Abstract
Significant progress has been made in understanding the neurobiological mechanisms through which exercise protects and restores the brain. In this feature review, we integrate animal and human research, examining physical activity effects across multiple levels of description (neurons up to inter-regional pathways). We evaluate the influence of exercise on hippocampal structure and function, addressing common themes such as spatial memory and pattern separation, brain structure and plasticity, neurotrophic factors, and vasculature. Areas of research focused more within species, such as hippocampal neurogenesis in rodents, also provide crucial insight into the protective role of physical activity. Overall, converging evidence suggests exercise benefits brain function and cognition across the mammalian lifespan, which may translate into reduced risk for Alzheimer’s disease (AD) in humans.
Animal and human perspectives on physical activity and brain function
Abundant data suggests that physical activity reduces the risk of various diseases, including those associated with compromised cognition and brain function (e.g., heart disease, stroke, obesity) and, in turn, independence and quality of life [1]. Exercise protects the brain from the adverse effects of aging (Box 1) [2,3]. Our ability to capitalize on physical activity as a lifestyle change for improved brain health critically depends on a better understanding of neurobiological mechanisms through which physical activity protects and restores the brain.
Box 1. So how is the science relevant to me?
An often-asked question with regard to the physical activity and exercise literature is ‘so how does the science impact my life and that of my family?’ Of course, one can answer this question by stating that explicating the basic mechanisms underlying exercise effects on brain and behavior will enhance our understanding of cognitive plasticity over the lifespan and in the face of disease. However, although such an answer may be satisfying to a scientist, it is much less satisfying to a citizen who desires a more practical answer.
Although certainly much remains to be learned about the influence of exercise on body, brain, and cognition, we can, on the basis of the extant literature, provide at least some tentative answers. For example, there are epidemiological studies that have examined the relationship between reports of physical activity and exercise at one point in time and the diagnosis of AD and other forms of dementia years later. One such example is a study by Larson et al. [22], who asked 1740 men and women over the age of 65 years how many times per week, over the course of 1 year, they participated in various physical activities for at least 15 min. per episode. They then examined the relationship between the amount of physical activity and the diagnosis of AD an average of 6.2 years later. The incidence of AD was substantially greater in individuals who exercised less than three times per week than it was for those who exercised more frequently (see also [20,23,215,218,239]).
Indeed, a meta-analysis of prospective observational studies, which included 163,797 non-demented participants at baseline and 3219 diagnosed cases at follow-up found that the relative risk of dementia in the highest physical activity category compared with the lowest was 0.55 for AD and 0.82 for Parkinson’s disease [240]. The studies described thus far suggest an important, practical physical activity-related benefit, albeit from correlational data, for older adults. However, the evidence is consistent with animal models of AD [225,241–249] and the concept that human exercise training benefits brain plasticity for those with mild cognitive impairment and patients with probable AD [186–188] (see Table 1 in main text).
It is also important to consider whether exercise can provide practical benefits beginning in development. There are several studies that report a positive association between physical activity and exercise and academic achievement in adolescents [250,251]. In a 2-year randomized controlled trial with 1490 preadolescent children, Donnelly et al. [252] found improvements in overall academic achievement for the physically active but not for the control group children. A recent study of street-crossing behavior in a virtual-reality environment has also found a benefit for children with greater cardiovascular fitness, in terms of safer crossing behavior particularly under challenging conditions, compared with children of low fitness [253]. Interestingly, the fitter children were not quicker at crossing streets than the less-fit children, but instead made better decisions about when to cross.
In summary, physical activity has benefits throughout the lifespan. It reduces the risk of dementia and is also associated with real-world, practical outcomes starting in development, such as better academic performance and decision making.
This problem has been approached from two perspectives: animal and human neuroscience. Animal research enables a reductionist mechanistic understanding of how exercise can induce changes at the molecular, cellular, and neural circuit levels and how these may impact cognitive function. However, whereas understanding microscale molecular and cellular changes is relatively limited in humans, higher-level cognition, as well as macro and systems-level changes in the central nervous system (CNS), can be evaluated. New neuroimaging technologies have enabled the field of Cognitive Neuroscience to begin to bridge the gap between animal and human studies. In particular, changes in brain structure and function as a result of exercise and other types of interventions such as cognitive training, nutrition, and social interaction can now be addressed (for a review, see [4]).
In this feature review, we identify areas of overlap between studies with different species and suggest how cross-species, multi-method research might further address important issues in the study of neural and cognitive plasticity, with a focus on exercise and the broader domain of physical activity. We start by examining evidence that exercise rather than other environmental factors (cognitive stimulation, enrichment, social interaction) has profound effects on brain function. Next, the types of cognition influenced by physical activity in animals and humans are explored with a focus on the hippocampus, a brain area important for learning and memory. Thereafter, mechanistic aspects are discussed in both species including neurotrophins, synaptic plasticity, adult neurogenesis, angiogenesis, and functional imaging, as well as further modulation of physical activity outcomes by (epi)genetic factors. Overall, exercise is a simple, low-cost lifestyle intervention that can be quantified in a straightforward manner in both animals and humans.
Exercise and environmental enrichment
Extensive animal research has shown that the CNS responds to external stimuli, producing molecular, cellular, and structural modifications responsible for functional plasticity. An enriched environment (EE) is a complex combination of social, cognitive, and physical stimulation. In a classic study, it was shown that housing rodents in an EE comprising a large cage with varying sets of toys such as balls, tunnels, and ladders improved learning and memory [5]. Beneficial effects of EE on behavior and brain function have since been reported in a multitude of studies using rodent spatial memory, neuroanatomical, cellular, and molecular assays [6,7]. In particular, changes such as increased brain weight, neurotransmitter content, gliogenesis, synaptic plasticity, and dendritic spine growth as well as upregulation of neuronal signaling molecules, neurotrophin levels, and adult hippocampal neurogenesis have been reported and associated with cognitive enhancement (for reviews, see [8,9]). A major theoretical formulation of EEs applicable to humans has been the intellectual engagement hypothesis [4,10]. This hypothesis proposes that greater complexity of the environment as characterized by diverse stimuli, demand for complex decisions, and social and physical stimulation is related to enhanced cognitive development across the lifespan. Studies have addressed this hypothesis by examining the contribution of environmental complexity, or ‘enrichment’, to brain development and healthy aging [4,11–15]. A common theme from these studies is that engaging in a variety of activities that are novel, cognitively challenging, and multimodal (e.g., combine physical and mental stimulation) may be associated with more protection against age-related cognitive decline and dementia than focusing on any one type of activity [13–16].
Interestingly, in animal studies, evaluation of the different aspects of EE revealed that exercise alone can elicit many of the observed changes. Running (RUN) increases the birth of new neurons in the hippocampus, neuronal spine density, synaptic plasticity, neurotrophin levels, and spatial memory function in mice (for a review, see [17]) compared with sedentary controls. Furthermore, housing rodents in identical-sized caging in either social or isolated conditions indicates that an EE without a running wheel does not enhance neurogenesis, neurotrophin levels [18], or Morris water maze learning [19], whereas conditions that included exercise (RUN and EER) increased these parameters (Figure 1). Of course, it is far more difficult to examine the individual components of enrichment, such as physical activity, with humans. A recent attempt to do so was a study that approached this question with an experimental design that compares exercise combined with cognitive engagement with exercise alone. Ander-son-Hanley et al. [16] compared the effects of 3 months of stationary cycling with stationary cycling during engagement in a virtual bicycle ride (cybercycle) and found evidence suggesting that the cybercycle may have been more beneficial for executive function. Unfortunately, a group exposed only to cognitive enrichment (i.e., the virtual environment) was not included in this study. Nevertheless, the experimental comparison of exercise only, with activity plus cognitive challenge is similar to the manipulations that have been used in animal models of environmental enrichment and offers a needed complementary approach to correlational studies. Overall, the findings in human studies are consistent with rodent research suggesting that physical activity may provide lasting benefits for brain structure and function [4,16,20–23]. Moreover, exercise is a simple intervention that can be quantified by standard scientific measurements such as distance, intensity, and maximal oxygen consumption (VO2max) in a similar manner for animals and humans.
Figure 1.

