Abstract
Aim
In patients with cardiopulmonary arrest, brain cooling may improve neurological outcome, especially if applied prior to or during early reperfusion. Thus it is important to develop feasible cooling methods for pre-hospital use. This study examines cerebral and compartmental thermokinetic properties of nasopharyngeal cooling during various blood flow states.
Methods
Ten swine (40 ± 4 kg) were anesthetized, intubated and monitored. Temperature was deter mined in the frontal lobe of the brain, in the aorta, and in the rectum. After the preparatory phase the cooling device (RhinoChill™ system), which produces evaporative cooling in the nasopharyngeal area, was activated for 60 min. The thermokinetic response was evaluated during stable anaesthesia (NF, n = 3); during untreated cardiopulmonary arrest (ZF, n = 3); during CPR (LF, n = 4).
Results
Effective brain cooling was achieved in all groups with a median cerebral temperature decrease of −4.7 °C for NF, −4.3 °C for ZF and −3.4 °C for LF after 60 min. The initial brain cooling rate however was fastest in NF, followed by LF, and was slowest in ZF; the median brain temperature decrease from baseline after 15 min of cooling was −2.48 °C for NF, −0.12 °C for ZF, and −0.93 °C for LF, respectively. A median aortic temperature change of −2.76 °C for NF, −0.97 for LF and +1.1 °C for ZF after 60 min indicated preferential brain cooling in all groups.
Conclusion
While nasopharyngeal cooling in swine is effective at producing preferential cerebral hypothermia in various blood flow states, initial brain cooling is most efficient with normal circulation.
Keywords: Cardiac arrest, Cardiopulmonary resuscitation, Therapeutic hypothermia, Temperature
1. Introduction
Sudden cardiac death remains a major cause of premature death in the US, with ~270,000 EMS-treated cases of out-of-hospital cardiac arrest annually but with only ~6% survival to hospital discharge.1-3 Of the many post-resuscitation care strategies evaluated, mild therapeutic hypothermia has demonstrated significant improvements in neurological outcome after cardiac arrest in both experimental settings as well as clinical studies.4-9 Of concern given our current cooling methods, the animal data suggest that the protection provided by therapeutic hypothermia decays with the time elapsed while achieving target temperature.10-15 Indeed the best reperfusion after ischaemia may be “cooled reperfusion”.16,17 Therefore, preferential brain cooling, especially if it could be delivered during the intra-arrest phase of treatment, may show a significant improvement in long term neurological outcome, given that the majority of out-of-hospital cardiac arrest victims admitted to ICU will suffer or die from consequences of neurological injury.18 Unfortunately, an effective pre-hospital cooling strategy is not easy to implement.19 Thus, it is important to develop practical feasible, non-invasive cooling methods for out-of-hospital use.
Nasopharyngeal cooling is a novel approach to therapeutic hypothermia that may overcome some of the limitations of current cooling methods. The cooling is achieved by actively spraying a mixture of perfluorocarbon liquid along with high flow oxygen into the nasal passages via a nasal cannula.20 The rapid evaporation of the liquid produces significant cooling of the nasal passages just under the base of the brain. The device can be made portable for the EMS setting, is simple to implement, non-invasive and is undergoing clinical evaluation.21 However, its ability to preferentially cool the brain over a wide range of circulatory states such as untreated cardiac arrest or during normal circulation is unknown. We designed a large animal study to determine the cerebral and compartmental thermokinetic properties of nasopharyngeal cooling during three different circulatory flow states that ranged from normal circulation over CPR to untreated cardiac arrest.
2. Methods
The study was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. All animals received treatment and care in compliance with the 1996 Guide for the Care and Use of Laboratory Animals by the National Research Council in accord with the USDA Animal Welfare Act, PHS Policy, and the American Association for Accreditation of Laboratory Animal Care. All studies were conducted by qualified personnel.
2.1. Animal preparation
Ten female domestic swine (40 4.4 kg) were sedated with intramuscular ketamine (20 mg kg−1)±and xylazine (2 mg kg−1), followed by induction of general anaesthesia by mask administration of 4% isoflurane in 100% oxygen. After endotracheal intubation with a cuffed tracheal tube (ID = 7 mm), a surgical plane of anaesthesia was maintained on isoflurane and a mixture of air and oxygen, adjusted to achieve an inspiratory oxygen fraction of 0.4. The animals were mechanically ventilated with a pressure controlled ventilator (Modulus SE 7900; Datex-Ohmeda Inc., USA) to maintain an end-tidal carbon dioxide partial pressure (EtCO2) of 45 mm Hg at baseline. Circulating warm water blankets (T/Pump; Gaymar Industries, NY) were used to maintain a rectal temperature between 37 and 38 °C during the preparatory phase.
