol: Minute 5, 30, and 60. The animals were observed for a total of 2 hours after the start of the protocol. Mortality risk and survival time were analyzed using Fisher’s exact test and Kaplan Meier survival analysis with the log rank test.
RESULTS
Overall, 1/15 (7%) rats in the saline-treated group survived to 120 minutes compared with 5/15 (33%) rats in the methylene blue-treated group (difference −26%, 95% CI –54%, 0.3%). The median survival time for the NS group was 42 min (95% CI, 28.1,55.9) and the methylene blue group was 109 min (95% CI, 93.9,124.1). Heart rate and MAP differences between groups were analyzed until 60 minutes. Heart rate was significantly higher in the methylene blue-treated group starting 25 min after the start of the amlodipine infusion (95% CI, 30–113) that was analyzed until 60 minutes. MAP was significantly higher in the methylene blue-treated group starting 25 min after the amlodipine infusion (95% CI, 2–30) that was analyzed up until 60 minutes.
CONCLUSIONS
Methylene blue did not result in a significant difference in mortality risk. There was an increase heart rate, MAP and median survival time in the methylene blue group.
Introduction
Acute drug poisoning is one of the leading causes of injury-related fatality in the United States and the leading cause of cardiac arrest in victims under the age of 40 years encountered in the emergency department.1,2 The cardiovascular drug class accounts for a significant proportion of the reported poisoning fatalities. Over the last 5 years, there were over 12 million exposures with over 7,000 poisoning-related deaths reported to the American Association of Poison Control Centers National Poisoning Data System (NPDS).3–7 Cardiovascular drugs were involved in over 4% of the reported exposures and accounted for nearly 18% of poisoning fatalities. Within this class, calcium channel blockers (CCBs) were the most common cardiovascular drugs involved in poisoning fatalities. CCBs accounted for over 50,000 cases reported over the past five years with 301 cases resulting in major effect and over 100 deaths.3–7 CCB-related fatalities typically result from the combination of refractory hypotension and bradycardia that produce multiorgan dysfunction.8
CCBs act with different degrees of selectivity on cardiac myocytes, conductive cardiac tissue, and vascular smooth muscle. CCBs are commonly categorized into two groups: dihydropyridines and non-dihydropyridines.9 The dihydropyridines such as nifedipine and amlodipine act predominantly on the vascular smooth muscle producing peripheral vasodilation and are commonly used as antihypertensive agents. The two non-dihydropyridines, verapamil and diltiazem, act predominantly on the cardiac myocytes and are more commonly used for treatment of arrhythmias. Historically, the non-dihydropyridines account for over 90% of all CCB-related fatalities. Although some may view dihydropyridine poisoning as inconsequential when compared to the non-dihydropyridines, morbidity and mortality with the dihydropyridine CCBs are not uncommon, perhaps due to their widespread therapeutic availability.10–13
Management of patients with severe CCB poisoning consists primarily of gastrointestinal decontamination, fluid resuscitation, and administration of calcium, glucagon, and vasopressors.14–16 Because mortality remains high despite supportive care, novel therapies are often utilized such as high dose insulin-euglycemic therapy, intravenous lipid emulsion, and levosimendan.17–19 Despite promising results from experimental models and case reports, there is still no consistently successful treatment for severe CCB poisoning, and deaths are still reported.20–22 There is therefore important need for new therapy in the treatment of patients with severe CCB poisoning. One such therapy that has been used for other shock states such as sepsis and anaphylaxis is methylene blue.23–26 The aim of this study was to determine the efficacy of methylene blue in an animal model of amlodipine-induced shock.
Methods
Study Design
This is an unblinded controlled laboratory investigation using methylene blue in the setting of severe amlodipine toxicity. Adult Sprague-Dawley rats (300–400 grams) were chosen because they have been used in multiple studies of calcium blocker poisoning and shock.20,27,28 The animal care and use committee of the institution approved this protocol and the care and handling of the animals were in accordance with National Institutes of Health guidelines. The animals were housed in plastic cages with 12-hour light and dark cycles and allowed free access to food and water.
