Abstract
Aims
To examine the effects of reduced nicotine cigarettes on smoking behavior, toxicant exposure, dependence and abstinence.
Design
Randomized, parallel arm, semi-blinded study.
Setting
University of Minnesota Tobacco Use Research Center.
Interventions
Six weeks of: (i) 0.05 mg nicotine yield cigarettes; (ii) 0.3 mg nicotine yield cigarettes; or (iii) 4 mg nicotine lozenge; 6 weeks of follow-up.
Measurements
Compensatory smoking behavior, biomarkers of exposure, tobacco dependence, tobacco withdrawal and abstinence rate.
Findings
Unlike the 0.3 mg cigarettes, 0.05 mg cigarettes were not associated with compensatory smoking behaviors. Furthermore, the 0.05 mg cigarettes and nicotine lozenge were associated with reduced carcinogen exposure, nicotine dependence and product withdrawal scores. The 0.05 mg cigarette was associated with greater relief of withdrawal from usual brand cigarettes than the nicotine lozenge. The 0.05 mg cigarette led to a significantly higher rate of cessation than the 0.3 mg cigarette and a similar rate as nicotine lozenge.
Conclusion
The 0.05 mg nicotine yield cigarettes may be a tobacco product that can facilitate cessation; however, future research is clearly needed to support these preliminary findings.
Keywords: Biomarkers of exposure, compensatory smoking, nicotine dependence, reduced nicotine cigarettes, tobacco cessation, tobacco withdrawal
INTRODUCTION
In recent years, tobacco companies have renewed their efforts to manufacture and market potential reduced exposure tobacco products (called PREPs) to cigarette smokers. These products include cigarettes modified to reduce toxicants but maintain levels of nicotine [1,2]. However, to date these modified cigarettes have not shown great promise for reducing exposure to toxicants significantly [3,4]. Alternatively, cigarettes with significantly reduced nicotine (the major known addictive constituent in cigarettes) may have promise in dramatically reducing cigarette use [5]. Unlike ‘light’ or ‘mild’ cigarettes that reduce nicotine yields through filter ventilation but which lead to similar levels of cotinine and toxicants as regular cigarettes due to compensatory smoking behavior [6–8], reduced nicotine in cigarette tobacco makes compensatory smoking more difficult. Limited data from previous studies of such products suggest that compensatory smoking does not occur, toxicant exposure does not increase and abstinence may be facilitated [9,10]. Theoretically, reducing levels of nicotine to the point of non-reinforcement would lead to extinction or cessation of smoking as well as unlearning cues associated with reinforcement.
No clinical trial has examined the effects of smoking reduced nicotine cigarettes on smoking behavior, on resulting toxicant exposure, on withdrawal symptoms and craving, on dependence scores or on abstinence rates compared with medicinal nicotine products.
To address these questions, we conducted a study in which smokers were randomized to 6 weeks of 0.3 mg nicotine yield cigarettes, 0.05 mg nicotine yield cigarettes or to 4 mg medicinal nicotine lozenges. Medicinal nicotine has been recommended as the comparator to which PREPs should be tested for toxicant exposure [11] and also serves as a usual care condition to compare abstinence rates across products. As primary outcomes, we hypothesized that smoking behavior, toxicant exposure, withdrawal and craving upon product discontinuation and product dependence would be relatively less for the 0.05 mg nicotine yield cigarette compared to the 0.3 mg nicotine yield cigarette, because the 0.05 mg cigarette would lead to less reinforcement from smoking than the 0.3 mg cigarette. As a secondary outcome, we hypothesized that pre-treatment with 0.05 mg cigarettes will produce favorable quit rates that are similar to nicotine lozenges and better than 0.3 mg cigarettes.
Our goal was to examine the feasibility of using these cigarettes as a method to reduce smoking behavior significantly and as a potential cessation tool, which would lead subsequently to reduction in harm. The results also provide information on the role that different aspects of tobacco use (nicotine versus sensory aspects of smoking) contributes to tobacco addiction.
METHODS
Subjects
Smokers of ‘light’ cigarettes (0.7–1.0 mg nicotine/cigarette) between the ages of 18 and 70 years who were interested in quitting smoking were recruited via advertisement. To be eligible smokers had to (i) have smoked 10–40 cigarettes daily for the past year (the range was instituted to reduce heterogeneity); (ii) be in good physical health; (iii) be in good psychiatric health; and (iv) have no contraindications for medicinal nicotine use. Subjects using other tobacco or nicotine products were excluded, as were subjects who were pregnant or nursing.
Study design
After a telephone screening to determine preliminary eligibility, an orientation session was held at which the study was explained further, written informed consent was obtained and a more thorough screening for eligibility was performed.
After a 2-week period during which baseline measurements were collected while subjects smoked ad libitum, subjects were assigned to one of three conditions: (i) 0.3 mg nicotine yield cigarettes, (ii) 0.05 mg nicotine yield cigarettes or (iii) nicotine lozenges (4 mg). Quest cigarettes (manufactured by Vector; Vector Tobacco Inc., Durham, NC, USA) were chosen because they are commercially available reduced nicotine cigarettes (nicotine yield as measured in mainstream smoke by the Federal Trade Commission method) with reduced levels of tobacco-specific carcinogens compared to conventional cigarettes [12]. Subjects assigned to the cigarette conditions were blinded as to which cigarette they received (i.e. 0.05 mg versus 0.3 mg). Subjects were instructed to use their assigned treatment for 6 weeks (after which time they were to discontinue product use) and to not use other nicotine or tobacco products during the treatment or any products during the follow-up period. Subjects were seen weekly during the 6-week treatment period and at 1, 2, 4 and 6 weeks after cessation. Subjects who completed the study were paid up to $345.
To allow for compensatory smoking, at each visit subjects assigned to either cigarette condition were provided a supply equivalent to 150% of their baseline smoking rate and were told to smoke ad libitum. Subjects assigned to receive the 4 mg nicotine lozenge were asked to quit smoking and to use at least six to eight pieces per day, the mean number of lozenges used among smokers enrolled in a clinical trial [13]. If side effects suggested that the dose was too high, the 2 mg nicotine lozenge was substituted at that time. Subjects maintained a daily smoking diary in which they recorded any cigarettes smoked (either those assigned to them or their own). If they smoked cigarettes other than those assigned, they were to note when that cigarette was smoked. They were not penalized for smoking that cigarette, but told that although we do not encourage them to smoke cigarettes other than those assigned, it is crucial to the study that they indicate to us whenever they smoked any other cigarettes.