After aseptic preparation of the surgical sites, a cranial bolt (Licox®IMC IM3 Bolt System; Integra Neuroscience, Plainsboro, NJ) was placed through a single burr hole in the left cranium; an 18-gauge thermocouple probe (IT-18, Physitemp Instruments, Clifton, NJ) was advanced via the bolt for continuous measurement of brain temperature. The length of the probe was chosen such that its tip extended 8-10 mm beyond the inner end of the cranial bolt into the left forebrain. Subsequently, the animal was placed in dorsal recumbency and the right and left femoral artery, the left femoral vein and the left external jugular vein were cannulated. Solid state pressure transducer catheters (Micro-Tip® Transducer, Millar Instruments, Houston, TX) were introduced through left femoral artery and vein for continuous measurement of right atrial and ascending aortic pressure. An 18-gauge thermocouple probe was advanced through the right femoral cannula into the abdominal aorta. Another temperature probe (RET-1, Physitemp Instruments, Clifton, NJ) was placed into the rectum to a depth of 10 cm.
EtCO2, tidal volume, respiratory rate and pulse oximetry (SpO2) were continuously measured with a NICO® cardiopulmonary management system (Respironics Inc., Murrysville, PA). A three-lead ECG was monitored continuously. All data was recorded at a sampling rate of 1000 points/s by a commercially available data acquisition system (PowerLab® 16/30 and Chart™ 5.0, ADInstruments, Bella Vista, Australia) and stored on the hard drive of a computer for later analysis.
The nasopharyngeal cooling device (RhinoChill™ System, Benechill, San Diego, CA) was positioned prior to collecting baseline data. The device (Fig. 1) consists of two blind-ended plastic tubes that are advanced through nostrils and the ventral passages of the nasal cavities until their tips reside in the rhinopharynx. Upon activation of the device, a mixture of oxygen (40 L/min) and a perfluorochemical (16 mL/min) is conducted through the tubes and escapes through small dorsolateral openings in the nasopharyngeal area as mist. Immediate evaporation of the volatile perfluorochemical leads to cooling of the area.
Fig. 1.
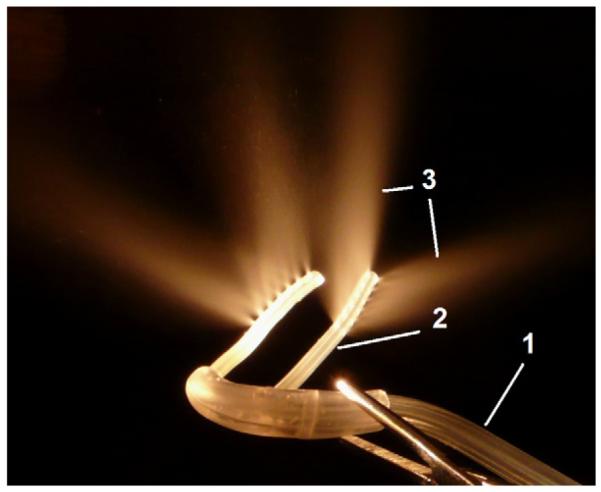
Photograph of the tubing used for nasopharyngeal cooling. The perfluorochemical (PFC)-oxygen mixture is delivered from the oxygen tank and the PFC reservoir in a single tube (1) that then bifurcates into a left and right nasopharyngeal cannula (2). The perfluorochemical-oxygen spray (3) exits in dorsal and lateral direction from the distal end of the cannulas.