Animal Preparation
All animals were prepared and instrumented in a similar manner. The animals were allowed access to food and water ad libitum until the night prior to the study protocol. After fasting overnight, intravenous access was obtained and general anesthesia was induced with 5% isoflurane and maintained with 1.5% isoflurane for the duration of the protocol. Animals were given a tracheostomy and ventilated with a Harvard rodent ventilator (Model: 683, Harvard Apparatus Inc., Holliston, MA). Bilateral femoral cutdowns were performed, and each femoral vein cannulated with a 24 g catheter for infusion of amlodipine in one femoral vein and treatments in the other side. The right external carotid was cannulated with a 22 g catheter for hemodynamic monitoring. Heart rate (HR) and mean arterial pressure (MAP) tracings were recorded using a Power Lab 4/20 ML 840 (ADI Instruments, Houston, TX).
Study Protocol
The dose of amlodipine that produced death within 60 minutes was 17 mg/kg/hour (LD50) based on a dose-finding study.29 Rats were divided into 2 groups by block randomization: amlodipine followed by methylene blue or amlodipine followed by 0.9% sodium chloride solution (NS). At 15 rats per group our study had an 81% power to detect an absolute 50% difference in percent survival. Rats received methylene blue at 2 mg/kg (1 cc) over 5 minutes or an equivalent volume of (1 cc) NS in three intervals from the start of the protocol: Minute 5, 30, and 60. The animals were observed for a total of 2 hours after the start of the protocol. The primary outcome was survival to a 2-hour endpoint. The secondary outcomes were HR and MAP.
Measurements
The cardiac rhythm (three-lead), MAP, and HR were monitored continuously using a Power Lab 4/20 ML 840.
Data Analysis
Mortality risk was compared using Fischer’s exact test. The survival times were compared using the Kaplan–Meier method, and the two groups were compared using the log-rank test. Medians were used for non-parametric testing and median differences were given with 95% confidence intervals (CIs). One-way analysis of variance with repeated measures was used to analyze continuous variables (HR and MAP) over time to compare differences between groups and data were reported as mean SEM. All statistical tests were two-tailed. Data were analyzed using SPSS statistical software (version 8.0; SPSS Inc., Chicago, IL).
Results
Mortality and Survival Times
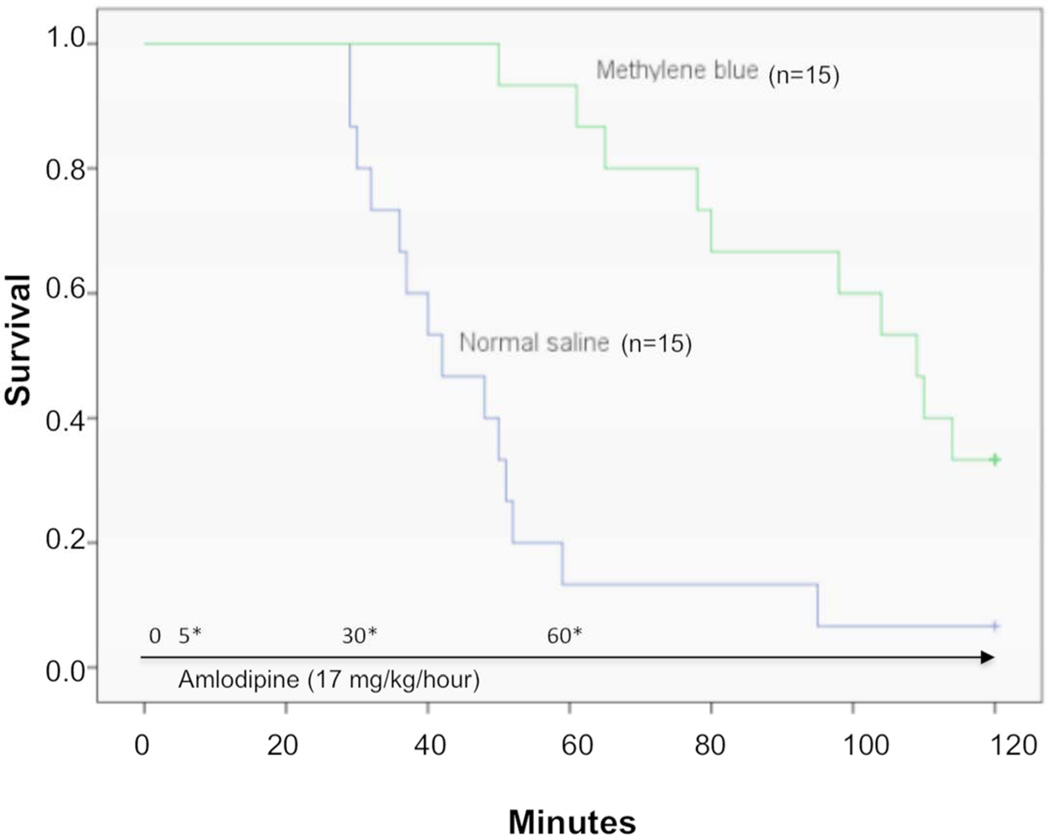
Overall, 1/15 (7%) rats in the saline-treated group survived to 120 minutes compared with 5/15 (33%) rats in the methylene blue-treated group (difference −26%, 95% CI –54%, 0.3%). The median survival time for the NS group was 42 min (95% CI, 28.1–55.9) and the methylene blue group was 109 min (95% CI, 93.9–124.1) Figure 1 shows the Kaplan–Meier analysis comparing survival times and mortality rates in the methylene blue and control groups. The log-rank test revealed a statistically significant difference between the survival rates over time (p = 0.002).
