Abstract
Published reports examining lipid levels during pregnancy and preeclampsia have been inconsistent. The objective of this meta-analysis was to test the association between preeclampsia and maternal total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), non-HDL-C, and triglyceride levels measured during pregnancy. We conducted a systematic search for studies published between the index date until July 2013 reporting maternal lipid levels in women with preeclampsia and normotensive pregnant women. Seventy-four studies met all eligibility criteria and were included in the meta-analysis. Weighted mean differences in lipid levels were calculated using a random-effects model. Statistical heterogeneity was investigated using the I2 statistic. Meta-regression was used to identify sources of heterogeneity. Preeclampsia was associated with elevated total cholesterol, non-HDL-C, and triglyceride levels, regardless of gestational age at the time of blood sampling, and with lower levels of HDL-C in the third trimester. A marginal association was found with LDL-C levels. Statistical heterogeneity was detected in all analyses. Meta-regression analyses suggested that differences in body mass index (weight (kg)/height (m)2) across studies may be partially responsible for the heterogeneity in the triglyceride and LDL-C analyses. This systematic review and meta-analysis demonstrates that women who develop preeclampsia have elevated levels of total cholesterol, non-HDL-C, and triglycerides during all trimesters of pregnancy, as well as lower levels of HDL-C during the third trimester.
Keywords: body mass index, cholesterol, hyperlipidemia, hypertriglyceridemia, meta-analysis, preeclampsia, systematic review, triglycerides
Preeclampsia is a potentially devastating disease of pregnancy that complicates 2%–8% of all pregnancies in the United States and can threaten the life of both the mother and her unborn child (1, 2). Manifesting after 20 weeks of gestation, preeclampsia is a multiorgan disorder defined as de novo hypertension (systolic blood pressure ≥140 mm Hg; diastolic blood pressure ≥90 mm Hg) combined with proteinuria (≥300 mg/24 hours), as defined by the American Congress of Obstetricians and Gynecologists (3). Without intervention, the mother is at substantial risk for seizures (eclampsia), renal and liver failure, pulmonary edema, stroke, and death (1). For the fetus, preeclampsia poses increased risks of intrauterine growth restriction, prematurity, and death (4). Preeclampsia is also recognized as a major risk factor for cardiovascular disease later in life for both the woman and her child (5). Despite considerable research, the only effective treatment for preeclampsia is to deliver the baby, placenta, and all products of conception (4).
Maternal endothelial dysfunction is a classic hallmark of preeclampsia (6). Many markers of endothelial dysfunction have been reported in preeclamptic women, including an imbalance of anticoagulation and procoagulation factors and increased levels of fibronectin, endothelial cell adhesion molecules, and other factors in the coagulation cascade (7). Increased levels of circulating lipids result in their accumulation within endothelial cells. This accumulation decreases the release of prostacyclin, resulting in oxidative stress via endothelial dysfunction (8), a key mechanism in the proposed pathophysiology of preeclampsia (9).
Recently, a meta-analysis was performed on studies evaluating the relationship between maternal serum triglyceride levels and preeclampsia, and the authors found that women with preeclampsia had significantly higher levels of triglycerides than normotensive women (10). Although numerous studies suggest that a dyslipidemic pattern of increased total cholesterol, triglycerides, and low-density lipoprotein cholesterol (LDL-C), along with decreased high-density lipoprotein cholesterol (HDL-C) concentrations may be associated with an increased risk of preeclampsia, results are inconsistent (11–15). Many of these studies have had small sample sizes, and the gestational age at the time of the lipid measurements has varied, making it difficult to compare findings across studies. The relationship between preeclampsia and non-HDL-C, a lipid measurement reflecting atherogenic, triglyceride-rich lipoproteins (approximately 75% of which are LDL-C) (16), has not often been evaluated. Thus, we conducted a systematic review and meta-analysis to examine the associations of total cholesterol, LDL-C, HDL-C, and non-HDL-C during pregnancy with the subsequent risk of preeclampsia and to confirm associations reported in a previous meta-analysis of triglyceride levels using more recent studies and a broader search strategy. Subgroup analyses focusing on potential differences between lipid levels in both the mild and severe forms of preeclampsia are also presented.
METHODS
Search strategy
This study consisted of a systematic literature review followed by a meta-analysis, both conducted taking into account the Meta-analysis of Observational Studies in Epidemiology: a Proposal for Reporting Criteria and Preferred Reporting Items for Systematic Reviews and Meta-analyses group guidelines (17, 18). A systematic literature search of eligible studies was conducted in the PubMed/MEDLINE and Scopus databases from the index date through July 2013 for studies evaluating the association between lipid levels during pregnancy and preeclampsia using the following search strategy: (“preeclampsia” OR “eclampsia” OR “toxemia” OR “pregnancy-induced hypertension” OR “gestational hypertension”) AND (“cholesterol” OR “lipid” OR “HDL” OR “LDL” OR “triglycerides” OR “dyslipidemia” OR “hyperlipidemia” OR “hypertriglyceridemia”). The search was not restricted by language, and no limits or filters were placed on the search to ensure maximal sensitivity. References from these publications were also manually searched for potentially relevant citations not detected by the electronic search. A research librarian assisted in creating the most efficient and effective search strategy possible.
Study selection
Records identified from the literature search were screened for duplicates. Titles and abstracts were screened, and potentially relevant articles were selected for full-text review. Studies were considered for inclusion in our meta-analysis if they met the following criteria: 1) an original study that examined the association between lipid levels during pregnancy and preeclampsia; 2) raw lipid levels presented as a group mean with either a standard error or standard deviation or as a median with the 95% confidence interval; and 3) a proper control group of normotensive pregnant women.
Data abstraction
Full manuscripts were obtained for studies that appeared to examine lipid levels in women with preeclampsia. Full-text review was performed by 2 independent investigators (C.N.S. and C.J.S.) using a piloted data abstraction form and resulting in 91% concordance. Information collected included study characteristics (author, year of publication, study location, dates, and design); participant characteristics (definitions of the preeclampsia and control groups, diagnostic criteria, mean age, mean prepregnancy body mass index (BMI) (weight (kg)/height (m)2), and mean gestational age at blood sampling); lipid measurements (mean or median, along with standard error (SE), standard deviation (SD), or 95% confidence interval (CI)); number of subjects in each group; and statistical significance for tests between the groups). Inconsistencies between the 2 reviewers were adjudicated by a third, independent reviewer (K.K.R.).
Quality assessment
The quality assessment was performed by applying the Newcastle-Ottawa Scale (19). In tailoring the scale to fit this study, we took into account the sampling methods of the studies and the similarities between the study groups on age and BMI, exposure and outcome ascertainment, and study design. Our abstracting instrument included a total of 8 questions with 11 points possible. While abstracting the data for the meta-analysis, C.N.S. and C.J.S. independently performed the quality assessment. Overall, the publications were classified as high quality (scoring ≥5 points) or low quality (scoring <5 points). Only studies scoring 5 or more points were included, ensuring only high-quality research articles were included in this meta-analysis.
Data synthesis and analysis
Lipid measurements originally reported in millimolars were converted to milligrams per deciliter. When standard errors for the means were provided, they were converted to standard deviations. Because our meta-regression results suggest that variation in trimester of lipid measurement is a potential source of heterogeneity, studies were categorized by trimester on the basis of the mean gestational age reported at blood sampling. First, second, and third trimesters were defined as 1–13, 14–26, and 27 or more weeks, respectively. Blood collection times that overlapped 2 trimesters were classified as the latter of the 2 trimesters. Because of the low number of studies with lipid measurements in the first trimester, the first and second trimesters were combined for all analyses. If measured non-HDL-C was not available, non-HDL-C was calculated as total cholesterol minus HDL-C (20).
Lipid levels among women with preeclampsia and healthy pregnant controls were compared by calculating weighted mean differences (WMDs) and 95% confidence intervals, stratified by trimester of lipid measurement. Because significant heterogeneity was observed in all comparisons (P < 0.05), random-effects models were used to allow for the inherent heterogeneity found between studies (21). We also performed similar meta-analyses stratified by preeclampsia severity. Statistical heterogeneity was assessed using the Mantel-Haenszel Q statistic and the I2 statistic. An I2 value of more than 50% is considered moderate heterogeneity, and an I2 value greater than 75% is considered high heterogeneity. Random-effects meta-regression analyses were conducted to assess whether BMI, trimester of blood sampling, and fasting blood sampling status were acting as potential modifiers of the association between maternal lipid levels and preeclampsia, causing the observed high level of heterogeneity. Possible publication bias was evaluated using funnel plots and the Egger test. All statistical analyses were 2-sided and were performed using Stata, version 9.0, software (StataCorp LP, College Station, Texas).