Brief (approximately 10 minutes) standardized counseling was provided at each of the visits during the treatment phase of the study. Subjects assigned to the cigarette conditions were counseled to consider the use of these products as a step towards quitting. They discussed any difficulties they experienced with switching cigarettes and behavioral strategies to resist smoking other (non-Quest) cigarettes. Subjects assigned to the nicotine lozenge condition were provided with treatment tools recommended by the US Clinical Practice Guideline [14]. During the abstinence phase, all subjects received counseling similar to that received by the subjects assigned to the nicotine lozenge condition. Therefore, all three treatment groups received similar amounts of behavioral support.
Outcome measures
Biomarkers of tobacco toxicant exposure measures included: (i) urinary cotinine plus cotinine–glucuronide (total cotinine), a direct measure of nicotine exposure (product nicotine delivery and amount consumed) [15,16]; (ii) alveolar carbon monoxide (CO) measured using the Bedfont Micro Smokerlyzer® (Bedfont Scientific Limited, Kent, UK); (iii) urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides (total NNAL), metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone ((NNK; [17]); (iv) urinary N′-nitrosonornicotine and its glucuronide (total NNN), metabolites of the tobacco-specific carcinogen N′-nitrosonornicotine [18]; (v) urinary 1-hydroxypyrene and its glucuronide and sulfate (total 1-HOP), a metabolite of pyrene which is an accepted biomarker for uptake of carcinogenic polycyclic aromatic hydrocarbons (PAH; [19]); (vi) urinary 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of the toxicant, acrolein [20]; and (vii) S-phenylmercapturic acid (S-PMA), a metabolite of the human leukemogen, benzene [21]. These biomarkers reflect exposure to particulate or smoke constituents in cigarettes. All measures were assessed at baseline. Additionally, carbon monoxide was assessed at each treatment clinic visit, cotinine at weeks 2 and 6 of treatment and at follow-up visits (except at 1 week post-treatment) and biomarkers for other exposure measures at weeks 2 and 6 of treatment. CO, 1-HOP, 3-HPMA, S-PMA are influenced by factors other than tobacco while total cotinine, total NNAL and total NNN are tobacco-specific.
Subjective measures included: (i) a tobacco use questionnaire that asked about current tobacco use status (cigarettes and other tobacco products), number of ≥24-hour quit attempts and duration of abstinence during these quit attempts; (ii) a daily diary detailing the number of cigarettes smoked; (iii) the Minnesota Nicotine Withdrawal Scale, a widely used scale that assesses withdrawal from cigarettes [22–24], nicotine gum [25,26] and smokeless tobacco [25,27]; (iv) the Fagerstrom Test for Nicotine Dependence (FTND, [28]), the most widely used and psychometrically tested scale for nicotine dependence; and (v) perceived health risk, a ladder involving rating risk for addiction of a product on a scale ranging from 1 to 10. All these measures were assessed at baseline. Cigarette or product use was assessed daily, the tobacco use questionnaire and Minnesota Nicotine Withdrawal Scale at each clinic visit, and the FTND and perceived health risk at weeks 2 and 6.
This study was approved by the University of Minnesota Research Subjects Protection Programs Institutional Review Board.
Statistical analysis
Subjects’ baseline characteristics including demographics and smoking history were compared among three treatment groups. Discrete variables were analyzed using Pearson's χ2 test or Fisher's exact test. Continuous variables were analyzed using either one-way analysis of variance (ANOVA) or Kruskall–Wallis test.
For outcome variables measured at each baseline visit, the average was used as the baseline measurement. Repeated-measures ANOVA was used for outcomes that had been measured repeatedly from baseline to the end of the treatment phase. Each repeated-measures ANOVA model contained five terms: treatment effect, visit effect, interaction effect between treatment and visit, random subject effect (between-subject error) and random error (within-subject error). The variance–covariance structure was specified as the first-order autoregression, and variance parameters were estimated using restricted maximum likelihood method with Satterthwaite approximation. The P-values reported for multiple comparisons were unadjusted. Biomarkers including cotinine, NNN, NNAL, 1-HOP, 3-HPMA and S-PMA were analyzed in a natural log scale for repeated-measures ANOVA such that the model assumptions of normality and equal variances can hold, and geometric means in original units were also calculated. The differences in the point prevalence (no smoking in past 7 days) and continuous abstinence (no smoking in past 4 weeks) rates during the follow-up period between treatment groups were evaluated using χ2 tests, as were dropout rates. SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) was used. A P-value <0.05 indicated statistical significance.
RESULTS
Subjects
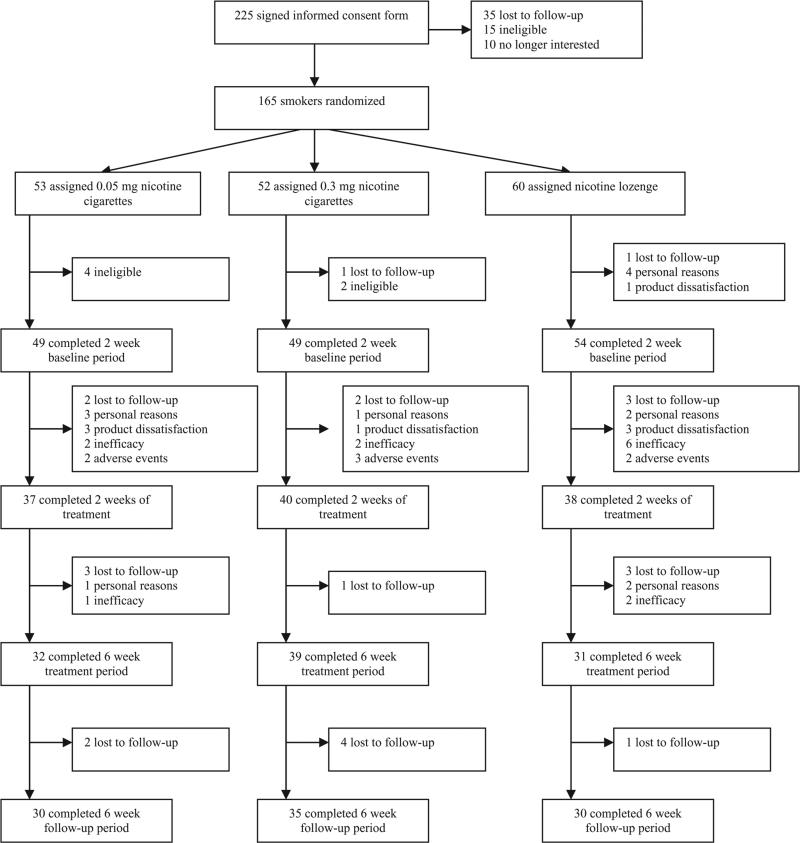
Of 883 subjects screened over the telephone, 462 were considered eligible for participation. Primary reasons for ineligibility were smoking outside the range of eligible cigarettes per day, smoking ineligible cigarettes or unstable illness. Two hundred and twenty-eight attended the orientation meeting, 225 signed the informed consent form and 165 were assigned randomly to treatment (53 to 0.05 mg cigarettes; 52 to 0.3 mg cigarettes and 60 to nicotine lozenges). Dropout rates throughout the study were highest in those assigned to nicotine lozenges and lowest in those assigned to 0.3 mg nicotine yield cigarettes, with significant differences observed between groups at the end of the 6-week treatment period (48.3% dropout rate for nicotine lozenge versus 39.6% for 0.05 mg nicotine cigarettes versus 25.0% for 0.3 mg nicotine cigarettes; χ2 = 6.49, P = 0.0389). Figure 1 illustrates the number of dropouts in each group at various stages throughout the study with reasons for dropouts indicated. The demographics and smoking history of smokers are shown in Table 1, with no significant differences among the experimental groups except age of becoming a regular smoker (P = 0.0195). There were no significant differences in demographics between subjects who dropped out of the study after randomization and those who completed the entire study.