Figure 1.
Kaplan-Meier Survival curve comparing methylene blue and normal saline for treatment of amlodipine toxicity in a rodent model with protocol summary and timeline.
5*, 30*, 60* - Infusion of methylene blue 2 mg/kg (1 mL total) or normal saline (1 mL) over 5 minutes
Hemodynamic Parameters
Heart rate was significantly higher in the methylene blue-treated group starting 25 min after the start of the amlodipine infusion (95% CI, 30–113) which was analyzed up until 60 minutes as the majority of the subjects expired within an hour. The MAP was significantly higher in the methylene blue-treated group starting 25 min after the amlodipine infusion (95% CI, 2–30) that was analyzed up until 60 minutes.
Limitations
Our study utilized a rodent model of amlodipine toxicity with multiple dosing of methylene blue. This may not be the optimal dosing strategy of methylene blue for CCB toxicity. However, there is reported variability in the dosing of methylene blue for refractory septic shock.30,31
Our study did not demonstrate a change in mortality risk at 120 minutes. This may be because we used a large effect size and were underpowered to detect smaller effects. While we did perform block randomization we were not blind to the treatments. Blinding the treatments was difficult due to the readily recognizable blue color of methylene blue compared to NS.
We did not employ other antidotal therapies or fluid resuscitation commonly used for CCB toxicity, as we wanted isolate methylene blue effects. We also used a continuous infusion of amlodipine to simulate oral ingestion with continued GI absorption. These conditions may not mimic the actual reality of clinical medicine.
Dimethyl sulfoxide (DMSO) may hypotension. In our own experience we have observed very high concentrations of DMSO (100%) resulting in hypotension in canines. Prior to the initiation of our study we used varying concentrations of DMSO alone (up to 60%) in rats and observed no alterations in hemodynamics. As we utilized a 20% DMSO solution to dissolve the amlodipine, it is unlikely DMSO had an interaction with the results of this study.
We did not specifically perform baseline blood gases on the animals in this study. In our prior studies using similar models of CCB toxicity with nifedipine and verapamil, all animals at baseline have similar pH, pCO2, pO2 and base excess. The animals from prior studies were ventilated with oxygen and never became hypoxemic. In our prior studies, when the animals became hypotensive from the CCB poisoning, they developed a metabolic acidosis with increasingly negative base excess. However, they compensated for the metabolic acidosis by decreasing their pCO2 to produce a respiratory alkalosis.