RESULTS
Literature search
Results from our search strategy are summarized in Figure 1. We identified 3,993 publications. Of these, 898 were duplicates between the 2 databases, and an additional 2,823 were excluded on the basis of review of their titles and abstracts. The remaining 141 articles were eligible for abstraction. After reviewing the full texts, we excluded an additional 67 articles. The most common reason for exclusion after full-text review was the inability to obtain or extract the data necessary for analysis. Sixteen studies were excluded on the basis of their low quality scores (<5 points). Six studies using the same patient data were identified (22–28). To ensure the data were represented only once, we excluded 5 of the studies from the analysis (22, 24, 25, 27, 28). In addition, because we intended to stratify the analysis on the basis of trimester of triglyceride measurement, we also excluded 5 studies that did not report the gestational age at the time of blood sampling. After final exclusions, 74 original articles were included in our meta-analyses: 64 for total cholesterol, 60 for HDL-C, 54 for LDL-C, 70 for triglycerides, and 46 for non-HDL-C. Flow diagrams for the studies included in the subgroup analyses of preeclampsia severity are provided in Web Figures 1–4, available at http://aje.oxfordjournals.org/.
Figure 1.
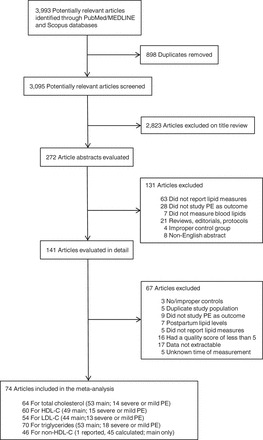
Flow diagram of study selection for the meta-analysis of the association between lipid measurements during pregnancy and risk of preeclampsia (PE). “Main” refers to the main meta-analysis of lipid levels during pregnancy and risk of any PE. “Severe or mild” refers to the subanalysis of lipid levels during pregnancy and the risk of PE, stratified by PE severity. Overlap between the 2 meta-analyses is possible. Studies were published from the index date of the databases until July 2013. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Study characteristics
The studies that met the eligibility criteria for inclusion in the main preeclampsia meta-analysis examined a total of 7,369 participants—1,975 women with preeclampsia and 5,394 healthy pregnant women. Studies that evaluated lipid levels by severity of preeclampsia (for severe preeclampsia, n = 19; for mild preeclampsia, n = 15) included 568 women with severe preeclampsia compared with 1,004 normotensive pregnant controls, as well as 427 women with mild preeclampsia compared with 667 normotensive healthy pregnant women. Characteristics of the studies included in the meta-analyses are shown in Table 1. The 73 included studies were conducted in Asia (52%), Europe (25%), and North America (10%) with sample sizes ranging from 20 to 1,000 pregnant women. The majority of the included studies measured serum lipid levels during the third trimester, and fasting measurements were obtained in 62% of the studies. Within each study, controls were commonly matched to women with preeclampsia on maternal age, gestational age at blood sampling, and/or maternal BMI. In preeclamptic women, mean lipid levels ranged from 162–345 mg/dL, 29–79 mg/dL, 116–300 mg/dL, 96–197 mg/dL, and 100–436 mg/dL for total cholesterol, HDL-C, non-HDL-C, LDL-C, and triglycerides, respectively. In normotensive women, values ranged from 111–317 mg/dL, 33–93 mg/dL, 90–243 mg/dL, 96–197 mg/dL, and 111–269 mg/dL for total cholesterol, HDL-C, non-HDL-C, LDL-C, and triglycerides, respectively. Characteristics of the studies included in the subanalyses of severe and mild preeclampsia can be found in Web Table 1.
Table 1.
Characteristics of Studies Included in the Main Meta-Analysis of Lipid Measurements During Pregnancy and Preeclampsia, 1950–July 2013
| First Author, Year (Reference No.) | Country | Trimester of Blood Sampling | No. of Cases | No. of Controls | Fasting Status | BMIa Similar Between Cases and Controls | Mean Lipid Levels, mg/dL |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cholesterol |
HDL-C |
LDL-C |
Triglycerides |
|||||||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |||||||
| Adiga, 2007 (48) | India | Third | 25 | 25 | Yes | Yes | 246 | 203 | 48 | 66 | ||||
| Ahenkorah, 2008 (49) | Ghana | Third | 30 | 50 | Yes | No | 244 | 248 | 73 | 77 | 172 | 149 | 304 | 225 |
| Akiibinu, 2013 (50) | Nigeria | Third | 32 | 40 | Unknown | No | 176 | 111 | ||||||
| Aziz, 2007 (51) | Pakistan | Third | 16 | 16 | Yes | Yes | 178 | 184 | 40 | 51 | 118 | 108 | 232 | 113 |
| Babacan, 2011 (52) | Turkey | Third | 34 | 11 | Unknown | Unknown | 345 | 182 | 46 | 62 | 162 | 109 | 281 | 204 |
| Barden, 2001 (53) | Australia | Third | 21 | 19 | Unknown | Unknown | 270 | 278 | 61 | 68 | 154 | 166 | 323 | 227 |
| Belo, 2002 (23) | Portugal | Third | 51 | 67 | No | Yes | 268 | 285 | 54 | 62 | 140 | 146 | 239 | 186 |
| Caruso, 1999 (54) | Italy | Third | 10 | 10 | Yes | Yes | 79 | 59 | 116 | 135 | 261 | 237 | ||
| Cekmen, 2003 (55) | Turkey | Third | 32 | 34 | Yes | Unknown | 249 | 225 | 32 | 43 | 171 | 132 | 274 | 234 |
| Chalas, 2002 (56) | France | Third | 24 | 25 | Yes | Yes | 253 | 252 | 73 | 68 | 206 | 172 | ||
| Dane, 2009 (57) | Turkey | Third | 10 | 97 | Yes | Unknown | 52 | 61 | 379 | 221 | ||||
| Demir, 2011 (58) | Turkey | Third | 35 | 35 | Yes | Yes | 173 | 177 | 64 | 52 | 124 | 132 | 131 | 133 |
| Demirci, 2011 (59) | Turkey | Second | 30 | 320 | Yes | No | 220 | 201 | 60 | 60 | 158 | 121 | ||
| Dey, 2013 (60) | India | Second | 24 | 279b and 282c | Yes | Yes | 200b and 230c | 198b and 192c | 49b and 48c | 49b and 47c | 119b and 118c | 117b and 119c | 167b and 177c | 167b and 172c |
| Duan, 2011 (61) | China | Third | 72 | 72 | Yes | No | 251 | 226 | 57 | 58 | 123 | 116 | 354 | 229 |
| Enquobahrie, 2004 (62) | United States | First | 27 | 510 | No | Unknown | 198 | 191 | 64 | 65 | 108 | 97 | 138 | 121 |
| Francoual, 2002 (63) | France | Third | 24 | 25 | Unknown | Unknown | 254 | 244 | 70 | 65 | 123 | 107 | 253 | 208 |
| Garzetti, 1993 (64) | Italy | Third | 20 | 20 | Unknown | Unknown | 232 | 219 | ||||||
| Gohil, 2011 (65) | India | Third | 50 | 40 | Unknown | Unknown | 238 | 209 | 42 | 60 | 136 | 116 | 270 | 215 |
| Harsem, 2007 (66) | Norway | Third | 38 | 41 | Yes | Yes | 186 | 169 | ||||||
| Iftikhar, 2010 (12) | Pakistan | Third | 45 | 45 | Unknown | Unknown | 282 | 279 | 33 | 35 | 162 | 148 | 245 | 186 |