2.2. Experimental protocol
The influence of the nasopharyngeal cooling device on fore-brain, oesophageal, aortic and rectal temperatures was examined under three different global blood flow conditions: normal flow (NF), no flow (ZF), and low flow (LF). All groups were anaesthetized as described above. The first group (NF, n = 3) was evaluated during stable anaesthesia; this group represented a normal circulatory blood flow state. After collecting baseline data, the nasopharyngeal cooling device was activated for 60 min while temperatures were monitored and recorded. In the second group of animals (ZF, n = 3), a cardiac pacing lead (Bipolar pacing catheter, Edwards Lifescience LLC, Irvine, CA) was advanced through the jugular introducer sheath into the right ventricle. After collection of baseline values, ventricular fibrillation (VF) was electrically induced. Cooling was started upon loss of pulse and was continued for 60 min; cardiopulmonary arrest (CPA) remained untreated for the entire 60 min period. This group represented a state with complete absence of blood flow. In group 3 (LF, n = 4), CPA was identically induced, however immediately after induction of VF and concurrently with the activation of cooling, a mechanical chest compression device (LUCAS™, Jolife, Lund, Sweden) was used to deliver 60 min of precordial chest compressions with ventilator settings of 10 breaths per minute delivered with an inspiratory time of 1 s and a peak inspiratory pressure of 20 mm Hg. This group receiving CPR during VF represented a low blood flow state.
2.3. Statistical analyses
Temperature changes over time and temperature deviations from baseline were analyzed using descriptive statistics and data is presented as median or mean standard deviation, as appropriate. Student’s t-test was used ± to identify differences between groups (α < 0.05). Repeated measures analysis of variance (ANOVA) was used to identify significant temperature decreases from baseline (α < 0.01). Statistical analysis was performed with JMP (SAS Institute Inc., Cary, NC).
3. Results
3.1. Cooling in the brain with nasopharyngeal cooling
The decrease in brain temperature was initially more pronounced in NF compared to LF and ZF, with a statistically significant decrease from baseline being evident after 3, 7 and 12 min, respectively. The temperature changes for animals within NF and ZF were rather homogeneous (Fig. 2). The course of temperature decrease in LF was intermediate to the other groups, but the temperature decline was more varied among individual animals in this group. While the initial cooling rate varied by blood flow state the total median brain cooling effect after 60 min was close to 4 °C in all groups and was not statistically different among NF, ZF or LF (Table 1).
Fig. 2.
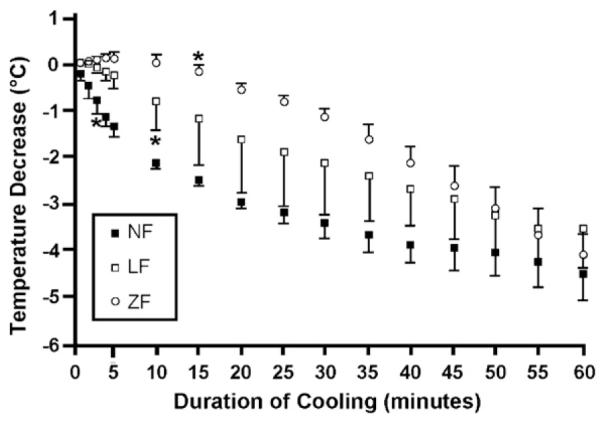
Change in brain temperatures from baseline (mean ± SD) during untreated cardiopulmonary arrest (ZF; n = 3), CPR (LF; n = 4) and anaesthesia (NF; n = 3) over the course of 60 min of nasopharyngeal cooling. *indicates first significant decrease from baseline (α<0.01).
Table 1.
Brain cooling under different blood flow conditions.
| Temperature decrease (°C) from baseline |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5min | 10 min | 15 min | 20 min | 25 min | 30 min | 45 min | 60 min | ||
| ZF (n=3) | Median (range) |
0.21 (0.22) | 0.14 (0.28) | −0.12 (0.28) | −0.43 (0.24) | −0.86 (0.26) | −1.28 (0.45) | −2.70 (0.82) | −4.28 (1.52) |
| Mean (STDV) |
0.15 (0.12)** | 0.06 (0.15)**,*** | −0.15 (0.14)** | −0.45 (0.12)** | −0.80 (0.14)** | −1.18 (0.24)** | −2.60 (0.42)** | −4.29 (0.76) | |
| LF (n= 4) | Median (range) |
−0.13 (0.58) | −0.65 (1.25) | −0.93 (2.16) | −1.20 (2.52) | −1.56 (3.17) | −1.94 (2.68) | −2.62 (1.87) | −3.40 (1.98) |
| Mean (STDV) |
−0.24 (0.27)* | −0.77 (0.57)*,*** | −1.16 (1.00)* | −1.51 (1.13)* | −1.86 (1.35) | −2.12 (1.14) | −2.90 (0.85) | −3.58 (0.88) | |
| NF (n=3) | Median (range) |
−1.25 (0.43) | −2.09 (0.15) | −2.48 (0.10) | −2.84 (0.07) | −3.13 (0.40) | −3.41 (0.64) | −3.88 (0.94) | −4.66 (1.04) |
| Mean (STDV) |
−1.33 (0.23)*,** | −2.10 (0.08)* ,** | −2.51 (0.06)*,** | −2.86 (0.04)*,** | −3.20 (0.21)** | −3.46 (0.32)** | −3.94 (0.47)** | −4.44 (0.55) | |
Change in cerebral temperature from baseline during untreated cardiac arrest (ZF, zero blood flow state), during CPR (LF, low blood flow state) and during anaesthesia only (NF, normal blood flow state) during 60 min of nasopharyngeal cooling. Values are presented as medians (range) and mean (STDV).
