Abstract
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality. With the ageing population, the prognostic determinants among others include frailty, health status, disability, and cognition. These constructs are seldom measured and factored into clinical decision-making or evaluation of the prognosis of these at-risk older adults, especially as it relates to high-risk interventions. Addressing this need effectively requires increased awareness and their recognition by the treating cardiologists, their incorporation into risk prediction models when treating an elderly patient with underlying complex CVD, and timely referral for comprehensive geriatric management. Simple measures such as gait speed, the Fried score, or the Rockwood Clinical Frailty Scale can be used to assess frailty as part of routine care of elderly patients with CVD. This review examines the prevalence and outcomes associated with frailty with special emphasis in patients with CVD.
Keywords: Frailty, Assessment, Prognosis, Cardiovascular disease
Introduction
Cardiovascular diseases (CVDs) are the leading causes of death and hospitalization and represent an enormous clinical and public health burden which disproportionately affects older adults.1 There has been a demonstrable shift in the burden of CVD towards older persons in the past two decades. During the same period, novel therapeutic approaches have improved their survival.2–4 The overall health and prognosis of the older adults is influenced by frailty, comorbid conditions, and general health status as well as CVD. Current guidelines by the American College of Cardiology/American Heart Association (ACC/AHA) underscore the need to assess general health, comorbidity, cognitive status, and life expectancy,5,6 but surprisingly do not mention frailty.
Despite their conceptual prognostic importance, these constructs are seldom formally evaluated in clinical practice and the treatment of CVD remains geared primarily towards the timely treatment of the underlying condition. A better understanding of the impact of these variables on outcomes may improve the care of patients with CVD. Identifying persons at increased risk among those with advanced chronologic age may foster care effectiveness by focusing on global health and by optimizing the use of finite healthcare resources. Addressing this need requires increased awareness and recognition by treating cardiologists, incorporation of frailty into risk prediction models, and timely referral to manage frailty and other age-related problems. This review describes tools to assess frailty, and it is relevance to the prognosis and clinical management of patients with CVD.
Implications of cardiovascular disease in ageing populations
There are currently ∼4.1 million deaths from CVD in Europe each year, with ∼82% of these deaths in persons older than 65 years, and ∼46% in persons older than 75 years.7 The temporal declines in cardiovascular mortality, while varying widely between countries, have on average have been similar for older (>65 years) compared with younger individuals within most European countries.7,8 While age-specific cardiovascular mortality has been decreasing, in part because of lower case fatality rates, hospital admissions for CVD have continued to rise.7 Improvements in life expectancy, more effective treatments and better outcomes are therefore changing the epidemiology of CVD, towards older persons who are more likely to be frail and to have greater comorbidity, creating enormous clinical, societal and economic challenges.9
Improved treatments and prevention of CVD are also increasing the proportion of patients who die from non-cardiac causes. In a large US cohort, there was a 33% decline in cardiac deaths at 5 years after hospital discharge following percutaneous coronary intervention (PCI), but a 57% increase in non-cardiac deaths.10 Non-cardiac deaths also predominate in patients undergoing trans-cutaneous aortic valve replacement (TAVR) who, even after successful treatment, have high 1 year mortality, nearly 30% in the Placement of AoRTic TraNscathetER (PARTNER) Valve trial.11 These observations emphasize the importance of considering non-cardiac predictors of death and quality of life in patients with CVD.
Frailty
‘Frailty’ represents a complex clinical syndrome of increased vulnerability to stressors (Figure 1) which results from multiple impairments across different systems,12 and accounts, at least in part, for the heterogeneity between biological and chronological age (Figure 2).13,14 Individuals who have a lower functional capacity and fewer physiological reserves are at higher risk for homoeostatic disruption with stress such as acute myocardial infarction (MI), heart failure, or cardiac surgery. Frailty has multiple contributors including age-related loss of muscle mass, reduced nutritional intake, low physical activity, as well as cardiovascular and non-CVD. Frailty has been associated with raised inflammatory markers including interleukin 6, C-reactive protein, low vitamin D, and low testosterone levels.15–19 A clinically useful definition of frailty includes slowness, weakness, low physical activity, exhaustion, and weight loss (Supplementary material online, FigureS1). Frailty overlaps but is distinct from co-morbidity, depression, poor quality of life, cognitive decline, and dependency in activities of daily living (Table 1).20,21
Figure 1.
