Abstract
Accumulating evidence from animal and human research shows exercise benefits learning and memory, which may reduce the risk of neurodegenerative diseases, and could delay age-related cognitive decline. Exercise-induced improvements in learning and memory are correlated with enhanced adult hippocampal neurogenesis and increased activity-dependent synaptic plasticity. In this present chapter we will highlight the effects of physical activity on cognition in rodents, as well as on dentate gyrus (DG) neurogenesis, synaptic plasticity, spine density, neurotransmission and growth factors, in particular brain-derived nerve growth factor (BDNF).
Keywords: Adult neurogenesis, Dentate gyrus, Running, Learning and memory, Neurotrophic factors, Synaptic plasticity, Endurance factors
1 Exercise and Cognition
Cognition studies in adult rodents have shown that both voluntary and forced exercise enhance spatial memory in the Morris water maze, Y-maze, T-maze and radial arm maze tests (Fordyce and Farrar 1991; van Praag 2008). Running also improves performance on tasks that require minimal spatial navigation. For example, contextual fear conditioning, passive avoidance learning and novel object recognition are enhanced by running (Baruch et al. 2004; Falls et al. 2010; Liu et al. 2008; Mello et al. 2009; O'Callaghan et al. O'Callaghan et al. 2007; Hopkins and Bucci 2010; Fahey et al. 2008; Griffin et al. 2009). It remains to be determined whether the beneficial effects of exercise for performance on these tasks is mediated by the hippocampus and/or whether other brain areas are involved to a similar extent. For example, contextual fear conditioning depends on both the hippocampus and amygdala (Phillips and LeDoux 1992; Myers and Gluck 1994; Wiltgen et al. 2006; Maren 2001, 2008). The hippocampus is critical for memory of context; whereas the amygdala stores the context–shock association (Huff and Ruddy 2004). However, the effects of physical activity on the amygdala are still unclear. Greenwood et al. (2009) showed that 6 weeks of running before conditioning improves hippocampus dependent memory for context, but not extinction (amygdala-dependent), suggesting that voluntary physical activity selectively increased hippocampus-dependent memory. Subsequent studies showed that physical activity improved learning and consolidation of cued conditioned fear, an amygdala-dependent process, but not the retrieval or performance of conditioned fear (Falls et al. 2010; Lin et al. 2012). Interestingly, exercise increases BDNF mRNA, and synaptic proteins such as TrkB and SNAP-25 levels in both the dentate gyrus (DG) and amygdala (Greenwood et al. 2009; Lin et al. 2012), albeit more so in the DG (Greenwood et al. 2009). Thus, physical activity may differentially affect amygdala and hippocampus-dependent plasticity.
Within the hippocampal subfields (area CA1, area CA3, DG), physical activity likely has differential functional effects. Indeed, it has become increasingly well-established that the subfields of the hippocampus may mediate different aspects of memory formation. Area CA1 is deemed important for encoding, area CA3 for pattern completion and the DG is considered to mediate pattern separation (McHugh et al. 2007; Leutgeb et al. 2007; Leutgeb and Leutgeb 2007; Gilbert et al. 2001; Bakker et al. 2008; Gold and Kesner 2005; Kesner 2007; Schmidt et al. 2012). The DG is one of the brain regions with substantial addition of new neurons throughout the lifetime of mammals, (Altman and Das 1965) and it has been suggested that these new neurons contribute significantly to pattern separation. Ablation of adult neurogenesis results in deficits in fine pattern discrimination in the touchscreen (Clelland et al. 2009) and the ability to distinguish between two similar contexts in fear conditioning (Tronel et al. 2010). Spatial pattern separation was evaluated recently in running versus sedentary mice (Creer et al. 2010). Specifically, mice were tested on a spatial discrimination task, where stimuli were presented in close or distal proximity using a touchscreen method that requires minimal motor activity (Morton et al. 2006). There was no difference between the groups when the separation between stimuli was large, however, runners outperformed sedentary mice when the difference between stimuli was small (Creer et al. 2010). The observed improvement in making fine spatial distinctions may be due at least in part to the increase in adult neurogenesis that is observed in the hippocampus with exercise (van Praag et al. 1999a). Indeed, in a transgenic mouse with increased adult hippocampal neurogenesis there is improved differentiation between overlapping contextual representations, indicative of enhanced pattern separation (Sahay et al. 2011).
2 Neurogenesis and Exercise
Many different extrinsic and intrinsic factors can regulate the production of new neurons. Housing mice in an enriched environment (EE) resulted in the first evidence that adult neurogenesis could be enhanced (Kempermann et al. 1997, 1998). Increased neurogenesis was correlated with improved performance in a hippocampus-dependent spatial learning task, the Morris water maze (Kempermann et al. 1997). However, an enriched environment is a complex combination of inanimate and social stimulation, learning and physical activity. Separation of the different elements of the EE showed that running per se increases cell proliferation, neurogenesis, and synaptic plasticity, as well as spatial memory function in the mouse DG (van Praag et al. 1999a, b; Kronenberg et al. 2003; van der Borght et al. 2007). A potential confound in the initial study was that the positive control, the EE also contained a running wheel (van Praag et al. 1999a). However, at that time the finding that running increased neurogenesis was completely unexpected and so an EE group without a wheel was not included. Subsequent research suggested that running enhanced the number of bromodeoxyuridine (BrdU) positive cells more so than EE without running wheels, but that EE still differed from standard housing conditions (Ehninger and Kempermann 2003), even though only marginally so (Fabel et al. 2009). However, in these studies the control mouse cages were typically smaller than those of the EE group, allowing less activity.