Exercise increases the production of new neurons in the dentate gyrus (DG) of the hippocampus. In two independent studies [18,19], mice were housed under (A,I) control (CON), (B,J) enriched environment only (EEO), (C,K) running (RUN), or (D,L) enriched environment and running (EER) conditions in (A–D) single or (I–L) group housing. Confocal images of bromodeoxyuridine (BrdU)-positive cells in the DG in sections derived from mice housed under (E) CON, (F) EEO (G) RUN, and (H) EER conditions. Sections were immunofluorescently double-labeled for BrdU (green) and NeuN (red) indicating neuronal phenotype (adapted from [18]). Panels (A–D) are reproduced with permission from [19]. Both studies show that adult DG neurogenesis is increased under the RUN and EER conditions but not under CON or EEO, indicating that running is the neurogenic stimulus.
Spatial learning and relational memory
Physical activity elicits functional and structural changes throughout the brain. However, its effects on the hippocampus are of particular interest because this is a brain area essential for memory formation [24] and spatial navigation [25]. Although this structure is especially susceptible to aging and neurodegenerative disease-related atrophy and dysfunction [26], it is also highly plastic and responsive to exercise [27,28]. In particular, exercise has been shown to enhance hippocampus-dependent spatial memory in rodents in paradigms including the Morris water maze, the Y-maze, and the radial arm maze [17,29] (Table 1). Running also improved performance in tasks with low motor demand, such as contextual fear conditioning, passive avoidance learning, spatial pattern separation, and novel object recognition [30–34]. In addition, a recent study showed that subthreshold learning of object location using a very brief acquisition trial is enhanced by exercise [35]. This non-reinforced form of learning may be similar to human learning during daily activities without explicit immediate reward.
Table 1.
Physical activity effects on the brain, across animal and human studiesa
| Neurotrophins | Cognition and behavior | Synaptic plasticity hippocampus (DG) |
Neurogenesis and brain structure | Others | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Factor | Brain area | Periphery | Factor | |||||||
| Adults | Animal | ↑BDNF ↑proBDNF ↑TrkB receptor |
Hippocampus, DG, perirhinal cortex, amygdala | Lumbar spinal cord, soleus muscle | ↑Learning and memory ↑Pattern separation ↑Discrimination ↑Adaptation ↓Anxiety |
↑LTP ↑Proteins associated (CREB, synapsin I, syntaxin, synaptotagmin, NMDAR2A, NMDAR2B, CaMKII, MAPK, GAP-43) |
↑Proliferation ↑Survival ↑Neurogenesis |
↑Early gene expression (Arc, c-Fos, Zif268) Notch1 modulation | ↑Angiogenesis | [27,30,32,34,48, 66–75,78–80,87, 106,110,116,136, 151,161,163,174, 175,201,207–209] | |
| ↑VEGF | Hippocampus | Lung, skeletal muscle | |||||||||
| ↑IGF-1 | Hippocampus, striatum, septum, cerebellum, cortex, thalamus, red nucleus, hypothalamus, brain stem nuclei | Cerebrospinal fluid | |||||||||
| Human | ↑BDNF (transient) | Blood plasma and serum, skeletal muscle | ↑Executive function ↑Visuospatial memory ↑Relational memory (children only) ↑Pattern separation ↑Academic achievement (children) |
↑Hippocampal volume | ↑Hippocampal blood volume (DG only) | [39,40,55,57,98, 254,255,259] | |||||
| Aging | Animal | ↑BDNF ↑TrkB receptor ↑IGF-1 |
Hippocampus, DG, perirhinal cortex | ↑Learning and memory ↔Pattern separation ↑Adaptation ↑Motor ability ↓Anxiety |
↑LTP | ↑Neurogenesis | ↑Immune factors (GRO-KC, IL-18, leptin, IL-1β, MCP-1, VEGF) | ↑Angiogenesis | [34,76,80,81,116,165] | ||
| Human | ↑BDNF (transient) | Blood plasma and serum, skeletal muscle | ↑Executive function ↑Attention and processing speed ↑Memory ↑ADAS-Cog (with subjective memory impairment) |
↑Hippocampal volume ↑Gray matter density in prefrontal cortex (voxel wise) ↑Gray matter density in lateral temporal cortex ↑Distributed frontal and temporal white matter integrity associated with increased fitness from walking |
↑Hippocampal blood flow | ↑Transient BDNF (peripheral) ↔Basal BDNF, IGF-1, VEGF (peripheral) |
↑Functional synchrony of frontal and temporal aspects of the DMN | [28,57,61–63,98,122, 124,187,189,256–258] | |||
| Alzheimer’s disease | Animal | ↑BDNF ↑TrkB receptor |
Hippocampus | ↑Learning and memory ↓Anxiety ↑Sensorimotor function ↑Exploratory behavior |
↑LTP ↑Proteins associated (synaptophysin) |
↑Neurogenesis | ↓IL-1β ↓TNF-α ↑CD40 ↑MHCII ↓Aβ40 ↓Aβ |
↓Aβ load ↓Tau AT100 epitope ↑NPC1 ↑NPC2 ↓APP ↓MAP2ab ↑SOD1 |
[183,184,225,241–249] | ||
| Human | ↑Global cognition | [186–188] | |||||||||
Abbreviations: GAP-43, growth-associated protein 43; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; ADAS-Cog, AD Assessment Scale – Cognitive Subscale; TNF-α, tumor necrosis factor alpha; MHCII, major histocompatibility complex class II; APP, amyloid precursor protein; MAP2ab, microtubule-associated protein 2ab; NPC1 and NPC2, Niemann–Pick type C1 and C2; SOD1, superoxide dismutase 1.
Only evidence from randomized controlled trials (RCTs) or intervention trials are shown for human studies, because this most closely parallels experimental control in animal studies. ↑, Increase; ↓, decrease; ↔, no change.
Data from human studies support the observation that fitness and exercise training is beneficial for relational mnemonic functions that critically depend on the hippocampus compared with other types of memory such as item memory. For example, a series of studies found an association between cardiovascular fitness and performance on relational binding (e.g., remembering both the name of a person you recently met and where you met them), but not on item recognition performance, in preadolescent children [36,37]. Another study, with 15–18 year olds, found a positive association between fitness and spatial learning performance on a Virtual Morris Water Maze task but no correlation with verbal list learning [38]. Two of these studies found a positive association between associative memory and hippocampal volume [36,38]. Indeed, aerobic training is associated with improvements in memory tasks that are theorized to require the hippocampus, such as those that require relational binding [39] and visuospatial memory for relationships between landmarks on a map [40].
It should be noted that the hippocampus comprises three main subfields: the dentate gyrus (DG), area CA3, and area CA1. Each of these regions has specific cell types and plasticity contributing to learning and memory processes [41–44] and may respond and contribute in different ways to the effects of exercise on hippocampal memory function. The DG is unique in its ability to generate new neurons [45] in mammals, including humans [46,47], which can be doubled or tripled by exercise in rodents [17,48]. The neurogenic DG and to some extent area CA3 are deemed important for pattern separation, or the differential storage of highly similar stimuli and experiences [49,50]. In a recent study, sedentary and running mice were tested on a spatial discrimination task where identically shaped stimuli were presented in close or distal proximity on a touch screen [51]. There was no difference between the groups when the separation between stimuli was large; however, runners outperformed sedentary mice when the difference between stimuli was small [34]. The observed improvement in making fine spatial distinctions may be due, at least in part, to the exercise-induced increase in adult neurogenesis. Indeed, in a transgenic mouse with enhanced adult hippocampal neurogenesis, there is improved differentiation between overlapping contextual representations, indicative of enhanced pattern separation [52], and removal of important cortical inputs to new neurons, such as the lateral and perirhinal cortex, is detrimental to task performance [53].
Similar tasks evaluating the ability to distinguish between highly similar stimuli have been used to test memory function in humans. In combination with imaging studies, it was shown that functional MRI (fMRI) activity in area CA3 and the DG coincided with pattern separation performance [54]. In a recent study, young adults who participated in a long-term aerobic exercise regime demonstrated enhanced performance on a visual pattern separation task, especially in those individuals who experienced a proportionally large change in fitness [55]. Conversely, subjects that scored high on the Beck Depression Index performed poorly [55], consistent with the hypothesis that stress and depression reduce adult neurogenesis [56]. In another study, using MRI in humans (21–45 years old), increased DG blood volume correlated with improved cognitive function, proposing an indirect measurement of neurogenesis in humans [57,58]. It should be noted, however, that direct in vivo imaging of newly born neurons in the DG in humans has not yet been established. There are many technical difficulties associated with the resolution needed to image a small set of cells that are evolving over a relatively prolonged time (weeks and months) into neurons [59].