Discussion
Methylene blue is used for many purposes such as the treatment of acquired methemoglobinemia and less commonly for the treatment of shock from sepsis and anaphylaxis.32 Although the mechanism of action of methylene blue for the treatment of shock from sepsis and anaphylaxis is not established, suggested mechanisms implicate the nitric oxide pathway, which includes inhibition of certain isoforms nitric oxide synthase, scavenging nitric oxide, and inhibition of guanylate cyclase.33–35 Inhibition of excessive production and activity of both nitric oxide and cGMP may be critical in the treatment of refractory shock that occurs in cardiac bypass, sepsis, and anaphylaxis.30,36,37
Experimental studies demonstrate that certain calcium channel blockers may also modulate blood pressure through a nitric oxide mechanism in addition to blockade of L-type calcium channels. One study demonstrated that amlodipine and some other dihydropyridine CCBs release nitric oxide (measured as an increase in nitrite) in a dose-dependent fashion from canine coronary microvessels.38 The R+ enantiomer of amlodipine induced nitric oxide release whereas the S− enantiomer only blocked the L-type calcium channels with no release of nitric oxide.39 Although the exact mechanism is uncertain, amlodipine appears to increase nitric oxide production by increasing endothelial nitric oxide synthase activity through phosphorylation of this enzyme.40 Another in vivo study demonstrated that rabbit femoral artery vasodilation from amlodipine was partly dependent on nitric oxide generation through the mediation of bradykinin B2-receptors.41
Nitric oxide synthase plays an important role in regulating vascular tone and is an important mediator of shock. There are 3 isoforms of nitric oxide synthase: neuronal nitric oxide synthase, inducible nitric oxide synthase, and endothelial nitric oxide synthase. Endothelial nitric oxide synthase generates nitric oxide in blood vessels and is involved with regulating vascular function.42 Inducible nitric oxide synthase is produced in vascular smooth muscle cells and cardiac myocytes by mediators such as tumor necrosis factor, various cytokines, and oxidative stress. Activation of either endothelial or inducible nitric oxide synthase isoform increases production of nitric oxide, which in turn increases the generation of cyclic guanosine monophosphate (cGMP) by activation of guanylate cyclase.43 The accumulation of cGMP leads to vasodilatation and decreased systematic vascular resistance. Another effect of increased cGMP concentration is decreased contractile response to vasoconstrictors such as norepinephrine.44 This dysregulation of nitric oxide synthesis and release occurs in conditions such as anaphylaxis, sepsis, and cardiac bypass and is believed to be the main mechanism responsible for refractory distributive shock.45
Non-specific competitive nitric oxide synthase inhibitors such as N-monomethyl-L-arginine increase mean arterial pressure in sepsis, however no mortality benefit has been demonstrated. In fact, the use of non-specific nitric oxide synthase inhibitors results in increased mortality in both animals and humans.46,47 Non-specific inhibition of nitric oxide synthase may be detrimental in part because nitric oxide is also vital in other important physiological pathways. More selective inhibition along the NO-cGMP pathway, with methylene blue use to inhibit guanylate cyclase to decrease the actual production of cyclic guanosine monophosphate may avoid the detrimental effects with general NO inhibition. Studies demonstrate decreased vasopressor requirement and an increase in systemic vascular resistance with the use of methylene blue when used for the treatment of both sepsis and anaphylaxis.30,48–52 At this time there are only a few case reports and no experimental evidence to support the use of methylene for the treatment of shock from acute drug poisoning.53,54
Using a rodent model of severe amlodipine poisoning we demonstrated that methylene blue increased survival time and was associated with an increase in HR and MAP. One theory for these results may be related to the nitric oxide pathway. Certain CCBs such as amlodipine lower blood pressure when used in the therapeutic setting through the combination L-type calcium channel blockade and nitric oxide formation.
The dose for methylene blue utilized for shock from sepsis and anaphylaxis varies depending on the study. The dosing regimen for methylene blue used in our study is based on both experimental and clinical data and is similar to what is used for the treatment of methemoglobinemia: 1–2 mg/kg. The dosing used for refractory septic shock has included a single bolus, repeated bolus based on response, low-dose infusion, and infusions following a bolus.30,31 Based on other studies, a cumulative dose of methylene blue greater than 7 mg/kg is associated with adverse effects such as paradoxical induction of methemoglobinemia, acute hemolytic anemia, and impaired pulmonary function.55
There are several complications to consider when methylene blue is used as a treatment for shock. Methylene blue may cause acute hemolytic anemia with high doses, blue discoloration of body fluids, and shortness of breath.55,56 Another adverse effect is the precipitation of serotonin syndrome. Serotonin syndrome is a potentially fatal condition characterized by autonomic instability that includes agitation, muscle rigidity, and hyperthermia due to excessive stimulation at the 5-HT 2A receptor.57 In 2011, the FDA issued a safety announcement that methylene blue, even at therapeutic doses, may cause serotonin toxicity which may be due to both the parent compound and metabolite, azure B, which inhibits monoamine oxidase (MAO-A).58–62
Our results indicate that methylene blue did not result in a significant difference in mortality risk at two hours but there was an increase in median survival time in the methylene blue group. There was also an increase in HR and MAP in the methylene blue group. Further studies with other models and different CCBs should be done before methylene blue is routinely implemented for the care of CCB-poisoned humans.