In a recent study, we aimed to dissociate the effects of physical activity and enrichment in young female C57Bl/6 mice housed in identically sized cages under control, running, and EE with or without running wheels. Cell proliferation, neuron survival, and neurotrophin levels were enhanced only when running wheels were accessible, suggesting that exercise is the critical factor mediating increased BDNF levels and adult hippocampal neurogenesis (Kobilo et al. 2011a); Fig. 1). This study has been replicated and extended in individually housed male C57Bl/6 mice. Specifically, the authors added more enrichment in the form of dietary treats and continuous addition of novel objects. Even so, only running and running plus enrichment increased neurogenesis. The greatest amount of physical activity was observed in the running only condition and these mice exhibited improved spatial learning in the Morris water maze (Mustroph et al. 2012). Altogether, these findings do not preclude the effects of EE only on behavior. Indeed, EE only has effects on synaptic plasticity, such as increased dendritic complexity of young granule neurons (Beauquis et al. 2010), enhanced arborization of cortical neurons (Ip et al. 2002), and elevated synaptophysin levels in hippocampus and cortex (Lambert et al. 2005). Moreover, EE only has been shown to have profound effects on dendritic spines and synapses in cortex and cerebellum (Greenough et al. 1978, 1985, 1986). Therefore, these findings suggest that EE and physical activity share common features pertaining to synaptic plasticity (van Praag et al. 2000), but that the increment in adult neurogenesis and BDNF levels in the hippocampus is specific to physical activity.
Fig. 1.
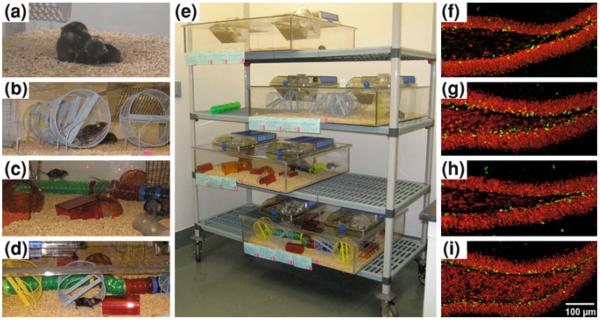
Exercise but not enrichment increases DG neurogenesis. Female C57Bl/6 mice (n = 10 per group) were housed in large cages (30”×33”×8”) as follows: a Control (CON). b Running (RUN). c Enriched environment only (EEO). d Enrichment and running (EER), this cage contained enrichment objects similar to (c), as well as 10 running wheels. e Overview of the experimental cages. (f–g) Confocal images of BrdU-positive cells in the dentate gyrus in sections derived from mice housed in (f) CON, (g) RUN, (h) EEO, or (i) EER conditions. Sections were immunofluorescent double-labeled for BrdU (green) and NeuN (red) indicating neuronal phenotype (Kobilo et al. 2011a)
Reduced neurogenesis occurs naturally with aging and is observed in certain mouse models of neurodegenerative disease. Exercise may ameliorate or reverse this change in some but likely not all conditions. Indeed, it has been shown that neurogenesis declines as early as middle-age (Kuhn et al. 1996), and may contribute to age-related reductions in cognitive function (Erickson and Barnes 2003). However, the robust effect of exercise on neurogenesis is maintained throughout life in rodents. It has been shown that exercise from 3 to 9 months of age significantly reduced the age-dependent decline in cell proliferation and led to a consecutive increase in the number of more mature cells (Kronenberg et al. 2006). Moreover, in mice that started wheel running in middle age (Wu et al. 2008; Marlatt et al. 2012) or old age (van Praag et al. 2005), new neuron number was elevated. Furthermore, recent studies have shown that physical activity can reverse radiation-treatment-related decline in hippocampal neurogenesis (Naylor et al. 2008; Wong-Goodrich et al. 2010). In addition, running can ameliorate the genetically reduced generation of proliferating hippocampal cells and enhance the dendritic arborization of newly generated neurons in synRas mice (Lafenetre et al. 2010).
The effects of exercise have also been evaluated in mouse models of neurodegenerative disease. In certain mouse models of Alzheimer's disease (AD), exercise is able to reduce pathology and enhance cognition and adult neurogenesis. In TgCRND8 mice (which express APP695swe, Ind), running improved spatial memory and reduced extracellular Aβ plaque load (Adlard et al. 2005). Exercise was also found to be beneficial in Tg2576 mice (which express APP695swe), even after the onset of pathology (Nichol et al. 2007). Similarly, APOE4 mice have been shown to benefit from regular physical activity (Nichol et al. 2009). Furthermore, in the 3xTG model of the disease, physical activity reduced disease symptoms and improved synaptic plasticity in a recent study (Garcia-Mesa et al. 2011). Reduced neurogenesis in this model (Rodriguez et al. 2008) was improved by running (Rodriguez et al. 2011). Altogether, the effects of exercise appears to be promising in mouse models of AD and is consistent with positive data emerging from studies in humans (Buchman et al. 2012; Lautenschlager et al. 2012).
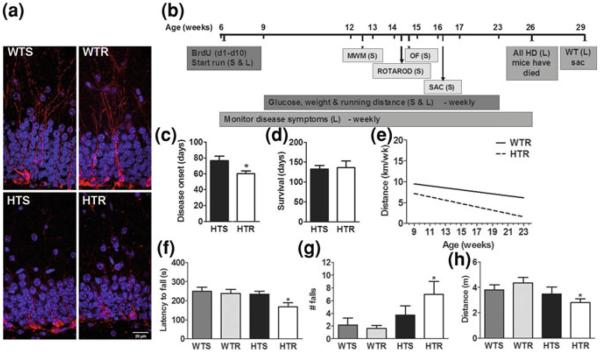
A notable exception to the beneficial effects of exercise on brain and behavior appears to be in mouse models of Huntington's disease (HD). While initial research showed that EE delays symptom onset in R6/1 transgenic mice (van Dellen et al. 2000), physical activity had equivocal results. In R6/1 mice, running normalized rearing behavior and delayed the onset of deficits in rear-paw clasping, motor coordination and spatial working memory. However, rotarod performance, ubiquitinated protein aggregates, hippocampal BDNF protein levels (Pang et al. 2006; van Dellen et al. 2008), and hippocampal neurogenesis in R6/2 mice (Kohl et al. 2007) were unchanged by exercise. In a recent study, running started in presymptomatic 6-week-old male HD (N171-82Q) mice did not improve function and appeared to accelerate disease onset (shaking, hunched back and poor grooming), reduced striatal volume, and impaired motor behavior compared to sedentary controls. Furthermore, weight loss, reduced lifespan, hyperglycemia, Morris water maze learning deficits, diminished hippocampal neurogenesis, deficits in immature neuronal morphology, intranuclear inclusions, and decreased DG volume were refractory to physical activity (Potter et al. 2010; Fig. 2). It remains to be determined whether similar observations will be made in other mouse models of the disease. Interestingly, a case study in humans also has indicated that physical exercise may not prevent or delay disease onset or progression. In humans, the number of polyglutamine repeats can predict disease onset to some extent (Langbehn et al. 2004). A marathon runner presented with myopathy 20 years before the predicted disease onset for 41 CAG repeats (Altschuler 2006; Kosinski et al. 2007). Thus, the neurogenic and cognitive effects of physical activity should be evaluated carefully across the spectrum of neurological diseases.
