Summary
Objective
To evaluate the potential of ADAMTS-4 (aggrecanase -1) activity in synovial fluid (SF) as a biomarker of knee injury and joint disease.
Design
We have measured ADAMTS-4 activity in the synovial fluid of 170 orthopaedic patients with different degrees of joint pathology, using a commercial ADAMTS-4 fluorescence resonance energy transfer (FRET) substrate assay. Patients were classified at arthroscopy as (i) macroscopically normal, (ii) with an injury of the meniscus, anterior cruciate ligament or chondral/osteochondral defects or (iii) with osteoarthritis, and the influence of independent factors (age, patient group, effusion and synovial inflammation) on ADAMTS-4 activity levels was assessed.
Results
In most patients (106/170) ADAMTS-4 activity was undetectable; ADAMTS-4 ranged from 0 to 2.8 ng/mL in synovial fluid from patients with an injury, 0–4.1 ng/mL in osteoarthritic patients and 4.0–12.3 ng/mL in patients with large effusions. Four independent variables each significantly influenced ADAMTS-4 activity in synovial fluid (all P < 0.001): age (concordance = 0.69), presence of osteoarthritis (OA) (concordance = 0.66), level of effusion (concordance = 0.78) and inflammation (concordance = 0.68). Not only did effusion influence the amount of ADAMTS-4 activity most strongly, but it also did this in an ordered manner (P < 0.001).
Conclusions
The main finding of this study is that ADAMTS-4 levels in synovial fluid are most strongly correlated with inflammation and severity of effusion in the knee. Further study is required to determine if it could provide a useful tool to aid clinical diagnoses, indicate treatment, to monitor progression of joint degeneration or OA or alternatively the success of treatment.
Keywords: ADAMTS-4 activity, Synovial fluid, Inflammation, Effusion, Knee osteoarthritis
Introduction
The lack of valid biomarkers with a good relationship to joint structural pathology and symptomatic disease in individual patients is suggested as being partly responsible for the slow pace of innovation in developing novel, effective treatments for osteoarthritis1. Osteoarthritis (OA) of the knee can develop secondary to several disorders or injury in the joint and manifests as alteration of the joint structure, with progressive degradation of any or all of the tissues within it, such as cartilage, menisci and ligaments, as well as inflammation of the synovium and changes to the subchondral bone. Measuring biomarkers in fluids proximal to the site of the pathology, such as the synovial fluid (SF) in the knee, can be both more informative about the disease state in that particular joint and more sensitive, by virtue of their higher concentration than, for example, in blood or urine2,3.
The best candidates for biomarkers in OA are suggested to be structural molecules or enzymes linked to cartilage, bone or synovium degradation4. One of the earliest and most striking biochemical changes to articular cartilage following injury or in degenerative joint diseases, such as OA, is degradation and loss of aggrecan. Matrix metalloproteinases (MMPs) and aggrecanases (or A Disintegrin And Metalloprotease with Thrombospondin motifS (ADAMTS)) are the enzymes attributed with degrading the majority of the aggrecan. The most common ‘pathological’ ADAMTS-generated cleavage site in the aggrecan core protein is between the 392glutamate and the 393alanine bond in the interglobular domain (reviewed in 5). Many studies have been undertaken to develop and trial measuring the presence of these ‘neo-epitopes’ generated by enzyme activity to assess their potential as biomarkers with an ARGS-aggrecan assay showing considerable promise when measured in blood or synovial fluid6.
ADAMTS-5 (aggrecanase-2) is the predominant member of the ADAMTS family in mice models of degenerative joint disease or OA. The situation in humans is less clear and ADAMTS-4 (aggrecanase-1) is likely to be a significant player5. In this study we have used a commercially available assay that can detect ADAMTS-4 activity independently of any contribution from ADAMTS-5. We have measured the extent of ADAMTS-4 activity in synovial fluid from a cohort of heterogeneous patients, typical of those attending an orthopaedic outpatient clinic with different degrees of joint pathology, ranging from macroscopically normal to end stage OA. Our objective was to investigate if measuring ADAMTS-4 activity could provide a useful tool to aid the clinician.