Figure 1.
Flow of subjects through study
Table 1.
Baseline demographics and smoking history of subjects in each of the three treatment groups. In instances where data are missing, the total number of subjects used in calculating values is fewer than the 165 randomized to treatment.
| Demographics | 0.3 mg nicotine cigarettes (n = 52) | 0.05 mg nicotine cigarettes (n = 53) | Nicotine lozenges (n = 60) | P-value | |||
|---|---|---|---|---|---|---|---|
| Age (years) | 51 | 39.4 ± 14.0 | 53 | 40.7 ± 13.3 | 59 | 43.1 ± 12.4 | 0.3312 |
| Female | 26 | 50.0% | 23 | 43.4% | 29 | 48.3% | 0.6967 |
| Non-Hispanic whites | 47 | 90.4% | 44 | 83.0% | 49 | 81.7% | 0.7653 |
| Education | 0.8782 | ||||||
| Less than high school graduate | 2 | 3.9% | 2 | 3.8% | 3 | 5.0% | |
| High school graduate | 12 | 23.1% | 11 | 20.8% | 17 | 28.3% | |
| Greater than high school graduate | 37 | 71.2% | 40 | 75.5% | 39 | 65.0% | |
| Marital status | 0.1052 | ||||||
| Never married | 22 | 42.3% | 25 | 47.2% | 22 | 36.7% | |
| Currently married | 22 | 42.3% | 17 | 32.1% | 16 | 26.7% | |
| Currently not married | 8 | 15.4% | 11 | 20.8% | 21 | 35.0% | |
| Cigarettes per day | 52 | 19.8 ± 7.8 | 53 | 21.1 ± 8.1 | 60 | 21.3 ± 9.6 | 0.6066 |
| Duration of having smoked at this rate (years) | 52 | 15.4 ± 13.0 | 53 | 14.1 ± 12.6 | 59 | 15.7 ± 14.1 | 0.7960 |
| Age smoking first cigarette (years) | 52 | 14.4 ± 2.9 | 53 | 15.5 ± 4.8 | 60 | 14.5 ± 2.9 | 0.2518 |
| Age becoming a regular smoker (years) | 52 | 16.5 ± 3.0 | 52 | 19.4 ± 7.3 | 58 | 17.7 ± 4.1 | 0.0195 |
| Motivation to quit (0–10 scale) | 52 | 9.0 ± 1.1 | 53 | 9.2 ± 1.0 | 59 | 9.2 ± 1.3 | 0.6928 |
| Number of quit attempts | 0.4482 | ||||||
| 1–2 | 13 | 25.0% | 17 | 32.1% | 13 | 21.7% | |
| 3–5 | 14 | 26.9% | 14 | 26.4% | 24 | 40.0% | |
| 6–10 | 12 | 23.1% | 10 | 18.9% | 13 | 21.7% | |
| 11+ | 10 | 19.2% | 4 | 7.6% | 9 | 15.0% | |
| Spouse or significant other smokes | 20 | 38.5% | 20 | 37.7% | 21 | 35.0% | 0.9515 |
Product use during treatment
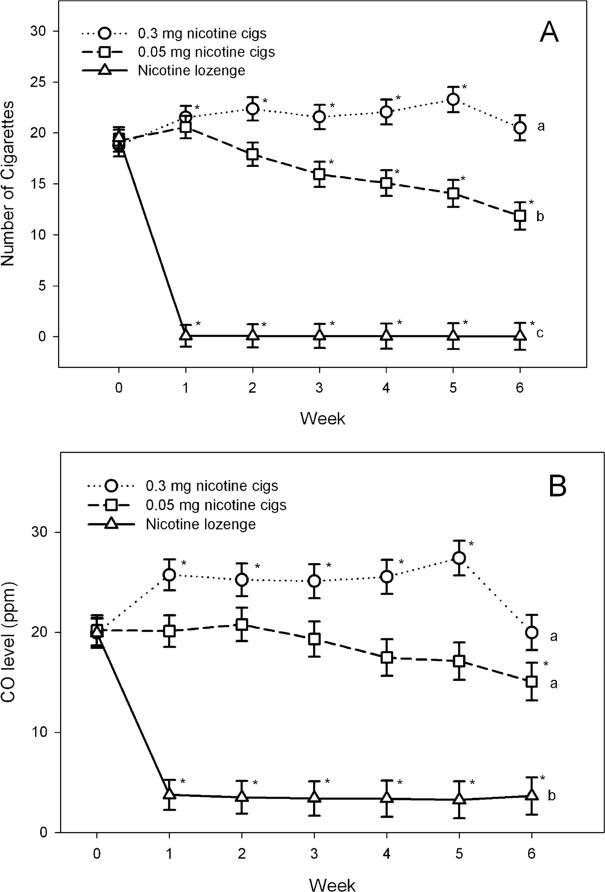
The number of assigned cigarettes smoked per day during the treatment period is illustrated in Fig. 2a. Significant treatment (F(2, 180) = 102.48, P < 0.0001), time (F(6, 648) = 37.77, P < 0.0001) and treatment × time (F(12, 648) = 62.38, P < 0.0001) effects were observed. In those smoking 0.3 mg cigarettes, the number of cigarettes smoked per day increased significantly (P = 0.0127 to P < 0.0001) at each of the first 5 weeks of treatment compared to the number of usual brand cigarettes they were smoking at baseline (Fig. 2a). This is in contrast to the significantly decreased (P = 0.0043 to P < 0.0001) number of cigarettes smoked per day (relative to baseline) observed after week 2 in those assigned 0.05 mg cigarettes. At week 6, the mean number of 0.3 mg cigarettes smoked per day was significantly greater than that of 0.05 mg cigarettes smoked (t = 4.73, P < 0.0001). Nicotine lozenge use (among those assigned to this condition) remained relatively stable throughout the 6-week treatment period and ranged from a mean of 5.9 [standard deviation (SD = 2.4] lozenges per day at week 6 to a mean of 6.9 (SD = 3.5) lozenges per day at week 3.