Fig. 2.
Exercise is not beneficial in a mouse model of Huntington's disease (HD) N171-82Q. a Morphology of Doublecortin (DCX) expressing immature neurons in the DG of WT and HD mice. Dendritic branching complexity of DCX-labeled (red) immature neurons was reduced in the transgenic mice, HD sedentary (HTS) and HD runner (HTR) compared to Wildtype sedentary (WTS) and Wildtype runner (WTR). Granular cell layer neurons were labeled with DAPI (blue). b Timeline of experiment in weeks of age for the subset of mice tested for behavior (S) and mice evaluated over their lifespan (L) mice. BrdU was injected over the first 10 days of the study. Behavioral testing was carried out from week 12 to 15 and mice (S) were sacrificed (SAC) at approximately 16 weeks of age. Survival analysis indicated that HD mice died between 11 and 26 weeks of age. c Onset of disease symptoms such as hunched back, poor grooming and involuntary shaking occurred earlier in HD runners; * p<0.03. d Lifespan did not change as a result of exercise. e There was no significant difference between the groups in running distance over the duration of the experiment. (f–g) Exercise may exacerbate locomotor deficits in HD mice. (f) The latency to fall off an accelerating rotarod was shorter in HTR mice than in all other groups; * p<0.03. g The total number of falls from the rotarod over 5 min was increased in HD runners compared to WT mice; * p<0.02, HTR versus WTS and WTR. h The total distance traveled over 30 min in an open field was reduced in HD mice compared to WT mice, specifically in HTR mice compared to WTR mice; * p<0.03. Abbreviations: MWM Morris water maze; OF Open field; S Subset tested for behavior; L Lifespan group; SAC sacrificed (Potter et al. 2010)
3 Synaptic Plasticity: Effects of Exercise On Long-Term Potentiation/Depression, Dendritic/Spine Size and Morphology
Running can influence neural plasticity on many levels, including modifications in synaptic function. Induction of long-term potentiation (LTP), a physiological model of certain forms of learning and memory (Bliss and Collingridge 1993), was measured in hippocampal slices from running and control mice. Field recordings showed a significantly greater LTP in the DG of running mice as compared to controls (van Praag et al. 1999b; Vasuta et al. 2007). Recordings from CA1 region in the same mice showed no differences between groups, suggesting that the changes observed in the DG were the direct result of increased neurogenesis (van Praag et al. 1999b). Subsequent studies have shown similarly enhanced LTP in vivo in anesthetized rats subject to voluntary (Farmer et al. 2004) and forced running (O'Callaghan et al. 2007). Field recordings in vivo showed a significantly enhanced LTP in the DG induced with a weak theta patterned stimulation which did not produce LTP in control subjects, as well as more short-term potentiation in running rats as compared to controls (Farmer et al. 2004).
Thus exercise modifies the synaptic plasticity of the DG likely as a result of the enhanced neurogenesis. Indeed, recordings of individual newborn dentate granule cells in hippocampal slices have shown that they display a low threshold for LTP induction and enhanced LTP compared to mature granule cells (Wang et al. 2000; Schmidt-Hieber et al. 2004; Ge et al. 2007). This enhanced plasticity was observed in a specific time window (1–1.5 months old) of their maturation process and was dependent on transiently increased synaptic expression of NR2B containing N-methyl-D-asparate (NMDA) receptors (Ge et al. 2007). Together, these results support the hypothesis that newborn granule cells have a unique role in synaptic plasticity of the hippocampus and that their contribution can be enhanced with exercise. Indeed, protective effects induced by exercise on hippocampal plasticity have been observed in middle age to old age rodents. The age-related impairment in expression of LTP was reversed in middle age rodents exposed to regular exercise (O'Callaghan et al. 2009), which correlates with improved memory, enhanced hippocampal neurogenesis and increments of BDNF levels in middle-age rodents exposed to long periods of running (O'Callaghan et al. 2009; Marlatt et al. 2012). It should also be noted that in contrast to the enhanced LTP observed in running rodents, long-term depression (LTD), another type of synaptic plasticity (Bear and Abraham 1996), induced by low frequency stimulation was relatively unaffected by exercise. However, the involvement of NR2A containing NMDA receptors was increased by exercise compared to non-runner mice (Vasuta et al. 2007) suggesting that exercise can alter the contribution of NMDA subunits to LTD.
Modifications in synaptic plasticity have also been associated with morphological changes in response to neural activity (Nägerl et al. 2004). In particular, morphological changes in DG have been observed with exercise (Eadie et al. 2005; Redila and Christie 2006; Zhao et al. 2006; Stranahan et al. 2007). Analysis of individual granule cells revealed that exercise significantly increased the total length, complexity, and spine density of granule cell dendrites (Eadie et al. 2005). Upon classification of individual DG cells by their position in the layer, it was shown that exercise enhances dendritic complexity in all the zones of the granule cell layer (subgranular, inner and outer granule cell zones) (Redila and Christie 2006). Moreover, long-term exercise (2 months) induced morphological changes not only in the DG, but also in the entorhinal cortex and CA1 pyramidal cells (Stranahan et al. 2007).