| Islam, 2010 (67) | Bangladesh | Third | 30 | 40 | Yes | Unknown | 271 | 263 | 42 | 56 | 134 | 115 | 226 | 166 |
| Jamalzei, 2013 (68) | Iran | Third | 100 | 100 | Yes | No | 180 | 182 | 52 | 49 | 178 | 186 | 260 | 220 |
| Kaaja, 1995 (69) | Finland | Third | 8 | 21 | Yes | Yes (matched) | 196 | 190 | 31 | 43 | 124 | 151 | 328 | 177 |
| Kalar, 2012 (70) | Pakistan | Third | 22 | 22 | Unknown | Yes | 193 | 203 | 37 | 51 | 13 | 99 | 254 | 117 |
| Kandimalla, 2011 (71) | Trinidad and Tobago | Second | 11 | 91 | No | Yes | 236 | 259 | 59 | 68 | 107 | 101 | 146 | 106 |
| Kashinakunti, 2010 (72) | India | Third | 90 | 90 | Yes | Yes | 289 | 160 | 43 | 67 | 111 | 114 | 215 | 188 |
| Kim, 2007 (73) | Korea | Third | 32 | 57 | Unknown | Unknown | 232 | 227 | 46 | 67 | 128 | 121 | 280 | 226 |
| Koçyigit, 2004 (13) | Turkey | Third | 45 | 30 | Yes | Yes | 270 | 294 | 29 | 51 | 192 | 112 | 256 | 111 |
| Lei, 2011 (74) | China | Third | 33 | 200 | Yes | Yes | 228 | 217 | 50 | 57 | 108 | 120 | 353 | 269 |
| Llurba, 2004 (75) | Spain | Third | 53 | 30 | Yes | Yes | 302 | 286 | 269 | 221 | ||||
| Lorentzen, 1995 (76) | Norway | Second | 19 | 19 | Yes | Yes (matched) | 115d and 372e | 80d and 213e | ||||||
| Madazli, 1999 (77) | Turkey | Third | 22 | 21 | Yes | Unknown | 308 | 277 | 39 | 46 | 157 | 177 | 341 | 253 |
| Maksane, 2011 (78) | India | Third | 20 | 20 | Yes | Unknown | 162 | 149 | 46 | 60 | 136 | 116 | 278 | 215 |
| Mihu, 2009 (79) | Romania | Third | 25 | 25 | Yes | Yes | 261 | 261 | 58 | 61 | 268 | 221 | ||
| Mohindra, 2002 (80) | India | Third | 54 | 33 | Yes | Unknown | 233 | 186 | ||||||
| Mori, 2010 (81) | Japan | Third | 15 | 17 | Yes | Yes | 309 | 317 | 73 | 93 | 130 | 139 | 289 | 174 |
| Mukherjee, 2010 (44) | India | Third | 62 | 54 | Yes | Yes | 233 | 186 | 39 | 52 | 139 | 102 | 279 | 158 |
| Murai, 1997 (82) | United States | Third | 31 | 31 | No | No | 249 | 192 | ||||||
| Negrato, 2009 (83) | Brazil | Second | 19 | 180 | Yes | No | 61 | 63 | 224 | 200 | ||||
| Nelson, 1966 (84) | United States | Third | 10 | 12 | Unknown | Unknown | 309 | 317 | 191 | 159 | ||||
| Pecks, 2012 (85) | Germany | Third | 14 | 28 | Unknown | Unknown | 232 | 201 | 69 | 75 | 130 | 146 | 271 | 191 |
| Peng, 1985 (86) | China | Third | 40 | 40 | Yes | Unknown | 254 | 230 | 55 | 73 | 128 | 125 | ||
| Powers, 1998 (87) | United States | Third | 20 | 32 | Yes | Yes (matched) | 286 | 183 | ||||||
| Reyna-Villasmil, 2008 (88) | Venezuela | Third | 35 | 35 | Unknown | Unknown | 251 | 263 | 51 | 67 | 113 | 133 | 300 | 196 |
| Rodie, 2004 (89) | United Kingdom | Third | 23 | 23 | No | Yes | 198 | 201 | 153 | 141 | ||||
| Rosing, 1989 (90) | Sweden | Third | 26 | 21 | Yes | Unknown | 293 | 172 | 28 | 35 | 345 | 221 | ||
| Rudra, 2006 (91) | United States | Second | 22 | 711 | No | Unknown | 238 | 214 | 104 | 102 | 164 | 144 | ||
| Sahu, 2009 (14) | India | Third | 30 | 30 | Yes | Unknown | 175 | 168 | 50 | 66 | 197 | 89 | 234 | 87 |
| Sharami, 2012 (92) | Iran | Third | 41 | 41 | Yes | Yes | 220 | 193 | 43 | 49 | 138 | 124 | 340 | 203 |
| Stefanović, 2009 (93) | Serbia | Third | 17 | 20 | Unknown | Yes | 57 | 53 | 111 | 118 | 323 | 173 | ||
| Takahashi, 2008 (94) | Brazil | First | 9 | 39 | Unknown | Yes | 212f and 183g | 186f and 191g | 59 | 59 | 87f and 113g | 89f and 99g | 110f and 205g | 115f and 165g |
| Uzun, 2005 (15) | Turkey | Third | 41 | 33 | Yes | Unknown | 252 | 237 | 42 | 52 | 122 | 106 | 235 | 140 |
| Vanderjagt, 2004 (95) | Nigeria | Third | 43 | 130 | Unknown | Unknown | 266 | 263 | 55 | 63 | 145 | 153 | 203 | 190 |
| Ware-Jauregui, 1999 (96) | Peru | Third | 125 | 179 | No | Yes | 339 | 233 | 39 | 42 | 146 | 144 | 301 | 249 |
| Wetzka, 1999 (97) | Germany | Third | 9 | 24 | Yes | Yes | 231 | 234 | 64 | 72 | 105 | 130 | 436 | 234 |
| Yamaguchi, 1988 (98) | Japan | Third | 29 | 29 | Yes | Unknown | 260 | 241 | 33 | 33 | 96 | 75 | 339 | 233 |
| Zhou, 2012 (99) | China | Second | 61 | 939 | Yes | No | 197 | 191 | 79 | 85 | 105 | 107 | 246 | 214 |
| Ziaei, 2006 (100) | Iran | Third | 25 | 25 | Unknown | Yes (matched) | 260 | 241 | 62 | 66 | 146 | 132 | 302 | 213 |
| Ziaei, 2012 (101) | Iran | Second | 14 | 127 | Yes | Yes (matched) | 197 | 191 | 57 | 55 | 125 | 111 | 203 | 144 |
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
a Weight (kg)/height (m)2.
b At 15 weeks.
c At 19 weeks.
d At 17 weeks.
e In the third trimester.
f Before 13 weeks.
g At 25 weeks.
Meta-analyses
Results from the WMD meta-analyses of lipid measurements during pregnancy and preeclampsia are presented in Table 2. Total cholesterol levels measured in the first or second trimester were significantly higher in women who developed preeclampsia than in normotensive pregnant women (WMD = 12.49 mg/dL, 95% CI: 3.44, 21.54). Total cholesterol levels measured in the third trimester were also significantly higher in preeclamptic women compared with normotensive pregnant women (WMD = 20.20 mg/dL, 95% CI: 8.70, 31.70). Although no relationship with preeclampsia was observed for HDL-C levels measured in the first trimester, preeclamptic women had significantly lower HDL-C levels in the third trimester than normotensive women (WMD = −8.86 mg/dL, 95% CI: −11.50, −6.21). This relationship was also observed when stratifying by mild and severe preeclampsia. Non-HDL-C measured in both the first/second trimesters (WMD = 11.57, 95% CI: 3.47, 19.67) and third trimester (WMD = 29.59, 95% CI: 12.13, 47.06) was significantly higher among preeclamptic women than among normotensive pregnant women. LDL-C levels measured in the first/second trimesters (WMD = 3.89 mg/dL, 95% CI: −0.19, 7.97) and third trimester (WMD = 10.92, 95% CI: −0.59, 22.42) were greater among women who developed preeclampsia than among those who remained normotensive throughout pregnancy, though these relationships were only marginally significant (P = 0.06). Triglyceride levels measured in the first/second trimesters were significantly higher in women who developed preeclampsia than in normotensive pregnant women (WMD = 25.08 mg/dL, 95% CI: 14.39, 35.77). Triglyceride levels measured in the third trimester were also significantly higher in preeclamptic women when compared with normotensive pregnant women (WMD = 80.29 mg/dL, 95% CI: 51.45, 109.13). This relationship was also observed when stratifying by mild and severe preeclampsia. Forest plots for all meta-analyses can be found in Web Figures 5–21.
Table 2.