Figure 2.
Least squares (LS) mean (±standard error) of number of cigarettes smoked per day and exhaled carbon monoxide (CO). *P < 0.05 at that visit compared to baseline (within-group comparison). Groups with different letters were significantly different (P < 0.05) at the week 6 treatment visit (between-group comparison). For example, cigarettes per day are significantly different between each of the groups, but CO concentrations are significantly different between the nicotine lozenge group and each of the two cigarette groups, but the two cigarette groups are not different from each other
Of those subjects who had not dropped out at the visit in question, subjects were most likely to use a nicotine or tobacco product other than what was assigned to them during the first week of treatment with 30.4% of those assigned to 0.05 cigarettes, 22.9% of those assigned to 0.3 mg and 40.8% assigned to nicotine lozenges reporting such use. After week 1, the percentage who reported using a non-study-assigned nicotine or tobacco product ranged from 0 to 12.5% (during weeks 2–6) in the 0.05 mg cigarette group, 5.0 to 10.5% in the 0.3 mg cigarette group and 8.3 to 21.9% in the nicotine lozenge group. No significant difference between groups was observed in the percentage of subjects using tobacco or nicotine products other than those assigned at any weekly visit except for week 6. At the week 6 visit, 5.3% in the 0.05 mg cigarette group, 0% in the 0.3 mg cigarette group and 21.9% in the nicotine lozenge group (P = 0.0056) reported using such products. Among those reporting smoking usual brand cigarettes during the treatment period, the mean number of cigarettes smoked ranged from 0.3 to at most 6.4 per week. Distribution of cotinine concentrations at week 6 demonstrates that, in the 0.05 mg nicotine cigarette group, very few individuals had substantial cotinine concentrations, suggesting that most did not use non-study nicotine-containing products during the study. The distribution shows that 22 subjects had cotinine concentrations between 0 and 250 ng/ml, four between 251 and 500 ng/ml, three between 501 and 1000 ng/ml and three had >5000 ng/ml.
Effects of products on biomarkers of exposure during treatment
Biomarker concentrations during the treatment period for exhaled CO are illustrated in Fig. 2b. Urinary total cotinine, total NNAL, total NNN, total 1-HOP, 3-HPMA and S-PMA are presented in Table 2.
Table 2.
Geometric means of biomarkers at baseline and weeks 2 and 6 of treatment period by treatment groups. Values are for all subjects from whom data were collected at the visit in question.
| Geometric mean (95% confidence interval) |
|||
|---|---|---|---|
| Biomarkers | Baseline | Week 2 | Week 6 |
| Total cotinine1 | |||
| 0.3 mg cigarettes | 4057 (3323, 4952) | 2150 (1696, 2725)* | 2093 (1611, 2719)*a |
| 0.05 mg cigarettes | 4216 (3492, 5090) | 278 (174, 442)* | 188 (111, 319)*b |
| Nicotine lozenge | 3917 (3399, 4514) | 2291 (1708, 3073)* | 2154 (1312, 3536)*a |
| Total NNAL2 | |||
| 0.3 mg cigarettes | 0.96 (0.73, 1.26) | 0.54 (0.41, 0.69)* | 0.47 (0.30, 0.73)*a |
| 0.05 mg cigarettes | 0.92 (0.70, 1.21) | 0.34 (0.20, 0.57)* | 0.20 (0.11, 0.34)*b |
| Nicotine lozenge | 1.06 (0.84, 1.35) | 0.24 (0.18, 0.32)* | 0.14 (0.07, 0.26)*b |
| Total NNN2 | |||
| 0.3 mg cigarettes | 0.10 (0.06, 0.16) | 0.09 (0.06, 0.14) | 0.06 (0.04, 0.10)a |
| 0.05 mg cigarettes | 0.09 (0.05, 0.15) | 0.06 (0.04, 0.11) | 0.03 (0.02, 0.07)*ab |
| Nicotine lozenge | 0.08 (0.05, 0.12) | 0.02 (0.01, 0.04)* | 0.02 (0.01, 0.04)*b |
| Total 1-HOP2 | |||
| 0.3 mg cigarettes | 0.84 (0.70, 1.02) | 0.95 (0.58, 1.53) | 0.73 (0.59, 0.90)a |
| 0.05 mg cigarettes | 0.89 (0.71, 1.12) | 0.75 (0.56, 1.01) | 0.57 (0.42, 0.78)*a |
| Nicotine lozenge | 0.94 (0.71, 1.24) | 0.40 (0.29, 0.56)* | 0.34 (0.21, 0.57)*b |
| 3-HPMA2 | |||
| 0.3 mg cigarettes | 3662 (2868, 4674) | 2838 (2226, 3619) | 2732 (2110, 3537)a |
| 0.05 mg cigarettes | 3320 (2667, 4134) | 1639 (1215, 2211)* | 1453 (1039, 2032)*b |
| Nicotine lozenge | 3445 (2539, 4673) | 911 (670, 1239)* | 1062 (749, 1508)*b |
| S-PMA2 | |||
| 0.3 mg cigarettes | 2.21 (1.54, 3.18) | 1.30 (0.88, 1.92)* | 1.35 (0.94, 1.93)*a |
| 0.05 mg cigarettes | 2.46 (1.68, 3.62) | 1.54 (1.03, 2.31)* | 0.76 (0.48, 1.20)*b |
| Nicotine lozenge | 2.69 (1.95, 3.72) | 0.33 (0.22, 0.49)* | 0.48 (0.30, 0.78)*b |
ng/ml.
pmol/mg creatinine.
P < 0.05 at that visit compared to baseline (within-group comparison).