Use of retrovirus-mediated labeling of newborn neurons has made it possible to examine and characterize morphological details of these cells throughout their lifetime (van Praag et al. 2002). Retroviral labeling showed that exercise does not modify spine density of newborn granule cells in young compared to aged runner mice (van Praag et al. 2005). A subsequent detailed study in young mice showed that running increased the motility of the dendritic spines of newborn neurons (21 days postvirus-injection) and accelerated their maturation, as quantified by the presence of mushroom spines (28–52 days postvirus-injection), without modifying dendritic complexity (Zhao et al. 2006). Thus, exercise modifies the morphology of dentate granule cells and other parameters related to memory function, and may also influence the rate of integration of newborn granule cells into the hippocampal circuitry.
4 Exercise and Neurotrophic Factors (BDNF, FGF-2, NGF, VEGF and IGF)
Several cellular and molecular systems important for maintaining neuronal function and plasticity, such as neurotrophins may be instrumental for positive effects of exercise on the brain. In particular, brain-derived neurotrophic factor (BDNF) is well known to play an important role in the adult brain in synaptic plasticity (Kuipers and Bramham 2006), learning (Yamada and Nabeshima 2003), and neurogenesis (van Praag 2008; Bekinschtein et al. 2011) and is considered to be the most important factor upregulated by physical activity (Cotman et al. 2007). The first study showing exercise-induced increases in neurotrophins in the hippocampus was by Neeper and colleagues (Neeper et al. 1995). Specifically, they described that 2–7 days of running increases the level of BDNF mRNA in specific brain areas, including the hippocampus. Indeed, BDNF gene and protein expression in the hippocampus were elevated as long as the animals were housed with the running wheels (Berchtold et al. 2005). Furthermore BDNF levels remained upregulated for 2 weeks after running wheels were no longer accessible (Berchtold et al. 2010). Many studies have now demonstrated exercise-dependent changes in BDNF expression (see Cotman and Berchtold 2002, Cotman et al. 2007; Vaynman and Gomez-Pinilla 2005).
A detailed analysis of the hippocampal subfields showed that exercise increases BDNF mRNA levels in the DG but not in CA1 (Farmer et al. 2004). Moreover, exercise induced an increment in the DG of synapsin I, a vesicle-associated phosphoprotein that modulates transmitter release as well as the formation and maintenance of presynaptic structures, suggesting that exercise may induce plasticity in a specific hippocampal subfield, the DG (Vaynman et al. 2004a, b). Changes in the levels of BDNF mRNA and protein, NMDA receptor subunit NR2B mRNA, GluR5 and synapsin I, (Farmer et al. 2004; Vaynman et al. 2004a, b; O'Callaghan et al. 2007) may be associated with the enhanced LTP and neurogenesis observed in the DG of running mice (van Praag et al. 1999b; Farmer et al. 2004; O'Callaghan et al. 2007). Indeed, BDNF is proposed to regulate the synaptic transmission and activity-dependent synaptic plasticity by pre- and postsynaptic mechanisms (Poo 2001; Binder and Scharfman 2004; Kuipers and Bramham 2006). Genetic deletion of BDNF in mice showed disruption of normal induction of LTP (Korte et al. 1995), a defect that was rescued with BDNF replacement, either through the BDNF-expressing adenovirus (Korte et al. 1996) or by supplying exogenous BDNF (Patterson et al. 1996). Moreover, blockade of tyrosine receptor kinase B (TrkB), the high-affinity receptor for BDNF, with a specific immune-adhesin chimera (TrkB-IgG; Vaynman et al. 2004b) or a TrkB inhibitor (K252a; Liu et al. 2008) was sufficient to inhibit BDNF actions and its beneficial effects on learning and memory.
Over the past years, the effects of exercise on adult neurogenesis and BDNF levels have been extensively studied (for review see van Praag 2008, 2009). Selective ablation of the gene encoding TrkB in hippocampal neural progenitor cells has been shown to prevent the exercise-induced increase in neurogenesis (Li et al. 2008) and the neurogenesis-dependent LTP (Bergami et al. 2008). Using BDNF knockdown by RNA interference using lentiviral vectors injected into the DG has also resulted in reduced neurogenesis (Taliaz et al. 2010). Furthermore, intracerebral infusion of BDNF increases neurogenesis in the DG (Scharfman et al. 2005) and mimics exercise-induced changes in learning (Griffin et al. 2009). The effects of exercise on hippocampal neurogenesis, and BDNF levels have been suggested to be mediated by activation of NMDA receptor containing ε1 subunit (Kitamura et al. 2003). BDNF's neurogenic effects are area-specific, since BDNF infusion does not induce neurogenic changes in the subventricular zone (Galvão et al. 2008), consistent with data showing that the neurogenic effect of exercise is limited to the hippocampus (Brown et al. 2003).
The beneficial effects of exercise be may to some extent age-dependent. In a recent study, Titterness and colleagues (Titterness et al. 2011) have shown that 13 days of exercise did not increase BDNF protein levels in the DG of adolescent female and male mice (postnatal 22), which correlated with no differences in LTP induction between sedentary and runner female mice. Interestingly, enhanced LTP was observed in male runner mice suggesting a differential sensitivity to exercise in adolescent female and male mice, possibly due to differences in development. However, in male and female adult and aged mice the effects are generally positive. Five weeks (Wu et al. 2008) and 8 months of forced exercise on a treadmill (O'Callaghan et al. 2009) as well as voluntary exercise (Marlatt et al. 2012) restored reduced BDNF and TrkB levels in the DG of middle-aged mice (Hattiangady et al. 2005; Wu et al. 2008; O'Callaghan et al. 2009). This effect correlates with enhanced neurogenesis and improvement of cognitive function (Wu et al. 2008; O'Callaghan et al. 2009; Marlatt et al. 2012). Effects of exercise on proBDNF levels, important for synaptic plasticity (Ding et al. 2006) remain to be determined.