Summary Weighted Mean Differences From Meta-Analyses of the Association Between Lipid Levels During Pregnancy and Preeclampsia, 1950–July 2013
| Lipid Stratification | No. of Studies | Random-Effects Model |
Heterogeneity |
Egger P Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| WMD, mg/dL | 95% CI | P Value | χ2 | P Value | τ2 | I2, % | |||
| Total Cholesterol | |||||||||
| All preeclampsia | |||||||||
| First/second trimesters | 11 | 12.49 | 3.44, 21.54 | 0.007 | 27.14 | 0.002 | 122.38 | 56 | 0.82 |
| Third trimester | 46 | 20.20 | 8.70, 31.70 | 0.001 | 3,892.87 | <0.0001 | 1,500.00 | 99 | 0.06 |
| Severe preeclampsia | |||||||||
| First/second trimesters | 4 | −2.19 | −14.90, 10.52 | 0.74 | 13.01 | 0.005 | 115.78 | 77 | 0.60 |
| Third trimester | 13 | 26.17 | −0.37, 52.72 | 0.05 | 223.86 | <0.0001 | 2,100.0 | 95 | 0.07 |
| Mild preeclampsia | |||||||||
| First/second trimesters | 3 | 8.01 | −3.17, 19.19 | 0.16 | 1.60 | 0.45 | 0.00 | 0 | 0.58 |
| Third trimester | 11 | 18.17 | −16.77, 53.11 | 0.31 | 394.21 | <0.0001 | 3,200.0 | 97 | 0.24 |
| HDL-C | |||||||||
| All preeclampsia | |||||||||
| First/second trimesters | 10 | −0.48 | −3.31, 2.34 | 0.74 | 32.96 | <0.0001 | 12.91 | 73 | 0.71 |
| Third trimester | 41 | −8.86 | −11.50, −6.21 | <0.0001 | 771.48 | <0.0001 | 63.33 | 95 | 0.27 |
| Severe preeclampsia | |||||||||
| First/second trimesters | 2 | −3.12 | −15.06, 8.82 | 0.61 | 15.96 | <0.0001 | 69.76 | 94 | |
| Third trimester | 14 | −5.60 | −10.59, −0.62 | 0.03 | 382.51 | <0.0001 | 80.52 | 97 | 0.80 |
| Mild preeclampsia | |||||||||
| First/second trimesters | |||||||||
| Third trimester | 11 | −5.92 | −11.61, −0.24 | 0.04 | 361.66 | <0.0001 | 79.90 | 97 | 0.57 |
| LDL-C | |||||||||
| All preeclampsia | |||||||||
| First/second trimesters | 9 | 3.89 | −0.19, 7.97 | 0.06 | 9.21 | 0.33 | 5.11 | 13 | 0.80 |
| Third trimester | 36 | 10.92 | −0.59, 22.42 | 0.06 | 1,012.02 | <0.0001 | 1,100.0 | 97 | 0.19 |
| Severe preeclampsia | |||||||||
| First/second trimesters | |||||||||
| Third trimester | 12 | 14.14 | −13.73, 42.01 | 0.32 | 319.12 | <0.0001 | 2,200.0 | 97 | 0.04 |
| Mild preeclampsia | |||||||||
| First/second trimesters | |||||||||
| Third trimester | 10 | 4.09 | −33.36, 41.55 | 0.83 | 724.43 | <0.0001 | 3,400.0 | 99 | 0.08 |
| Non-HDL-Ca | |||||||||
| All preeclampsia | |||||||||
| First/second trimesters | 9 | 11.57 | 3.47, 19.67 | 0.005 | 34.18 | <0.0001 | 98.4 | 76 | 0.50 |
| Third trimester | 38 | 29.59 | 12.13, 47.06 | 0.001 | 8,388.4 | <0.0001 | 2,900 | 99 | 0.85 |
| Triglycerides | |||||||||
| All preeclampsia | |||||||||
| First/second trimesters | 13 | 25.08 | 14.39, 35.77 | <0.0001 | 28.49 | 0.01 | 196.16 | 58 | 0.12 |
| Third trimester | 44 | 80.29 | 51.45, 109.13 | <0.0001 | 18,831.98 | <0.0001 | 9,000 | 99 | 0.10 |
| Severe preeclampsia | |||||||||
| First/second trimesters | 5 | 35.65 | 11.20, 60.10 | 0.004 | 38.65 | <0.0001 | 645.02 | 90 | 0.52 |
| Third trimester | 15 | 65.22 | 28.75, 101.69 | <0.0001 | 249.22 | <0.0001 | 4,200 | 94 | 0.71 |
| Mild preeclampsia | |||||||||
| First/second trimesters | 4 | 39.06 | 11.09, 67.03 | 0.006 | 13.31 | 0.004 | 610.61 | 77 | 0.11 |
| Third trimester | 12 | 54.53 | 12.55, 96.51 | 0.01 | 347.20 | <0.0001 | 4,600.0 | 97 | 0.86 |
Abbreviations: CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; WMD, weighted mean difference.
a If measured non-HDL-C was not available, non-HDL-C was calculated as total cholesterol minus HDL-C.
We detected moderate to significant heterogeneity in nearly all of the meta-analyses we conducted (P ≤ 0.01) with I2 values ranging from 56% to 99% (Table 2), with the exception of the first/second-trimester total cholesterol and mild preeclampsia analysis (P = 0.45, I2 = 0%) and the first/second-trimester LDL-C analysis (P = 0.33, I2 = 13%). Table 3 shows the results from the univariate and multivariate meta-regression analyses designed to identify potential factors that could explain the high heterogeneity. BMI imbalance between the comparison groups is likely an important source of heterogeneity in the LDL-C analysis (P = 0.04, R2 = 15.2%) and triglyceride analysis (P = 0.08, R2 = 9.8%) such that, for each 1.0 unit increase in BMI WMD between groups, decreases in the WMD of 5.50 mg/dL and 11.02 mg/dL are expected, respectively. In multivariate meta-regression models with trimester of lipid measurement and fasting status at blood sampling, BMI remained significant as a potential source of heterogeneity for LDL-C but not for triglycerides. Additionally, trimester of lipid measurement was identified as another potential source of heterogeneity in meta-analyses of HDL-C and triglycerides. However, neither BMI imbalance between the comparison groups nor trimester of lipid measurement was detected as a possible source of heterogeneity in the total cholesterol analysis. Fasting status at the time of lipid measurement was not identified as a potential source of heterogeneity for any of the lipid analyses.
Table 3.
Univariate and Multivariate Meta-Regression Results From the Meta-Analysis of Lipid Levels During Pregnancy and the Risk of Preeclampsia, 1950–July 2013
| Covariate | No. | β Coefficienta | 95% CI | P Value | I2, % | R2, % |
|---|---|---|---|---|---|---|
| Total Cholesterol | ||||||
| BMIb | 30 | 4.48 | −2.53, 11.50 | 0.20 | 98.56 | 2.16 |
| Fasting status | 60 | |||||
| Nonfasting | −1.18 | −33.12, 30.76 | 0.94 | |||
| Unknown | 0.65 | −21.06, 22.37 | 0.30 | |||
| Trimester | 57 | 10.15 | −14.64, 34.95 | 0.42 | 98.60 | −0.78 |
| Multivariate | 29 | 98.75 | −2.90 | |||
| BMI | 4.37 | −3.25, 11.98 | 0.25 | |||
| Fasting status | ||||||
| Nonfasting | 28.53 | −17.60, 74.66 | 0.21 | |||
| Unknown | 8.20 | −30.08, 46.48 | 0.90 | |||
| Trimester | 8.67 | −24.72, 42.06 | 0.60 | |||
| LDL-C | ||||||
| BMI | 25 | −5.50 | −10.93, −0.07 | 0.04 | 89.8 | 15.20 |
| Fasting status | 49 | 95.7 | −2.44 | |||
| Nonfasting | −8.79 | −31.25, 13.68 | 0.44 | |||
| Unknown | −5.35 | −21.80, 11.09 | 0.52 | |||
| Trimester | 45 | 6.804 | −12.81, 26.42 | 0.49 | 95.8 | −0.79 |
| Multivariate | 24 | 89.3 | 7.60 | |||
| BMI | −6.80 | −13.65, 0.05 | 0.05 | |||
| Fasting status | ||||||
| Nonfasting | 4.79 | −23.21, 32.79 | 0.72 | |||
| Unknown | 2.740 | −21.57, 27.04 | 0.82 | |||
| Trimester | 0.89 | −21.18, 22.96 | 0.93 | |||
| HDL-C | ||||||
| BMI | 28 | 0.23 | −1.76, 2.21 | 0.82 | 93.4 | −4.92 |
| Fasting status | 54 | 94.9 | −4.42 | |||
| Nonfasting | 1.36 | −7.68, 10.39 | 0.77 | |||
| Unknown | 0.69 | −4.86, 6.23 | 0.81 | |||
| Trimester | 51 | −8.82 | −14.20, −3.44 | 0.002 | 93.9 | 21.58 |
| Multivariate | 27 | 92.7 | 12.23 | |||
| BMI | 0.33 | −1.68, 2.34 | 0.74 | |||
| Fasting status | ||||||
| Nonfasting | 5.36 | −12.84, 23.55 | 0.55 | |||
| Unknown | 6.10 | −3.67, 15.87 | 0.21 | |||
| Trimester | −8.31 | −17.08, 0.46 | 0.06 | |||
| Triglycerides | ||||||
| BMI | 32 | −11.021 | −23.341, 1.299 | 0.08 | 98.1 | 9.84 |
| Fasting status | 57 | 98.3 | 0.04 | |||
| Nonfasting | −30.843 | −70.804, 9.118 | 0.13 | |||
| Unknown | −2.845 | −32.268, 26.578 | 0.85 | |||
| Trimester | 57 | 47.424 | 20.60, 74.247 | 0.001 | 99.7 | 19.52 |
| Multivariate | 32 | 94.2 | 29.73 | |||
| BMI | −5.589 | −18.007, 6.828 | 0.36 | |||
| Fasting status | ||||||
| Nonfasting | −11.357 | −69.504, 46.790 | 0.69 | |||
| Unknown | 19.280 | −26.851, 65.410 | 0.40 | |||
| Trimester | 56.297 | 18.750, 93.844 | 0.005 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
a Reference groups were first/second trimesters and fasting.
