Abstract
Two methods for the fast separation of arsenic species are presented. The general approach is to modify existing methodology utilizing carbonate eluents for a small particle size, short column length Hamilton PRPX100 column which is interfaced with the Agilent 8800 ICP-QQQ using oxygen as reaction gas and detection of AsO at m/z 91. Using H2O2 in the extractant to oxidize As(III) to As(V) it is possible to separate arsenobetaine from DMA, MMA and As(V) in 1.5 minutes. Such a method may be useful where a measure of total inorganic As is sufficient, for example for regulatory compliance in food or beverage testing. It is possible to separate six As species. i.e the four above and arsenocholine and As(III) in 4.5 minutes using a gradient separation. Such a method could be useful analysis of urinary arsenic species. Coupling with high sensitivity of ICP-QQQ yields equivalent or better detection limits than conventional methods with run times up to 5 times faster, which is a significant benefit for sample throughput and method development.
Introduction
Arsenic can exist in a number of different compounds in environment and biological systems, and a variety of different chromatographic and non-chromatographic methods have been developed to separate and quantify individual or groups of arsenic species1. The current interest in human exposure to arsenic has increased interest in methods to separate and quantify inorganic arsenic in foods2, 3 and juices and to quantify inorganic arsenic and metabolites in urine4. The typical approach for these analyses is anion exchange chromatography coupled to ICP-MS. Separation of common arsenic anions requires from ca. 5-19 minutes depending on the suite of species under consideration. Recent developments in chromatography columns of smaller particle size and shorter column length are reducing chromatographic runtimes without compromising resolving power. In this brief report I illustrate the use of small particle size (5μm) short column length (50 mm) Hamilton PRP X100 to provide faster chromatographic run times for arsenic speciation. I also use the ICP-QQQ as the element specific detector with oxygen as reaction gas and mass shifting arsenic to AsO at m/z 91 which provides lower detection limits and freedom from polyatomic and doubly charged interferences5 and thus allow reduced chromatographic injection volumes which are more appropriate for these fast chromatographic methods.
Materials and methods
A 5μm stationary phase, 2.1 X 50 mm length Hamilton PRP 100X anion exchange column (Reno, NV) was used. All separations used carbonate based eluents which were achieved by an eluent A of 3% methanol in deionized water and eluent B: 50 mM ammonium carbonate (NH4)2CO3 ((pH ca 9) in 3% methanol. As has been shown by many, addition of methanol increases ICPMS sensitivity for arsenic analysis6, 7. The chromatography system (Agilent 1260, Santa Clara, CA) was interfaced directly to an 8800 ICP-QQQ (Agilent, Santa Clara, CA) with due attention to minimizing dead volume of tubing between column and spray chamber. The ICP-QQQ was operated with oxygen as a reaction gas and measurement of arsenic as AsO at m/z 91. Instrument operation parameters are given in Table 1. Inorganic As(III) and As(V) were obtained from inorganic ventures (Christiansburg, VA), monomethyl sodium arsonate, MMA, dimethylsodium arsenate, DMA, (Chem Service, West Chester, PA) arsenobetaine, AsB, (Sigma Aldrich, St Louis, MO), while arsenocholine, AsC, was a kindly donated by Kevin Kubachka at US FDA. Standard urine NIST 2669 (Gaithersburg, MD) was also utilized. Urine samples were diluted 10X in deionized water prior to analysis.
Table 1.
ICP-QQQ operating conditions
| Plasma flow rate (L/min) | 15 | Cell focus (V) | 0 |
| RF Power | 1550 | Cell entrance (V) | −50 |
| sampler depth (mm) | 8 | Cell exit (V) | −60 |
| carrier gas flow (L/min) | 0.95 | Deflect (V) | 3 |
| peri pump (rps) | 0.4 | Plate bias (V) | −60 |
| S/C temp (C) | 2 | Q1 bias (V) | 0 |
| dilution gas (L/min) | 0.1 | Q1prefilter bias (V) | −10 |
| Extract 1 (V) | 0 | Q1 postfilter bias (V) | −10 |
| Extract 2 (V) | −180 | Oxygen flow rate (%) | 30 |
| Omega Bias (V) | −100 | Energy Discrimination (V) | −7 |
| Omega Lens (V) | 5 | OctP bias (V) | −5 |
| Q1 Entrance (V) | −6 | Q1 m/z | 75 |
| Q1 Exit (V) | 0 | Q2 m/z | 91 |
| Wait time offset (msec) | 2 | Dwell time (msec) | 200 |
Results
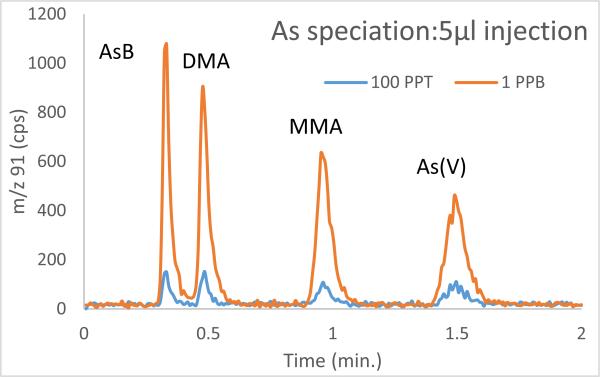
Anion exchange chromatography of arsenobetaine, As(III), DMA, MMA, and As(V) involves dilute eluents and/or lower flow rates to separate the first three species followed by increase in eluent strength and/or flow rate to elute the final two compounds at reasonable retention times. The relative interchangeability of As(III) and As(V) during extraction from a solid or during storage of the resultant extractant, coupled with the fact that many methods simply require a measure of total inorganic As, has led to extraction methods which utilize H2O2 to oxidize all As(III) to As(V) prior to chromatographic separation8. Because As(III) pKa1 is 9, also the pH of the eluent, the As(III) peak tails and closely elutes with DMA so oxidizing As(III) to As(V) makes for quicker separation regardless of column length. In this instance, I use isocratic elution with 50mM carbonate (100% eluent B) and a flow rate of 1.5 ml/min. To assess sensitivity at low sample injection volume a 5μl injection was used. The chromatogram is shown in figure 1.