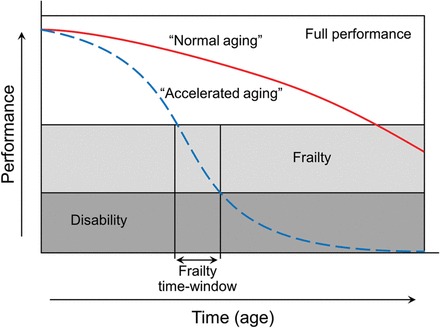
Trajectories of health and functioning with ageing. On the ‘Y’ axis is the global measure of performance which may be physical, cognitive, social, or quality of life. Performance is divided into the meaningful levels. Individuals with full performance and high functional reserve can face environmental perturbations with ease. In contrast frail, individuals have a high risk from homoeostasis disruption and negative health outcomes including disability and death, due to exhaustion of functional reserve. With disability assistance is needed to function. The trajectory of decline with ageing varies widely between individuals. In some it is much steeper, and crosses the threshold of disability years before death. It may be precipitous after stroke, myocardial infarction, or fracture. Effective treatments of the presenting condition, avoiding complications and interventions which reduce frailty (arrow) may decrease the rate of decline or improve performance (modified from source).15
Figure 2.
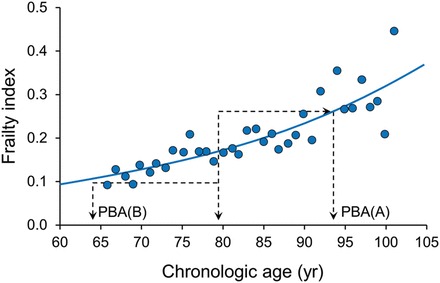
Relationship between frailty, age and the risk of death. This figure demonstrates a relationship between deficit accumulation as an estimate of biologic age and its correlation with the risk of death. Consider two people, A and B, of the same chronologic age. At 78 years, the mean value of the frailty index is 0.16. Person A has a frailty index value of 0.26 that is higher than the mean value by 0.1 corresponding to the mean value of the frailty index at age 93 years. In essence, person A has the life expectancy of 93 years old; thus, although chronologically 78 years old, person A can be considered to be biologically 93 years old. In contrast, person B has a frailty index value of 0.1 that is lower than the mean value by 0.06 corresponding to the mean value of the frailty index at age 63 years. In essence, person B has the life expectancy of 63 years old; thus, although chronologically 78 years old, person B can be considered to be biologically 63 years old.14
Table 1.
Frailty and other multidimensional concepts which influence health, independence and quality of life in elderly patients with cardiovascular disease
| Components | Tools for measurement | |
|---|---|---|
| Frailty | Exercise capacity | Time to walk 4–10 m |
| Muscle strength | Grip strength by dynamometer | |
| Nutritional status | Reported weight loss, | |
| Frail screening tool | ||
| Fried criteria | ||
| Disability | ||
| Dependency | Ability to undertake activities of daily living—shopping, finances, cleaning, cooking etc. | Nagi scale |
| Dependent for basic cares—eating, toileting, washing, etc. | Katz | |
| Cognitive function | Memory and executive function | MMSE, Montreal Cognitive Assessment Tool |
| Mood | Center for Epidemiological Studies Depression scale | |
| Physiological function | Measures of organ system function | Creatinine clearance, albumin, haemoglobin, forced expiratory volume in 1 s |
| Comorbidity | Number and severity of chronic conditions | Charlson index |
| Social support | Family or community support | |
| Financial resources | ||
Measuring frailty
Frailty is often assessed clinically from the ‘end of the bed’, using subjective approaches such as the ‘eye ball test’. Lower body mass index values have been related to lower muscle mass.22 These patients with sarcopenia are known to have adverse long-term outcomes.23 However, this approach is unreliable and prone to bias. Simple, objective, and standardized methods provide quantitative estimates and reliable information on frailty to guide clinical decisions.
A number of simple tools are available to measure frailty using some or all of the key frailty criteria—by questionnaires or simple measurements.24 Gait speed measured over 5–10 m, is easy to perform, reproducible, correlates with outcomes, is not confounded by fitness or cardiopulmonary symptoms.25,26 Grip strength, measured by dynamometer (Jamar, Patterson Medical, IL, USA), is also easy to perform and predicts adverse outcomes.27 The Short Physical Performance Battery and Up and Go test measures gait speed, chair rises, balance.28 The Fried scale (Table 2) is based on the presence or absence of five frailty criteria—slowness assessed by walk speed, weakness assessed by hand grip strength, and self-reported low physical activity, exhaustion, and unintentional weight loss.29 The few studies which compared frailty indices suggest that simple measures perform as well as the more complex multi-item tools.30,31
Table 2.
| 1 | Unintentional weight loss | >4.5 kg in the past year |
| 2 | Exhaustion | For at least 3 days during the last week ‘I felt that everything I did was an effort’ or ‘I could not get going’ |
| 3 | Physical activity | No physical activity, spend most of the time sitting or rarely a short walk during the last year |
| 4 | Walk time | Time to walk 4 m >6 s |
| 5 | Grip strength | Grip strength by dynamometer |
Frail = 3 or more criteria present, pre-frail = 1 or 2 criteria.