b Weight (kg)/height (m)2.
On the basis of Egger's test, publication bias was not present in most of the meta-analyses with P values ranging from 0.06 to 0.82 (Table 2), with the exception of the analysis of LDL-C from the third trimester and severe preeclampsia (P = 0.04). However, visual inspection of the funnel plots suggests that publication bias may be present for the total cholesterol, LDL-C, and HDL-C analyses of third-trimester measurements stratified by preeclampsia severity. Funnel plots for all analyses can be found in Web Figures 22–41.
DISCUSSION
Our meta-analysis shows that maternal serum total cholesterol, non-HDL-C, and triglyceride levels during pregnancy are elevated during the first/second and third trimesters in women who subsequently develop preeclampsia compared with women who remain normotensive during pregnancy. Additionally, our results suggest that women who subsequently develop preeclampsia likely have increased levels of LDL-C in the first/second and third trimesters compared with normotensive women, though these results were of marginal significance. Finally, maternal HDL-C levels during the third trimester of pregnancy are lower in preeclamptic women compared with normotensive pregnant women.
A recent meta-analysis of hypertriglyceridemia and preeclampsia reported that maternal triglyceride levels during pregnancy were elevated in women who subsequently developed preeclampsia (10); however, our methodological approach was substantially more inclusive and comprehensive. The authors of that study located 24 case-control and 5 cohort studies for inclusion in their meta-analysis, whereas our search strategy led us to 86 articles (13 of which we excluded because of low quality scores), 3 times the number of articles found in the previous analysis. However, despite the additional articles, we found very similar WMDs in our analysis when we stratified by trimester of measurement. The authors also detected significant heterogeneity after stratification by trimester, which they attributed to difference in BMI; this hypothesis, however, was not tested. We performed meta-regression analyses to assess BMI as a possible source of heterogeneity and found that BMI differences across studies were possible sources of heterogeneity for triglycerides and LDL-C.
During the course of a normal pregnancy, total cholesterol, HDL-C, triglycerides, and LDL-C levels rise markedly (29, 30). Cholesterol is necessary for placental steroid synthesis, and increases in cholesterol levels during pregnancy promote the accumulation of maternal fat stores in the first two-thirds of pregnancy to serve as a source of calories for the mother and fetus during the later stages of pregnancy and lactation (29, 31–33). Results from this meta-analysis suggest that women with preeclampsia experience greater changes in lipid metabolism than normotensive women. For example, the difference in triglycerides between preeclamptic and normotensive pregnancies is substantially greater during the third trimester (WMD = 80.29) compared with the first/second trimesters (WMD = 25.08). This same trend of greater differences in the third trimester compared with the first/second trimesters was also observed for total cholesterol, HDL-C, LDL-C, and non-HDL-C, suggesting that preeclamptic women experience larger changes in lipid levels during pregnancy than normotensive women.
Dyslipidemia in preeclamptic women is characteristic of what occurs in insulin-resistant, hyperglycemic women who are not pregnant, many of whom also have the clustering of metabolic syndrome characteristics that include hypertension (34, 35). This suggests that a similar pathophysiological process may be occurring in women with preeclampsia and could be contributing to the dyslipidemic changes. Insulin resistance and type 2 diabetes are characterized by the increased overproduction of the triglyceride-rich very-low-density lipoprotein cholesterol and subsequent increased levels of other triglyceride-rich lipoproteins, which are included in non-HDL-C and reflected in elevated triglyceride levels (36).
Preeclampsia has been proposed to have a 3-stage disease process that stems from an imbalance between placental factors and maternal adaptation to them (37). The disease begins with incomplete maternal tolerance to the allogeneic trophoblasts (stage 1), followed by poor placentation that leads to reduced placental perfusion and poor spiral artery remodeling (stage 2) (37). As a result, the oxidatively stressed placenta releases a number of trophoblast-derived antiangiogenic (e.g., soluble fms-like tyrosine kinase-1, soluble vascular endothelial growth factor, and soluble endoglin) and proangiogenic (e.g., placenta growth factor) factors that contribute to an exaggerated maternal inflammatory response (stage 3) (38). The imbalance of angiogenic factors is thought to increase maternal vascular inflammation with generalized endothelial dysfunction (39). Women with elevated lipid levels likely have preexisting endothelial dysfunction that is worsened as a result of the physiological burden of pregnancy (40); this condition may be further exacerbated by increased maternal vascular inflammation. It is possible that preeclamptic women have higher baseline levels of total cholesterol, triglycerides, and LDL-C and lower levels of HDL-C prepregnancy, but only a handful of studies have taken prepregnancy measurements in preeclamptic women, and we were not able to assess the impact of prepregnancy lipid levels on the risk of preeclampsia (41, 42).
Recent research has demonstrated that LDL-C is not the only form of cholesterol associated with adverse outcomes. In fact, it has been shown that the inclusion of other atherogenic lipoproteins, referred to as non-HDL, which include very-low-density lipoproteins and other apolipoprotein B–containing lipoproteins, is more predictive of cardiovascular disease than LDL-C levels alone (20). Previous research suggests that LDL-C levels are higher among women who develop preeclampsia than among those who remain normotensive throughout pregnancy (42); however, our results suggest only a moderate difference in LDL-C levels between the 2 groups. Alternatively, we did find that levels of very-low-density lipoproteins (results not shown) and non-HDL-C are significantly higher among preeclamptic women than among normotensive women, suggesting that, although LDL-C levels may not be the most useful measure for preeclampsia prediction, a combined measure of all types of non-HDL-C may be useful.
Previous studies evaluating the association between lipid levels during pregnancy and preeclampsia have suggested measuring lipid levels in all pregnant women as a means of early-pregnancy “screening” of women who may be at higher risk for development of the disease (43, 44). However, results from our meta-analysis indicate that LDL-C and HDL-C levels measured during pregnancy would not be as useful in predicting preeclampsia as other lipid types. HDL-C levels were significantly different between preeclamptic and normotensive women during only the third trimester of pregnancy, which may be too late for an effective prediction tool. Preeclamptic and normotensive women showed marginally significant differences in LDL-C levels during both the second and third trimesters of pregnancy; however, with a WMD of only 3.89 mg/dL in the second trimester, this marker may not be clinically useful as a prediction tool.
Women who developed preeclampsia did have significantly elevated total cholesterol, triglyceride, and non-HDL-C measurements as early as the second trimester; thus, these lipid measurements obtained early in pregnancy may be helpful in identifying women at higher risk of developing preeclampsia. Although a WMD of 12.49 (for total cholesterol), 25.08 (for triglycerides), or 11.57 (for non-HDL-C) may not be clinically significant as an individual marker, when combined with other biomarkers known to differ in preeclamptic women, such as increased mean arterial pressure (45), soluble fms-like tyrosine kinase-1 (46), and placental growth factor (46), total cholesterol, triglyceride, and/or non-HDL-C measurements could be clinically useful in identifying women at higher risk for developing preeclampsia. The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy recently revised the guidelines for the diagnosis of preeclampsia in an effort to increase the diagnostic sensitivity and specificity for preeclampsia by allowing for its diagnosis in the absence of proteinuria when any 1 of 6 severe features of preeclampsia is found (47). Because these severe features are relatively infrequent during pregnancy, implementation of the revised definition is unlikely to alter the conclusions of the meta-analysis or lessen the potential clinical utility of including lipid measurements in preeclampsia prediction algorithms.