Groups with different letters were significantly different (P < 0.05) at the week 6 treatment visit (between-group comparison). For example, total cotinine is significantly different between the 0.05 mg cigarette group with 0.3 mg cigarette group and with nicotine lozenge group, but the 0.3 mg cigarette group is not significantly different from the nicotine lozenge group. Total NNAL: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides; total NNN: N′-nitrosonornicotine and its glucuronide; total 1-HOP: 1-hydroxypyrene and its glucuronide and sulfate; 3-HPMA: 3-hydroxypropylmercapturic acid; S-PMA: S-phenylmercapturic acid.
For all seven biomarkers of exposure, significant treatment effects (P-values 0.0131–<0.0001), time effects (P-values 0.0001–<0.0001) and treatment × time interaction effects (P-values 0.0045–<0.0001) were found.
As illustrated in Fig. 2b, exhaled CO concentrations followed a similar pattern as seen for number of cigarettes smoked per day. Exhaled CO concentrations increased during the first 5 weeks of treatment in those using 0.3 mg cigarettes, whereas in those receiving 0.05 mg cigarettes exhaled CO decreased gradually, with a statistically significant decrease observed at week 6 of treatment when compared with baseline (P = 0.0247). At week 6, exhaled CO concentrations were nearly significantly different (P = 0.0569) between the two cigarette groups. Urinary cotinine concentrations decreased significantly in all treatment groups, with the greatest decrease observed in the 0.05 mg cigarette group and moderate decreases occurring in the 0.3 mg cigarette and nicotine lozenge groups (Table 2). For the other biomarkers assessed, greatest decreases from baseline were found in the group receiving nicotine lozenge with the smallest changes in biomarker concentrations observed mainly in the group receiving 0.3 mg cigarettes (Table 2).
Effects of products on subjective responses during treatment
Dependence
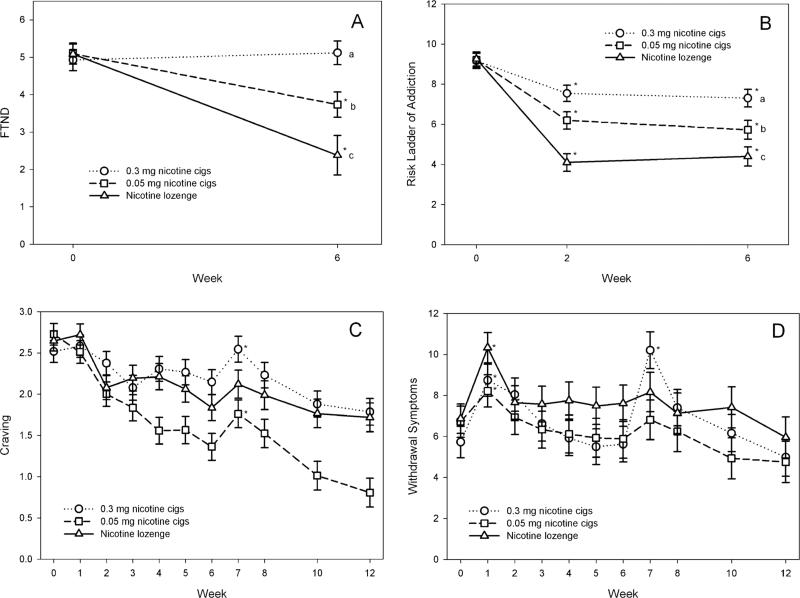
FTND score and perceived risk for addiction score during treatment are illustrated in Fig. 3a,b. Both these measures showed significant treatment (P = 0.0124 and <0.0001, respectively), time (P-values <0.0001) and treatment × time interaction (P-values <0.0001) effects. Significant decreases in FTND and perceived risk of addiction scores were observed for the 0.05 mg cigarette and nicotine lozenge groups (all P-values ≤ 0.001) at week 6 compared to baseline. For the 0.3 mg cigarette group, perceived risk of addiction decreased significantly between baseline and week 6 (P < 0.0001); however, FTND score did not (P = 0.4810). At week 6, significant differences between groups were found in FTND (P-values 0.0315–<0.0001) and perceived risk of addiction (P-values 0.0456–<0.0001), with the highest levels observed in those assigned to 0.3 mg cigarettes and the lowest in those assigned to nicotine lozenge.
Figure 3.
Least squares (LS) mean [±standard error (SE)] of Fagerstrom Test for Nicotine Dependence (FTND) score and perceived risk for addiction score (Panels A and B). *P < 0.05 at that visit compared to baseline (within-group comparison). Groups with different letters were significantly different (P < 0.05) at the week 6 treatment visit (between-group comparison). Least squares (LS) mean (±SE) of craving and withdrawal symptoms craving and withdrawal symptoms (Panels C and D). *P < 0.05 at that visit compared to the previous visit (i.e. week 1 versus week 0 and week 7 versus week 6)
Nicotine craving and withdrawal symptoms during treatment and follow-up are illustrated in Fig. 3c,d. Nicotine craving and withdrawal symptoms at the time of switching to products (week 1) and cessation from products (week 7) were examined. Upon cessation of usual brand cigarettes and switching to the products, there was a significant increase in withdrawal symptoms (P-values 0.0188–<0.0001) and no significant change in craving in all three treatment groups. Increase in nicotine withdrawal scores upon cessation of usual brand cigarettes (week 1 compared to baseline) was significantly smaller for the group assigned to 0.05 mg cigarettes compared to the group assigned nicotine lozenges (P = 0.0253) and nearly significantly smaller (P = 0.0917) than for those assigned to 0.3 mg cigarettes. Upon cessation of the product (week 7 compared to week 6), significant increase in craving (P = 0.0079) and withdrawal symptoms (P < 0.0001) were observed for the 0.3 mg cigarette group. In those discontinuing 0.05 mg cigarettes, craving increased significantly (P = 0.0138), but withdrawal symptoms did not (P = 0.2297). In those discontinuing nicotine lozenge, neither changes in craving (P = 0.0814) nor withdrawal symptoms (P = 0.4856) were increased significantly. Change in withdrawal symptoms was significantly lower in those discontinuing 0.05 mg cigarettes (P = 0.0006) or nicotine lozenges (P = 0.0002) compared to those discontinuing 0.3 mg cigarettes, with no significant differences in craving observed between groups.