In addition to BDNF, other neurotrophins, such as fibroblast growth factor 2 (FGF-2) (Gomez-Pinilla et al. 1997) and nerve growth factor (NGF) (Neeper et al. 1996) show a marked upregulation of mRNA in the hippocampus as a result of exercise. However, the increase in these factors is transient and less pronounced than exercise-induced increases in BDNF. In particular, upregulation of FGF-2 mRNA level was found after four nights of running and only observed in the hippocampus (Gomez-Pinilla et al. 1997). Similarly, NGF mRNA increased after the second night of running, mainly in the DG and hilus (Neeper et al. 1996). Furthermore, 8 weeks of treadmill exercise restored NGF mRNA levels in the hippocampus of aged rats (Chae and Kim 2009), as well as long-term forced exercise (8 months) in the DG of middle-aged mice (O'Callaghan et al. 2009). Both FGF-2 and NGF, have been implicated in adult neurogenesis. Using mice genetically deficient in FGF-2 (FGF-2−/− mice), intraventricular application of FGF-2 by viral gene transfer showed increased proliferation of progenitor cells in the DG (Yoshimura et al. 2001). Similarly, intracerebroventricular infusion of FGF-2 has been found enhance the neurogenesis and the dendritic complexity of the newborn granule cells (Rai et al. 2007). In a recent study, it was shown that FGF-2 deficiency in mice (FGF-2−/− mice) does not produce alterations in cell proliferation, but causes defects in neural differentiation in the adult DG. This defect was not rescued by exogenous FGF-2. Neutralization of FGF-2 with an antibody to FGF-2 did not interfere with neurogenesis, suggesting that FGF-2 may operate synergistically in combination with other mechanisms/growth factors to mediate the maturation of new neurons in the adult DG (Werner et al. 2011).
Other trophic factors that have been shown to be regulated by exercise and influence adult neurogenesis include vascular endothelial growth factor (VEGF) and insulin like growth factor I (IGF-I). Recent evidence indicates that VEGF can act as a neurotrophic factor (Ogunshola et al. 2002) and produces neurogenic effects on progenitor cells (Jin et al. 2002; Cao et al. 2004). Interestingly, it has been shown adult hippocampal neurogenesis occurs near the local microvasculature of hippocampus (Palmer et al. 2000; Fabel et al. 2003). Angiogenic changes associated with exercise have been shown to occur in the hippocampus (Fabel et al. 2003; van Praag et al. 2005; Clark et al. 2009; Van der Borght et al. 2009) that may be mediated by VEGF. Indeed, VEGF is a hypoxia-inducible protein that promotes angiogenesis through receptor tyrosine kinases on endothelial cells (Krum et al. 2002). Fabel and colleagues (Fabel et al. 2003) have shown that blockade of peripheral VEGF inhibits the increase in neurogenesis observed with running. Moreover, it has been shown that 50 days of exercise increases neurogenesis, density of blood vessels in the DG, but not other hippocampal subfields, and enhances performance in the water maze (Clark et al. 2009). Changes in neurogenesis and angiogenesis can be observed as early as 3 days of running (Van der Borght et al. 2009). Changes in cerebral blood volume measured using MRI imaging have been shown to be correlated with enhanced neurogenesis in exercising mice; these changes were specific to the DG. As in mice, exercise increased cerebral blood volume in humans and correlated with cognitive function, suggesting that this measurement may be an indirect measure for levels of neuro-genesis in humans (Pereira et al. 2007). However, it should also be noted that an increase in angiogenesis is not necessarily linked to increased neurogenesis (van Praag et al. 2007).
Vasculature changes associated with exercise in the adult brain are also mediated by IGF-1 (Lopez–Lopez et al. 2004). Exercise enhances IGF-1 levels in the periphery (Trejo et al. 2001) and brain (Carro et al. 2000). At least part of the increase in the brain reflects elevated transport from the periphery across the blood–brain barrier (Reinhardt and Bondy 1994). It has been shown that systemic injection of IGF-1 in sedentary rats mimics the effects of exercise, including enhanced neurogenesis, patterns of neuronal accumulation of IGF-1, c-Fos and BDNF expression in the hippocampus, while subcutaneous infusion of a blocking IGF-1 antibody produces the opposite effects (Carro et al. 2000; Trejo et al. 2001). Peripheral infusion of IGF-1 also increased adult neurogenesis (Aberg et al. 2000), and reversed the aging related reduction in new neuron production (Lichtenwalner et al. 2001). Because IGF-1 induced-modifications correlated with BDNF levels, it has been suggested that BDNF may be a potential downstream target that mediates some of the protective effects of IGF-1 (Ding et al. 2006). In addition, IGF-1 is able to increase the spontaneous firing in neurons accumulating IGF-1; therefore, peripheral IGF-1 may initiate growth factor cascades in the brain that can alter synaptic plasticity.
5 Exercise and Neurotransmitters
Physical activity influences many neurotransmitter systems in the brain including the glutamatergic (Farmer et al. 2004; Kitamura et al. 2003; Lou et al. 2008; Vasuta et al. 2007), GABAergic (Molteni et al. 2002), endocannabinoid (Hill et al. 2010), opioidergic (Sforzo et al. 1986), and monoaminergic systems (Chaouloff 1989). One of the main components of the glutamatergic system that has been linked to neurogenesis and synaptic plasticity is the NMDA receptor. The expression of the NR2A and NR2B subunits of this receptor were found to be significantly increased after physical activity (Farmer et al. 2004; Kitamura et al. 2003; Lou et al. 2008; Vasuta et al. 2007). However, genes related to the gamma-aminobutyric acid (GABA) (GABAA receptor, glutamate decarboxylase GAD65) system were downregulated after physical activity (Molteni et al. 2002). Knowledge of the role of the AMPA receptor in exercise induced plasticity is limited, though a recent study revealed exercise-induced changes to the AMPA receptor subunits, GluR1 and GluR2/3 (Real et al. 2010).