An alternative approach to assessing frailty is to quantify the accumulation of deficits.14,29 The frailty index score calculates deficits based on symptoms, signs, disabilities, diseases, and laboratory measurements.14,32 A standard Comprehensive Geriatric Assessment records ∼40 items, some of which are self-reported (e.g. ‘how would you rate your health’), while others are assessed by tests, (e.g. Mini-Mental State Examination), clinical evaluation (e.g. congestive heart failure) or laboratory measurement (diabetes mellitus).14,33 A fraction is given for the deficits present to a limited extent (e.g. health as good = 0, fair = 0.5, poor = 1). The number of deficits is expressed as a proportion of the total possible. Research suggests cumulative deficits predict adverse outcomes more reliably than chronological age, and treatments which improve specific deficits may improve outcomes.34
A number of tools combine different components of physical function, cumulative deficits, and dependency. They include the FRAIL questionnaire35 (Table 3), the Clinical Frailty Scale,36 the Gérontopôle Frailty Screening Tool,37 the Edmonton Frailty Scale,30,38 and the Study of Osteoporotic Fracture index.30,39 A global clinical assessment of frailty based on physical function and level of independence with activities of daily living has been proposed by Rockwood et al.36 (Table 4).
Table 3.
The simple ‘FRAIL’ Questionnaire Screening Tool101
| 3 or greater = frailty; 1 or 2 = pre-frail |
|---|
| Fatigue: are you fatigued? |
| Resistance: cannot walk up one flight of stairs? |
| Aerobic: cannot walk one block |
| Illness: do you have more than five illnesses? |
| Loss of weight: have you lost >5% of your weight in the past 6 months? |
Table 4.
A global clinical measure of fitness and frailty in elderly people
| 1 Very fit— robust, active, energetic, well-motivated and fit; these people commonly exercise regularly and are in the most fit group for their age |
| 2 Well—without active disease, but less fit than people in category 1 |
| 3 Well, with treated comorbid disease—disease symptoms are well controlled compared with those in category 4 |
| 4 Apparently vulnerable—although not frankly dependent, these people commonly complain of being ‘slowed up’ or have disease symptoms |
| 5 Mildly frail—with limited dependence on others for instrumental activities of daily living |
| 6 Moderately frail—help is needed with both instrumental and non-instrumental activities of daily living |
| 7 Severely frail—completely dependent on others for the activities of daily living, or terminally ill |
In general, these assessments are easy to administer. While simpler tools may be limited by focusing on only a few aspects of a multidimensional problem, they are useful to identify individuals for a more comprehensive geriatric assessment. Screening for frailty has been recommended by a 2013 frailty consensus statement for all persons 70 years or older and those with significant (>5 lb; 2.3 kg) annual unintentional weight loss.40 At present few large studies have compared different approaches,30,41 and there is no clear consensus on which tools best inform clinical decisions. It is likely the optimal approach will vary depending on the clinical situation.
Even though tests to assess frailty are simple to administer, frailty measures are not included in most contemporary models of outcome assessment. The reasons for the non-inclusion are not certain, but could relate to limited familiarity, concerns about the complexity of measurement, or to lack of widely accepted and standardized approaches. In addition some clinicians may not be aware of the importance of frailty, comorbidity, and quality of life as predictors of mortality and morbidity, or are uncertain of their relevance to clinical management.
We recommend using Fried criteria, the Rockwood clinical frailty scale or gait speed routinely in all patients with CVD who are 65 years or older. Gait speed, a component of Fried criteria cannot be measured in immobile or moribund patient and that is a limitation of the model. In these cases, deficit index can be calculated by the Rockwood clinical frailty scale.
Prevalence of frailty
Varying degrees of frailty are common in elderly patients with CVD, but because different definitions and instruments are used, it is difficult to make simple comparisons of prevalence between studies and populations.42 In a systematic review of 21 studies, the weighted prevalence of frailty in community dwellers older than 65 years was ∼10% for ‘physical frailty’ and ∼14% for a broader frailty phenotype.43 In general, the prevalence of frailty increases with age, and is greater in females and in residents of long-term care facilities.29,44 Frailty is about three times more prevalent among persons with compared with those without heart disease.45 In the Cardiovascular Health Study, frail subjects were more likely to have subclinical CVD,46 and subjects with subclinical CVD were more likely to have impaired physical or mental function during the follow-up.47 Frailty has been reported in 20% of patients aged ≥65 years undergoing PCI,48,49 and in 27% of patients aged ≥70 years with significant coronary artery disease at cardiac catheterization.31,48 Frailty is particularly common in patients undergoing TAVR.11 Frailty is also prevalent in patients with heart failure, which directly contributes to frailty by reducing exercise capacity and skeletal muscle function. Patients with congestive heart failure are more prone to falls and cognitive decline due to cerebral hypoperfusion, accelerating development of frailty and disability.50–52
Frailty and prognosis
Frail patients with CVD have a worse prognosis than non-frail patients.46,47,53–58 In 628 patients ≥65 years who underwent PCI at the Mayo Clinic, 3-year mortality was 28% for frail patients compared with 6% for non-frail patients using the Fried criteria.48 Frailty, quality of life, and co-morbidity each improved prediction of mortality in addition to the conventional Mayo Clinic risk score. In 4671 patients aged >65 years with an acute coronary syndrome managed medically who participated in the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY-ACS) trial,59 25% were pre-frail (one to two items) and 5% frail (≥3 items) by a questionnaire based on the Fried frailty score.29 Frail participants were more likely to suffer cardiovascular death, MI, or stroke after adjusting for the Global Registry of Acute Coronary Events (GRACE) score.