Strengths and limitations
A key strength of our meta-analysis is the sensitive search strategy that we developed in consultation with a research librarian. Specifically, we searched 2 databases and did not apply any language, country, or date restrictions to the search to increase our chances of identifying all possible publications related to the topic. Additionally, the high yield of articles eligible for inclusion allowed us to limit our analysis to the studies of higher quality. Finally, we were able to perform meta-regression to illustrate that fasting status at the time of lipid measurement did not affect our results. This is an important finding for studies of lipid measurements and pregnancy outcomes, because it is particularly difficult to obtain fasting measurements longitudinally throughout pregnancy. If incorporated with routinely measured markers in pregnancy, such as pregnancy-associated plasma protein A and glucose, most, if not all, samples could be collected during a nonfasting state. Therefore, we demonstrate that lipid measurements may be incorporated with routine clinical analysis of other biomarkers, which is particularly important when evaluating the feasibility of universal screening for lipid levels during pregnancy.
This meta-analysis also included a separate analysis of severe and mild preeclampsia. It should be noted, however, that we did not use our own specific criteria for the definitions of severity. Preeclampsia definitions have varied slightly over time, and many countries have their own definitions of severity. Thus, some of the heterogeneity between the severity studies could be explained by varying definitions. Of the studies included in this subanalysis, 2 did not report their classification criteria for severe preeclampsia. Among those that did, the most common criteria for diagnosis of severe preeclampsia were systolic blood pressure of 160 mm Hg or higher, diastolic blood pressure of 110 mm Hg or higher, proteinuria of either 2 g/24 hours or more or 5 g/24 hours or more, or the presence of another severe symptom. Further, it is increasingly believed that early- and late-onset preeclampsia may be different diseases with different pathophysiologies; however, we did not locate an adequate number of studies that distinguished between early- and late-onset preeclampsia.
Although we were able to perform meta-regression to identify BMI as a potential confounder and source of heterogeneity, we were unable to incorporate this factor into our mean difference meta-analysis. This was not possible because the included studies would have to have presented their mean lipid levels stratified by BMI, and those levels of stratification would have to have been equivalent across all studies.
This meta-analysis could not be performed with all of the studies located in the systematic literature review because of the lack of information about the mean and standard deviation in each group (or lack of information necessary to convert the information into means and standard deviations) (n = 17). These studies, if added to the meta-analysis, might have altered our findings. However, a comparison of our triglyceride results to those of the previous meta-analysis on the topic that included one-third of the studies included here shows very similar results, indicating that the addition of further studies to this meta-analysis is unlikely to alter our findings.
Conclusion
Total cholesterol, triglyceride, non-HDL-C, and HDL-C levels measured during pregnancy are significantly related to the risk of preeclampsia. This finding is clinically useful because maternal lipid levels can be easily measured in all clinical laboratories with routine, well-established lipid panels; thus, inexpensive lipid panels could serve as a cost-effective method for identifying pregnant women at risk for developing preeclampsia. Future research is needed to understand the role of dyslipidemia and other components of metabolic syndrome, such as insulin resistance and obesity, in the pathogenesis of preeclampsia and the mechanisms by which this relationship could be moderated.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, University of Iowa College of Public Health, University of Iowa, Iowa City, Iowa (Cassandra N. Spracklen, Caitlin J. Smith, Audrey F. Saftlas, Jennifer G. Robinson, Kelli K. Ryckman).
No funding or financial support was received for this study.
We thank University of Iowa education and outreach librarian, Chris Childs, for his assistance in constructing the most effective and efficient search strategy for the systematic review.
Conflict of interest: none declared.
REFERENCES
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357(9249):53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Committee on Practice Bulletins—Obstetrics ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 4.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222(3):222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 5.Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28(1):1–19. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 6.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Granger JP, Alexander BT, Llinas MT, et al. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38(3 Pt 2):718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 8.Ghio A, Bertolotto A, Resi V, et al. Triglyceride metabolism in pregnancy. Adv Clin Chem. 2011;55:133–153. doi: 10.1016/b978-0-12-387042-1.00007-1. [DOI] [PubMed] [Google Scholar]
- 9.Taylor R, Roberts J. Endothelial Cell Dysfunction. 3rd ed. San Diego, CA: Elsevier; 2009. [Google Scholar]
- 10.Gallos ID, Sivakumar K, Kilby MD, et al. Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: a meta-analysis. BJOG. 2013;120(11):1321–1332. doi: 10.1111/1471-0528.12375. [DOI] [PubMed] [Google Scholar]
- 11.Bayhan G, Koçyigit Y, Atamer A, et al. Potential atherogenic roles of lipids, lipoprotein(a) and lipid peroxidation in preeclampsia. Gynecol Endocrinol. 2005;21(1):1–6. doi: 10.1080/09513590500097382. [DOI] [PubMed] [Google Scholar]
- 12.Iftikhar U, Iqbal A, Shakoor S. Relationship between leptin and lipids during pre-eclampsia. J Pak Med Assoc. 2010;60(6):432–435. [PubMed] [Google Scholar]
- 13.Koçyigit Y, Atamer Y, Atamer A, et al. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol. 2004;19(5):267–273. doi: 10.1080/09513590400018108. [DOI] [PubMed] [Google Scholar]
- 14.Sahu S, Abraham R, Vedavalli R, et al. Study of lipid profile, lipid peroxidation and vitamin E in pregnancy induced hypertension. Indian J Physiol Pharmacol. 2009;53(4):365–369. [PubMed] [Google Scholar]
- 15.Uzun H, Benian A, Madazli R, et al. Circulating oxidized low-density lipoprotein and paraoxonase activity in preeclampsia. Gynecol Obstet Invest. 2005;60(4):195–200. doi: 10.1159/000087205. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed December 14, 2013.