The psychological changes associated with prolonged physical activity are often described as a `runner's high'. Exercise induced changes in psychological functions are frequently reported to being as a direct consequence of alterations in the endogenous opioid system (Schwarz and Kindermann 1992). More recently, an alternative hypothesis to explain this phenomenon was proposed, showing that exercise increases blood concentrations of endogenous endocannabinoids (Heyman et al. 2012). Voluntary wheel running increased the agonist binding site density of the cannabinoid CB(1) receptor, CB(1) receptor-mediated GTPgammaS binding, and the levels of anandamide in the hippocampus (Hill et al. 2010). These alterations were required for the exercise-induced increase in progenitor cell proliferation in the hippocampus (Hill et al. 2010), and may play a role in the well-documented antidepressant effects of exercise (for review see Ota and Duman 2012). Indeed, the changes in monoamines with exercise are of particular interest as physical activity has been shown to lead to recovery from depression (Lawlor and Hopker 2001). The antidepressant effect of exercise for mild depression in humans (Ernst et al. 2006) has been shown to be just as potent as that of serotonergic medications (Babyak et al. 2000). Therefore, it is of interest that serotonergic agonists, including antidepressants such as fluoxetine (Encinas et al. 2006; Malberg et al. 2000), have been suggested to enhance cell genesis, whereas administration of the serotonin 5-HT (1A) receptor antagonists, decreases cell proliferation in the DG (Radley and Jacobs 2002).
In a recent study, we aimed to evaluate to what extent the effects of antidepressants on neurogenesis are comparable to those of voluntary wheel running. Specifically, neurogenesis was evaluated in 2-month-old female C57Bl/6 mice after 28 days of treatment with either fluoxetine, a selective serotonin reuptake inhibitor which has been shown to enhance neurogenesis (Santarelli et al. 2003), duloxetine, a dual serotoninergic-noradrenergic reuptake inhibitor, or exercise. Interestingly, only exercise enhanced (by 200 %) the BrdU positive cell survival. In addition, only fluoxetine and exercise resulted in a phenotype shift with a greater percentage of BrdU-positive cells becoming new neurons (Fig. 3). Thus, the neurogenic response to exercise is much stronger than to antidepressants, and it is not very likely that anxiolytic effects of these drugs are mediated by adult neurogenesis (Marlatt et al. 2010). Recent research by others supports our findings in this regard (Hanson et al. 2011).
Fig. 3.
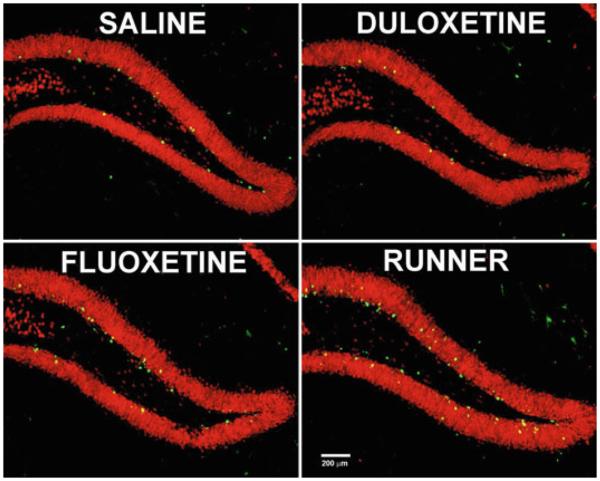
Running but not antidepressants enhances neurogenesis. Photomicrographs of the dentate gyrus in sections derived from mice injected with saline, duloxetine 6 mg/kg, fluoxetine 18 mg/kg, or housed with a running wheel for 28 days. BrdU labeled cells (green) and cells labeled with the neuronal marker NeuN (red). Co-labeling analysis indicated that running mice had more than 2-fold increase in the number of new neurons compared to all the other groups (Marlatt et al. 2010)
6 Endurance Factors
Another logical approach toward identifying pharmacological ways to enhance neurogenesis is the investigation into whether skeletal muscle activation as a result of exercise or pharmacological agents underlies neurogenic and cognitive effects of aerobic activity. Indeed, much research pertaining to the effects of exercise on brain function has focused on cellular, structural, and biochemical changes in the brain without much consideration for the peripheral factors that may elicit changes in synaptic plasticity, angiogenesis, neurogenesis, and cognition (Cotman et al. 2007; Gomez-Pinilla et al. 2008; Hillman et al. 2008; van Praag 2008). Physical activity has also shown effects on neurotropic factors in mammalian skeletal muscle (for review see Sakuma and Yamaguchi 2011), an abundant source of neurotrophins (Chevrel et al. 2006). Increased BDNF levels and BDNF mRNA expression in the peripheral system have been described following exercise (Gomez-Pinilla et al. 2002; Allard et al. 2004; Ferris et al. 2007). These increments may play a role in enhancing glucose metabolism and may act as a myokine, producing neurotrophic effects in the brain (Sakuma and Yamaguchi 2011). Indeed, the possibility that skeletal muscle activation as a result of exercise or pharmacological agents underlies cognitive effects of aerobic activity has just begun to be explored.
Recently, transcriptional factors regulating muscle fiber contractile and metabolic genes have been identified (Wang et al. 2004) and led to the identification of compounds that can increase the ability of cells to burn fat and enhance exercise endurance (Narkar et al. 2008). The peroxisome proliferator activated receptor delta (PPARδ) is a transcription factor that regulates fast-twitch muscle fiber contraction and metabolism. Overexpression of this factor increased oxidative muscle fiber number. In addition, administration of the selective agonist GW501516 increased exercise stamina when combined with training (Narkar et al. 2008). PPARδ is controlled by AMP-activated protein kinase (AMPK), a master metabolic regulator important for glucose homeostasis, appetite, and exercise physiology (Hardie 2004). Treatment with AMPK agonist AICAR enhanced running endurance by 45 % in sedentary mice (Narkar et al. 2008).