Abstinence
Abstinence rates were calculated using the intent-to-treat sample (at the point of random assignment to the product and before baseline measures). Dropouts were considered treatment failures. Biochemically verified [CO < 8 parts per million (p.p.m.)] point prevalence rates of abstinence from cigarettes at each of the follow-up visits and 4-week continuous abstinence rates are shown in Table 3. In this analysis, subjects were allowed to use lozenges. Similarly, biochemically verified (CO < 8 p.p.m. and total cotinine <35 ng/ml) abstinence from all nicotine-containing products (including lozenges) is also listed in Table 3. For abstinence from cigarettes, 4-week continuous abstinence rates were highest in those receiving 0.05 mg nicotine cigarettes and lowest in those receiving 0.3 mg nicotine cigarettes, with the difference across the three groups nearly significant (Table 3). Point prevalence abstinence rates followed a similar pattern, with significant differences between groups observed in abstinence rates verified by both CO and urinary cotinine concentrations. CO verified point prevalence abstinence rates were statistically significant only at the week 6 post-treatment visit and nearly significant at the weeks 2 and 4 post-treatment visits.
Table 3.
Continuous (past 4 weeks) and point-prevalence (past 1 week) post-treatment abstinence rates. Products with different superscript letters were significantly different (P < 0.05).
| Treatments |
|||||||
|---|---|---|---|---|---|---|---|
| 0.3 mg nicotine cigarettes (n = 52) |
Nicotine lozenges (n = 60) |
0.05 mg nicotine cigarettes (n = 53) |
|||||
| # abstinent | % | # abstinent | % | # abstinent | % | P-value | |
| Continuous abstinence | |||||||
| 1CO verified | 11 | 21.2 | 21 | 35.0 | 23 | 43.4 | 0.0508 |
| 2CO and cotinine verified | 7 | 13.5 | 11 | 18.3 | 16 | 30.2 | 0.0913 |
| CO verified point prevalence abstinence | |||||||
| Follow-up week | |||||||
| 1 | 18 | 34.6 | 25 | 41.7 | 22 | 41.5 | 0.6954 |
| 2 | 17 | 32.7 | 25 | 41.7 | 29 | 54.7 | 0.0719 |
| 4 | 12 | 23.1 | 22 | 36.7 | 23 | 43.4 | 0.0829 |
| 6 | 12 | 23.1a | 22 | 36.7a,b | 25 | 47.2b | 0.0357 |
| CO and cotinine verified point prevalence abstinence | |||||||
| Follow-up week | |||||||
| 2 | 12 | 23.1a | 16 | 26.7a | 24 | 45.3b | 0.0298 |
| 4 | 8 | 15.4a | 13 | 21.7a | 21 | 39.6b | 0.0120 |
| 6 | 7 | 13.5a | 12 | 20.0a,b | 19 | 35.9b | 0.0192 |
Carbon monoxide (CO) verified abstinence represents abstinence from cigarettes but usage of nicotine lozenge is allowed.
CO and cotinine verified represents abstinence from all nicotine-containing products, including nicotine lozenge.
Unlike the cigarette conditions, the nicotine lozenge condition involved complete cessation from cigarettes from the onset of treatment, providing a potentially unfair advantage to the cigarette conditions. Therefore, to determine if duration of cigarette abstinence had an impact on abstinence rates in the nicotine lozenge condition, the continuous CO verified abstinence rates from the last 4 weeks of product use without smoking was compared with the 4-week continuous CO verified abstinence rates at the end of the follow-up period. The results were identical (35%). In addition, the point prevalence rate at the end of 6 weeks of product use was compared with the rate at the end of the follow-up period and the rates were similar (40.0% versus 36.7%).
DISCUSSION
This study showed that, unlike the 0.3 mg nicotine yield cigarettes, 0.05 mg nicotine yield cigarettes were not associated with compensatory smoking behavior. Thus, although increased smoking and exhaled CO were observed with the 0.3 mg cigarettes, decreases in cigarette intake and eventually in exhaled CO were observed for the 0.05 mg cigarettes, a finding similar to another study [29]. The 0.05 mg cigarettes were also associated with reduced exposure biomarker levels (e.g. total NNAL, total NNN, 3-HPMA, S-PMA), reduced nicotine dependence and withdrawal scores. Conversely, the 0.3 mg cigarettes did not result in significant decreases in most exposure biomarkers, led to persistent self-reported dependence and higher levels of withdrawal from this product compared to the other products. As expected, nicotine lozenge was associated with the most consistent reductions in toxicant exposure, dependence on cigarettes and perceived risk for addiction. It is important to note that levels of total 1-HOP, 3-HPMA, and SPMA did not attain zero values for the nicotine lozenge group because there are environmental and endogenous sources of pyrene, acrolein and benzene other than tobacco smoke exposure. [30] Furthermore, total NNAL has a long half-life [31] and there is evidence for endogenous formation of NNN is some users of nicotine replacement therapy [32] In addition, the results showed that a few subjects in the nicotine lozenge group had reported using cigarettes or other non-assigned tobacco products during the first 6 weeks (8.3–21.9%). Interestingly, the 0.05 mg cigarette led to the highest abstinence rates of the three products tested, although difference in continuous abstinence rates did not reach statistical significance.
The slope of the decline in cigarette smoking rate was slightly faster than the decline in exhaled CO concentrations for the 0.05 mg condition. This result may reflect the use of usual cigarettes among some subjects during the first few weeks of treatment, or may reflect subjects’ engagement in some compensatory smoking behavior during the initial period of adjustment to the product. None the less, CO decreased over time, as the number of cigarettes smoked decreased and the minimal compensatory smoking observed when subjects were converted to the 0.05 mg cigarettes is consistent with the results of several small studies in which limited or no compensatory smoking was found when subjects smoked either a single reduced nicotine cigarette in a laboratory setting or smoked one of five progressively lower nicotine content cigarettes for a week [9,10]. Conversely, studies examining use of highly ventilated low-yield cigarettes have found that substantial compensation occurs [33].
The reduction in levels of urinary total NNAL and NNN is consistent with reduced tobacco specific nitrosamine levels found in these products [12]. For example, Marlboro and Camel ‘light’ cigarettes have NNK levels of 0.68 and 0.55 μg/g wet weight and NNN levels of 2.8 and 2.7 μg/g wet weight, respectively, while the corresponding values for the 0.3 mg and 0.05 mg Quest products were 0.19 and 0.054 μg/g wet weight NNK and 0.82 and 0.83 μg/g wet weight NNN. For the 0.05 mg cigarettes, additional reduction in carcinogen and toxicant exposures (to acrolein and benzene) is probably attributable to the observed reduction in cigarette intake such that, by the end of treatment, most biomarker levels in this group were not significantly different from those in the nicotine lozenge group. Therefore, the observed reduction in biomarker levels is due probably to differences in the amount of the constituents related to the biomarkers in the product itself and in the case of the cigarettes, the amount of product use. On the other hand, no reductions were observed for 1-HOP, which may indicate that exposure to polycyclic aromatic hydrocarbons may be significant when using both the 0.3 mg and 0.05 mg cigarettes and not affected by the degree of reduction in smoking behavior observed in this study.