Method
Patient samples
Synovial fluid was collected from patients presenting with clinical symptoms who were undergoing a routine diagnostic arthroscopy of the knee and consented to take part in this study (99∖61∖RJ approved by Shropshire Research Ethics Committee). Patients were classified into three groups according to their appearance at arthroscopy as assessed by the treating surgeon:
-
(i)
those with macroscopically normal knees and no obvious abnormality,
-
(ii)
those with injury of the meniscus, anterior cruciate ligament or chondral/osteochondral lesions and
-
(iii)
those with OA (i.e., with features, such as the presence of osteophytes and fragmenting articular cartilage in one or more compartments of the knee (Outerbridge Grade 37) or exposed subchondral bone (Outerbridge Grade 4).
The presence of synovial inflammation was noted when the synovium was more reddened, swollen and convoluted than one would expect in a normal knee joint and the extent of joint effusion (none, small, moderate or large) was also assessed, using the sweep and patella tap tests8. A small effusion was recorded if the sweep test was positive but the patella tap test negative, a moderate effusion was recorded if the sweep and patella tap tests were positive and a large effusion was recorded if sweep and patella tap tests were negative due to over-distention of the joint with effusion.
The synovial fluid was collected by injecting 20 mLs of 0.9% saline into the synovial cavity prior to arthroscopy; the knee was flexed and extended 20 times to allow mixing of the saline with the joint fluid. A needle was then reinserted into the knee and the synovial fluid aspirated; in the laboratory it was centrifuged for 15 min at 3000 g and the supernatant stored at −80°C until use.
Plasma was collected at the same time and a dilution factor of the synovial fluid was obtained by measuring the ratio of urea in SF:plasma (normally ∼0.9 SF:serum)9. Urea was measured with a colorimetric assay QuantiChrom™ Urea assay Kit (BioAssay Systems DIUR-500).
ADAMTS-4 activity
ADAMTS-4 activity was measured in SF with the Sensolyte™ 520 ADAMTS-4 assay kit (Anaspec Inc) which uses a substrate for fluorescence resonance energy transfer (FRET), 5-FAM and TAMRA as the donor–acceptor pair. This acts as a substrate for active ADAMTS-4, which cleaves it into two separate fragments resulting in an increase of 5-FAM fluorescence which can be monitored at excitation and emission of 490 nm and 520 nm, respectively. The ADAMTS-4 assay was carried out according to the manufacturer's instructions. Fifty microlitres of each sample of SF lavage were pipetted into wells of a 96-well black microplate (Costar®, Corning Life Sciences). In addition, a range of concentrations (2–10 ng) of human recombinant ADAMTS-4 were pipetted into other wells to provide a standard curve to relate ADAMTS-4 activity in the SF samples to ng/mL of enzyme. There was also a well containing assay buffer only, to provide a substrate control. Fifty microlitres of 5-FAM fluorescence reference standards (70–2500 nM) were set up to calibrate the plate reader and act as an indicator of the amount of final product of the enzymatic reaction. The substrate solution (5-FAM/5-TAMRA) was added after pre-incubation at 37°C and the fluorescence intensity was measured following 60 min incubation at 37°C. Relating the amount of fluorescence intensity from each sample to that of the standard curve obtained from the recombinant ADAMTS-4 and multiplying it by the appropriate dilution factor for each SF sample, calculated the amount of ADAMTS-4 protein in each sample.
Statistics
The lower limit of detection (LLOD) and lower limit of quantification (LLOQ) of ADAMTS-4 were calculated from the standard deviation of blank samples and the slope of the calibration curve. QQ-plots were used to assess the distribution of continuous variables; age was normally distributed but ADAMTS-4 level was not. ADAMTS-4 levels were therefore summarised using medians and quartiles and compared between groups using non-parametric tests whereas parametric tests were used for age. Fisher's exact test was used to compare distributions in categorical variables (e.g., presence of effusion by patient classification). ADAMTS-4 levels below the LLOD were classified as having 0 ng/mL of ADAMTS-4. We used a censored or Tobit regression model10 to investigate the influence of the various independent factors (age, patient group, effusion and inflammation) on ADAMTS-4 levels, with the left limit set at the LLOD. This method requires a normal distribution for ADAMTS-4 levels, which was achieved by a logarithmic transformation, and minimises potential bias from the presence of an LLOD compared to traditionally used methods such as imputing half the LLOD for values below the LLOD10,11. The association between the independent factors (e.g., disease/injury, effusion and the presence of an inflamed synovium) and ADAMTS-4 levels was assessed by the concordance c (a generalisation of the area under the curve (AUC) of a receiver operating characteristic (ROC) curve) and Nagelkerke's R2, with 95% confidence intervals calculated on the basis of 999 bootstrap samples.