Results from older adults are similar but have not yet utilized multiple measures to demonstrate specificity within the domain of memory. For example, Erickson and colleagues demonstrated that cardiovascular fitness was associated with faster and more accurate spatial short-term memory performance in 165 healthy older adults. Left hippocampal volume accounted for a statistically significant portion of variance in the accuracy rates of the most difficult task condition [60]. In a follow-up longitudinal analysis, improvements in fitness were not correlated with improvements in task performance for either training group; however, changes in hippocampal volume were associated with improved accuracy for the aerobic walking group only [28]. By contrast, caudate and thalamus volume change was not positively correlated with spatial memory improvement, demonstrating some selectivity to the hippocampus among subcortical structures. Importantly, the walking group showed a 1–2% increase in hippocampal volume, whereas the stretching group showed a 1–2% decrease in hippocampal volume over the 1-year intervention. However, this follow-up study does not imply that the hippocampus is the sole causal agent influencing changes in spatial memory performance. If this were true, the stretching group should have shown decrements in performance. Indeed, it is likely that other cortical regions and systems played a role in changes in spatial memory performance for both groups. Thus, although studies with human populations have shown that fitness and exercise training seems to benefit pattern separation, spatial, and relational memory performance (Table 1), which critically involve the hippocampus, it is likely that other brain regions play important roles (Box 2) [61–63]. Clarifying the distributed nature of brain region-specific changes that mediate performance improvements across species will be an important avenue for future research.
Box 2. Effects of exercise on human cognitive abilities.
Animal models cannot measure cognitive capacities that are uniquely (or largely) human, such as verbal learning and memory, and are limited in assessing abilities that heavily rely on the prefrontal cortices, such as fluid intelligence (e.g., inductive reasoning) and executive functions. These aspects of cognition are enhanced with exercise training [254,256,257,259] and are relevant for predicting functional independence in the elderly. Therefore, it is important to understand the mechanism through which exercise enhances these uniquely human abilities.
Several studies have examined verbal and visual memory using standardized neuropsychological tests such as word-list learning, story memory, and figural copy tests (see [257] for a review). However, the overall effects of aerobic exercise training on performance in the healthy elderly, as indexed by these tasks, have been somewhat weak, possibly because neuropsychological tests are less sensitive to individual differences in the range of normal functioning. Greater sensitivity to higher-level cognition may come from tasks designed to parametrically increase workload for a given cognitive ability. Several studies have shown greater performance for more highly fit participants on cognitive tasks that specifically contrast difficult and relatively easier conditions, particularly with regard to tasks that tap executive control processes such as multitask performance, inhibiting inappropriate responses, and attending selectively to task-relevant information [62,256] (for reviews, see [254,255,258,260–262]). In particular, using fMRI to examine neural activity coupled with the task shows that greater fitness is associated with increases in prefrontal cortex activity during more difficult task conditions [62,260]. Overall, aerobic fitness and aerobic training are associated with better performance when prefrontal cortex involvement is critical for task success. Additionally, meta-analyses have clearly suggested that exercise and physical activity benefits various perceptual, cognitive, and motor skills [256]. Indeed, exercise has a much broader beneficial effect on cognition than does cognitive training. Thus, future research is needed for understanding of the mechanisms through which exercise affects prefrontal cortex function and associated cognitive abilities.
Another approach is to examine the functional coupling of the hemodynamic BOLD signal during either task states or what is known as the ‘resting state’ when participants lie quietly with no engagement in controlled task conditions. The neural basis of functional coupling has been widely studied and data suggest it reflects coupling of fluctuations in the power of high-frequency neuronal activity (e.g., [263]). The method has been applied to human populations to understand how fitness and exercise training are associated with individual differences in the functional coupling of macroscale brain systems relevant to cognition and clinical status [61,264,265]. Aerobic exercise may be beneficial for functional coupling of a network known as the Default Mode Network (DMN), a functional brain network that is highly metabolically active during mind wandering, which is significant because the DMN is regarded as a functional biomarker of cognitive aging and a host of clinical pathologies across the lifespan (for a review, see [266]). In addition, aerobic exercise training may increase the specificity of functional networks that are vulnerable to dedifferentiation, or lack of specificity, with aging [61]. A similar concept has been shown with functional coupling during verbal associative encoding in postadolescent teenage males [265]. Importantly, although task-related neuroimaging of the whole brain is likely to remain a human neuroscience endeavor, there may be an opportunity for bridging across animal and human models with resting-state neuroimaging [267,268] to understand the cellular and molecular basis of the mechanisms through which exercise affects functional coupling at the system level (see Figure 2 in main text).
The literature described above suggests that physical activity affects hippocampal structure and function and that this can be measured at both cellular and macroscopic levels. Measures of cardiovascular fitness, brain blood flow, and analog paradigms for assessing hippocampal function such as virtual navigation and pattern separation tasks have enabled researchers to begin to bridge between animal and human research (e.g., Figure 2). Future studies will be needed to further examine how the brain circuits that support perceptual and memory processes are impacted by exercise across the lifespan of different species.
Figure 2.
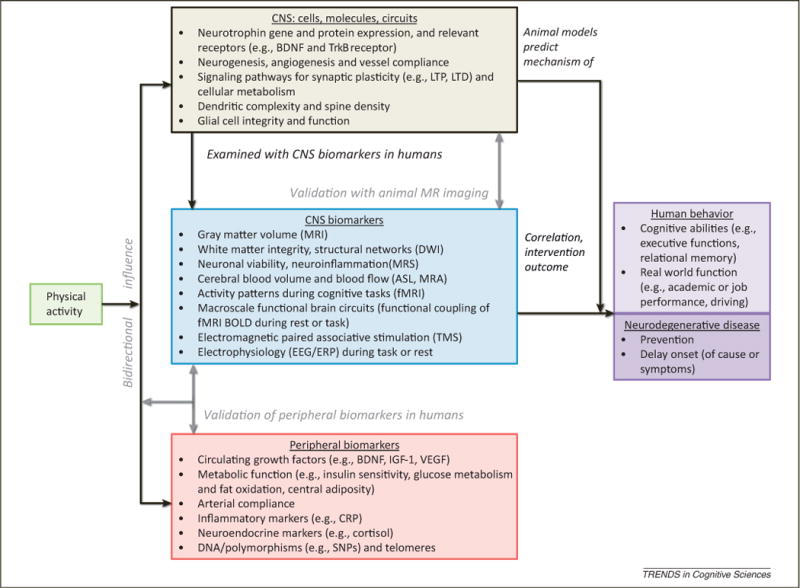
In search of the neurobiological mechanisms mediating physical activity benefits on cognition, behavior, and neurodegenerative diseases. Physical activity influences both the peripheral nervous system and the central nervous system (CNS), which interact with each other in a bidirectional manner. Animal models provide a means to measure the effects of physical activity directly and at a microscale, whereas human studies mostly depend on noninvasive neuroimaging methods that measure biomarkers of cellular and molecular processes at a macroscale. There may be opportunities to bridge between animal and human measures with conceptually parallel experimental designs that assess the relationship between the effects of physical activity on central and peripherally measured outcomes and/or utilize analogous imaging methods to measure CNS outcomes. Ultimately, understanding the neurobiological mechanisms that mediate the effects of physical activity on human behavior and disease will improve public health recommendations that outline what types of physical activity produce the most neuroprotection and real-world benefit. It will also provide insight how this varies across the lifespan, different genetic profiles and neurodegenerative disorders. Abbreviations: BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin receptor kinase B; LTP, long-term potentiation; LTD, long-term depression; DWI, diffusion-weighted imaging; MRS, magnetic resonance spectroscopy; ASL, arterial spin labeling; MRA, magnetic resonance angiography; fMRI, functional MRI; BOLD, blood oxygen level dependent; TMS, transcranial magnetic stimulation; EEG/ERP, electroencephalography/event-related potentials; IGF-1, insulin-like growth factor 1; VEGF, vascular endothelial growth factor; CRP, C-reactive protein; SNP, single-nucleotide polymorphism.