Acknowledgments
This study is supported by the 2011 AACT Junior Investigator Grant.
The project described was supported by Award Number-K12 HL109009 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
David H. Jang, Contribution: Principal investigator and primary grantee; manuscript preparation and data analysis
Sean Donovan, Contribution: Performed significant parts of the experiments, data analysis, manuscript preparation
Lewis S. Nelson, Contribution: Mentor, data analysis, manuscript preparation
Theodore C. Bania, Contribution: Performed significant portion of the study, data analysis, manuscript prep
Robert S. Hoffman, Contribution: Mentor, data analysis, manuscript preparation
Jason Chu, Contribution: Co-investigator, performed significant portion of the study, data analysis, manuscript prep
Contributor Information
David H. Jang, University of Pennsylvania Perelman School of Medicine, Department of Emergency Medicine, National Heart, Lung, and Blood Institute (NHLBI) K12 Scholar.
Sean Donovan, Emergency Medicine Resident, Albany Medical Center, sdonovan31@yahoo.com.
Lewis S. Nelson, New York University School of Medicine, Professor of Emergency Medicine, lnelsonmd@gmail.com.
Theodore C. Bania, Mt Sinai Roosevelt Hospital, Mt Sinai St Luke's Hospital, Assistant Professor in Emergency Medicine, Icahn School of Medicine at Mt Sinai, toxtod@aol.com.
Robert S. Hoffman, Division of Medical Toxicology, Department of Emergency Medicine, NYU School of Medicine, Professor of Emergency Medicine, bobhoffmd@gmail.com.
Jason Chu, Mt Sinai Roosevelt Hospital, Mt Sinai St Luke's Hospital, Assistant Professor in Emergency Medicine, Icahn School of Medicine at Mt Sinai, jasonchu99@gmail.com.
References
- 1.Centers for Disease C, Prevention. Increases in age-group-specific injury mortality--United States, 1999–2004. MMWR Morbidity and mortality weekly report. 2007;56:1281–1284. [PubMed] [Google Scholar]
- 2.McCaig LF, Burt CW. Poisoning-related visits to emergency departments in the United States, 1993–1996. J Toxicol Clin Toxicol. 1999;37:817–826. doi: 10.1081/clt-100102460. [DOI] [PubMed] [Google Scholar]
- 3.Bronstein AC, Spyker DA, Cantilena LR, Jr, et al. 2007 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila) 2008;46:927–1057. doi: 10.1080/15563650802559632. [DOI] [PubMed] [Google Scholar]
- 4.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2008 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol (Phila) 2009;47:911–1084. doi: 10.3109/15563650903438566. [DOI] [PubMed] [Google Scholar]
- 5.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2009 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila) 2010;48:979–1178. doi: 10.3109/15563650.2010.543906. [DOI] [PubMed] [Google Scholar]
- 6.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Dart RC. 2010 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 28th Annual Report. Clin Toxicol (Phila) 2011;49:910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- 7.Bronstein AC, Spyker DA, Cantilena LR, Jr, Rumack BH, Dart RC. 2011 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila) 2012;50:911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 8.Ramoska EA, Spiller HA, Winter M, Borys D. A one-year evaluation of calcium channel blocker overdoses: toxicity and treatment. Ann Emerg Med. 1993;22:196–200. doi: 10.1016/s0196-0644(05)80202-1. [DOI] [PubMed] [Google Scholar]
- 9.Abernethy DR, Schwartz JB. Calcium-antagonist drugs. N Engl J Med. 1999;341:1447–1457. doi: 10.1056/NEJM199911043411907. [DOI] [PubMed] [Google Scholar]
- 10.Jang DHSM, Nelson LS, et al. An 11-year retrospective comparison of dihydropyridines and non-dihydropyridine calcium channel blockers-Meeting ABSTRACT. Clin Tox. 2012;50:580. [Google Scholar]