Frailty is a strong predictor of mortality in patients with chronic heart failure. In patients admitted to hospital with acute decompensated heart failure, simple measures of physical function have been associated with length of hospital stay, reduced activities of daily living, higher readmissions, and mortality.60 In one community-based study, the attributable-risk associated with frailty in patients with heart failure was 35% for emergency department visits and 19% for hospitalizations.61 In patients referred for cardiac surgery, frailty has been associated with postoperative mortality and morbidity, and greater need for rehabilitation and institutional care following the procedure.62,63 In patients with severe symptomatic aortic stenosis treated by TAVR, frailty predicts need for institutional care and mortality 6–12 months after a successful procedure.64–66
Relevance of frailty to clinical care
Identifying frailty has important implications for clinical care. The presence of frailty, worse health status, and more comorbid conditions identify a subset of elderly patients at higher risk of dying during the follow-up, even after a successful procedure (Table 5).67,68 The magnitude of risk associated with the frailty, comorbidity, and poor quality of life is greater than predicted from the risk models derived from conventional risk variables.65,67,68 For example, in the study from the Mayo Clinic, the presence of frailty in a patient undergoing PCI increased the risk of mortality five-fold and mortality/MI ∼2.5-fold compared with patients not determined frail at the time of the coronary intervention.48 Frailty is also a strong, independent predictor of emergency department visits and hospitalizations in community dwellers with heart failure.61 Identifying these conditions may be useful when counselling patients regarding their prognosis following a procedure. For example, if a patient is at moderate risk for long-term worse outcomes, they may decide against the procedure if they know the incremental risk from associated frailty and other age-associated determinants. Alternatively, a person at high risk estimated by conventional risk factors may be a better candidate if they are not frail and have good functional status. Assessment of frailty may therefore reclassify individuals to new and clinically meaningful risk categories. Identifying frailty can also prompt more comprehensive geriatric evaluation, and interventions to improve functional status. Reducing frailty is likely to both improve clinical outcomes and decrease healthcare utilization and costs.
Table 5.
Reasons for evaluating whether frailty is present in patients with cardiovascular diseases
| 1 | Population ageing is increasing the number of frail patients with CVD |
| 2 | Eye ball or end of the bed assessments of frailty may not be reliable |
| 3 | Frailty increases the risks of cardiac surgery and other cardiovascular interventions |
| 4 | Frailty increases the risk of cardiovascular and non-cardiovascular mortality and the need for future institutional care |
| 5 | Frail patients may have more complications from medical treatments |
| 6 | The benefits of some cardiac interventions may be less in frail elderly patients because of competing risks. Non-cardiac deaths dominate following TAVR, PCI, and CABG |
Management of patients diagnosed with frailty
In several observational studies, frail patients were less likely to receive cardiac catheterization or cardiac surgery (Figure 3).31,69 Despite observed differences in care, there is currently limited evidence on how treatment and management should be altered for frail patients. Individualized approaches will be needed, depending on the patient and the treatment options. Treatment decisions may raise ethical dilemmas, particularly when it is uncertain how much benefit a frail patient will obtain from an intervention. It is important to distinguish frailty from futility, where attempts to improve prognosis are useless.11 Frail patients may benefit greatly from treatments which reduce symptoms of limiting angina, and those related to heart failure or arrhythmia. Because frail patients have an increased risk of complications from procedures,67,68 a less invasive strategy may be preferred, for example, trans-cutaneous rather than surgical aortic valve replacement, or PCI rather than coronary artery bypass graft (CABG) for multi-vessel coronary artery disease. In some patients with a high mortality despite intervention, medical management may be more appropriate. In addition to frailty, quality of life, dependency, co-morbidity, dementia, and patient preference are relevant to these decisions.
Figure 3.
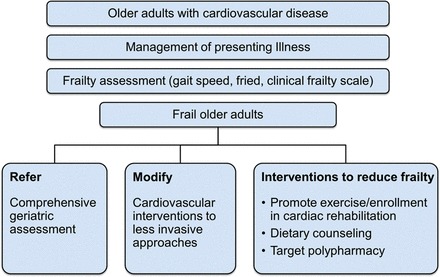
Proposed algorithm for older adults with cardiovascular disease.