We investigated the effects of endurance factors, PPARδ agonist GW501516 and AICAR, activator of AMPK on memory and neurogenesis. Specifically, mice were injected with GW or AICAR for 7 days and concurrently with BrdU to label dividing cells. Both AICAR and GW improved retention of spatial memory in the Morris water maze. Moreover, AICAR significantly and GW modestly elevated DG neurogenesis. Thus, pharmacological activation of skeletal muscle may mediate cognitive effects of aerobic exercise (Kobilo et al. 2011b) and provide a possible therapeutic approach for conditions in which exercise is limited. Interestingly, although these compounds mimic some of the results of exercise their effects are not identical to physical activity itself. Prolonged administration of AICAR (14 days) no longer enhances spatial memory function or neurogenesis (Kobilo et al. 2011b). Similarly, short-term AICAR treatment promoted sirtuin 1 protein expression in skeletal muscle whereas 14 days of treatment did not (Suwa et al. 2011).
7 Conclusions
While previous studies suggested that both EE and exercise increase adult neurogenesis, recent research has shown only exercise enhances the production of new DG neurons. The positive effects of exercise are likely the result of a combination of factors including, but not limited to enhanced neurogenesis, modifications in synaptic plasticity, spine density, neurotrophins, and angiogenesis that may mediate the beneficial effects on learning and memory, reduction of the risk of neurodegenerative diseases and delay age-related cognitive decline. Within the hippocampus, the most pronounced changes with physical activity are in the DG subfield, with an increased production of new neurons and BDNF levels, which are associated with improved performance on tasks presumably mediated by the DG. Furthermore, running, but not antidepressants that block serotonergic or noradrenergic reuptake, triggers the production of new neurons in the DG. However, we also suggest that caution should be used when applying exercise to conditions of brain injury and neurodegenerative disease as the consequences could be detrimental as found in a mouse model of Huntington's disease. While further research is needed to understand the cellular mechanisms underlying effects of aerobic activity on the brain, exercise is a powerful lifestyle intervention that could be used to augment and maintain cognitive function throughout the lifespan.
Acknowledgments
This work was supported by the National Institute on Aging, Intramural Research Program.
References
- Aberg MA, Aberg ND, Hedbäcker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363(1):43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Adlard PA, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Altschuler EL. Strenuous, intensive, long-term exercise does not prevent or delay the onset of Huntington's disease. Med Hypotheses. 2006;67:1429–1430. doi: 10.1016/j.mehy.2006.04.068. [DOI] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;5870(319):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118:1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Beauquis J, Roig P, De Nicola AF, Saravia F. Short-term environmental enrichment enhances adult neurogenesis, vascular network and dendritic complexity in the hippocampus of type 1 diabetic mice. PLoS One. 2010;5(11):e13993. doi: 10.1371/journal.pone.0013993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Chae CH, Kim HT. Forced, moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochem Int. 2009;55:208–213. doi: 10.1016/j.neuint.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- Chevrel G, Hohlfeld R, Sendtner M. The role of neurotrophins in muscle under physiological and pathological conditions. Muscle Nerve. 2006;33(4):462–476. doi: 10.1002/mus.20444. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38:61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Ernst C, Olson AK, Pinel JP, Lam RW, Christie BR. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatr Neurosci. 2006;31:84–92. [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:50. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey B, Barlow S, Day JS, O'Mara SM. Interferon-alpha-induced deficits in novel object recognition are rescued by chronic exercise. Physiol Behav. 2008;95:125–129. doi: 10.1016/j.physbeh.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behav Brain Res. 2010;207:321–331. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neurosci. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39(4):728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46:123–133. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- Galvão RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mesa Y, López-Ramos JC, Giménez-Llort L, Revilla S, Guerra R, Gruart A, Laferla FM, Cristòfol R, Delgado-García JM, Sanfeliu C. Physical exercise protects against Alzheimer's disease in 3xTg-AD mice. J Alzheimers Dis. 2011;24(3):421–454. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang C, Hsu K, Ming G, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;6(11):626–633. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15(6):808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88(5):2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci J. 2008;9(7):568–758. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Hwang HM, Gorman C. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc Natl Acad Sci U S A. 1985;82:4549–4552. doi: 10.1073/pnas.82.13.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978;202(4372):1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Greenough WT, McDonald JW, Parnisari RM, Camel JE. Environmental conditionsmodulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Res. 1986;380:136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19(10):988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36:2589–2602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans–possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, Gorzalka BB, Hillard CJ, Christie BR. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. Interpreting the effects of exercise on fear conditioning: the influence of time of day. Behav Neurosci. 2010;124:868–872. doi: 10.1037/a0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Rudy JW. The amygdala modulates hippocampus-dependent context memory formation and stores cue-shock associations. Behav Neurosci. 2004;118(1):53–62. doi: 10.1037/0735-7044.118.1.53. [DOI] [PubMed] [Google Scholar]
- Ip EY, Giza CC, Griesbach GS, Hovda DA. Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. J Neurotrauma. 2002;19:573–585. doi: 10.1089/089771502753754055. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8:939–942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14(11):771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res. 2003;47:55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011a;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011b;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl Z, Kandasamy M, Winner B, Aigner R, Gross C, Couillard-Despres S, Bogdahn U, Aigner L, Winkler J. Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington's disease. Brain Res. 2007;1155:24–33. doi: 10.1016/j.brainres.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci U S A. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski CM, Schlangen C, Gellerich FN, Gizatullina Z, Deschauer M, Schiefer J, Young AB, Landwehrmeyer GB, Toyka KV, Sellhaus B, Lindenberg KS. Myopathy as a first symptom of Huntington's disease in a Marathon runner. Mov Disord. 2007;22:1637–1640. doi: 10.1002/mds.21550. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–563. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Krum JM, Mani N, Rosenstein JM. Angiogenic and astroglial responses to vascular endothelial growth factor administration in adult rat brain. Neuroscience. 2002;110:589–604. doi: 10.1016/s0306-4522(01)00615-7. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006;9:580–586. [PubMed] [Google Scholar]