- 11.Spiller HA, Milliner BA, Bosse GM. Amlodipine fatality in an infant with postmortem blood levels. J Med Toxicol. 2012;8:179–182. doi: 10.1007/s13181-011-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams BD, Browne WT. Amlodipine overdose causes prolonged calcium channel blocker toxicity. Am J Emerg Med. 1998;16:527–528. doi: 10.1016/s0735-6757(98)90011-0. [DOI] [PubMed] [Google Scholar]
- 13.Sklerov JH, Levine B, Ingwersen KM, Aronica-Pollack PA, Fowler D. Two cases of fatal amlodipine overdose. J Anal Toxicol. 2006;30:346–351. doi: 10.1093/jat/30.5.346. [DOI] [PubMed] [Google Scholar]
- 14.Doyon S, Roberts JR. The use of glucagon in a case of calcium channel blocker overdose. Ann Emerg Med. 1993;22:1229–1233. doi: 10.1016/s0196-0644(05)80997-7. [DOI] [PubMed] [Google Scholar]
- 15.Dolan DL. Intravenous calcium before verapamil to prevent hypotension. Ann Emerg Med. 1991;20:588–589. doi: 10.1016/s0196-0644(05)81624-5. [DOI] [PubMed] [Google Scholar]
- 16.Bailey B. Glucagon in beta-blocker and calcium channel blocker overdoses: a systematic review. J Toxicol Clin Toxicol. 2003;41:595–602. doi: 10.1081/clt-120023761. [DOI] [PubMed] [Google Scholar]
- 17.Osthoff M, Bernsmeier C, Marsch SC, Hunziker PR. Levosimendan as treatment option in severe verapamil intoxication: a case report and review of the literature. Case Rep Med. 2010;2010 doi: 10.1155/2010/546904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kline JA, Tomaszewski CA, Schroeder JD, Raymond RM. Insulin is a superior antidote for cardiovascular toxicity induced by verapamil in the anesthetized canine. J Pharmacol Exp Ther. 1993;267:744–750. [PubMed] [Google Scholar]
- 19.Engebretsen KM, Kaczmarek KM, Morgan J, Holger JS. High-dose insulin therapy in betablocker and calcium channel-blocker poisoning. Clin Toxicol (Phila) 2011;49:277–283. doi: 10.3109/15563650.2011.582471. [DOI] [PubMed] [Google Scholar]
- 20.Abraham MK, Scott SB, Meltzer A, Barrueto F., Jr Levosimendan does not improve survival time in a rat model of verapamil toxicity. J Med Toxicol. 2009;5:3–7. doi: 10.1007/BF03160973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crump BJ, Holt DW, Vale JA. Lack of response to intravenous calcium in severe verapamil poisoning. Lancet. 1982;2:939–940. doi: 10.1016/s0140-6736(82)90912-6. [DOI] [PubMed] [Google Scholar]
- 22.Sim MT, Stevenson FT. A fatal case of iatrogenic hypercalcemia after calcium channel blocker overdose. J Med Toxicol. 2008;4:25–29. doi: 10.1007/BF03160947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paciullo CA, McMahon Horner D, Hatton KW, Flynn JD. Methylene blue for the treatment of septic shock. Pharmacotherapy. 2010;30:702–715. doi: 10.1592/phco.30.7.702. [DOI] [PubMed] [Google Scholar]
- 24.Buzato MA, Viaro F, Piccinato CE, Evora PR. The use of methylene blue in the treatment of anaphylactic shock induced by compound 48/80: experimental studies in rabbits. Shock. 2005;23:582–587. [PubMed] [Google Scholar]
- 25.Evora PR, Roselino CH, Schiaveto PM. Methylene blue in anaphylactic shock. Ann Emerg Med. 1997;30:240. doi: 10.1016/s0196-0644(97)70152-5. [DOI] [PubMed] [Google Scholar]
- 26.Koch HJ. Effects of methylene blue in septic shock. Crit Care Med. 1996;24:1093–1094. doi: 10.1097/00003246-199606000-00043. [DOI] [PubMed] [Google Scholar]
- 27.Perez E, Bania TC, Medlej K, Chu J. Determining the optimal dose of intravenous fat emulsion for the treatment of severe verapamil toxicity in a rodent model. Acad Emerg Med. 2008;15:1284–1289. doi: 10.1111/j.1553-2712.2008.00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Perez E, Chu J, Bania T, Medlej K. L-carnitine increases survival in a murine model of severe verapamil toxicity. Acad Emerg Med. 2011;18:1135–1140. doi: 10.1111/j.1553-2712.2011.01217.x. [DOI] [PubMed] [Google Scholar]
- 29.Jang DHDS, Bania TC, et al. The novel development of an experimental model of dihydropyridine calcium channel blocker poisoning using intravenous amlodipine. Int J Cardiovasc Res. 2013;2 [PMC free article] [PubMed] [Google Scholar]