The higher mortality of frail patients may reduce their ability to benefit from interventions when benefits accrue over time. Examples include elective repair of thoracic or abdominal aortic aneurysm, surgery for asymptomatic heart valve or coronary artery disease, and implantable cardioverter defibrillators. In a secondary analysis from the Surgical Treatment for Ischemic Heart Failure (STICH) trial which compared CABG with medical therapy in patients with ischaemic left ventricular dysfunction, patients with low exercise capacity, a marker of frailty, had a higher early mortality related to surgery if randomized to CABG, while mortality during ∼5-year follow-up was similar by treatment. In contrast, patients with better exercise capacity had a lower risk from surgery and lower mortality during the follow-up if randomized to CABG compared with medical therapy.70
Recognizing frailty is also important for patient care. Closer attention may be needed to avoid complications related to dosing of medication, and to reduce the risk of falls when in unfamiliar environments. Planning of care can consider the likelihood of longer hospital admission and greater need for long-term support after discharge. For some elective procedures ‘prehabilitation’, which would include optimal treatment of medical conditions and interventions to reduce frailty, could reduce procedural risks. Clinical trials are needed to evaluate this approach.
Interventions to reduce frailty
Frailty is dynamic and its earlier stages are potentially reversible.40 Adverse outcomes are likely to be less in frail patients when treatment of the presenting cardiovascular and associated medical conditions is optimized, and complications avoided. In addition, a number of interventions have been proposed to reduce frailty. Two issues are important clinically: first, identification of the causes of frailty and its association with chronic inflammation and vascular disease; and second, establishment of the possibilities for prevention and their effectiveness.12
Exercise prescription
The 2013 consensus statement on frailty focused on four interventions which have shown some efficacy in the treatment of frailty.40 The most consistent benefit has been demonstrated with interventions related to exercise.71–73 In a randomized trial, Singh et al.74 demonstrated that exercise-based rehabilitation decreased hospitalization and nursing home placement following hip fractures in frail patients. Enrolment in cardiac rehabilitation improves outcomes of patients with CVD,75,76 and may be particularly beneficial for frail patients. In addition to encouraging greater physical activity, specific deficits can be identified and prescription targeted to prevent and treat frailty. Patients with acute MI, stable angina, heart failure, cardiac transplant, or following major procedures such as PCI, CABG, or TAVR are eligible for cardiac rehabilitation. This facility, however, remains underutilized despite demonstration of improvement in outcomes.77,78 To improve outcomes of patients with CVD, cardiac rehabilitation services need to be optimally utilized and the protocols modified to cater for frail patients and to monitor their progress over the course of the treatment.
Dietary counselling may also be important. Nutritional supplements or a dietary plan that includes 25–30 g of high-quality protein per meal have been proposed to slow or prevent sarcopenic muscle loss.79 Nutritional supplements can increase muscle mass, improve grip strength, and work synergistically with the benefits of resistance exercises in older adults.72,80–84 Individual dietary prescription and supplements tailored to the needs of CVD patients with frailty presents the potential for an exciting new advance and current research efforts include the addition of branched amino acid leucine to resistance exercise in frail, older women (Clinical Trials, NCT 01922167).
Vitamin D supplements have been reported to improve muscle function, reduce falls,85 and fractures,86 and when combined with calcium to improve survival in elderly populations with vitamin D deficiency.87,88 However, other meta-analyses suggest calcium supplements with or without vitamin D may increase the risk of MI.89 There is currently uncertainty on whether vitamin D supplements benefit frail patients with CVD. The VITamin D and OmegA-3 TriaL (VITAL) which is currently enrolling 20 000 men and women in the USA to daily dietary supplements of vitamin D3 (2000 IU) or omega-3 fatty acids (Omacor® fish oil, 1 g) will provide more information on the role of vitamin D supplements.90
Polypharmacy or the use of multiple or duplicative medications increases the risk drug–drug and drug–disease interactions and contributes to adverse health outcomes. Reducing unnecessary medications can reduce costs and side effects.91–94 In 30 136 patients with non-ST elevation MI in the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) registry, excessive dosing of unfractionated or low-molecular weight heparin or glycoprotein IIb/IIIa inhibitors occurred in 42% of subjects, and was associated with more bleeding, increased length of hospital stay and higher mortality.94 Fifteen per cent of major bleeding was attributed to dosing errors which were more frequent in older subjects. Criteria proposed by Beers,95 the Screening Tool of Older Persons Potentially Inappropriate Prescriptions, and Screening Tool to Alert Doctors to the Right Treatment (STOPP/START criteria),96,97 can be used to guide reductions in poly-pharmacy.
Currently, evidence that interventions designed to improve frailty result in better outcomes in elderly patients with CVD is limited. Large randomised clinical trials are needed to evaluate the optimal management of these patients. The costs of care of the elderly are very high, and interventions which maintain independence of frail patients are more likely to be cost-effective. Evaluation of cost-effectiveness is an important aspect of future clinical trials.
Conclusion
Improved life expectancy and population ageing are increasing the number of frail adults with CVD. Current evidence-based treatment guidelines do not usually account for frailty and other measures of overall health, and data on the efficacy and safety of evidence-based treatments in frail elderly patients are currently limited. Identifying frailty is important because it is associated with an increased risk of both cardiovascular and non-cardiovascular morbidity and mortality, dependency, and complications from cardiovascular procedures and medical treatments. Including an objective assessment of frailty using simple tools will better inform the optimal care of older patients with CVD.