Growth factors in exercise-induced changes in brain and cognition
To begin to understand the cellular and molecular mechanisms underlying the benefits of exercise for the brain, animal models are used. Important candidates in this regard are the neurotrophins (see Glossary). Brain-derived neurotrophic factor (BDNF) is of particular interest because it supports neural survival, growth, and synaptic plasticity [64,65]. Neeper and colleagues [27] were the first to show a positive correlation between physical activity and BDNF mRNA levels. Specifically, using a voluntary wheel-running paradigm, which allows rats to determine running time, speed, and distance, mimicking, to some extent, human choices, they showed increased BDNF mRNA levels in the cerebellum, caudal cortex (analogous to the entorhinal and visual cortex in humans), and hippocampus [27,66]. Both BDNF gene and protein expression are increased in the hippocampus after short (2–7 days) [27,66–72] or long (1–8 months) periods of exercise with either continuous or alternating running days [18,67–70,73–77] and can remain elevated at least 2 weeks after exercise has ended [74]. Analysis of the hippocampal sub-fields showed that changes in BDNF mRNA levels were localized to the neurogenic DG rather than area CA1 [78]. Forced animal exercise protocols (treadmill running), comparable to some human exercise regimes, also increased hippocampal BDNF gene expression after short [30,79] or long [77,80,81] periods of exercise. Additionally, animal studies have shown that physical activity elevates BDNF gene expression in other parts of the nervous system such as the lumbar spinal cord [82], cerebellum [66], amygdala [77], caudal neocortex [27,66], and perirhinal cortex [75,83]; the latter is a brain area important for visual discrimination and novel object recognition [84].
Interestingly, many genes that are upregulated with exercise have a recognized interaction with BDNF, supporting a central role for this neurotrophin in brain plasticity [85,86]. BDNF utilizes the tropomyosin receptor kinase B (TrkB) receptor to activate signal transduction cascades [65]. Indeed, exercise increases both BDNF and TrkB receptor levels in the hippocampus [67,69,71, 77,78,80,81]. Concurrently, voluntary running elevates the expression of other genes involved in synaptic trafficking (synapsin I, synaptotagmin, syntaxin), signal transduction pathways (CaMKII, MAPK/ERK I and II, protein kinase C [PKC], PKC-δ), or transcription (CREB), as well as genes associated with the glutamatergic system (NMDA receptor [NMDAR]2A/B) [67,69,71,77]. In particular, in the DG, exercise elevates BDNF mRNA as well as expression of NMDAR2A/B, GluR5, and synapsin I, which may mediate enhanced synaptic plasticity and neurogenesis [30,73, 78,87].
Cognitive function has been observed to decline with age in both humans and rodents [88]. Although there are multiple pathways by which cognitive deterioration may occur late in life, decreased neurotrophin levels correlate with age-related hippocampal dysfunction and memory impairment [2,89] (for a review, see [90]). Exercise in aging animals is apparently less effective at increasing BDNF levels than in young rodents [91]. Although after 4 weeks of voluntary wheel running, no change occurred [92] (see, however, [93]), longer-term voluntary or forced treadmill running may reduce the age-related decline in BDNF and TrkB levels [76,80,81].
Given the multitude of animal studies that have demonstrated exercise-dependent changes in BDNF levels in the brain, BDNF has been of central interest to translational research. Similar to upregulation of central BDNF expression in rodents, physical activity increases circulating BDNF levels in healthy humans [94–96] (for a review, see [97,98]). It should be noted that measurement of BDNF in the periphery is an indirect means of inferring central expression. It has been estimated that the brain contributes 70–80% of circulating BDNF both at rest and during exercise, suggesting that the brain is the major but not the sole contributor to circulating BDNF [99]. In skeletal muscle, exercise elevates BDNF mRNA and protein levels [100,101]; however, muscles are not a source of circulating BDNF [100]. In blood, more than 90% of the BDNF is stored in platelets and is released during clotting processes [102]. Therefore, serum seems to reflect stored and freely circulating BDNF in the blood [102], whereas plasma seems to reflect only freely circulating BDNF [103]. Thus, peripherally generated BDNF and other factors that modulate platelet storage and release are challenging methodological considerations for making inferences between peripherally measured BDNF and central expression. In particular, these parameters may influence the temporal dynamics of changes in peripheral BDNF levels following single training bouts of aerobic exercise [98,104].
In rodents, in addition to BDNF, other trophic factors such as fibroblast growth factor 2 (FGF-2) [105] and nerve growth factor (NGF) [66] are upregulated in the hippocampus as a result of exercise, albeit less pronouncedly than BDNF. Exercise also increases insulin-like growth factor 1 (IGF-1) and vascular endothelial growth factor (VEGF) levels. IGF-1 is elevated in several brain areas such as the hippocampus, striatum, septum, cortex, cerebellum, thalamus, red nucleus, hypothalamus, and brain stem nuclei, as well as the cerebrospinal fluid [106–108] and skeletal muscle [109], whereas VEGF is specifically increased in the hippocampus and peripheral areas such as skeletal muscle and lung [110]. Neurons accumulating IGF-1 increase spontaneous firing and are more sensitive to afferent stimulation [106]. Moreover, systemic injection of IGF-1 mimics the beneficial effects of exercise in sedentary rats, including enhanced DG adult neurogenesis [107,111]. However, the effects of IGF-1 may be mediated by BDNF, a potential downstream target [112]. VEGF, by contrast, is a hypoxia-inducible protein that promotes angiogenesis through receptor tyrosine kinases on endothelial cells [113]. Interestingly, exercise-induced changes in hippocampal vasculature are associated with adult neurogenesis [114–117] and may be mediated by VEGF and IGF-1 that are both produced in the periphery. IGF-1 is transported across the blood–brain barrier [118], whereas there is limited permeability to VEGF [115,119]. Moreover, blockade of peripheral IGF-1 and VEGF precludes the neurogenic effects of exercise [107,115]. IGF-1 is also produced locally in the brain, probably by microglia, as has been shown in aged rodents [120]. In addition, 6 weeks of wheel running elevated VEGF protein levels in the cortex and hippocampus of middle-aged female mice [121].
In humans, the impact of 1 year of moderate aerobic exercise on circulating BDNF, IGF-1, and VEGF in older adults who were healthy but had low activity levels has been researched [122]. Changes in basal circulating growth factor serum levels as well as in the functional coupling of brain regions previously shown to be responsive to aerobic training in older adults were evaluated[61]. Basal circulating BDNF, IGF-1, and VEGF did not change from before to after the 1-year walking intervention (see also [28]). However, there was a positive correlation between changein circulating BDNF, IGF-1, and VEGF, and fMRI measured change in functional coupling between the bilateral parahippocampal and bilateral middle temporal gyrus [122]. These results are consistent with findings from animal studies described above and suggest that these neurotrophins contribute to the positive effects of exercise on learning and memory in rodents [123]. In future studies, multiple measures over time, perhaps through burst measurement techniques, could generate a more refined assessment of the time course of circulating growth factor changes relative to alterations in human cognition, brain structure, and function. It will also be important to account for the contribution of structural changes [28,124,125].
Given the importance of BDNF, a factor that should be considered in human exercise studies is ValMet polymorphisms of the BDNF gene. This single-nucleotide polymorphism (SNP) in the BDNF gene occurs in approximately 20–30% of Caucasians [126] and leads to a valine-to-methionine change at position 66 (Val66Met) in the prodomain of BDNF. This SNP has been found to decrease activity-dependent BDNF secretion [127,128] and is associated with increased susceptibility to depression and anxiety-related disorders [129,130] and reduced memory function [131]. In addition, in mouse models of these polymorphisms, neural plasticity was impaired and refractory to treatment with antidepressants [132]. The interaction between this polymorphism and exercise in humans is further described in the epigenetics section. Overall upregulation of neurotrophins by exercise may facilitate other neural plasticity processes.