All statistical analysis was performed using R vs 3.0.3 (The R Foundation for Statistical Computing) using the packages “censReg” and “rms”. A P-value of 0.05 or below was assumed to denote statistical significance. In cases of multiple pairwise tests, P-values were adjusted using Holm's procedure to maintain the 0.05 alpha-level.
Results
Samples were collected from 170 patients, presenting with symptoms for varying times, ranging from 1 month to 16 years. The mean age of the macroscopically normal group was 28.6 years (8.5SD; n = 22), the injury group 33.2 years (10.3SD; n = 96) and the OA group 48.7 years (16.9SD; n = 10). A number of patients combined an injury and OA (mean age 48.9 years ± 11.6SD; n = 31). Mean age differed significantly between patients with OA (alone or combined with injury) and those with ‘macroscopically normal’ joints (both P < 0.001) or injured joints (both P < 0.001, all pairwise t-tests). Eleven patients were not classified. Effusions were present in 6% of ‘normal’ joints, 40% of those with injuries, 71% of those with OA and 64% of those with injuries and OA. Effusions were less common in ‘macroscopically normal’ joints than any of the other types (P < 0.05) but otherwise the incidence rates did not differ between the groups (P > 0.25, all pairwise Fisher's exact tests). Inflammation was noted in the knee joints of 25% of patients categorised as ‘macroscopically normal’, 54% of the injury group, 67% of the OA group and 83% of those with injuries and OA. These rates were higher in joints with OA and injury compared to ‘normal’ joints or those with only injury (P < 0.05), otherwise no differences were found (P > 0.09, all pairwise Fisher's exact tests).
The LLOD and LLOQ of the ADAMTS-4 assay were 0.14 ng/mL and 0.41 ng/mL, respectively. In most patients ADAMTS-4 activity did not reach detectable levels (106 out of 170). It was detectable in only 5% of samples from the ‘normal’ group, in 33% of the injury group, in 60% of the OA group and in 67% of the combined injury and OA group. These rates were lower in the ‘normal’ group compared to each of the three others (P < 0.03) and lower in the injury group compared to the combined injury and OA group (P = 0.004), but otherwise no differences were found (P > 0.32, all pairwise Fisher's exact tests). The measured level of ADAMTS-4 ranged between 0.2 and 34.9 ng/mL SF (with a mean dilution factor for the SF samples of 3.6 (±2.2, range 1.1–12.1)). The median level of ADAMTS-4 was 0 ng/mL in the ‘normal’ group (with one patient having a detectable amount of 0.3 ng/mL), 0 ng/mL in the injury group (range 0–29.8, with 32 patients having detectable levels), 1.2 ng/mL in the OA samples (range 0–11.5, with six patients having detectable levels) and 1.3 ng/mL in the combined injury and OA group (range 0–15.4, with 21 patients having detectable levels). There was significantly less ADAMTS-4 in the ‘normals’ than any of the other three patient groups and the injury group had less than the combined injury and OA group (both P < 0.005; pairwise Mann–Whitney tests).
Further analysis was simplified by separating the patient group qualifier into two: having OA (No/Yes) and having an injury (No/Chondral/Other; Table I). No significant interaction effect of injury and OA on ADAMTS-4 levels was found (P = 0.13; Tobit regression), justifying their further analysis as separately additive.
Table I.