p < 0.05, between NF and LF at the same time point.
p < 0.05 between NF and ZF at the same time point.
p < 0.05 between LF and ZF at the same time point.
3.2. Cooling in body compartments with nasopharyngeal cooling
By contrast to brain temperature, rectal and aortic temperatures changed little in ZF or LF and there was even a trend towards increase in aortic temperature in the untreated CPA group (Fig. 3). The systemic cooling effect was most pronounced in the animals with normal circulation with aortic and rectal cooling rates varying between −0.1 and −0.03 °C min−1, −0.05 and 0.02 °C min−1, respectively (Table 2).
Fig. 3.
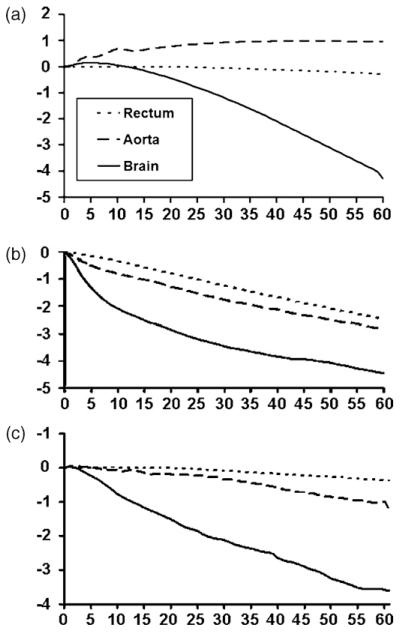
Changes in mean cerebral, aortic and rectal temperatures during untreated cardiac arrest (a), anaesthesia only (b) and CPR (c) over the course of 60 min of nasopharyngeal cooling.
Table 2.
Compartmental cooling rates under different blood flow conditions.
| Cooling rates (°Cmin−1) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 5min | 10 min | 15 min | 20 min | 25 min | 30 min | 45 min | 60 min | |
| Brain | ||||||||
| ZF (n = 3) | 0.03 | −0.02 | −0.04 | −0.06 | −0.07 | −0.08 | −0.10 | −0.13 |
| LF (n = 4) | −0.05 | −0.11 | −0.08 | −0.07 | −0.07 | −0.05 | −0.05 | −0.01 |
| NF (n = 3) | −0.27 | −0.15 | −0.08 | −0.07 | −0.07 | −0.05 | −0.02 | −0.03 |
| Aorta | ||||||||
| ZF (n = 3) | 0.08 | 0.06 | −0.01 | 0.02 | 0.02 | 0.01 | 0.00 | 0.00 |
| LF (n = 4) | 0.05 | 0.03 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 |
| NF (n = 3) | −0.10 | −0.07 | −0.04 | −0.05 | −0.05 | −0.04 | −0.04 | −0.03 |
| Rectum | ||||||||
| ZF (n = 3) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.01 | −0.01 | −0.01 |
| LF (n = 4) | 0.00 | 0.00 | 0.00 | 0.00 | −0.01 | −0.01 | −0.01 | 0.00 |
| NF (n = 3) | −0.02 | −0.04 | −0.05 | −0.04 | −0.05 | −0.05 | −0.04 | −0.04 |
Cerebral, aortic and rectal mean cooling rates (°C min−1) based on the temperature change of each preceding 5-min time interval during untreated cardiac arrest (ZF: no blood flow), CPR (LF: low blood flow) and anaesthesia only (NF: normal blood flow).