Figure 1.
Separation of four As species using isocratic conditions, 50 mM carbonate eluent and 1.5ml/min flow rate
Even with a small injection volume of 5μl, low detection limits are achieved, ranging from 15 ng/l for AsB and DMA to 20 ul/l for MMA and 30 ul/l As(V). Omitting As(III) from the chromatogram allows for isocratic separation, as only the separation of this species from DMA requires a more dilute eluent. An isocratic separation means no additional equilibration time to initial conditions and thus a total runtime of < 2 minutes for these common As species that one might measure in, for example, food matrices. This fast separation compares favorably with other fast separations developed for the PRP X100 column. Raber et al.8 used an isocratic 5 mM malonic acid mobile phase and report a retention time for As(V) of 5 min.
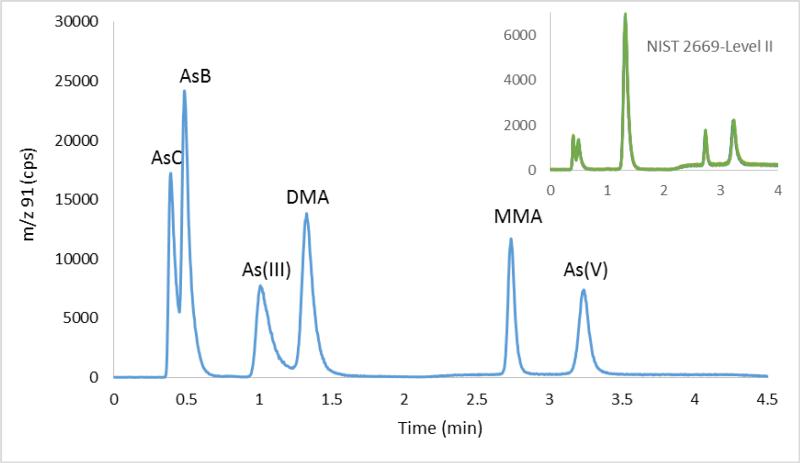
It is possible to include As(III) in the separation by starting with a dilute (10 mM carbonate) eluent as initial conditions. The eluent strength and flow rate are increase when the closely resolved species, As(III) and DMA have been separated. The example chromatogram for 5 μg/l of each As species is shown in Figure 2 and gradient conditions given in Table 2. In this case, AsC is also included in the separation, being partially resolved from AsB. The total runtime is 4.5 minutes, allowing for column reequilibration after elution of the As(V).
Figure 2.
Gradient separation of six As species. Inset: 6 within-run replicate analyses of NIST 2669 level II, diluted 10X in DI, run after batches of 8 samples analyses. The six chromatograms are overlaid.
Table 2.
Gradient Conditions for separation of six As species
| time (min) | A (%) | B (%) | flow (ml/min) |
|---|---|---|---|
| 0 | 80 | 20 | 1 |
| 1.49 | 80 | 20 | 1 |
| 1.5 | 100 | 2 | |
| 3.5 | 100 | 2 | |
| 3.51 | 80 | 20 | 2 |
| 3.99 | 80 | 20 | 2 |
| 4 | 80 | 20 | 1 |
Detection limits for a 20 μl injection volume based on 3σ baseline noise are ca 10 ng/l for AsC, AsB, DMA, MMA and 15 ng/l for As(III) and As(V). This method can be compared with that of Verdon et al9 which has a total runtime of 16 minutes, albeit the Verdon method has basesline resolution of AsC and AsB and partial resolution of TMAO, which is not separated from AsB in the method presented here. Similarly, a method by Cubadda et al.10, using a similar carbonate gradient elution on an IC-SEP 120 column, reported elution of As(V) at 12 minutes and a total chromatographic runtime of 19 minutes. The method reported here was used to quantify urinary As species and the repeated analysis of NIST 2669 Level II standard. (six replicates over 4 hrs analysis) showed excellent repeatability and quantitative recovery, although in all aliquots of this SRM sample, which was stored frozen, As(III) had transformed to As(V), also AsB as reported by this method is actually the sum of AsB and TMAO. AsC, AsB and TMAO are not fully resolved by this method, nevertheless the sum of the ASC and AsB peaks by this method, 7.14 μg/l, is in good agreement with the certified total for AsC, AsB, and TMAO of 7.11 μg/l. Many studies simply seek to separate these species from the more toxicologically important later eluting species and this method achieves that while also being quantitative with reference to the total urinary As concentration.