Univariable and multivariable predictors of ADAMTS-4 in synovial fluid of the knee
| n∗ | Median ADAMTS-4 ng/mL (IQR) | P-value† | Coefficient‡ (95% CI) | Concordancec (95% CI) | Nagelkerke'sR2 (95% CI) | P-value§ | |
|---|---|---|---|---|---|---|---|
| Univariable analysis | |||||||
| Age | 170 | 0.08 (0.04–0.13) | 0.69 (0.62–0.78) | 0.10 (0.03–0.19) | <0.001 | ||
| OA | 159 | <0.001 | 0.66 (0.59–0.73) | 0.11 (0.04–0.22) | <0.001 | ||
| No | 118 | 0.0 (0–0.3) | 2.4 (1.2–3.6) | ||||
| Yes | 41 | 1.3 (0–4.1) | |||||
| Injury | 160 | 0.10 | 0.58 (0.52–0.65) | 0.03 (0.00–0.12) | 0.10 | ||
| None | 32 | 0 (0–0) | |||||
| Chondral | 95 | 0 (0–2.2) | 1.7 (0.2–3.3) | ||||
| Other | 33 | 0 (0–2.8) | 1.4 (−0.4–3.3) | ||||
| Effusion | 122 | <0.001‖ | 0.78 (0.69–0.86) | 0.31 (0.17–0.49) | <0.001 | ||
| No | 71 | 0.0 (0.0–0.0) | |||||
| Small | 28 | 0.0 (0.0–0.75) | 1.8 (0.4–3.2) | ||||
| Moderate | 14 | 1.7 (0.2–3.4) | 3.6 (1.9–5.3) | ||||
| Large | 9 | 8.5 (4.0–12.3) | 5.4 (3.4–7.3) | ||||
| Inflammation | 144 | <0.001 | 0.68 (0.60–0.76) | 0.11 (0.03–0.24) | <0.001 | ||
| No | 62 | 0.0 (0.0–0.0) | |||||
| Yes | 82 | 0.2 (0.0–2.8) | 2.4 (1.1–3.6) | ||||
| Multivariable analysis | |||||||
| Full model | 104 | 0.88 (0.83–0.96) | 0.46 (0.36–0.66) | ||||
| Age | 0.72¶ | 0.13¶ | 0.07 | ||||
| OA | 0.65 | 0.10 | 0.64 | ||||
| Injury | 0.54 | 0.01 | 0.86 | ||||
| Effusion | 0.79 | 0.34 | <0.001 | ||||
| Inflammation | 0.70 | 0.15 | 0.04 | ||||
| Reduced model | 106 | 0.88 (0.83–0.95) | 0.46 (0.34–0.63) | ||||
| Age | 0.05 (0.01–0.09) | 0.72¶ | 0.13¶ | 0.02 | |||
| Effusion | 0.79 | 0.34 | <0.001 | ||||
| No | |||||||
| Small | 1.7 (0.4–3.0) | ||||||
| Moderate | 3.3 (1.7–4.8) | ||||||
| Large | 4.4 (2.6–6.2) | ||||||
| Inflammation | 0.71 | 0.16 | 0.006 | ||||
| No | |||||||
| Yes | 1.8 (0.5–3.0) | ||||||
Notes (IQR: interquartile range; CI: confidence intervals).
Number of patients for each analysis, which vary due to data missingness.
P-values from univariate non-parametric statistical analyses.
Coefficients from censored regression.
P-values from censored regression.
P-value from Jonckheere trend test.
Concordance and Nagelkerke's R2 values for individual predictors in the full and reduced models are the univariable values when calculated using each model's dataset. They are therefore NOT partial concordance or R2 coefficients.
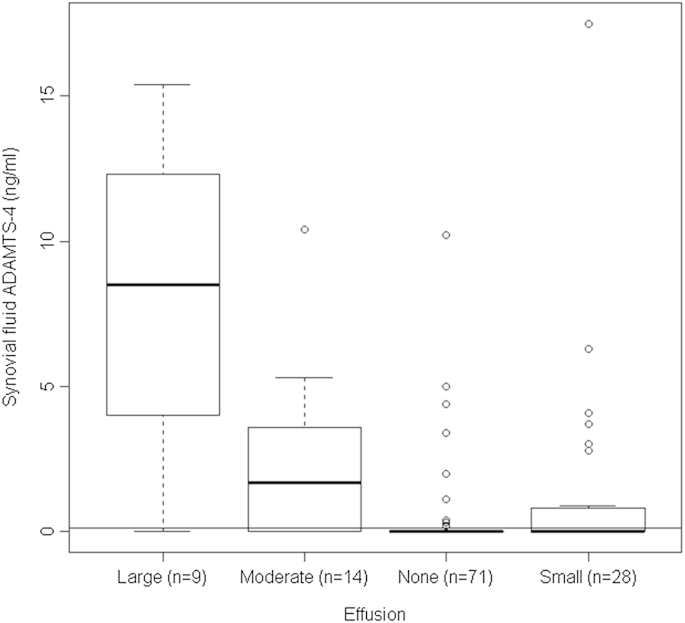
Four independent variables each significantly influenced ADAMTS-4 levels (all P < 0.001; Table I, univariable analysis), namely age (concordance = 0.69; Nagelkerke's R2 = 0.10), having OA (concordance = 0.66; Nagelkerke's R2 = 0.11), effusion level (concordance = 0.78; Nagelkerke's R2 = 0.31) and presence of an inflamed synovium (concordance = 0.68; Nagelkerke's R2 = 0.11). Effusion had the best predictive value, with ADAMTS-4 levels rising in an ordered fashion with increased effusion (P < 0.001, Jonckheere test; Fig. 1). In all, 14% of patients with no effusion, 36% of patients with a small effusion, 71% with a moderate effusion and 89% of those with a large effusion had detectable levels of ADAMTS-4. The significance levels from the univariable Tobit regression and conventional non-parametric tests were in complete agreement (Table I).