- 20.Robinson JG, Wang S, Jacobson TA. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol. 2012;110(10):1468–1476. doi: 10.1016/j.amjcard.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-analysis. 1st ed. West Sussex, United Kingdom: Wiley; 2009. [Google Scholar]
- 22.Belo L, Caslake M, Santos-Silva A, et al. Lipoprotein(a): a longitudinal versus a cross-sectional study in normal pregnancy and its levels in preeclampsia. Atherosclerosis. 2002;165(2):393–395. doi: 10.1016/s0021-9150(02)00208-3. [DOI] [PubMed] [Google Scholar]
- 23.Belo L, Caslake M, Gaffney D, et al. Changes in LDL size and HDL concentration in normal and preeclamptic pregnancies. Atherosclerosis. 2002;162(2):425–432. doi: 10.1016/s0021-9150(01)00734-1. [DOI] [PubMed] [Google Scholar]
- 24.Belo L, Gaffney D, Caslake M, et al. Apolipoprotein E and cholesteryl ester transfer protein polymorphisms in normal and preeclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 2004;112(1):9–15. doi: 10.1016/s0301-2115(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 25.Belo L, Santos-Silva A, Caslake M, et al. Oxidized-LDL levels in normal and pre-eclamptic pregnancies: contribution of LDL particle size. Atherosclerosis. 2005;183(1):185–186. doi: 10.1016/j.atherosclerosis.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Reyes LM, García RG, Ruiz SL, et al. Angiogenic imbalance and plasma lipid alterations in women with preeclampsia from a developing country. Growth Factors. 2012;30(3):158–166. doi: 10.3109/08977194.2012.674035. [DOI] [PubMed] [Google Scholar]
- 27.Reyes L, Garcia R, Ruiz S, et al. Nutritional status among women with pre-eclampsia and healthy pregnant and non-pregnant women in a Latin American country. J Obstet Gynaecol Res. 2012;38(3):498–504. doi: 10.1111/j.1447-0756.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- 28.Reyes LM, García RG, Ruiz SL, et al. Risk factors for preeclampsia in women from Colombia: a case-control study. PLoS One. 2012;7(7):e41622. doi: 10.1371/journal.pone.0041622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–948. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 30.Mahendru AA, Everett TR, Wilkinson IB, et al. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. J Hypertens. 2014;32(4):849–856. doi: 10.1097/HJH.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 31.Basaran A. Pregnancy-induced hyperlipoproteinemia: review of the literature. Reprod Sci. 2009;16(5):431–437. doi: 10.1177/1933719108330569. [DOI] [PubMed] [Google Scholar]
- 32.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71(5 suppl):1256S–1261S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 33.Lorentzen B, Henriksen T. Plasma lipids and vascular dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):33–39. doi: 10.1055/s-2007-1016250. [DOI] [PubMed] [Google Scholar]
- 34.McLaughlin T, Abbasi F, Lamendola C, et al. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167(7):642–648. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 35.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59(7):635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 36.Adiels M, Olofsson SO, Taskinen MR, et al. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 37.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63(6):534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 38.Staff AC, Benton SJ, von Dadelszen P, et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61(5):932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 39.Roberts JM, Taylor RN, Musci TJ, et al. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161(5):1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 40.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175(5):1365–1370. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 41.Magnussen EB, Vatten LJ, Lund-Nilsen TI, et al. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335(7627):978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charlton F, Tooher J, Rye KA, et al. Cardiovascular risk, lipids and pregnancy: preeclampsia and the risk of later life cardiovascular disease. Heart Lung Circ. 2014;23(3):203–212. doi: 10.1016/j.hlc.2013.10.087. [DOI] [PubMed] [Google Scholar]
- 43.Carty DM, Delles C, Dominiczak AF. Novel biomarkers for predicting preeclampsia. Trends Cardiovasc Med. 2008;18(5):186–194. doi: 10.1016/j.tcm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukherjee R, Ray CD, Chakraborty C, et al. Clinical biomarker for predicting preeclampsia in women with abnormal lipid profile: statistical pattern classification approach. Presented at the International Conference on Systems in Medicine and Biology; December 16–18, 2010; Kharagpur, India. [Google Scholar]
- 45.Poon LC, Kametas NA, Pandeva I, et al. Mean arterial pressure at 11(+0) to 13(+6) weeks in the prediction of preeclampsia. Hypertension. 2008;51(4):1027–1033. doi: 10.1161/HYPERTENSIONAHA.107.104646. [DOI] [PubMed] [Google Scholar]
- 46.Hassan MF, Rund NM, Salama AH. An elevated maternal plasma soluble fms-like tyrosine kinase-1 to placental growth factor ratio at midtrimester is a useful predictor for preeclampsia. Obstet Gynecol Int. 2013;2013:202346. doi: 10.1155/2013/202346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 48.Adiga U, D'souza V, Kamath A, et al. Antioxidant activity and lipid peroxidation in preeclampsia. J Chin Med Assoc. 2007;70(10):435–438. doi: 10.1016/S1726-4901(08)70034-0. [DOI] [PubMed] [Google Scholar]
- 49.Ahenkorah L, Owiredu WKBA, Laing EF, et al. Lipid profile and lipid peroxidation among Ghanaian pregnancy-induced hypertensives. J Med Sci. 2008;8(8):691–698. [Google Scholar]
- 50.Akiibinu MO, Kolawole TO, Ekun OA, et al. Metabolic dysfunctions in Nigerian pre-eclamptics. Arch Gynecol Obstet. 2013;288(5):1021–1026. doi: 10.1007/s00404-013-2854-4. [DOI] [PubMed] [Google Scholar]
- 51.Aziz R, Mahboob T. Preeclampsia and lipid profile. Pak J Med Sci. 2007;23(5):751–754. [Google Scholar]
- 52.Babacan F, Isik B, Bingol B. Changes in serum paraoxonase activity, calcium and lipid profiles in pre-eclampsia, a preliminary study. J Turk Soc Obstet Gynecol. 2011;8(3):169–174. [Google Scholar]
- 53.Barden A, Ritchie J, Walters B, et al. Study of plasma factors associated with neutrophil activation and lipid peroxidation in preeclampsia. Hypertension. 2001;38(4):803–808. doi: 10.1161/hy1101.092969. [DOI] [PubMed] [Google Scholar]
- 54.Caruso A, Ferrazzani S, De Carolis S, et al. Gestational hypertension but not pre-eclampsia is associated with insulin resistance syndrome characteristics. Hum Reprod. 1999;14(1):219–223. doi: 10.1093/humrep/14.1.219. [DOI] [PubMed] [Google Scholar]
- 55.Cekmen MB, Erbagci AB, Balat A, et al. Plasma lipid and lipoprotein concentrations in pregnancy induced hypertension. Clin Biochem. 2003;36(7):575–578. doi: 10.1016/s0009-9120(03)00099-7. [DOI] [PubMed] [Google Scholar]
- 56.Chalas J, Audibert F, Francoual J, et al. Concentrations of apolipoproteins E, C2, and C3 and lipid profile in preeclampsia. Hypertens Pregnancy. 2002;21(3):199–204. doi: 10.1081/PRG-120015846. [DOI] [PubMed] [Google Scholar]
- 57.Dane B, Dane C, Kiray M, et al. A new metabolic scoring system for analyzing the risk of hypertensive disorders of pregnancy. Arch Gynecol Obstet. 2009;280(6):921–924. doi: 10.1007/s00404-009-1029-9. [DOI] [PubMed] [Google Scholar]
- 58.Demir B, Demir S, Atamer Y, et al. Serum levels of lipids, lipoproteins and paraoxonase activity in pre-eclampsia. J Int Med Res. 2011;39(4):1427–1431. doi: 10.1177/147323001103900430. [DOI] [PubMed] [Google Scholar]
- 59.Demirci O, Tuğrul AS, Dolgun N, et al. Serum lipids level assessed in early pregnancy and risk of pre-eclampsia. J Obstet Gynaecol Res. 2011;37(10):1427–1432. doi: 10.1111/j.1447-0756.2011.01562.x. [DOI] [PubMed] [Google Scholar]
- 60.Dey M, Arora D, Narayan N, et al. Serum cholesterol and ceruloplasmin levels in second trimester can predict development of pre-eclampsia. N Am J Med Sci. 2013;5(1):41–46. doi: 10.4103/1947-2714.106198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duan DM, Niu JM, Lei Q, et al. Serum levels of the adipokine chemerin in preeclampsia. J Perinat Med. 2011;40(2):121–127. doi: 10.1515/JPM.2011.127. [DOI] [PubMed] [Google Scholar]
- 62.Enquobahrie DA, Williams MA, Butler CL, et al. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17(7):574–581. doi: 10.1016/j.amjhyper.2004.03.666. [DOI] [PubMed] [Google Scholar]
- 63.Francoual J, Audibert F, Trioche P, et al. Is a polymorphism of the apolipoprotein E gene associated with preeclampsia? Hypertens Pregnancy. 2002;21(2):127–133. doi: 10.1081/PRG-120004768. [DOI] [PubMed] [Google Scholar]