4. Discussion
This study shows that non-invasive nasopharyngeal cooling is effective at preferentially cooling the brain compared to other regions of the body. The findings suggest that significant cerebral cooling occurs eventually independent of the subject’s blood flow condition but that the time course of brain cooling rates markedly varies among the three global blood flow conditions examined in this study. We observed the most rapid onset of brain cooling in the animals with normal flow, the slowest onset during no flow, and low flow cooling rates were intermediate. The anaesthetized swine in NF demonstrated a rapid initial drop in forebrain temperature with a cooling rates as high as 0.27 °C min−1 during the first 5 min, followed by a continuous decline in the cooling rate, decreasing to only 0.02–0.03 °C min−1 towards the end of the study. This two-phase course of cerebral cooling has been described in another study using a different nasopharyngeal cooling device.22 The lowest initial cooling rates were seen in the ZF animals, where the onset of brain cooling was delayed by 10 min compared to the normal circulation group, followed by a steady progressive temperature decline over the remainder of the study and ending in a cooling rate of more than 0.1 °C min−1. In this study, statistically significant forebrain cooling during CPR was achieved after 7 min, which is of particular relevance given the current evidence that suggests importance of intra-arrest hypothermia for neurologically intact survival.13,14 The marked difference in the initial cooling rate between ZF and NF highlights the importance of blood flow on immediate brain cooling when using nasopharyngeal hypothermia devices. It appears likely that, at least in swine, transmission of the cold via direct conduction plus circulation of blood from the nasopharyngeal tissue to the brain is essential for earliest effective cerebral cooling. Consistent with this finding is the observation that LF had initial cooling rates that were intermediate between the other two groups. However, the effect of blood flow on brain temperature diminishes as nasopharyngeal cooling progresses, and our data shows a similar decrease in brain temperature after 60 min of cooling in all groups. Importantly, control animals for each treatment group in which no cooling is applied were not examined. Since the absolute temperature decrease may well be a composite effect of both passive and nasopharyngeal cooling, the absolute effect of the nasopharyngeal device alone cannot be determined and has to be interpreted with caution. This limitation however does not invalidate comparative observations between groups.
Heat flux is dependent on not only the temperature gradient from one point to another, but also on the thermal conductivity and the thickness of the tissue.23 In this study, brain temperature probe insertion methodology was chosen such that the thermocouple tip is located 8-10 mm into the forebrain, and therefore quite distant from the cooling source. However, the spatial temperature distribution in the brain may be quiet heterogeneous and would need to be described in future studies. The coldest regions of the brain in the first few minutes of nasopharyngeal cooling are likely to be those nearest the source of cooling, namely the inferior frontal lobe, the preoptic region and hypothalamus and posteriorly, the brain stem. In the absence of circulation, cold will accumulate in these regions rather than being dissipated by blood borne convection.
We note that the brain is preferentially cooled compared with the other compartments of the body. The temperature gradient was greatest in the ZF where there was a nearly 4 °C difference between brain and aorta temperatures after 60 min, while the smallest gradient was 2 °C between brain and aorta temperatures during normal blood flow. In contrast it appears that the preferential cooling to the brain is limited under normal blood flow conditions, as suggested by the waning brain cooling rate in NF. Prolonged nasopharyngeal cooling in the presence of intact global circulation, leads to a thermodynamic steady state of the brain, in which cold-inflow and cold-removal are in balance, the net effect being a relatively constant level systemic-to-brain temperature gradient. This would explain the nearly parallel decrease of aortic and brain temperature in NF during the last 30 min of the experiment with a relatively constant mean aorta-to-brain temperature difference of 1.66 0.05 °C, which is similar to another study using nasopharyngeal cooling ± in swine.22 In the absence of blood flow as a thermodynamic bridge between brain and body, the cerebral and systemic (e.g. aortic and rectal) temperatures are entirely dissociated.
Collectively the thermokinetic data from this study demonstrates that nasopharyngeal cooling can achieve relatively rapid brain cooling during periods of no flow, low flow and normal blood flow states. Whether these observations are equally true in humans remains to be evaluated and we caution extrapolation of this data, because of dissimilar nasopharyngeal and cerebral vascular anatomy, and large differences in brain weight between swine and humans.24 However, the notion of preferential cooling of brain with nasopharyngeal cooling appears feasible with the right methods and conditions, and therefore this concept may be of value in human therapeutic hypothermia.