Although blind to the nicotine content of their assigned cigarettes, only smokers in the 0.05 mg group appeared to experience a reduction on a scale measuring nicotine dependence. However, the perceived risk for addiction decreased for both cigarette products. The reduced nicotine dependence associated with the 0.05 mg cigarettes is consistent with other studies which show reduced FTND scores [9] or decreased motivation to smoke [29] after smoking low nicotine content or denicotinized cigarettes. Another indicator of reduced dependence is the reduction in withdrawal symptoms experienced after cessation from the 0.05 mg cigarettes and the nicotine lozenge compared to withdrawal from 0.3 mg cigarettes. It is notable that, although the 0.3 mg cigarettes and nicotine lozenges were associated with similar cotinine levels, less withdrawal was observed after nicotine lozenge discontinuation. This suggests that withdrawal may be affected by the nicotine pharmacokinetics of the discontinued product. On the other hand, craving increased for both 0.3 mg and 0.05 mg cigarette conditions after cessation of these products, but not for nicotine lozenge. This finding would indicate that craving for cigarettes has a different abstinence pattern than total withdrawal [34] and may be affected by different aspects of smoking (e.g. missing the sensory aspects of smoking as opposed to primarily nicotine).
Our finding that use of 0.05 mg cigarettes led to greater withdrawal symptom relief than use of nicotine lozenge and no difference than the relief with use of 0.3 mg cigarettes suggests further that non-nicotine components of cigarette dependence (e.g. other tobacco constituents, sensory aspects of smoking) contribute to the relief of withdrawal symptoms. On the other hand, all products appeared to relieve craving equally. These findings are consistent with other studies demonstrating that use of denicotinized cigarettes reduce craving, negative affect and in some studies, withdrawal symptoms or a subset of symptoms during periods of short-term abstinence [9,35–46]. The use of 0.05 mg nicotine cigarettes, by reducing dependence and withdrawal symptoms, may therefore be a promising tool for achieving smoking cessation. Indeed, our study demonstrated that smoking cessation rates in those receiving 0.05 mg cigarettes were equivalent to (if not slightly higher than) cessation rates in those receiving nicotine lozenges. A study by Benowitz et al. found that 4 weeks following the end of a progressive reduction in nicotine content of cigarettes 20% of subjects attained abstinence [9]. This rate is surprisingly high, given that these subjects were not enrolling in a cessation study.
Our study suggests that significantly reducing nicotine content of cigarettes may facilitate abstinence by making smoking cessation easier to achieve. For the subpopulation of smokers who rely on nicotine for self-medication the use of medicinal products, either in its current form or in a form that results in faster delivery, greater amounts or in other ways that are more satisfying could be considered [47]. This approach is supported by several prior studies suggesting that the use of denicotinized cigarettes in combination with nicotine patch for smoking cessation show promise [48–50].
A major limitation of the current study was the large number of dropouts. About a third to almost half the population dropped out before the end of follow-up and about one-fifth to more than a third dropped out during treatment, with dropout rates lowest in the 0.3 mg cigarette group and highest in the nicotine lozenge group. Another limitation was the inability to determine if smokers were compliant with the study procedures (i.e. that they used the assigned products solely), although the observed cotinine levels are generally consistent with what would be expected with each product (i.e. larger decreases in the 0.05 mg nicotine cigarette group than the other two groups). Despite the fact that the data may be contaminated by smokers who smoked usual brand cigarettes during intervention, the results show that the 0.05 mg cigarette does not lead to greater toxicant exposure and it seems to reduce dependence and to support to abstinence. A third limitation was that the study was underpowered to examine abstinence difference among treatment conditions and the duration of follow-up was short. However, these preliminary results indicate that a future larger trial with longer follow-up is warranted. Finally, this study is generalizable to only one type of near nicotine-free cigarettes.
In summary, reduced nicotine content cigarettes of at least 0.05 mg nicotine yield can lead to reductions in toxicant exposure by way of changing smoking behavior and in dependence and can possibly facilitate abstinence among smokers interested in quitting. These cigarettes can be used potentially as a cessation tool. More research should be conducted on the threshold dose for nicotine addiction during the extinction phase and factors that moderate the threshold dose, the effects of reduced nicotine content cigarettes on vulnerable populations and adjunctive methods that might facilitate cessation.
Acknowledgements
This study was funded by P50 DA013333.
Footnotes
Clinical trial registration number
Declarations of interest
Peter Shields has served as an expert witness on behalf of plaintiffs in litigation cases against tobacco companies and Stephen Hecht is an expert witness for the plaintiff in ‘Kelly Hill et al. v. U.S. Smokeless Tobacco Company’. Dorothy Hatsukami has consulted for Pfizer, Abbott Laboratories and Novartis (travel expenses only) and has received a research grant from Nabi Biopharmaceuticals.
