Abstract
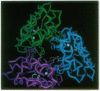
Phospholipase A2 (PLA2) from Indian cobra venom (Naja naja naja) was crystallized from ethanol in space group P4(3)2(1)2 in the presence of Ca2+. The x-ray crystal structure was determined to 2.3-A resolution by molecular replacement techniques using a theoretical model constructed from homologous segments of the bovine pancreatic, porcine pancreatic, and rattlesnake venom crystal structures. The structure was refined to an R value of 0.174 for 17,542 reflections between 6.0- and 2.3-A resolution (F > 2 sigma), including 148 water molecules. The 119-amino acid enzyme has an overall architecture strikingly similar to the other known PLA2 structures with regions implicated in catalysis showing the greatest structural conservation. Unexpectedly, three monomers were found to occupy the asymmetric unit and are oriented with their catalytic sites facing the pseudo-threefold axis with approximately 15% of the solvent accessible surface of each monomer buried in trimer contacts. The majority of the interactions at the subunit interfaces are made by residues unique to PLA2 sequences from cobra and krait venoms. The possible relevance of this unique trimeric structure is considered.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunie S., Bolin J., Gewirth D., Sigler P. B. The refined crystal structure of dimeric phospholipase A2 at 2.5 A. Access to a shielded catalytic center. J Biol Chem. 1985 Aug 15;260(17):9742–9749. [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A. Evolutionary relationships and implications for the regulation of phospholipase A2 from snake venom to human secreted forms. J Mol Evol. 1990 Sep;31(3):228–238. doi: 10.1007/BF02109500. [DOI] [PubMed] [Google Scholar]
- Demaret J. P., Chwetzoff S., Brunie S. Dimeric character of a basic phospholipase A2 from cobra venom: experimental and modelling study. Protein Eng. 1990 Dec;4(2):171–176. doi: 10.1093/protein/4.2.171. [DOI] [PubMed] [Google Scholar]
- Dijkstra B. W., Drenth J., Kalk K. H. Active site and catalytic mechanism of phospholipase A2. Nature. 1981 Feb 12;289(5798):604–606. doi: 10.1038/289604a0. [DOI] [PubMed] [Google Scholar]
- Dijkstra B. W., Kalk K. H., Hol W. G., Drenth J. Structure of bovine pancreatic phospholipase A2 at 1.7A resolution. J Mol Biol. 1981 Mar 25;147(1):97–123. doi: 10.1016/0022-2836(81)90081-4. [DOI] [PubMed] [Google Scholar]
- Dijkstra B. W., Renetseder R., Kalk K. H., Hol W. G., Drenth J. Structure of porcine pancreatic phospholipase A2 at 2.6 A resolution and comparison with bovine phospholipase A2. J Mol Biol. 1983 Jul 25;168(1):163–179. doi: 10.1016/s0022-2836(83)80328-3. [DOI] [PubMed] [Google Scholar]
- Gros P., van Gunsteren W. F., Hol W. G. Inclusion of thermal motion in crystallographic structures by restrained molecular dynamics. Science. 1990 Sep 7;249(4973):1149–1152. doi: 10.1126/science.2396108. [DOI] [PubMed] [Google Scholar]
- Hamlin R. Multiwire area X-ray diffractometers. Methods Enzymol. 1985;114:416–452. doi: 10.1016/0076-6879(85)14029-2. [DOI] [PubMed] [Google Scholar]
- Hazlett T. L., Dennis E. A. Affinity chromatography of phospholipase A2 from Naja naja naja (Indian cobra) venom. Toxicon. 1985;23(3):457–466. doi: 10.1016/0041-0101(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Hazlett T. L., Dennis E. A. Aggregation studies on fluorescein-coupled cobra venom phospholipase A2. Biochemistry. 1985 Oct 22;24(22):6152–6158. doi: 10.1021/bi00343a018. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Krueger E. T., Keim P. S. Amino acid sequence of phospholipase A2-alpha from the venom of Crotalus adamanteus. A new classification of phospholipases A2 based upon structural determinants. J Biol Chem. 1977 Jul 25;252(14):4913–4921. [PubMed] [Google Scholar]
- Holland D. R., Clancy L. L., Muchmore S. W., Ryde T. J., Einspahr H. M., Finzel B. C., Heinrikson R. L., Watenpaugh K. D. The crystal structure of a lysine 49 phospholipase A2 from the venom of the cottonmouth snake at 2.0-A resolution. J Biol Chem. 1990 Oct 15;265(29):17649–17656. doi: 10.2210/pdb1ppa/pdb. [DOI] [PubMed] [Google Scholar]