Synaptic plasticity
Improvements in learning and memory induced by exercise have been directly associated with activity-dependent synaptic plasticity, modifications in gene expression, and improved neurogenesis [48,73,87,133,134]. Many of these changes have been mainly observed in the hippocampus, which we know is important for memory and learning processes [24]. Induction of long-term potentiation (LTP), a physiological model of certain forms of learning and memory [135] in hippocampal slices or in vivo resulted in significant potentiation of the synaptic response in the DG of running young rodents [30,73,87,136] and reversed the age-related decline in DG LTP in aged rats [81]. Recordings from another hippocampal subregion, area CA1, showed no changes in LTP mediated by exercise, suggesting that the changes observed in the DG may result from increased neurogenesis [87]. Indeed, recordings from individual newborn dentate granule cells in hippocampal slices revealed a lower induction threshold and enhanced LTP induction compared with mature granule cells [137–139]. Consistent with the enhanced LTP, mRNA levels of NMDAR2B were increased specifically in the DG of runner rats [73]. Previous studies have shown that NMDAR2B alters the capacity to exhibit LTP and that its overexpression results in increased LTP induction [140,141]. Interestingly, enrichment including running wheels (EER) also increased NMDAR2B in the hippocampus [140]. Conversely, ablation of neurogenesis or deletion of NMDAR2B from newborn dentate granule cells prevented the induction of LTP in the DG, suggesting that this synaptic potentiation is preferentially mediated by newborn dentate granule cells [142–144]. In addition to changes in NMDAR2B mRNA levels, GluR5 mRNA levels were also significantly elevated in the DG of runner rats, but not in other areas of the hippocampus [73], suggesting a specific role in the synaptic plasticity of the DG. The significant enhancement in LTP following exercise is also consistent with an increase of BDNF in the hippocampus [18,27,66–77], which may mediate synaptic plasticity through the activation of signal transduction cascades [65] (Table 1).
Another type of synaptic plasticity, long-term depression (LTD), is considered to model forgetting by reducing the capacity of one set of synapses to elicit a synaptic response in another [145]. LTD, induced by low-frequency stimulation, is relatively unaffected by exercise [136]. However, the induction of LTD in running mice was found to depend on the activation of NR2A-containing NMDA receptors but not in sedentary mice [136], suggesting that exercise can alter the contribution of NMDA receptor subunits to LTD.
Modifications in synaptic plasticity have also been associated with morphological changes that can occur in response to neural activity [146]. Long-term exercise (2 months) has been shown to increase spine density in the entorhinal cortex and CA1 pyramidal cells [147]. Fine morphological alterations in the DG are consistently induced by exercise [147–150]. Analysis of individual dentate granule cells revealed that running increased the total length, complexity, and spine density of their dendrites, independent of their position in the granule cell layer [148,149], as well as the volume of the granule cell layer [151]. Use of retrovirus-mediated labeling of newborn neurons has made it possible to examine and characterize their morphological details throughout their development and integration into the granule cell layer [152]. Research has shown that exercise increases the motility of the dendritic spines of newborn neurons and accelerates their maturation without modifying dendritic complexity in young mice [150]. In young and aged running mice, there was no difference in spine density of new neurons [116], suggesting that, although fewer cells are generated in the aging brain, they may be functionally equivalent to those produced at earlier time points [153].
Non-invasive methods of human neuroscience cannot examine synaptic activity at the level of the synapse. Instead, indirect measures of acquisition or strengthening of activity between neuronal populations may be used as a biomarker of synaptic plasticity. A physiological approach for this is paired associative stimulation (PAS), which uses transcranial magnetic stimulation (TMS) to measure experimentally induced synaptic plasticity [154]. Synaptic plasticity is induced by pairing electrical stimulation of a hand muscle with electromagnetic stimulation of a corresponding region of motor cortex. The magnitude of plasticity in the circuit is measured by the increase in reactivity of the hand muscle to activation of the motor cortex following repeated paired stimulations. For example, older adults show less reactivity following paired-pulse training than young adults, consistent with much evidence that aging is associated with decreased synaptic plasticity [155]. Using this TMS method as an outcome measure, Cirillo and colleagues [154] showed that more active adults (age 18–38 years) had greater synaptic plasticity in the left abductor pollicis brevis (APB) muscle motor circuit. Although this was a cross-sectional study that compared groups with extreme differences in exercise behavior, it presents complementary evidence for the link between exercise and aerobic fitness and enhanced synaptic plasticity that maybe a generalizable mechanism for the effect of physical activity on coordinated brain function and improved learning and performance. It may be fruitful for future studies to examine the replicability and generalizability of this measure in controlled experimental studies that are designed to examine the effect of exercise training on brain function and higher-level outcome measures thought to be associated with synaptic plasticity such as skill learning or pattern separation.
Neurogenesis and brain structure
It is well established that mammals, including humans, produce new DG neurons in the adult brain [45,46,156]. New hippocampal neurons may make specific contributions to learning and memory, in part as a result of their unique neural circuitry [157]. New neuron production can be regulated by both extrinsic and intrinsic factors. Enhanced neurogenesis is generally considered beneficial for cognition and a decrease is correlated with stress and aging [158,159]. Unfortunately, it is not possible (yet) to study neurogenesis noninvasively in humans. In rodents, however, exercise more than doubles the production of new neurons in the young and aged brain [48,87,116] (Figure 1). Running influences all aspects of new neuron maturation, including cell proliferation, survival, and neuronal differentiation in the DG [18,19,34,48,53,73,76,80,87,106,107,115–117,151,160–166]. The subventricular zone/olfactory bulb, the other neurogenic area in the adult brain [167], does not appear to respond to exercise [163], although this issue remains unresolved [161]. Indeed, the number of neurospheres isolated from the subventricular zone is increased with running in aged animals [168]. Enhanced neurogenesis correlates with improved synaptic plasticity and memory [19,34,73,76,87, 116,160,164,165]. Over the past decade, exercise-induced neurogenesis has been studied under normal, aging, and disease conditions (Figure 1 and Table 1).
The mechanisms underlying the effects of running on neurogenesis are unclear. Neurotrophins such as BDNF, the TrkB receptor, VEGF, and IGF-1 have been proposed to mediate the neurogenic effect of exercise [73,76,80,107, 169,170]. In addition, immune cells such as macrophage migration inhibitory factor [171] and microglia [165,172, 173] may play a role. Local ablation of microglia in the brain prevents the exercise-induced increase in new cell genesis [174]. In addition, the survival of progenitor cells induced by exercise may be mediated by Notch1 activity [175]. Running also increases induction of the expression of immediate early genes (c-Fos, Zif268, Arc) in new and preexisting dentate granule cells [151,160], suggesting increased synaptic activity by exercise. Neurotransmitter systems have also been examined. Blocking cannabinoid signaling precludes exercise-induced cell genesis [176]. Interestingly, tryptophan hydroxylase (TPH)2-deficient mice that lack brain serotonin show normal baseline hippocampal neurogenesis but impaired activity-induced proliferation [177]. In addition, serotonin reuptake inhibitors which are used as antidepressants, such as fluoxetine, have been suggested to increase neurogenesis [178] (see, however, [179,180]) but pale compared with running (Box 3).
Box 3. Exercise mimetics: do they exist?
‘May I simulate exercise
By using the right drug supplies?
I’d like to improve
Every cortical groove,
But from my couch don’t make me rise!’
(Larry Eisenberg)
(In Well, by Gretchen Reynolds [300])
Exercise benefits mood and cognition in both young and old animals and humans. For example, the antidepressant effect of exercise for mild depression in humans [276] has a similar potency to that of serotonergic medications [277]. However, not everyone may be able to be active due to circumstances, disease, or frailty. Would it be possible to mimic some of the cognitive, emotional, and neurogenic benefits of exercise for the brain using pharmacological agents? An obvious mimetic candidate in this regard is the monoaminergic system. Interestingly, serotonergic agonists, including antidepressants such as fluoxetine [178,278], have been suggested to enhance cell genesis and reduce some forms of anxiety (see, however, [279]). Administration of the serotonin 5-hydroxytryptamine (5-HT)1A receptor antagonists, by contrast, decreases cell proliferation in the DG [280]. Marlatt et al. [179] compared antidepressants with voluntary wheel running. Specifically, 2-month-old female C57Bl/6 mice were treated with fluoxetine, a selective serotonin reuptake inhibitor that is considered to enhance neurogenesis, or duloxetine, a dual serotoninergic–noradrenergic reuptake inhibitor, or housed with a running wheel for 1 month. Interestingly, only exercise enhanced new DG cell survival [179].