- Lafenêtre P, Leske O, Ma-Högemeie Z, Haghikia A, Bichler Z, Wahle P, Heumann R. Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis. Front Behav Neurosci. 2010;3:34. doi: 10.3389/neuro.08.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol Learn Mem. 2005;83:206–216. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. International Huntington's Disease Collaborative Group (2004) A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet. 65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox K, Cyarto EV. The influence of exercise on brain aging and dementia. Biochim Biophys Acta. 2012;1822(3):474–481. doi: 10.1016/j.bbadis.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322:763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14(11):745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lin TW, Chen SJ, Huang TY, Chang CY, Chuang JI, Wu FS, Kuo YM, Jen CJ. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol Learn Mem. 2012;97(1):140–147. doi: 10.1016/j.nlm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Liu YF, Chen HI, Yu L, Kuo YM, Wu FS, Chuang JI, Liao PC, Jen CJ. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90:81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Lopez–Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou SJ, Liu JY, Chang H, Chen PJ. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. 2008;1210:48–55. doi: 10.1016/j.brainres.2008.02.080. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28(8):1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Lucassen PJ, van Praag H. Comparison of neurogenic effects of fluoxetine, duloxetine and running in mice. Brain Res. 2010;1341:93–99. doi: 10.1016/j.brainres.2010.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis and BDNF levels in female C57Bl/6 J mice. Dev Neurobiol. 2012 doi: 10.1002/dneu.22009. doi:10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Mello PB, Benetti F, Cammarota M, Izquierdo I. Physical exercise can reverse the deficit in fear memory induced by maternal deprivation. Neurobiol Learn Mem. 2009;92:364–369. doi: 10.1016/j.nlm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Skillings E, Bussey TJ, Saksida LM. Measuring cognitive deficits in disabled mice using an automated interactive touchscreen system. Nat Methods. 2006;3:767. doi: 10.1038/nmeth1006-767. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6 J mice, Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.06.007. doi: http://dx.doi.org/10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed]
- Myers CE, Gluck MA. Context, conditioning, and hippocampal rerepresentation in animal learning. Behav Neurosci. 1994;108(5):835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- Nägerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AS, Bull C, Nilsson MK, Zhu C, Björk-Eriksson T, Eriksson PS, Blomgren K, Kuhn HG. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105:14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res. 2007;184(2):124–132. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K, et al. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan RM, Ohle R, Kelly AM. The effects offorced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- O'Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–10129. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, Madri JA, Ment LR. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- Ota KT, Duman RS. Environmental and pharmacological modulations of cellular plasticity: role in the pathophysiology and treatment of depression. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.05.022. http://dx.doi.org/10.1016/j.bbr.2011.03.031. [DOI] [PMC free article] [PubMed]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pang TY, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington's disease transgenic mice. Neuroscience. 2006;141:569–584. doi: 10.1016/j.neuroscience.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Potter MC, Yuan C, Ottenritter C, Mughal M, van Praag H. Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington's disease. PLoS Curr. 2010;2 doi: 10.1371/currents.RRN1201. RRN1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- Real CC, Ferreira AF, Hernandes MS, Britto LR, Pires RS. Exercise-induced plasticity of AMPA-type glutamate receptor subunits in the rat brain. Brain Res. 2010;1363:63–71. doi: 10.1016/j.brainres.2010.09.060. [DOI] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neurosci. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One 13. 2008;3(8):e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TD, Yeh CY, Verkhratsky A. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2011;8(7):707–717. doi: 10.2174/156720511797633214. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Yamaguchi A. The recent understanding of the neurotrophin's role in skeletal muscle adaptation. J Biomed Biotechnol. 2011;2011:201696. doi: 10.1155/2011/201696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: the dentate gyrus and pattern separation. Behav Brain Res. 2012;226(1):56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992;13:25–36. doi: 10.2165/00007256-199213010-00003. [DOI] [PubMed] [Google Scholar]
- Sforzo GA, Seeger TF, Pert CB, Pert A, Dotson CO. In vivo opioid receptor occupation in the rat brain following exercise. Med Sci Sports Exerc. 1986;18:380–384. [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Radak Z, Kumagai S. Short-term adenosine monophosphate-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside treatment increases the sirtuin 1 protein expression in skeletal muscle. Metabolism. 2011;60:394–403. doi: 10.1016/j.metabol.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci U S A. 2010;107(17):7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titterness AK, Wiebe E, Kwasnica A, Keyes G, Christie BR. Voluntary exercise does not enhance long-term potentiation in the adolescent female dentate gyrus. Neuroscience. 2011;183:25–31. doi: 10.1016/j.neuroscience.2011.03.050. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington's in mice. Nature. 2000;404:721–722. doi: 10.1038/35008142. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington's disease. BMC Neurosci. 2008;9:34. doi: 10.1186/1471-2202-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght K, Kóbor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, Meerlo P. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19:928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121(2):324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999b;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, Titterness AK, Christie BR. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus. 2007;17:1201–1208. doi: 10.1002/hipo.20349. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gómez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004a;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004b;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Scott BW, Martin JM. Heterogeneous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2(10):e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Unsicker K, von Bohlen und Halbach O. Fibroblast growth factor-2 deficiency causes defects in adult hippocampal neurogenesis, which are not rescued by exogenous fibroblast growth factor-2. J Neurosci Res. 2011;89:1605–17. doi: 10.1002/jnr.22680. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26(20):5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Pfau ML, Flores CT, Fraser JA, Williams CL, Jones LW. Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation. Cancer Res. 2010;70:9329–9338. doi: 10.1158/0008-5472.CAN-10-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, Lo CP, Kuo YM. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci USA. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]