- Howard A. J., Nielsen C., Xuong N. H. Software for a diffractometer with multiwire area detector. Methods Enzymol. 1985;114:452–472. doi: 10.1016/0076-6879(85)14030-9. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Keith C., Feldman D. S., Deganello S., Glick J., Ward K. B., Jones E. O., Sigler P. B. The 2.5 A crystal structure of a dimeric phospholipase A2 from the venom of Crotalus atrox. J Biol Chem. 1981 Aug 25;256(16):8602–8607. [PubMed] [Google Scholar]
- Kuipers O. P., Thunnissen M. M., de Geus P., Dijkstra B. W., Drenth J., Verheij H. M., de Haas G. H. Enhanced activity and altered specificity of phospholipase A2 by deletion of a surface loop. Science. 1989 Apr 7;244(4900):82–85. doi: 10.1126/science.2704992. [DOI] [PubMed] [Google Scholar]
- Lombardo D., Dennis E. A. Immobilized phospholipase A2 from cobra venom. Prevention of substrate interfacial and activator effects. J Biol Chem. 1985 Dec 25;260(30):16114–16121. [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Miller S., Lesk A. M., Janin J., Chothia C. The accessible surface area and stability of oligomeric proteins. 1987 Aug 27-Sep 2Nature. 328(6133):834–836. doi: 10.1038/328834a0. [DOI] [PubMed] [Google Scholar]
- Plückthun A., Dennis E. A. Activation, aggregation, and product inhibition of cobra venom phospholipase A2 and comparison with other phospholipases. J Biol Chem. 1985 Sep 15;260(20):11099–11106. [PubMed] [Google Scholar]
- Renetseder R., Brunie S., Dijkstra B. W., Drenth J., Sigler P. B. A comparison of the crystal structures of phospholipase A2 from bovine pancreas and Crotalus atrox venom. J Biol Chem. 1985 Sep 25;260(21):11627–11634. [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Dennis E. A. Dual role of interfacial phospholipid in phospholipase A2 catalysis. Proc Natl Acad Sci U S A. 1977 May;74(5):1950–1954. doi: 10.1073/pnas.74.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. A comparison of the heme binding pocket in globins and cytochrome b5. J Biol Chem. 1975 Sep 25;250(18):7525–7532. [PubMed] [Google Scholar]
- Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. Crystal structure of bee-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990 Dec 14;250(4987):1563–1566. doi: 10.1126/science.2274788. [DOI] [PubMed] [Google Scholar]
- Scott D. L., White S. P., Browning J. L., Rosa J. J., Gelb M. H., Sigler P. B. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science. 1991 Nov 15;254(5034):1007–1010. doi: 10.1126/science.1948070. [DOI] [PubMed] [Google Scholar]
- Scott D. L., White S. P., Otwinowski Z., Yuan W., Gelb M. H., Sigler P. B. Interfacial catalysis: the mechanism of phospholipase A2. Science. 1990 Dec 14;250(4987):1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaller C., Eichele G., Weaver L. H., Wilson E., Karlsson R., Jansonius J. N. Diffraction methods for biological macromolecules. Seed enlargement and repeated seeding. Methods Enzymol. 1985;114:132–135. doi: 10.1016/0076-6879(85)14011-5. [DOI] [PubMed] [Google Scholar]
- Thunnissen M. M., Ab E., Kalk K. H., Drenth J., Dijkstra B. W., Kuipers O. P., Dijkman R., de Haas G. H., Verheij H. M. X-ray structure of phospholipase A2 complexed with a substrate-derived inhibitor. Nature. 1990 Oct 18;347(6294):689–691. doi: 10.1038/347689a0. [DOI] [PubMed] [Google Scholar]
- Weis W. I., Brünger A. T., Skehel J. J., Wiley D. C. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol. 1990 Apr 20;212(4):737–761. doi: 10.1016/0022-2836(90)90234-D. [DOI] [PubMed] [Google Scholar]
- Wery J. P., Schevitz R. W., Clawson D. K., Bobbitt J. L., Dow E. R., Gamboa G., Goodson T., Jr, Hermann R. B., Kramer R. M., McClure D. B. Structure of recombinant human rheumatoid arthritic synovial fluid phospholipase A2 at 2.2 A resolution. Nature. 1991 Jul 4;352(6330):79–82. doi: 10.1038/352079a0. [DOI] [PubMed] [Google Scholar]
- White S. P., Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990 Dec 14;250(4987):1560–1563. doi: 10.1126/science.2274787. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Rini J. M., Fremont D. H., Fieser G. G., Stura E. A. X-ray crystallographic analysis of free and antigen-complexed Fab fragments to investigate structural basis of immune recognition. Methods Enzymol. 1991;203:153–176. doi: 10.1016/0076-6879(91)03009-6. [DOI] [PubMed] [Google Scholar]