In summary, the smaller particle size, short column length anion exchange column used in conjunction with injection volumes up to 20 μl and interfaced with the high sensitivity ICP-QQQ can provide equivalent or better detection limits than the conventional column at 2.5- 5X faster chromatographic runtimes which is a significant benefit for sample throughput and method development.
Table 3.
Accuracy and reproducibility of repeated measurements of NIST 2669 Level II urine standard.
| AsC | AsB + TMAO | DMA | MMA | AsIII + AsV | |
|---|---|---|---|---|---|
| Measured (μg/l) | 4.42 | 2.72 | 25.86 | 6.32 | 10.44 |
| RSD (n = 6) | 2.86% | 3.51% | 1.26% | 4.54% | 5.34% |
| certified | 3.74 | 3.28 | 25.3 | 7.18 | 11.2 |
| % recovery | 118.3% | 80.6% | 102.2% | 88.1% | 93.2% |
Acknowledgements
I acknowledge the support of NIEHS P42 ES007373, NIEHS P01 ES022832, EPA RD83544201.
References
- 1.Francesconi KA, Kuehnelt D. Determination of arsenic species: a critical review of methods and applications, 2000-2003. The Analyst. 2004;129(5):373–95. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- 2.de la Calle MB, Baer I, Robouch P, Cordeiro F, Emteborg H, Baxter MJ, Brereton N, Raber G, Velez D, Devesa V, Rubio R, Llorente-Mirandes T, Raab A, Feldmann J, Sloth JJ, Rasmussen RR, D'Amato M, Cubadda F. Is it possible to agree on a value for inorganic arsenic in food? The outcome of IMEP-112. Analytical and Bioanalytical Chemistry. 2012;404(8):2475–2488. doi: 10.1007/s00216-012-6398-4. [DOI] [PubMed] [Google Scholar]
- 3.de la Calle MB, Emteborg H, Linsinger TPJ, Montoro R, Sloth JJ, Rubio R, Baxter MJ, Feldmann J, Vermaercke P, Raber G. Does the determination of inorganic arsenic in rice depend on the method? Trac-Trends in Analytical Chemistry. 2011;30(4):641–651. [Google Scholar]
- 4.Cubadda F, Aureli F, D'Amato M, Raggi A, Turco AC, Mantovani A. Speciated urinary arsenic as a biomarker of dietary exposure to inorganic arsenic in residents living in high-arsenic areas in Latium, Italy. Pure and Applied Chemistry. 2012;84(2):203–214. [Google Scholar]
- 5.Jackson BP, Liba A, Nelson J. Advantages of reaction cell ICP-MS on doubly charged interferences for arsenic and selenium analysis in foods. Journal of Analytical Atomic Spectrometry. 2015 doi: 10.1039/C4JA00310A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen EH, Sturup S. Carbon-enhanced inductively-coupled plasma-mass spectrometric detection of arsenic and selenium and its application to arsenic speciation. Journal of Analytical Atomic Spectrometry. 1994;9(10):1099–1105. [Google Scholar]
- 7.Grindlay G, Mora J, de Loos-Vollebregt M, Vanhaecke F. A systematic study on the influence of carbon on the behavior of hard-to-ionize elements in inductively coupled plasma-mass spectrometry. Spectrochimica Acta Part B-Atomic Spectroscopy. 2013;86:42–49. [Google Scholar]
- 8.Raber G, Stock N, Hanel P, Murko M, Navratilova J, Francesconi KA. An improved HPLC-ICPMS method for determining inorganic arsenic in food: Application to rice, wheat and tuna fish. Food Chemistry. 2012;134(1):524–532. [Google Scholar]
- 9.Verdon CP, Caldwell KL, Fresquez MR, Jones RL. Determination of seven arsenic compounds in urine by HPLC-ICP-DRC-MS: a CDC population biomonitoring method. Analytical and Bioanalytical Chemistry. 2009;393(3):939–947. doi: 10.1007/s00216-008-2537-3. [DOI] [PubMed] [Google Scholar]
- 10.D'Amato M, Aureli F, Ciardullo S, Raggi A, Cubadda F. Arsenic speciation in wheat and wheat products using ultrasound- and microwave-assisted extraction and anion exchange chromatography-inductively coupled plasma mass spectrometry. Journal of Analytical Atomic Spectrometry. 2011;26(1):207–213. [Google Scholar]