- 30.Juffermans NP, Vervloet MG, Daemen-Gubbels CR, Binnekade JM, de Jong M, Groeneveld AB. A dose-finding study of methylene blue to inhibit nitric oxide actions in the hemodynamics of human septic shock. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2010;22:275–280. doi: 10.1016/j.niox.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Kirov MY, Evgenov OV, Evgenov NV, et al. Infusion of methylene blue in human septic shock: a pilot, randomized, controlled study. Crit Care Med. 2001;29:1860–1867. doi: 10.1097/00003246-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol. 2013;9:242–249. doi: 10.1007/s13181-013-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peer G, Itzhakov E, Wollman Y, et al. Methylene blue, a nitric oxide inhibitor, prevents haemodialysis hypotension. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16:1436–1441. doi: 10.1093/ndt/16.7.1436. [DOI] [PubMed] [Google Scholar]
- 34.Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochemical pharmacology. 1993;45:367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu S, Yamamoto T, Momose K. Inhibition by methylene blue of the L-arginine metabolism to L-citrulline coupled with nitric oxide synthesis in cultured endothelial cells. Research communications in chemical pathology and pharmacology. 1993;82:35–48. [PubMed] [Google Scholar]
- 36.Evora PR, Simon MR. Role of nitric oxide production in anaphylaxis and its relevance for the treatment of anaphylactic hypotension with methylene blue. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2007;99:306–313. doi: 10.1016/S1081-1206(10)60545-5. [DOI] [PubMed] [Google Scholar]
- 37.Kirov MY, Evgenov OV, Bjertnaes LJ. Combination of intravenously infused methylene blue and inhaled nitric oxide ameliorates endotoxin-induced lung injury in awake sheep. Crit Care Med. 2003;31:179–186. doi: 10.1097/00003246-200301000-00028. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels: an unexpected mechanism of action of a calcium channel-blocking agent. Circulation. 1998;97:576–580. doi: 10.1161/01.cir.97.6.576. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XP, Loke KE, Mital S, Chahwala S, Hintze TH. Paradoxical release of nitric oxide by an L-type calcium channel antagonist, the R+ enantiomer of amlodipine. J Cardiovasc Pharmacol. 2002;39:208–214. doi: 10.1097/00005344-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Lenasi H, Kohlstedt K, Fichtlscherer B, Mulsch A, Busse R, Fleming I. Amlodipine activates the endothelial nitric oxide synthase by altering phosphorylation on Ser1177 and Thr495. Cardiovasc Res. 2003;59:844–853. doi: 10.1016/s0008-6363(03)00505-4. [DOI] [PubMed] [Google Scholar]
- 41.Xu B, Xiao-hong L, Lin G, Queen L, Ferro A. Amlodipine, but not verapamil or nifedipine, dilates rabbit femoral artery largely through a nitric oxide- and kinin-dependent mechanism. British journal of pharmacology. 2002;136:375–382. doi: 10.1038/sj.bjp.0704753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. The Biochemical journal. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochemical Society transactions. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 44.Weingartner R, Oliveira E, Oliveira ES, et al. Blockade of the action of nitric oxide in human septic shock increases systemic vascular resistance and has detrimental effects on pulmonary function after a short infusion of methylene blue. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 1999;32:1505–1513. doi: 10.1590/s0100-879x1999001200009. [DOI] [PubMed] [Google Scholar]
- 45.Nathan CF, Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Current opinion in immunology. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 46.Watson D, Grover R, Anzueto A, et al. Cardiovascular effects of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) in patients with septic shock: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144-002) Crit Care Med. 2004;32:13–20. doi: 10.1097/01.CCM.0000104209.07273.FC. [DOI] [PubMed] [Google Scholar]