Another exercise mimetic may be so-called ‘endurance factors’. Research has focused on the cellular, structural, and biochemical changes resulting from physical activity in the brain without much consideration for the peripheral factors that may initiate and elicit these. Would it be possible to mimic the neurogenic and cognitive effects of aerobic exercise by pharmacological skeletal muscle activation? Recently, transcriptional factors regulating muscle fiber contractile and metabolic genes have been identified [281] and led to the identification of compounds that can increase the ability of cells to burn fat and enhance running endurance [282]. Peroxisome proliferator-activated receptor delta (PPARδ) is a transcription factor that regulates fast-twitch muscle fiber contraction and metabolism. Over-expression of this factor increases oxidative muscle fiber number [282]. In addition, administration of the selective agonist GW501516 increased exercise stamina when combined with training [282]. PPARδ is controlled by AMP-activated protein kinase (AMPK), a master metabolic regulator important for glucose homeostasis, appetite, and exercise physiology [283]. Treatment with the AMPK agonist AICAR enhanced running endurance by 45% in sedentary mice [282]. Kobilo et al. [284] investigated the effects of AICAR, an activator of AMPK, on memory and neurogenesis. Specifically, mice were injected with AICAR for 7 days and concurrently with BrdU to label dividing cells. AICAR improved retention of spatial memory in the Morris water maze and modestly elevated DG neurogenesis. Thus, pharmacological activation of skeletal muscle may underlie the cognitive benefits of aerobic exercise [284] and provide a possible therapeutic approach for conditions in which exercise is limited. It should be noted that administration of these pharmacological agents is not identical to physical activity itself. Prolonged administration of AICAR (14 days) does not enhance brain function [284], suggesting that this compound may only partially and temporarily mimic the effects of exercise.
Furthermore, small molecules [285] such as those that mimic the effects of neurotrophins have been shown to benefit cognition and neural plasticity in mouse models of AD [286,287]. In addition, polyphenols, found in fruits and green tea, may have neuroprotective effects that synergize with those of exercise [162].
Neurogenesis declines naturally with aging, as early as middle age [181], and may contribute to age-related decline in cognitive function [88]. Recent research suggests peripheral blood-borne cytokines may play an important role therein. Parabiosis studies of young and aged animals suggest that systemic chemokines may regulate the central production of new neurons [182]. Fortunately, the positive effects of exercise on neurogenesis are maintained throughout life in rodents [76,80,116,166], possibly via muscle-derived/blood-borne factors that are delivered from the periphery to the brain. In mice that had been sedentary until 18 months of age, running reversed new cell survival to that of sedentary young controls and increased the percentage of new cells that became neurons [116]. In mice that started running in middle age (9 months old), cells that were labeled with bromodeoxyuridine (BrdU) at the onset of the study as well as the endogenous marker for immature neurons, doublecortin (at the end of the study, 17 months old), were elevated, suggesting that the neurogenic effect of running is maintained over time in aging animals [76]. The benefits of exercise on neurogenesis extend to mouse models of degenerative diseases such as AD [183,184] and Down syndrome [185], consistent with studies that have shown that exercise can still benefit cognitive function in those with mild cognitive impairment or dementia [186–188] (Table 1).
In humans, hippocampal neurogenesis and changes in fine morphology (dendrites and spines) cannot be directly examined at the level of brain cells. However, noninvasive neuroimaging methods permit characterization of brain structure, including gray and white matter, at a more macroscopic level. Some studies have assessed changes in gray matter density across the brain using voxel-wise techniques, which consider 3D, arbitrarily sized cubes (‘voxels’) as the unit for summarizing anatomical patterns across the entire brain. One study using this method found that 6 months of aerobic training increased gray matter density in the midline areas of the anterior cingulate cortex and supplementary motor area, the right inferior frontal gyrus, and the left superior temporal gyrus and increased white matter density in the anterior corpus callosum (genu) [189]. An advantage of voxel-wise techniques is their ability to examine the whole brain in an exploratory manner. However, a disadvantage is that a voxel is not defined with respect to brain anatomy. Measures of how much gray or white matter are in a voxel are statistically condensed to probability estimates of how likely a given voxel is to comprise of each tissue type. In addition, the method is typically conducted with T1-contrast MRI, which does not have good contrast for blood vessels that are interwoven through gray and white matter (and with greater density through gray matter, presenting a potential confound). In sum, the meaning of these probability estimates in relation to what is actually changing at the cellular and molecular level remains unclear [190–193].
Other studies have examined the effect of exercise on white matter tissue by examining microstructural properties with diffusion tensor imaging (DTI) or white matter lesions on T2-weighted MRI. A primary measure from DTI, fractional anisotropy (FA), measures the coherence of the orientation of water diffusion in a voxel independent of rate. Therefore, FA is considered a measure of axonal integrity, myelination, and axon diameter and density. Cross-sectional studies that have examined the association between fitness and white matter integrity with DTI have used relatively small sample sizes and have yielded mixed findings [194,195]. One study examined the relationship between change in regional diffusivity and change in fitness in a randomized controlled trial (RCT) [124]. There was a greater positive correlation between change in fitness and change in FA distributed throughout the frontal and temporal lobes compared with the covariance of fitness and FA changes for a non-aerobic exercise control group. However, there was no mean-level change in FA in the frontal, temporal, parietal, or occipital lobar masks and there were no regionally local increases in FA within lobes for the walking group. The extant findings suggest the possibility that changes in the brain from exercise are not necessarily targeted locally in the way neurogenesis is and may sometimes manifest throughout a system in a more distributed manner.
A technique that could be promising for more direct measurement of neuronal integrity and viability with MRI is magnetic resonance spectroscopy (MRS). This imaging technique can be used to measure the biochemical profile of regions of interest in the brain. One metabolite that MRS can measure well is N-acetylaspartate (NAA), which is a nervous system-specific metabolite synthesized in the mitochondria of the cell bodies of neurons and distributed in cell bodies, dendrites, and axons. NAA levels should therefore not be sensitive to regional variation in vasculature. Using this method, Erickson et al. [196] measured NAA in an 18 × 18-mm2 voxel in the right frontal cortex, including portions of the insula, surrounding white matter, caudate nucleus, and putamen. They found that fitter 66–80 year old adults had greater NAA levels than their less-fit peers (compared on VO2peak); however, 55–65-year-olds of higher and lower fitness did not differ in NAA. These results suggest that aerobic fitness is protective for neuronal integrity or mitochondrial function to a greater extent as normal aging progresses. However, this age–fitness interaction may be regionally specific. Another research group found greater NAA in a similar sized anterior cingulate region of interest in fitter middle-aged adults (age 40–65 years) compared with their less-fit peers [197]. A limitation of this method is evident in these two studies; it is currently not possible to perform a whole-brain MRS scan, which compromises comparability across studies and across participants in longitudinal assessment. Nevertheless, a productive future direction will be a greater understanding of the cellular and molecular basis of MRS signals in conjunction with using the method across species to test the translation of specific changes seen in exercising animals to exercising humans [e.g., 192].
Another common technique for measuring the effects of exercise on brain structure that has been used in human populations is calculating the volume of individual brain structures as defined by anatomy and not by tissue type per se. Using these methods, studies have focused on examining the anatomical specificity of positive associations between fitness and brain structure. For example, a well-controlled longitudinal study found that 1 year of moderate-intensity aerobic training in healthy, less-active older adults increased hippocampal volume by 1–2% [28]. Cross-sectional studies have found that greater aerobic fitness is associated with greater hippocampal volume in elderly adults [60] and preadolescent children with an automated segmentation routine [36] and in 15–18-year-old males using a manual tracing procedure to define the hippocampus [38].
At present, animal and human research provide measures of brain structure at different levels of analysis. However, in the near future it should be possible to characterize the brain structure of animals with high levels of precision with high-field MRI systems. Changes in regional volume and integrity, particularly with regard to white matter tracts, as a function of exercise or other factors can then be associated with cellular and molecular changes through histological examination of brain tissue. Such animal–human bridging studies can, in turn, be useful in inferring the cellular and molecular changes that are engendered by exercise and physical activity in human study participants (Figure 2).
Angiogenesis
Vessel formation is completed during development, but brain angiogenesis is maintained mostly to respond to specific stimuli such as injury or physical exercise [116,162,198]. Physical activity increases the proliferation of brain endothelial cells [199] and angiogenesis [200,201] throughout the brain, including motor areas of the cerebral cortex, as a robust adaptation to prolonged exercise [201]. Some vasculature changes associated with exercise in the adult brain may be mediated byIGF-1 and VEGF [115,199]. It has been shown that running enhances IGF gene expression [202] and protein levels in the hippocampus [106]. Angiogenesis induced by exercise is also associated with an increase in the brain of VEGF mRNA and protein [203].