Conflict of interest: none declared.
Supplementary material
Supplementary material is available at European Heart Journal online.
References
- 1.2005 Heart disease and stroke: The nation's leading killers http://www.cdc.gov . [Google Scholar]
- 2.Roger VL, Jacobsen SJ, Weston SA, Bailey KR, Kottke TE, Frye RL. Trends in heart disease deaths in Olmsted County, Minnesota, 1979–1994. Mayo Clin Proc. 1999;74:651–657. doi: 10.4065/74.7.651. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Killian JM, Pfeifer EA, Belau PG, Kottke TE, Frye RL, Bailey KR, Jacobsen SJ. Time trends in the prevalence of atherosclerosis: a population-based autopsy study. Am J Med. 2001;110:267–273. doi: 10.1016/s0002-9343(00)00709-9. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 5.Alexander KP, Newby LK, Armstrong PW, Cannon CP, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- 6.Alexander KP, Newby LK, Cannon CP, Armstrong PW, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 7.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe: epidemiological update. Eur Heart J. 2013;34:3028–3034. doi: 10.1093/eurheartj/eht356. [DOI] [PubMed] [Google Scholar]
- 8.Nichols M, Townsend N, Scarborough P, Rayner M. Trends in age-specific coronary heart disease mortality in the European Union over three decades: 1980–2009. Eur Heart J. 2013;34:3017–3027. doi: 10.1093/eurheartj/eht159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.2012 https://www.census.gov/compendia//statab/2012/tables/12s0105 . [Google Scholar]
- 10.Spoon DB PP, Singh M, Holmes DR, Jr, Gersh BJ, Rihal CS, Lennon RJ, Moussa ID, Simari RD, Gulati R. Trends in causes of long-term death after percutaneous coronary intervention. Circulation. 2013;128:A16049. doi: 10.1161/CIRCULATIONAHA.113.006518. [DOI] [PubMed] [Google Scholar]
- 11.Holmes DR, Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D, Thomas JD. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200–1254. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Strandberg TE, Pitkala KH. Frailty in elderly people. Lancet. 2007;369:1328–1329. doi: 10.1016/S0140-6736(07)60613-8. [DOI] [PubMed] [Google Scholar]
- 13.Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53:40–47. doi: 10.1111/j.1532-5415.2005.53008.x. [DOI] [PubMed] [Google Scholar]
- 14.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, Corsi AM, Bandinelli S, Guralnik JM. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25(10 Suppl):10–15. [PubMed] [Google Scholar]
- 16.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 17.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 18.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 19.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 20.Walston J, Arking DE, Fallin D, Li T, Beamer B, Xue Q, Ferrucci L, Fried LP, Chakravarti A. IL-6 gene variation is not associated with increased serum levels of IL-6, muscle, weakness, or frailty in older women. Exp Gerontol. 2005;40:344–352. doi: 10.1016/j.exger.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 22.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 23.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, Tosato M, Bernabei R, Onder G. Sarcopenia and mortality among older nursing home residents. J Am Med Direct Assoc. 2012;13:121–126. doi: 10.1016/j.jamda.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 24.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling CH, Taekema D, de Craen AJ, Gussekloo J, Westendorp RG, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ. 2010;182:429–435. doi: 10.1503/cmaj.091278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Iersel MB, Munneke M, Esselink RA, Benraad CE, Olde Rikkert MG. Gait velocity and the Timed-Up-and-Go test were sensitive to changes in mobility in frail elderly patients. J Clin Epidemiol. 2008;61:186–191. doi: 10.1016/j.jclinepi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 30.Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, Dam TT, Marshall LM, Orwoll ES, Cummings SR. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 32.Hilmer SN, Perera V, Mitchell S, Murnion BP, Dent J, Bajorek B, Matthews S, Rolfson DB. The assessment of frailty in older people in acute care. Aus J Ageing. 2009;28:182–188. doi: 10.1111/j.1741-6612.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 33.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 34.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 36.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vellas B, Balardy L, Gillette-Guyonnet S, Abellan Van Kan G, Ghisolfi-Marque A, Subra J, Bismuth S, Oustric S, Cesari M. Looking for frailty in community-dwelling older persons: the Gerontopole Frailty Screening Tool (GFST) J Nutr Health Aging. 2013;17:629–631. doi: 10.1007/s12603-013-0363-6. [DOI] [PubMed] [Google Scholar]