- 47.Cobb JP, Natanson C, Hoffman WD, et al. N omega-amino-L-arginine, an inhibitor of nitric oxide synthase, raises vascular resistance but increases mortality rates in awake canines challenged with endotoxin. The Journal of experimental medicine. 1992;176:1175–1182. doi: 10.1084/jem.176.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andresen M, Dougnac A, Diaz O, et al. Use of methylene blue in patients with refractory septic shock: impact on hemodynamics and gas exchange. Journal of critical care. 1998;13:164–168. doi: 10.1016/s0883-9441(98)90001-6. [DOI] [PubMed] [Google Scholar]
- 49.Schneider F, Lutun P, Hasselmann M, Stoclet JC, Tempe JD. Methylene blue increases systemic vascular resistance in human septic shock. Preliminary observations. Intensive Care Med. 1992;18:309–311. doi: 10.1007/BF01706481. [DOI] [PubMed] [Google Scholar]
- 50.Jaskille AD, Jeng JC, Jordan MH. Methylene blue in the treatment of vasoplegia following severe burns. Journal of burn care & research : official publication of the American Burn Association. 2008;29:408–410. doi: 10.1097/BCR.0b013e31816677b5. [DOI] [PubMed] [Google Scholar]
- 51.Ghiassi S, Sun YS, Kim VB, et al. Methylene blue enhancement of resuscitation after refractory hemorrhagic shock. The Journal of trauma. 2004;57:515–521. doi: 10.1097/01.ta.0000136159.22721.3d. [DOI] [PubMed] [Google Scholar]
- 52.Zheng F, Barthel G, Collange O, et al. Methylene blue and epinephrine: a synergetic association for anaphylactic shock treatment. Crit Care Med. 2013;41:195–204. doi: 10.1097/CCM.0b013e318267667b. [DOI] [PubMed] [Google Scholar]
- 53.Aggarwal N, Kupfer Y, Seneviratne C, Tessler S. Methylene blue reverses recalcitrant shock in beta-blocker and calcium channel blocker overdose. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang DH, Nelson LS, Hoffman RS. Methylene blue in the treatment of refractory shock from an amlodipine overdose. Ann Emerg Med. 2011;58:565–567. doi: 10.1016/j.annemergmed.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 55.Goluboff N, Wheaton R. Methylene blue induced cyanosis and acute hemolytic anemia complicating the treatment of methemoglobinemia. The Journal of pediatrics. 1961;58:86–89. doi: 10.1016/s0022-3476(61)80064-4. [DOI] [PubMed] [Google Scholar]
- 56.Gachot B, Bedos JP, Veber B, Wolff M, Regnier B. Short-term effects of methylene blue on hemodynamics and gas exchange in humans with septic shock. Intensive Care Med. 1995;21:1027–1031. doi: 10.1007/BF01700666. [DOI] [PubMed] [Google Scholar]
- 57.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 58.McDonnell AM, Rybak I, Wadleigh M, Fisher DC. Suspected serotonin syndrome in a patient being treated with methylene blue for ifosfamide encephalopathy. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2012;18:436–439. doi: 10.1177/1078155211433231. [DOI] [PubMed] [Google Scholar]
- 59.Heritier Barras AC, Walder B, Seeck M. Serotonin syndrome following Methylene Blue infusion: a rare complication of antidepressant therapy. Journal of neurology, neurosurgery, and psychiatry. 2010;81:1412–1413. doi: 10.1136/jnnp.2009.172221. [DOI] [PubMed] [Google Scholar]
- 60.Ng BK, Cameron AJ. The role of methylene blue in serotonin syndrome: a systematic review. Psychosomatics. 2010;51:194–200. doi: 10.1176/appi.psy.51.3.194. [DOI] [PubMed] [Google Scholar]
- 61.Rowley M, Riutort K, Shapiro D, Casler J, Festic E, Freeman WD. Methylene blue-associated serotonin syndrome: a 'green' encephalopathy after parathyroidectomy. Neurocritical care. 2009;11:88–93. doi: 10.1007/s12028-009-9206-z. [DOI] [PubMed] [Google Scholar]
- 62.Petzer A, Harvey BH, Wegener G, Petzer JP, Azure B. a metabolite of methylene blue, is a high-potency, reversible inhibitor of monoamine oxidase. Toxicol Appl Pharmacol. 2012;258:403–409. doi: 10.1016/j.taap.2011.12.005. [DOI] [PubMed] [Google Scholar]