Fig. 1.
Levels of ADAMTS-4 (ng/mL) activity as a function of effusion size, shown as a boxplot. The thick horizontal lines represent the medians, the boxes represent upper and lower quartiles and the fences indicate the range up to 1.5 times the interquartile range below or above the lower or upper quartiles. Values higher than 1.5 times the interquartile range above the upper quartile are shown as open circles. The LLOD of 0.14 ng/ml is shown as a horizontal line.
The Tobit regression allows simultaneous investigation of multiple factors. Using all variables (full model; Table I) suggested that three independent variables simultaneously significantly influenced ADAMTS-4 levels, namely age, level of effusion and having an inflamed synovium (reduced model, Table I).
Discussion
Several international efforts have been and are addressing the challenge of developing reliable, sensitive and specific biomarkers for OA. Examples are those by the European Society for Clinical Aspects of Osteoporosis and Osteoarthritis (ESCEO)4 and the Osteoarthritis Research Society International – Federal Drugs Agency (OARSI-FDA) initiative (http://oarsi.org/education/oarsi-resources/fnih-osteoarthritis-biomarkers-consortium-project). Many groups have investigated the aggrecanase-generated neoepitope ARGS and assays are now available with great sensitivity12 (down to 0.025 pmol/mL6). This neoepitope can be measured not only in SF, but also in serum, plasma and urine, although results are less reliable in urine6,13. These assays, however, will not discriminate between the different forms of aggrecanase or ADAMTSs. The assay used in the present study measured activity of ADAMTS-4 (aggrecanase 1), with negligible cleavage of the FRET substrate by other members of the aggrecanase family, such as ADAMTS-1 and ADAMTS-5. Another ADAMTS-4 assay, using a fluorescent ‘turn-on’ peptide conjugated to gold nanoparticles, demonstrated differential levels in acute and chronic joint injuries14.
Our data suggests that effusion level, presence of synovial inflammation and age are the strongest determinants of level of ADAMTS-4, with injury or OA being less important. Mechanical loading is known to influence protease production, with the type of load switching production between MMPs and aggrecanases, e.g., in rat intervertebral disc cells15. In a murine model of OA it has been shown that gene expression of proteases, including ADAMTS-4 and -5, is both rapid and highly mechanosensitive16. This could explain the particularly strong relationship which we saw between effusion (and so swelling and increased pressure) in the joint and levels of ADAMTS-4 activity. Since ADAMTS-5 activity has not been assessed in the present study, it would be very interesting to measure this in the same or similar patient groups to determine if it also is increased with effusion.
The relative levels of MMP and aggrecanase production in a joint may be important not only to how the joint tissues degrade but also to the individual's ability to heal that injury and to their likelihood of developing OA. The DBA/1 strain of mice, which shows superior healing of injuries compared to the C57BL/6 strain, have less aggrecanase- but more MMP-induced aggrecan degradation than the C57BL/6 mice. In addition, the DBA/1 mice did not go on to develop subsequent signs of OA seen in the C57BL/6 mice17.