- 64.Garzetti GG, Tranquilli AL, Cugini AM, et al. Altered lipid composition, increased lipid peroxidation, and altered fluidity of the membrane as evidence of platelet damage in preeclampsia. Obstet Gynecol. 1993;81(3):337–340. [PubMed] [Google Scholar]
- 65.Gohil JT, Patel PK, Gupta P. Estimation of lipid profile in subjects of preeclampsia. J Obstet Gynaecol India. 2011;61(4):399–403. doi: 10.1007/s13224-011-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harsem NK, Roald B, Braekke K, et al. Acute atherosis in decidual tissue: not associated with systemic oxidative stress in preeclampsia. Placenta. 2007;28(8-9):958–964. doi: 10.1016/j.placenta.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Islam NAF, Chowdhury MAR, Kibria GM, et al. Study of serum lipid profile in pre-eclampsia and eclampsia. Faridpur Med Coll J. 2010;5(2):56–59. [Google Scholar]
- 68.Jamalzei B, Fallah S, Kashanian M, et al. Association of the apolipoprotein E variants with susceptibility to pregnancy with preeclampsia. Clin Lab. 2013;59(5-6):563–570. doi: 10.7754/clin.lab.2012.120304. [DOI] [PubMed] [Google Scholar]
- 69.Kaaja R, Tikkanen MJ, Viinikka L, et al. Serum lipoproteins, insulin, and urinary prostanoid metabolites in normal and hypertensive pregnant women. Obstet Gynecol. 1995;85(3):353–356. doi: 10.1016/0029-7844(94)00380-V. [DOI] [PubMed] [Google Scholar]
- 70.Kalar MU, Kalar N, Mansoor F, et al. Preeclampsia and lipid levels—a case control study. Int J Collab Res Internal Med Public Health. 2012;4(10):1738–1745. [Google Scholar]
- 71.Kandimalla BH, Sirjusingh A, Nayak BS, et al. Early antenatal serum lipid levels and the risk of pre-eclampsia in Trinidad and Tobago. Arch Physiol Biochem. 2011;117(4):215–221. doi: 10.3109/13813455.2010.543137. [DOI] [PubMed] [Google Scholar]
- 72.Kashinakunti SV, Sunitha H, Gurupadappa K, et al. Lipid profile in preeclampsia—a case control study. J Clin Diag Res. 2010;4:2748–2751. [Google Scholar]
- 73.Kim YJ, Park H, Lee HY, et al. Paraoxonase gene polymorphism, serum lipid, and oxidized low-density lipoprotein in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2007;133(1):47–52. doi: 10.1016/j.ejogrb.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 74.Lei Q, Lv LJ, Zhang BY, et al. Ante-partum and post-partum markers of metabolic syndrome in pre-eclampsia. J Hum Hypertens. 2011;25(1):11–17. doi: 10.1038/jhh.2010.29. [DOI] [PubMed] [Google Scholar]
- 75.Llurba E, Gratacós E, Martín-Gallán P, et al. A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic Biol Med. 2004;37(4):557–570. doi: 10.1016/j.freeradbiomed.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 76.Lorentzen B, Drevon CA, Endresen MJ, et al. Fatty acid pattern of esterified and free fatty acids in sera of women with normal and pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1995;102(7):530–537. doi: 10.1111/j.1471-0528.1995.tb11355.x. [DOI] [PubMed] [Google Scholar]
- 77.Madazli R, Benian A, Gümüştaş K, et al. Lipid peroxidation and antioxidants in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 1999;85(2):205–208. doi: 10.1016/s0301-2115(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 78.Maksane S, Ranka R, Maksane N, et al. Study of serum lipid profile and magnesium in normal pregnancy and in pre-eclampsia: a case control study. Asian J Biochem. 2011;6(3):228–239. [Google Scholar]
- 79.Mihu D, Georgescu C, Mihu C, et al. High maternal serum leptin and interleukin-6 levels in preeclampsia and relationship with clinical and metabolical parameters of disease severity and pregnancy outcome. Acta Endocrinol (Copenh) 2009;5(1):49–60. [Google Scholar]
- 80.Mohindra A, Kabi BC, Kaul N, et al. Vitamin E and carotene status in pre-eclamptic pregnant women from India. Panminerva Med. 2002;44(3):261–264. [PubMed] [Google Scholar]
- 81.Mori T, Shinohara K, Wakatsuki A, et al. Adipocytokines and endothelial function in preeclamptic women. Hypertens Res. 2010;33(3):250–254. doi: 10.1038/hr.2009.222. [DOI] [PubMed] [Google Scholar]
- 82.Murai JT, Muzykanskiy E, Taylor RN. Maternal and fetal modulators of lipid metabolism correlate with the development of preeclampsia. Metabolism. 1997;46(8):963–967. doi: 10.1016/s0026-0495(97)90088-3. [DOI] [PubMed] [Google Scholar]
- 83.Negrato CA, Jovanovic L, Tambascia MA, et al. Association between insulin resistance, glucose intolerance, and hypertension in pregnancy. Metab Syndr Relat Disord. 2009;7(1):53–59. doi: 10.1089/met.2008.0043. [DOI] [PubMed] [Google Scholar]
- 84.Nelson GH. Lipid metabolism in toxemia of pregnancy. Clin Obstet Gynecol. 1966;9(4):882–897. doi: 10.1097/00003081-196612000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Pecks U, Caspers R, Schiessl B, et al. The evaluation of the oxidative state of low-density lipoproteins in intrauterine growth restriction and preeclampsia. Hypertens Pregnancy. 2012;31(1):156–165. doi: 10.3109/10641955.2010.544805. [DOI] [PubMed] [Google Scholar]
- 86.Peng HQ, Yang SZ, Zhang GY, et al. Serum lipid and lipoprotein metabolism in toxemia of pregnancy. Chin Med J. 1985;98(12):905–908. [PubMed] [Google Scholar]
- 87.Powers RW, Evans RW, Majors AK, et al. Plasma homocysteine concentration is increased in preeclampsia and is associated with evidence of endothelial activation. Am J Obstet Gynecol. 1998;179(6 Pt 1):1605–1611. doi: 10.1016/s0002-9378(98)70033-x. [DOI] [PubMed] [Google Scholar]
- 88.Reyna-Villasmil E, Torres-Cepeda D, Pena-Paredes E, et al. Concentraciones de homocisteína y perfil lipídico en preeclámpticas [in Spanish] Gac Med Caracas. 2008;116(3):235–240. [Google Scholar]
- 89.Rodie VA, Caslake MJ, Stewart F, et al. Fetal cord plasma lipoprotein status in uncomplicated human pregnancies and in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Atherosclerosis. 2004;176(1):181–187. doi: 10.1016/j.atherosclerosis.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 90.Rosing U, Samsioe G, Olund A, et al. Serum levels of apolipoprotein A-I, A-II and HDL-cholesterol in second half of normal pregnancy and in pregnancy complicated by pre-eclampsia. Horm Metab Res. 1989;21(7):376–382. doi: 10.1055/s-2007-1009242. [DOI] [PubMed] [Google Scholar]
- 91.Rudra CB, Qiu C, David RM, et al. A prospective study of early-pregnancy plasma malondialdehyde concentration and risk of preeclampsia. Clin Biochem. 2006;39(7):722–726. doi: 10.1016/j.clinbiochem.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 92.Sharami SH, Tangestani A, Faraji R, et al. Role of dyslipidemia in preeclamptic overweight pregnant women. Iran J Reprod Med. 2012;10(2):105–112. [PMC free article] [PubMed] [Google Scholar]
- 93.Stefanović M, Vukomanović P, Milosavljević M, et al. Insulin resistance and C-reactive protein in preeclampsia. Bosn J Basic Med Sci. 2009;9(3):235–238. doi: 10.17305/bjbms.2009.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi WH, Martinelli S, Khoury MY, et al. Assessment of serum lipids in pregnant women aged over 35 years and their relation with pre-eclampsia. Einstein. 2008;6(1):63–67. [Google Scholar]
- 95.Vanderjagt DJ, Patel RJ, El-Nafaty AU, et al. High-density lipoprotein and homocysteine levels correlate inversely in preeclamptic women in northern Nigeria. Acta Obstet Gynecol Scand. 2004;83(6):536–542. doi: 10.1111/j.1600-0412.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 96.Ware-Jauregui S, Sanchez SE, Zhang C, et al. Plasma lipid concentrations in pre-eclamptic and normotensive Peruvian women. Int J Gynaecol Obstet. 1999;67(3):147–155. doi: 10.1016/s0020-7292(99)00161-7. [DOI] [PubMed] [Google Scholar]
- 97.Wetzka B, Winkler K, Kinner M, et al. Altered lipid metabolism in preeclampsia and HELLP syndrome: links to enhanced platelet reactivity and fetal growth. Semin Thromb Hemost. 1999;25(5):455–462. doi: 10.1055/s-2007-994950. [DOI] [PubMed] [Google Scholar]
- 98.Yamaguchi K. Triglycerides and apoproteins in toxemia of pregnancy. Nihon Sanka Fujinka Gakkai Zasshi. 1988;40(12):1875–1882. [PubMed] [Google Scholar]
- 99.Zhou J, Zhao X, Wang Z, et al. Combination of lipids and uric acid in mid-second trimester can be used to predict adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2012;25(12):2633–2638. doi: 10.3109/14767058.2012.704447. [DOI] [PubMed] [Google Scholar]
- 100.Ziaei S, Bonab KM, Kazemnejad A. Serum lipid levels at 28–32 weeks gestation and hypertensive disorders. Hypertens Pregnancy. 2006;25(1):3–10. doi: 10.1080/10641950500543756. [DOI] [PubMed] [Google Scholar]
- 101.Ziaei S, Jahanian S, Kazemnejad A. Lipid concentration in small for gestational age (SGA) pregnancies and hypertensive disorders. Pregnancy Hypertens. 2012;2(2):164–167. doi: 10.1016/j.preghy.2012.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