Several different methods have been applied to human populations for examining the regional distribution of differences or changes in cerebral vasculature as a function of exercise, including perfusion methods using the (invasive) injection of a tracer and (noninvasive) perfusion imaging based on a magnetization-induced tracer signal. Using arterial spin labeling (ASL) with MRI, the most noninvasive method, one study found greater cerebral blood flow (CBF) in the hippocampus in six healthy older adults who had participated in a 4-month aerobic exercise program compared with five demographically matched participants in a health education control group [63]. This study did not compare post-training with a baseline session, which will be an important step for future research. Using a more invasive approach that relies on gadolinium contrast imaging, another study quantified hippocampal cerebral blood volume (CBV) in distinct subfields before and after a 3-month aerobic training program in 11 participants ranging from 21 to 45 years of age [57]. Although there was no control group, the results showed that hippocampal CBV, especially in the DG, increased from before to after the exercise intervention. Moreover, elevated CBV was positively correlated with increases in aerobic fitness and cognitive function. The regional distribution of CBV increases paralleled patterns of CBV differences (also measured with gadolinium MRI contrast imaging) in exercised animals reported in the same paper. However, from these studies alone it cannot be concluded empirically that exercise induces angiogenesis in the human hippocampus, because both methods are sensitive to differences in blood flow.
Another method that has been applied to human populations in the context of exercise neuroscience research is magnetic resonance angiography (MRA). Using MRA without gadolinium injection, Bullitt and colleagues [204] segmented and traced blood vessels of the anterior cerebral circulation (ACA), the posterior circulation (PCA), and the left and right middle cerebral artery (MCA) circulation. They compared seven highly active older adults between 60 and 74 years old with demographically matched adults with low levels of activity. They found that greater fitness was associated specifically with a greater number of small-radius (<0.5 mm) vessels in all circulation systems but no difference in larger vessels. In addition, lower fitness was associated with greater vessel tortuosity (i.e., frequent changes in direction) in the left and right MCA circulation. Although MRA also cannot exclude blood flow from influencing measurements of vessel number and tortuosity, these results provide data to support the prediction that exercise will have a greater impact on small-vessel number. This is important because greater microvascular density could increase protection against white matter hyperintensities, which would in turn protect against gray matter atrophy and cognitive impairment, and collectively represent one mechanistic pathway through which exercise benefits the aging mind and brain [205].
Overall, perfusion-based imaging and MRA offer complementary techniques for measuring the effects of physical activity on cerebral vasculature. Perfusion-based imaging offers better access to the capillary beds involved in neurovascular coupling that are likely to be sites of the interaction between neurogenesis and angiogenesis. MRA, however, offers more specific quantification of vessel number, radius, and tortuosity and may offer improved resolution for small vessels with future advances in magnetic field strength.
The cellular, molecular, and structural changes associated with exercise described in the preceding sections should also be considered against the (epi)genetic background of the organism as described below.
Genetics and epigenetics
(Neuro)epigenetic modifications are changes in gene expression that are not coded in the DNA sequence itself but result from changes in chromatin structure. In mammals, epigenetic processes mainly include DNA methylation, histone modification, and noncoding RNA-mediated processes that, in the brain, are associated with cognitive function. Histone acetylation is associated with promoting memory formation and histone deacetylase inhibitors have begun to be tested as therapeutic agents for cognitive impairment [206]. Recent studies in young and aged rats indicate that 2 weeks of treadmill exercise may increase hippocampal DNA methylation [207] and histone acetylation while also improving memory function and reducing proinflammatory markers [208]. Interestingly, recent research shows that the gene mutated in human CHARGE syndrome, the ATP-dependent chromatin-remodeling factor CHD7, functions as a regulator of neurogenesis. It binds to promoters of the Sox family of transcription factors, which are important for new neuron differentiation, to facilitate open chromatin structure. Exercise was shown to ameliorate the neurogenic defects, reversing both reductions in new cell number and deficits in fine dendritic morphology caused by CHD7 mutation [209] (Table 1).
The neurogenic response to running in rodents is also influenced by genetic background. For example, evaluating the neurogenic response to exercise in different mouse strains showed an increase in all strains examined in a recent study [151]. However, the magnitude of change differed between strains following 6 weeks of voluntary exercise. The most responsive strains increased neurogenesis by four- to fivefold and included AKR/J, B6129SF1/J,BALB/cByJ, CAST/EiJ, and SM/J, whereas C57Bl/6J, a strain often used in neurogenesis experiments, showed a 1.6 times increase.
In humans, one way to examine the contribution of genetic background to exercise effects on the brain is to examine whether individual differences in genes, such as SNPs, moderate the effects of physical activity on brain and cognition. Although other types of polymorphism exist (e.g., sequence repeats), there have been an increasing number of human studies of physical activity that examined specific gene SNPs that influence cognition or neural systems. Thus far, most of these analyses have been conducted in prospective observational studies in which physical activity is assessed at one point in time and cognition or the diagnosis of dementia several years later.
The great majority of these studies have also focused on a single gene, APOE. The APOE gene, which creates lipoprotein and has an important role in cholesterol transport, has three different alleles: e2, e3 (the most frequent), and e4. The e4 allele has been found in approximately 14% of the population and has been implicated in atherosclerosis and AD. Indeed, individuals with an e4 allele have approximately four times the probability of developing AD as non-e4 carriers [210]. Furthermore, even in the absence of AD, carriers of e4 alleles have been shown to display cognitive deficits as early as middle age [211,212].
An example of such a study is provided by Schuit et al. [213] in which the amount of physical activity of 347 elderly Dutch men was assessed via a self-report questionnaire. Three years later, a general measure of cognitive function, the Mini-Mental State Exam (MMSE), was administered to this cohort. As expected, the risk of cognitive decline was substantially higher for individuals who possessed an e4 allele than for those who did not, whereas more active older adults (i.e., more than 1 h of physical activity per day) with an e4 allele were four times less likely to show cognitive decline than more sedentary e4 carriers. Indeed, reduced cognition was found mostly in e4 carriers with low activity levels and to a much lesser extent in highly active e4 carriers and non-e4 carriers. A similar pattern of results was found in a Finnish study of 1449 older adults by Rovio et al. [214]. In this case, the main outcome measure was diagnosis of AD.
Interestingly, however, not all observational studies have found that e4 carriers show the largest benefit for physical activity with regard to cognitive decline or dementia. For example, studies have reported no difference in the effects of self-reported physical activity on cognitive impairment or age-associated neurodegenerative disease as a function of APOE e4 status [22,215–217], with a total of over 6000 participants. A large longitudinal study by Podewils et al. [218], with 3375 older men and women, over the course of 5.4 years found that physical activity effects on dementia risk were larger for non-carriers than for carriers of e4.
In addition to the longitudinal observational studies discussed above, there have been several cross-sectional studies that examined physical activity and APOE status effects on behavioral measures of cognition and brain function. Several of these studies have reported larger positive effects of physical activity for e4 carriers than for non-carriers. For example, Deeny et al. [219] found superior performance on a Sternberg memory search task and greater temporal lobe activation in magnetoencephalography (MEG) data for highly active compared with less-active e4 carriers (see also [220]). Smith et al. [221] reported differences in fMRI activation patterns in brain regions that distinguished between famous and unfamiliar names in a semantic memory task and these effects were larger for highly active e4 carriers. Head et al. [222] found that less-active e4 carriers showed higher Pittsburgh Compound B (PiB) binding than more physically active carriers. PiB is a marker of amyloid, a precursor of plaques associated with AD. Although these results appear promising, other cross-sectional studies have found either no relation between APOE e4 status and exercise for regional brain volumes [223] or more prominent associations between physical activity and PiB levels for e4 non-carriers than for carriers [224].
Clearly such a mixed bag of results is difficult to interpret. However, it must be kept in mind that there were numerous potentially important differences among these studies, including culture (e.g., Box 4), gender, methods of assessing physical activity (which were self-reported) and cardiorespiratory fitness, sample size, the age range of the participants, and measures of dementia, cognition, and brain function and structure. Thus, although some of these results are intriguing we do not yet understand the boundary conditions on APOE e4–physical activity/exercise interactions.
Box 4. How well do we follow evidence-based advice?