References
- 1.Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Institute of Medicine, National Academy Press; Washington, DC: 2001. [Google Scholar]
- 2.Royal College of Physicians . A Report by the Tobacco Advisory Group of the Royal College of Physicians. Royal College of Physicians; London: 2007. Harm reduction in nicotine addiction. Helping people who can't quit. p. 176. [Google Scholar]
- 3.Hatsukami D, Hecht SS. Hope or hazard: what research tells us about ‘potentially reduced-exposure’ tobacco products. University of Minnesota Transdisciplinary Tobacco Use Research Center; Minneapolis: 2005. [9 December 2009]. Available from http://www.rwjf.org/files/research/Hope%20or%20Hazard.pdf. [Google Scholar]
- 4.Hatsukami D, Joseph AM, LeSage M, Jensen J, Murphy SE, Pentel PR, et al. Developing the science base for reducing tobacco harm. Nicotine Tob Res. 2007;9:S537–53. doi: 10.1080/14622200701679040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services (USDHHS) The Health Consequences of Smoking: Nicotine and Addiction. A Report of the Surgeon General. USDHHS; Rockville, MD: 1988. [Google Scholar]
- 6.National Cancer Institute . Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. Smoking and Tobacco Control Monograph no. 13. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: 2001. [Google Scholar]
- 7.Hecht SS, Murphy SE, Carmella SG, Li S, Jensen J, Le C, et al. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:693–8. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- 8.Bernert JT, Jain RB, Pirkle JL, Wang L, Miller BB, Sampson EJ. Urinary tobacco-specific nitrosamines and 4-aminobiphenyl hemoglobin adducts measured in smokers of either regular or light cigarettes. Nicotine Tob Res. 2005;7:729–38. doi: 10.1080/14622200500259762. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., 3RD Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL, Jacob P, III, Herrera B. Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther. 2006;80:703–14. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, et al. Evaluation of carcinogen exposure in people who used ‘reduced exposure’ tobacco products. J Natl Cancer Inst. 2004;96:844–52. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- 12.Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res. 2006;8:309–13. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- 13.Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Arch Intern Med. 2002;162:1267–76. doi: 10.1001/archinte.162.11.1267. [DOI] [PubMed] [Google Scholar]
- 14.Fiore MC, Bailey W, Cohen S, Dorfman S, Goldstein M, Gritz E, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. US Department of Health and Human Services Public Health Service; Rockville, MD: 2000. [Google Scholar]
- 15.Jacob P, III, Byrd GD. Use of chromatographic and mass spectrometric techniques for the determination of nicotine and its metabolites. In: Gorrod JW, Jacob P III, editors. Analytical Determination of Nicotine and Related Compounds and Their Metabolites. Elsevier; Amsterdam: 1999. pp. 191–224. [Google Scholar]
- 16.Hecht SS, Carmella SG, Murphy SE. Effects of watercress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev. 1999;8:907–13. [PubMed] [Google Scholar]
- 17.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12:1257–61. [PubMed] [Google Scholar]
- 18.Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their pyridine-N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers Prev. 2005;14:885–91. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 19.Carmella SG, Le KA, Hecht SS. Improved method for determination of 1-hydroxypyrene in human urine. Cancer Epidemiol Biomarkers Prev. 2004;13:1261–4. [PubMed] [Google Scholar]
- 20.Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol. 2007;20:986–90. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47:171–83. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Hatsukami D, McBride C, Pirie P, Hellerstedt W, Lando H. Effects of nicotine gum on prevalence and severity of withdrawal in female cigarette smokers. J Subst Abuse. 1991;3:427–40. doi: 10.1016/s0899-3289(10)80024-0. [DOI] [PubMed] [Google Scholar]
- 23.Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Arch Gen Psychiatry. 1991;48:52–9. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- 24.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 25.Hatsukami D, Anton D, Keenan R, Callies A. Smokeless tobacco abstinence effects and nicotine gum dose. Psychopharmacology. 1992;106:60–6. doi: 10.1007/BF02253589. [DOI] [PubMed] [Google Scholar]
- 26.Hatsukami D, Huber M, Callies A. Physical dependence on nicotine gum: effect of duration on use. Psychopharmacology. 1993;111:449–56. doi: 10.1007/BF02253535. [DOI] [PubMed] [Google Scholar]
- 27.Hatsukami D, Gust SW, Keenan R. Physiologic and subjective changes from smokeless tobacco withdrawal. Clin Pharmacol Ther. 1987;41:103–7. doi: 10.1038/clpt.1987.17. [DOI] [PubMed] [Google Scholar]
- 28.Heatherton TF, Koslowski LT, Frecker RC, Fagerström K-O. The Fagerström Test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 29.Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days [see Comment]. Addiction. 2007;102:324–34. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- 30.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–41. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–6. [PubMed] [Google Scholar]
- 32.Stepanov I, Carmella SG, Han S, Pinto A, Strasser AA, Lerman C, et al. Evidence for endogenous formation of N′-nitrosonornicotine in some long-term nicotine patch users. Nicotine Tob Res. 2009;11:99–105. doi: 10.1093/ntr/ntn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose J, Behm F. Effects of low nicotine content cigarettes on smoke intake. Nicotine Tob Res. 2004;6:309–19. doi: 10.1080/14622200410001676378. [DOI] [PubMed] [Google Scholar]
- 34.Hughes JR, Hatsukami DK. The nicotine withdrawal syndrome: a brief review and update. International Journal of Smoking Cessation. 1992;1:21–6. [Google Scholar]
- 35.Baldinger B, Hasenfratz M, Battig K. Effects of smoking abstinence and nicotine abstinence on heart rate, activity and cigarette craving under field conditions. Hum Psychopharmacol. 1995;10:127–36. [Google Scholar]
- 36.Breland AB, Buchhalter AR, Evans SE, Eissenberg T. Evaluating acute effects of potential reduced-exposure products for smokers: clinical laboratory methodology. Nicotine Tob Res. 2002;4:S131–40. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- 37.Buchhalter AR, Schrinel L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: comparison with own brand, not own brand, and de-nicotinized cigarettes. Nicotine Tob Res. 2001;3:111–8. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- 38.Butschky MF, Bailey D, Henningfield JE, Pickworth WB. Smoking without nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacol Biochem Behav. 1995;50:91–6. doi: 10.1016/0091-3057(94)00269-o. [DOI] [PubMed] [Google Scholar]
- 39.Dallery J, Houtsmuller EJ, Pickworth WB, Stitzer ML. Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharmacology (Berl) 2003;165:172–80. doi: 10.1007/s00213-002-1242-8. [DOI] [PubMed] [Google Scholar]
- 40.Eid NC, Fant RV, Moolchan ET, Pickworth WB. Placebo cigarettes in a spaced smoking paradigm. Pharmacol Biochem Behav. 2005;81:158–64. doi: 10.1016/j.pbb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Gross J, Lee J, Stitzer ML. Nicotine-containing versus de-nicotinized cigarettes: effects on craving and withdrawal. Pharmacol Biochem Behav. 1997;57:159–65. doi: 10.1016/s0091-3057(96)00309-7. [DOI] [PubMed] [Google Scholar]
- 42.Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine Tob Res. 1999;1:357–64. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- 43.Rose J, Behm FM, Westman EC, Johnson M. Dissociating nicotine and non-nicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 44.Brauer L, Behm F, Lane J, Westman E, Perkins C, Rose J. Individual differences in smoking reward from de-nicotinized cigarettes. Nicotine Tob Res. 2001;3:101–9. doi: 10.1080/14622200123249. [DOI] [PubMed] [Google Scholar]
- 45.Rose J, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav. 2003;76:243–50. doi: 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–9. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- 47.Henningfield J, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Council on Scientific Affairs, American Medical Association. Tob Control. 1998;7:281–93. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. 2008;10:1139–48. doi: 10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- 49.Rose J, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- 50.Rose J, Behm F, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components for satiation in cigarette smoking. Pharmacol Biochem Behav. 2003;76:243–50. doi: 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]