- 38.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Inter Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 40.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Direct Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 42.Singh M, Alexander K, Roger VL, Rihal CS, Whitson HE, Lerman A, Jahangir A, Nair KS. Frailty and its potential relevance to cardiovascular care. Mayo Clin Proc. 2008;83:1146–1153. doi: 10.4065/83.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 44.Kanwar A, Singh M, Lennon R, Ghanta K, McNallan SM, Roger VL. Frailty and health-related quality of life among residents of long-term care facilities. J Aging Health. 2013;25:792–802. doi: 10.1177/0898264313493003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Haehling S, Anker SD, Doehner W, Morley JE, Vellas B. Frailty and heart disease. Int J Cardiol. 2013;168:1745–1747. doi: 10.1016/j.ijcard.2013.07.068. [DOI] [PubMed] [Google Scholar]
- 46.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 47.Newman AB, Arnold AM, Naydeck BL, Fried LP, Burke GL, Enright P, Gottdiener J, Hirsch C, O'Leary D, Tracy R. ‘Successful aging’: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 48.Singh M, Rihal CS, Lennon RJ, Spertus JA, Nair KS, Roger VL. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circulation. 2011;4:496–502. doi: 10.1161/CIRCOUTCOMES.111.961375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh MRV, Rihal C, Lennon R, Jahangir A, Lerman A, Sloan J, Spertus J. Correlates of frailty in patients with coronary heart disease undergoing percutaneous coronary interventions. Circulation. 2007;115:E556. [Google Scholar]
- 50.Gerber Y, Melton LJ, III, McNallan SM, Jiang R, Weston SA, Roger VL. Cardiovascular and noncardiovascular disease associations with hip fractures. Am J Med. 2013;126:169 e19–26. doi: 10.1016/j.amjmed.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hubbard RE, O'Mahony MS, Calver BL, Woodhouse KW. Nutrition, inflammation, and leptin levels in aging and frailty. J Am Geriatr Soc. 2008;56:279–284. doi: 10.1111/j.1532-5415.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 52.Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol. 2007;16:171–174. doi: 10.1111/j.1076-7460.2007.06563.x. [DOI] [PubMed] [Google Scholar]
- 53.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 54.Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women's Health and Aging Studies I and II. J Gerontol A, Biol Sci Med Sci. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 55.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 56.Boxer RS, Wang Z, Walsh SJ, Hager D, Kenny AM. The utility of the 6-minute walk test as a measure of frailty in older adults with heart failure. Am J Geriatr Cardiol. 2008;17:7–12. doi: 10.1111/j.1076-7460.2007.06457.x. [DOI] [PubMed] [Google Scholar]
- 57.Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Cong Heart Fail. 2010;16:208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez E, Vidan MT, Serra JA, Fernandez-Aviles F, Bueno H. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart. 2011;97:1602–1606. doi: 10.1136/hrt.2011.227504. [DOI] [PubMed] [Google Scholar]
- 59.Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, Ardissino D, Nicolau JC, Boden WE, Gurbel PA, Ruzyllo W, Dalby AJ, McGuire DK, Leiva-Pons JL, Parkhomenko A, Gottlieb S, Topacio GO, Hamm C, Pavlides G, Goudev AR, Oto A, Tseng CD, Merkely B, Gasparovic V, Corbalan R, Cinteza M, McLendon RC, Winters KJ, Brown EB, Lokhnygina Y, Aylward PE, Huber K, Hochman JS, Ohman EM. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–1309. doi: 10.1056/NEJMoa1205512. [DOI] [PubMed] [Google Scholar]
- 60.Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R, Guralnik JM. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNallan SM, Singh M, Chamberlain AM, Kane RL, Dunlay SM, Redfield MM, Weston SA, Roger VL. Frailty and healthcare utilization among patients with heart failure in the community. JACC. 2013;1:135–141. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 63.Sundermann S, Dademasch A, Rastan A, Praetorius J, Rodriguez H, Walther T, Mohr FW, Falk V. One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact Cardiovasc Thorac Surg. 2011;13:119–123. doi: 10.1510/icvts.2010.251884. discussion 123. [DOI] [PubMed] [Google Scholar]
- 64.Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, Maurer MS, Kirtane AJ, Kodali S, Moses JW, Leon MB, Smith CR, Williams M. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schoenenberger AW, Stortecky S, Neumann S, Moser A, Juni P, Carrel T, Huber C, Gandon M, Bischoff S, Schoenenberger CM, Stuck AE, Windecker S, Wenaweser P. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI) Eur Heart J. 2013;34:684–692. doi: 10.1093/eurheartj/ehs304. [DOI] [PubMed] [Google Scholar]
- 66.Stortecky S, Schoenenberger AW, Moser A, Kalesan B, Juni P, Carrel T, Bischoff S, Schoenenberger CM, Stuck AE, Windecker S, Wenaweser P. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489–496. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Rumsfeld JS, MaWhinney S, McCarthy M, Jr, Shroyer AL, VillaNueva CB, O'Brien M, Moritz TE, Henderson WG, Grover FL, Sethi GK, Hammermeister KE. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. JAMA. 1999;281:1298–1303. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- 68.Singh M, Rihal C, Roger VL, Lennon R, Spertus J, Jahangir A, Holmes D., Jr Comorbid conditions and outcomes after percutaneous coronary intervention. Heart. 2008;94:1424–1428. doi: 10.1136/hrt.2007.126649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ekerstad N, Swahn E, Janzon M, Alfredsson J, Lofmark R, Lindenberger M, Carlsson P. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 70.Stewart RA ZD, She L, Lee K, Drazner M, Lubiszewska B, Hebeler R, Kosevic D, Ruengsakulrach P, Nicolau J, Coutu B, Choudhary S, Mark D, Cleland J, Pina I, Velazquez E, Rynkiewicz A, White H. Exercise capacity and mortality in patients with ischemic left ventricular dysfunction randomized to coronary artery bypass surgery or medical therapy: an analysis from the Surgical Treatment for Ischemic Heart Failure (STICH) trial. JACC Heart Fail. 2014 doi: 10.1016/j.jchf.2014.02.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ory MG, Schechtman KB, Miller JP, Hadley EC, Fiatarone MA, Province MA, Arfken CL, Morgan D, Weiss S, Kaplan M. Frailty and injuries in later life: the FICSIT trials. J Am Geriatr Soc. 1993;41:283–296. doi: 10.1111/j.1532-5415.1993.tb06707.x. [DOI] [PubMed] [Google Scholar]
- 72.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. The N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 73.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh NA, Quine S, Clemson LM, Williams EJ, Williamson DA, Stavrinos TM, Grady JN, Perry TJ, Lloyd BD, Smith EU, Singh MA. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Direct Assoc. 2012;13:24–30. doi: 10.1016/j.jamda.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. doi: 10.1161/CIRCULATIONAHA.110.983536. [DOI] [PubMed] [Google Scholar]
- 76.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Thomas RJ, Squires RW, Allison TG. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15:336–340. doi: 10.1097/HJR.0b013e3282f48348. [DOI] [PubMed] [Google Scholar]
- 77.Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 78.Pack QR, Goel K, Lahr BD, Greason KL, Squires RW, Lopez-Jimenez F, Zhang Z, Thomas RJ. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation. 2013;128:590–597. doi: 10.1161/CIRCULATIONAHA.112.001365. [DOI] [PubMed] [Google Scholar]
- 79.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, de Groot LC. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Direct Assoc. 2012;13:720–726. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Direct Assoc. 2012;13:713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 82.Tieland M, Borgonjen-Van den Berg KJ, van Loon LJ, de Groot LC. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr. 2012;51:173–179. doi: 10.1007/s00394-011-0203-6. [DOI] [PubMed] [Google Scholar]
- 83.Paddon-Jones D. Perspective: exercise and protein supplementation in frail elders. J Am Med Direct Assoc. 2013;14:73–74. doi: 10.1016/j.jamda.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 84.Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Direct Assoc. 2013;14:10–17. doi: 10.1016/j.jamda.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, Almandoz JP, Mullan RJ, Lane MA, Liu H, Erwin PJ, Hensrud DD, Montori VM. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- 86.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stahelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40–49. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 87.Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, Salovaara K, Cooper C, Smith HE, Jacobs ET, Torgerson D, Jackson RD, Manson JE, Brixen K, Mosekilde L, Robbins JA, Francis RM, Abrahamsen B. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012;97:2670–2681. doi: 10.1210/jc.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 89.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flaherty JH, Perry HM, III, Lynchard GS, Morley JE. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:M554–M559. doi: 10.1093/gerona/55.10.m554. [DOI] [PubMed] [Google Scholar]
- 92.Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, McLachlan AJ, Cumming RG, Handelsman DJ, Le Couteur DG. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–995. doi: 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 93.Jano E, Aparasu RR. Healthcare outcomes associated with beers’ criteria: a systematic review. Ann Pharmacother. 2007;41:438–447. doi: 10.1345/aph.1H473. [DOI] [PubMed] [Google Scholar]
- 94.Alexander KP, Chen AY, Roe MT, Newby LK, Gibson CM, Allen-LaPointe NM, Pollack C, Gibler WB, Ohman EM, Peterson ED. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA. 2005;294:3108–3116. doi: 10.1001/jama.294.24.3108. [DOI] [PubMed] [Google Scholar]
- 95.American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barry PJ, Gallagher P, Ryan C, O'Mahony D. START (screening tool to alert doctors to the right treatment)—an evidence-based screening tool to detect prescribing omissions in elderly patients. Age Ageing. 2007;36:632–638. doi: 10.1093/ageing/afm118. [DOI] [PubMed] [Google Scholar]
- 97.Gallagher P, O'Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing. 2008;37:673–679. doi: 10.1093/ageing/afn197. [DOI] [PubMed] [Google Scholar]
- 98.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63:984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 99.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, Sherrington C, Lord SR, Kurrle SE. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fairhall N, Aggar C, Kurrle SE, Sherrington C, Lord S, Lockwood K, Monaghan N, Cameron ID. Frailty intervention trial (FIT) BMC Geriatr. 2008;8:27. doi: 10.1186/1471-2318-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]