If the nasopharyngeal cooling methodology examined in this study with an overall mean brain cooling rate of 3.9 ± 0.7 °C h−1 is capable of generating similar cooling rates in humans, it will compare favorably with other methods of cooling. In most clinical trials using surface cooling methodologies, including the landmark randomized controlled trials substantiating the efficacy of mild therapeutic hypothermia after cardiac arrest, core cooling rates achieved are in the range of 0.3-1.5 °C h−1, while more recently developed surface cooling techniques hold promise for cooling rates around 3 °C h−1.5,6,25-27 Non-invasive preferential brain cooling by means of a cooling helmet achieved decrease in cerebral temperature by 0.9 °C h−1.28 Endovascular cooling was shown to lower core temperature at a rate between 0.8 and 1.2 °C h−1.29,30 Rapid intravenous infusion of ice cold crystalloid solution, e.g. 30-40 mL kg−1 of isotonic saline, leads to a decrease in core temperature by 1.6-2.0 °C, but the maximum achievable cooling is ultimately limited by the large volume requirement.31-33 Com bination therapies such as cooling helmets in conjunction with an effective carotid artery cooling mechanism could hold great promise in rapid achievement and maintenance of preferential cerebral hypothermia, but have not been well described in the literature.
Our studies with nasopharyngeal cooling revealed additional interesting thermodynamic processes that may warrant further exploration. We noted during cooling in ZF a paradoxical slight warming of the brain during the first 4-8 min of cardiac arrest despite the beginning of nasopharyngeal cooling. While the warming is slight (only about 0.25 °C), others have observed a similar extent of brain “warming” with complete lack of blood flow, and if this is validated it may have important clinical consequences.35 It seems logical from this data that the brain continues to generate heat during the first minutes of cardiac arrest and that in the absence of blood flow to remove the generated heat, the brain paradoxically heats up. In reverse, the cessation of further increase in brain temperature may indicate halt of cerebral metabolic activity, as it was shown that high energy phosphates in the brain are almost entirely depleted within the first 3-5 min of global brain ischaemia.34 Since this initial brain warming was an unexpected finding in this study and the primary aim of this research was to compare brain cooling among different flow rates, no control groups without cooling were included. Thus, it remains open whether the absence of the cerebral hyperthermia in LF is an effect of cooling or CPR or a combination thereof. Nevertheless, a recently published study using a swine model of cardiac arrest suggests that CPR initiated after 15 min of untreated cardiac arrest and in the absence of nasopharyngeal cooling does not lead to a decrease of brain temperature.35
We noted a high degree of heterogeneity in brain cooling rates among animals undergoing CPR. In light of the above discussion, it appears intriguing to assume that this variation is merely a reflection of the inter-individual variability of CPR efficacy, the consequence of which being large variations in cardiac output and cerebral blood flow, respectively. Inconsistent quality of CPR is well described in clinical studies, in which chest compression and ventilation are delivered manually, and much of it is due to inconsistent CPR technique applied by resuscitation personnel.36-38 CPR may also have generated variable levels of blood flow in our study, although the LUCAS® device has been previously described to surpass efficacy of standardized manual chest compressions in swine.39,40 Since neither cardiac output nor cerebral blood flow were determined herein, confirmation of the cause of the variable brain cooling rate in LF remains impossible. Inconsistent placement of the cerebral temperature probe or dislocation during CPR could be responsible for the temperature variation seen and probe positioning was not confirmed by imaging methodologies. However, the consistency of the brain cooling patterns in the other two groups suggests that the placement procedure was valid. Furthermore, position of the temperature probe within the cranial bolt was fixed and was verified at the end of each experiment. In short, we can only speculate on why the group receiving CPR had the most variability, but future studies may want to carefully examine these issues of inter-animal variability in CPR generated blood flow.
5. Conclusion
Nasopharyngeal cooling in swine achieves preferential cerebral hypothermia that would be clinically significant during blood flow states that vary from normal flow to no flow at all. The onset of brain cooling is fastest during normal blood flow, but most preferential to the brain in the absence of blood flow. CPR, a condition of low blood flow, takes an intermediate position in regards to both rate and preferentiability of brain cooling. Nasopharyngeal cooling may overcome some of the limitations of other current cooling methods including the need for preferential brain cooling and faster brain cooling when treatment time is critical.
Acknowledgments
Funding for this project was provided in part by Benechill, Inc., San Diego CA. Benechill had advisory function in designing the study but no influence on the collection, analysis and interpretation of data; on the writing of the manuscript; or on the decision to submit the manuscript for publication.
Footnotes
“A Spanish translated version of the abstract of this article appears as Appendix in the final online version at doi:10.1016/j.resuscitation.2010.04.005”.