If ADAMTS-4 proves useful as a biomarker, further information on the aetiopathogenesis of degenerative joint disease(s) may be possible by measuring a differentially spliced variant of ADAMTS-4 which appears to be produced predominantly by synovial cells, particularly in OA18. Certainly differentiating the tissue source is important for biomarkers as ADAMTS-4 has been shown to be involved in atherosclerosis and is produced by monocytes from patients with acute coronary syndrome19. In addition, identification that ADAMTS-4 is a key player can help identify patients who might benefit from specific ADAMTS-4 inhibitors which are being developed. In terms of the BIPED classification of biomarkers20 (Burden of disease, Investigative, Prognostic, Efficacy of intervention or Diagnostic), evidence from this study suggests that ADAMTS-4 may prove useful for investigating the aetiopathogenesis of disease, making a diagnosis of joint effusion or inflammation and perhaps assessing the burden of the disease. Conversely, our results also suggest that the extent of joint effusion might serve as a proxy for ADAMTS-4 levels in the joint fluid. The low concordance and large influence of confounding factors suggest ADAMTS-4 is unlikely to be suitable for OA diagnosis.
There are, of course, several limitations to this study. One such is the way we related the relative fluorescent units created by the ADAMTS-4 in the SF samples to protein levels (ng/mL). We made the assumption that the kinetic curve would be the same for the recombinant ADAMTS-4 as it would be for the ADAMTS-4 in the biological samples, which of course it may not be. Different truncations and portions of the enzyme give different kinetics18. Variation in ADAMTS-4 structure, such as has been seen in OA joints, warrants further investigation in this matter in future studies. Another limitation is that we have only assessed ADAMTS-4 activity levels in comparison to a limited number of variables and observations and there are always more which could be made. For example, it would be interesting to determine if there was a relationship with the duration of patients' symptoms. A further problem with the study is missing data. Ideally, we would have full data on all patients but as in most ‘real world’ clinical studies this was not possible and for some patients information is partly lacking (e.g., 11 patients (6.5%) were not classified by the surgeon into 1 of the 3 categories at the time of the arthroscopic assessments). We believe that this data is missing completely at random as a consequence of busy clinics and therefore ‘missingness’ should not add bias to the assessments.
In conclusion, identification and validation of reliable, sensitive biomarkers for injured or degenerate joints remain important goals, not only for patient diagnosis and prognosis, but also for furthering our understanding of disease progression and indicating therapies which can be applied at a stage when the disease can be alleviated or modulated. We believe that measuring the level of ADAMTS-4 shows promise as a potentially useful biomarker and is worthy of further study.
Author contributions
Sally Roberts: Conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, obtaining of funding, administrative, technical or logistic support, collection and assembly of data. She takes responsibility for the integrity of the work as a whole (sally.roberts@rjah.nhs.uk).
Helena Evans: Analysis and interpretation of the data, drafting of the article, final approval of the article, technical or logistic support, collection and assembly of data.
Karina Wright: Analysis and interpretation of the data, drafting of the article, final approval of the article.
Louw van Niekerk: Drafting of the article, final approval of the article, provision of study material or patients, collection and assembly of data.
Bruce Caterson: Conception and design, drafting of the article, critical revision of the article for important intellectual content, final approval of the article.
James B Richardson: Drafting of the article, final approval of the article, provision of study material or patients, collection and assembly of data.
Karadi Hari Sunil Kumar: Drafting of the article, final approval of the article, provision of study material or patients, collection and assembly of data.
Jan Herman Kuiper: Analysis and interpretation of the data, drafting of the article, final approval of the article, statistical expertise.
Conflict of interest
The authors have no conflict of interest.
Acknowledgements
We are very grateful to the Arthritis Research UK (17540 and 19429) and Medical Research Council (G0800248) for their support in carrying out this project.
Contributor Information
S. Roberts, Email: sally.roberts@rjah.nhs.uk.
H. Evans, Email: helena.evans@rjah.nhs.uk.
K. Wright, Email: karina.wright@rjah.nhs.uk.
L. van Niekerk, Email: shelly.cameron@stees.nhs.uk.
B. Caterson, Email: caterson@cardiff.ac.uk.
J.B. Richardson, Email: james.richardson@nhs.net.
K.H.S. Kumar, Email: drkhskumar@yahoo.com.
J.H. Kuiper, Email: jan.kuiper@nhs.net.