Conflicts of interest statement
Dr. Barbut is founder and CEO of Benechill Incorporated, the company that develops the nasopharyngeal cooling device examined in this study. She will benefit from the success of the device. Lance B. Becker receives funding from Benechill to the University of Pennsylvania as the PI for other ongoing research projects. He has received honoraria and consulting fees from Philips Medical Systems, Gaymar Industries, Zoll Medical, Medtronics, and the NIH Data Safety Monitoring Board and Protocol Review Committee; is an inventor on patents assigned to the University of Pennsylvania and the University of Chicago related to hypothermia induction and reperfusion therapies. The remaining authors do not declare any relevant conflicts of interest.
References
- 1.Nichol G, Rumsfeld J, Eigel B, et al. Essential features of designating outof-hospital cardiac arrest as a reportable event: a scientific statement from the American Heart Association Emergency Cardiovascular Care Committee; Council on Cardiopulmonary, Perioperative, and Critical Care; Council on Cardiovascular Nursing; Council on Clinical Cardiology; and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2008;117:2299–308. doi: 10.1161/CIRCULATIONAHA.107.189472. [DOI] [PubMed] [Google Scholar]
- 2.Geocadin RG, Koenig MA, Stevens RD, et al. Intensive care for brain injury after cardiac arrest: therapeutic hypothermia and related neuroprotective strategies. Crit Care Clin. 2006;22:619, 36. doi: 10.1016/j.ccc.2006.11.008. abstract viii. [DOI] [PubMed] [Google Scholar]
- 3.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 4.Leonov Y, Sterz F, Safar P, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10:57–70. doi: 10.1038/jcbfm.1990.8. [DOI] [PubMed] [Google Scholar]
- 5.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 6.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 7.Holzer M, Bernard SA, Hachimi-Idrissi S, et al. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005;33:414–8. doi: 10.1097/01.ccm.0000153410.87750.53. [DOI] [PubMed] [Google Scholar]
- 8.Belliard G, Catez E, Charron C, et al. Efficacy of therapeutic hypothermia after out-of-hospital cardiac arrest due to ventricular fibrillation. Resuscitation. 2007;75:252–9. doi: 10.1016/j.resuscitation.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Froehler MT, Geocadin RG. Hypothermia for neuroprotection after cardiac arrest: mechanisms, clinical trials and patient care. J Neurol Sci. 2007;261:118–26. doi: 10.1016/j.jns.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 10.Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation. 2008;77:242–9. doi: 10.1016/j.resuscitation.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abella BS, Zhao D, Alvarado J, et al. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–91. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 12.Kuboyama K, Safar P, Radovsky A, et al. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–58. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Nozari A, Safar P, Stezoski SW, et al. Mild hypothermia during prolonged cardiopulmonary cerebral resuscitation increases conscious survival in dogs. Crit Care Med. 2004;32:2110–6. doi: 10.1097/01.ccm.0000142700.19377.ae. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich WD, Busto R, Alonso O, et al. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–9. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- 15.Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76:431–42. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao ZH, Chang WT, Chan KC, et al. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol Heart Circ Physiol. 2007;292:H1995–2003. doi: 10.1152/ajpheart.01312.2005. [DOI] [PubMed] [Google Scholar]
- 17.Hale SL, Dae MW, Kloner RA. Hypothermia during reperfusion limits ‘noreflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res. 2003;59:715–22. doi: 10.1016/s0008-6363(03)00456-5. [DOI] [PubMed] [Google Scholar]
- 18.Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–8. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 19.Suffoletto BP, Salcido DD, Menegazzi JJ, et al. Use of prehospital-induced hypothermia after out-of-hospital cardiac arrest: a survey of the National Association of Emergency Medical Services Physicians. Prehosp Emerg Care. 2008;12:52–6. doi: 10.1080/10903120701707880. [DOI] [PubMed] [Google Scholar]
- 20.Wolfson MR, Malone DJ, Wu J, et al. Intranasal perfluorochemical spray for preferential brain cooling in sheep. Neurocrit Care. 2008;8:437–47. doi: 10.1007/s12028-008-9064-0. [DOI] [PubMed] [Google Scholar]
- 21.Busch H, Janata A, Eichwede F, et al. Abstract P63: safety and feasibility of a new innovative cooling approach for immediate induction of therapeutic hypothermia in patients after successful resuscitation. Trans-nasal cooling after cardiac arrest. Circulation. 2008;118:S 1459. [Google Scholar]
- 22.Covaciu L, Allers M, Enblad P, et al. Intranasal selective brain cooling in pigs. Resuscitation. 2008;76:83–8. doi: 10.1016/j.resuscitation.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Merrill TL. Thermodynamics and heat transfer. In: Mayer SA, Sessler DI, editors. Therapeutic hypothermia. 1st ed Marcel Dekker; New York: 2005. pp. 265–92. [Google Scholar]
- 24.Burbridge B, Matte G, Remedios A. Complex intracranial arterial anatomy in swine is unsuitable for cerebral infarction projects. Can Assoc Radiol J. 2004;55:326–9. [PubMed] [Google Scholar]
- 25.Uray T, Malzer R, on behalf of the Vienna Hypothermia After Cardiac Arrest (HACA) Study Group Out-of-hospital surface cooling to induce mild hypothermia in human cardiac arrest: a feasibility trial. Resuscitation. 2008;77:331–8. doi: 10.1016/j.resuscitation.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Flint AC, Hemphill JC, Bonovich DC. Therapeutic hypothermia after cardiac arrest: performance characteristics and safety of surface cooling with or without endovascular cooling. Neurocrit Care. 2007;7:109–18. doi: 10.1007/s12028-007-0068-y. [DOI] [PubMed] [Google Scholar]
- 27.Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation. 1998;39:61–6. doi: 10.1016/s0300-9572(98)00118-x. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Olivero W, Lanzino G, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg. 2004;100:272–7. doi: 10.3171/jns.2004.100.2.0272. [DOI] [PubMed] [Google Scholar]
- 29.Al-Senani FM, Graffagnino C, Grotta JC, et al. A prospective, multicenter pilot study to evaluate the feasibility and safety of using the CoolGard system and Icy catheter following cardiac arrest. Resuscitation. 2004;62:143–50. doi: 10.1016/j.resuscitation.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Holzer M, Müllner M, Sterz F, et al. Efficacy and safety of endovascular cooling after cardiac arrest: Cohort study and Bayesian approach. Stroke. 2006;37:1792–7. doi: 10.1161/01.STR.0000227265.52763.16. [DOI] [PubMed] [Google Scholar]
- 31.Kim F, Olsufka M, Carlbom D, et al. Pilot study of rapid infusion of 2 L of 4 degrees C normal saline for induction of mild hypothermia in hospitalized, comatose survivors of out-of-hospital cardiac arrest. Circulation. 2005;112:715–9. doi: 10.1161/CIRCULATIONAHA.105.544528. [DOI] [PubMed] [Google Scholar]
- 32.Virkkunen I, Yli-Hankala A, Silfvast T. Induction of therapeutic hypothermia after cardiac arrest in prehospital patients using ice-cold Ringer’s solution: a pilot study. Resuscitation. 2004;62:299–302. doi: 10.1016/j.resuscitation.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Bernard S, Buist M, Monteiro O, et al. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 34.Wagner SR, 4th, Lanier WL. Metabolism of glucose, glycogen, and high-energy phosphates during complete cerebral ischemia. A comparison of normoglycemic, chronically hyperglycemic diabetic, and acutely hyperglycemic nondiabetic rats. Anesthesiology. 1994;81:1516–26. doi: 10.1097/00000542-199412000-00028. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Barbut D, Tsai MS, et al. Intra-arrest selective brain cooling improves success of resuscitation in a porcine model of prolonged cardiac arrest. Resuscitation. 2010 doi: 10.1016/j.resuscitation.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305–10. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 37.Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during inhospital cardiac arrest. Circulation. 2005;111:428–34. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 38.Van Hoeyweghen RJ, Bossaert LL, Mullie A, et al. Belgian Cerebral Resuscitation Study Group Quality and efficiency of bystander CPR. Resuscitation. 1993;26:47–52. doi: 10.1016/0300-9572(93)90162-j. [DOI] [PubMed] [Google Scholar]
- 39.Steen S, Liao Q, Pierre L, et al. Evaluation of LUCAS, a new device for automatic mechanical compression and active decompression resuscitation. Resuscitation. 2002;55:285–99. doi: 10.1016/s0300-9572(02)00271-x. [DOI] [PubMed] [Google Scholar]
- 40.Rubertsson S, Karlsten R. Increased cortical cerebral blood flow with LUCAS; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation. 2005;65:357–63. doi: 10.1016/j.resuscitation.2004.12.006. [DOI] [PubMed] [Google Scholar]