References
- 1.Hunter D.J., Nevitt M., Losina E., Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol. 2014;28:61–71. doi: 10.1016/j.berh.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson D.T. The current and future status of biomarkers in osteoarthritis. J Rheumatol. 2014;41:834–836. doi: 10.3899/jrheum.140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco F.J. Osteoarthritis year in review 2014: we need more biochemical biomarkers in qualification phase. Osteoarthritis Cartilage. 2014;22:2025–2032. doi: 10.1016/j.joca.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Lotz M., Martel-Pelletier J., Christiansen C., Brandi M.L., Bruyere O., Chapurlat R. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72:1756–1763. doi: 10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fosang A.J., Rogerson F.M. Identifying the human aggrecanase. Osteoarthritis Cartilage. 2010;18:1109–1116. doi: 10.1016/j.joca.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Larsson S., Lohmander L.S., Struglics A. An ARGS-aggrecan assay for analysis in blood and synovial fluid. Osteoarthritis Cartilage. 2014;22:242–249. doi: 10.1016/j.joca.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Outerbridge R.E. The etiology of chondromalacia patellae. J Bone Jt Surg Br. 1961;43-B(4):752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 8.Sturgill L.P., Snyder-Mackler L., Manal T.J., Axe M.J. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sports Phys Ther. 2009;39:845–849. doi: 10.2519/jospt.2009.3143. [DOI] [PubMed] [Google Scholar]
- 9.Kraus V.B., Stabler T.V., Kong S.Y., Varju G., McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007;15:1217–1220. doi: 10.1016/j.joca.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helsel R. Reporting limits. In: Helsel Dennis R., editor. Statistics for Censored Environmental Data Using MiniTab and R. John Wiley & Sons, Inc; 2012. pp. 22–36. [Google Scholar]
- 11.Lubin J.H., Colt J.S., Camann D., Davis S., Cerhan J.R., Severson R.K. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swearingen C.A., Chambers M.G., Lin C., Marimuthu J., Rito C.J., Carter Q.L. A short term pharmacodynamic model for monitoring aggrecanase activity: injection of monosodium iodoacetate (MIA) in rats and assessment of aggrecan neoepitope release in synovial fluid using novel ELISAs. Osteoarthritis Cartilage. 2010;18:1159–1166. doi: 10.1016/j.joca.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Germaschewski F.M., Matheny C.J., Larkin J., Liu F., Thomas L.R., Saunders J.S. Quantitation of ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthritis Cartilage. 2014;22:690–697. doi: 10.1016/j.joca.2014.02.930. [DOI] [PubMed] [Google Scholar]
- 14.Peng S., Zheng Q., Zhang X., Dai L., Zhu J., Pi Y. Detection of ADAMTS-4 activity using a fluorogenic peptide-conjugated Au nanoparticle probe in human knee synovial fluid. ACS Appl Mater Interfaces. 2013;5:6089–6096. doi: 10.1021/am400854z. [DOI] [PubMed] [Google Scholar]
- 15.Latridis J.C., Godburn K., Wuertz K., Alini M., Roughley P.J. Region-dependent aggrecan degradation patterns in the rat intervertebral disc are affected by mechanical loading in vivo. Spine. 2011;36:203–209. doi: 10.1097/BRS.0b013e3181cec247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burleigh A., Chanalaris A., Gardiner M.D., Driscoll C., Boruc O., Saklatvala J. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64:2278–2288. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- 17.Eltawil N.M., De Bari C., Achan P., Pitzalis C., Dell'Accio F. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage. 2009;17:695–704. doi: 10.1016/j.joca.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wainwright S.D., Bondeson J., Caterson B., Hughes C.E. ADAMTS-4_v1 is a splice variant of ADAMTS-4 that is expressed as a protein in human synovium and cleaves aggrecan at the interglobular domain. Arthritis Rheum. 2014;65:2866–2875. doi: 10.1002/art.38102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zha Y., Chen Y., Xu F., Zhang J., Li T., Zhao C. Elevated level of ADAMTS4 in plasma and peripheral monocytes from patients with acute coronal syndrome. Clin Res Cardiol. 2010;99:781–786. doi: 10.1007/s00392-010-0183-1. [DOI] [PubMed] [Google Scholar]
- 20.Bauer D.C., Hunter D.J., Abramson S.B., Corr M., Felson D., Heinegard D. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]