Specific recommendations for physical activity and exercise for different portions of the population have been made. For example, the Physical Activity Guidelines for Americans published in 2008 [1] suggested that adults should undertake at least 150 min per week of moderate-intensity or 75 min per week of vigorous-intensity exercise to reap substantial health benefits. Recommendations for children and adolescents were 60 min or more of physical activity on a daily basis.
Do we follow such recommendations? Unfortunately, as a society we do not do a very good job of getting the minimum recommended physical activity. The State Indicator Report on Physical Activity, 2010 (http://www.cdc.gov/physicalactivity/downloads/PA_State_Indicator_Report_2010.pdf) provides data both on a state-by-state basis and for the US population (see also [288]). Sixty-four percent of adults report getting the recommended minimal level of physical activity (with a range across states of 56–73%), while high-school students present an even more disturbing pattern, with only 17% reporting that they get the recommended weekly amount of physical activity (with a range of 16–27% across states).
Interestingly, there is a substantial and growing literature on both barriers to and facilitators of healthy lifestyle behaviors, including physical activity and exercise. Although a thorough review of this literature is beyond the scope of this section, we briefly discuss some of the relevant factors. The State Indicator Report on Physical Activity, 2010 made several evidence-based suggestions for how children and teens’ physical activity could be enhanced, including: providing increased access to places for physical activity; enhancing physical education/activity in schools and child-care settings; and supporting urban design, land use, and transportation policies to encourage energy exertion. These factors are consistent with numerous recent surveys conducted throughout the world that describe both individual and environmental influences [289] on physical-activity behavior.
It also appears important to consider the needs of specific populations when examining barriers and enablers of physical activity. For example, Hinkley and colleagues [290] report that different factors influence preschool boys’ and girls’ participation in physical activity. It also appears that parents often overestimate the amount of activity that their children get [291]. Therefore, objective assessment of physical activity in children is important. With individuals in nursing homes, and those with chronic diseases, there is also the need to provide additional professional and caregiver support to facilitate physical-activity programs and to tailor them to abilities and disabilities [292–294].
Self-efficacy, self-regulatory behaviors, and executive control processes are also important in beginning lifestyle change and maintaining physical-activity behaviors. Self-efficacy reflects belief in the ability to perform behaviors such as walking and cycling. In a recent study, McAuley et al. [295] reported that measures of different aspects of executive function (e.g., the ability to multitask and ignore task-irrelevant information in the environment) had both direct and indirect (through self-efficacy, or the belief one has in one’s capabilities to successfully conduct a course of action) effects on the extent to which older adults participated in a 12-month exercise program. Therefore, future development of strategies to improve both self-efficacy and executive functions could enhance physical activity participation [296].
Animal studies provide experimental evidence to weigh in with the mixed results from human studies. Transgenic APOE4 mice, a mouse model for this risk gene associated with AD incidence, benefited from housing with a running wheel for 6 weeks, showing an improvement in spatial learning in a radial arm water maze, restoration of reduced TrkB receptor levels, and increased levels of synaptophysin. However, although the relative increase in TrkB receptor levels was greater in APOE4 than in APOE3 mice, the overall levels did not differ between the two strains after running [225]. Another transgenic animal, the 3×TG mouse model of AD, shows improved neurogenesis following exercise [184]. Interestingly, exercise may not offer general benefits across neurodegenerative diseases. For example, in mouse models of Huntington’s disease (HD), the neurogenic response to exercise appears to have been abolished [226,227]. Whether cognitive, motor, and affective symptoms do benefit from physical activity remains unclear from both mouse [227–229] and human [230,231] studies.
Two additional genes have been studied as potential moderators of physical activity effects on cognition, psychosocial function, or brain function in human studies. One such gene (and its associated protein of the same name) is BDNF, discussed in detail above as a neurotrophin instrumental in cell survival and plasticity in, among other regions, the hippocampus and cortex. Variants of this gene, in particular the Val/Val genotype, have been associated with higher levels of learning and performance on several cognitive tasks [232] and the Met allele has been associated with higher risk of depression [233] (but see [234]). Mata et al. [235] examined whether BDNF genotype interacted with self-reported physical activity to predict depressive symptoms in 82 healthy adolescent girls. Interestingly, higher levels of physical activity were associated with fewer depressive symptoms for girls with a met allele, but not for girls with the val/val genotype. In another study [236], the BDNF gene moderated the effects of a 30-min acute exercise bout on the self-reported mood of a group of 64 18–36-year-olds. Finally, a functional polymorphism related to dopamine metabolism, catechol-O-methyltransferase (COMT), was observed to moderate the effects of 17 weeks of running training on response speed in a working memory and inhibition task. Participants with val/val alleles showed a larger improvement in performance than those with the met allele [301].
In summary, although genetic studies conducted with human participants provide some intriguing results, interpretation is complicated by several methodological concerns including the small effect sizes of individual genes and small samples of study participants. Some of these concerns may be addressed through animal studies, which can enforce more experimental control over the environment of animals with different genetic profiles. Future human studies will also benefit from increased knowledge, from animal models, concerning molecular pathways that link exercise and cognition, as well as larger samples that enable the study of multiple genes (and their associated proteins) as moderators of the effects of exercise on cognition and brain function. Epigenetic linkages in humans may also be possible by including a subset of twins in studies with large sample sizes that can be followed over time and perhaps post mortem to examine regional gene expression as a function of physical activity.
Concluding remarks
We have reviewed evidence from animal models and human studies that collectively characterize the effect of physical activity on the brain and cognition across the lifespan. Animal studies utilize well-controlled experimental paradigms and measure changes in the CNS at the level of molecules, neurons, and signaling pathways involved in neurotrophin levels, neurogenesis, synaptic plasticity, metabolism and behavior (Figure 2). Rodent models of normal and pathological aging enable insight as to whether exercise benefits are generalizable across different populations. These experiments provide the foundation for predicting the mechanisms associated with observational studies and physical activity intervention outcomes across the lifespan in specific populations. Although animal studies can identify basic mechanisms and validate biomarkers for human CNS outcomes, such as circulating growth factors (Box 5 and Figure 2), they are not always directly translatable to human physiology, behavior, and outcomes. For instance, it is hard to directly translate exercise time and distance from rodents to humans. This makes it difficult to make specific public health recommendations without complementary human studies (Box 5). Moreover, real-world performance outcomes such as adherence and functional independence depend a great deal on higher-level cognitive abilities that critically involve the prefrontal cortices in addition to the hippocampus and are difficult to test in animals (Boxes 1, 2, and 4) (see, however [237]). Limitations of human studies include the significant cost and difficulty of designing experiments that can follow participants long enough to examine the outcomes of interventions that naturally develop over the lifespan [238]. Although there is a consensus from animal and human studies that physical activity benefits brain function, further research is needed to search for the neurobiological mechanisms mediating the benefits of physical activity on cognition, behavior, and neurodegenerative diseases.
Box 5. Outstanding questions.
What are the relevant principles for the timing, duration, sequence, and type of cognitive engagement that interact positively with exercise on micro- and macroscale outcomes that are important for cognitive and brain health?
Does physical activity have to increase your heart rate to be neuroprotective? Some studies suggest not (e.g., [61,92,297,23]), but other studies show a dose–response relationship between increases in fitness and brain outcomes (e.g., [28,55,124]). The answer to this question is important for informing public health recommendations for improvement and maintenance of brain health throughout the lifespan.
There is growing evidence that resistance exercise, which is targeted for improved muscle strength, is also beneficial for brain structure and function in older adults [298,299]. The neural mechanisms may be evaluated through the development of animal models of resistance training and then compared with aerobic training.
What is the time course of exercise-induced changes in the human brain and how does it vary as a function of age, disease, and presence of brain injury? Can a reliable mapping between time course in animals and humans be established?
Can small-animal imaging be used to examine the cellular and molecular basis of exercise-induced changes in brain structure and function (e.g., resting-state BOLD coupling) as measured via MRI methods comparable with human studies?
What is the relationship between exercise-induced changes in peripheral growth factors and regional changes in central expression?
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute on Aging. A.F.K. was supported by 5R37AG025667 from the NIA. M.W.V. was supported by start-up funds from the University of Iowa. We thank Linda Kitabayashi for preparation of the photomicrograph and Dr Justin Rhodes and Martina Mustroph for sharing images.
Glossary
- Aerobic training
exercise training that improves the efficiency of the aerobic energy-producing systems and can improve maximal oxygen uptake and cardiorespiratory endurance
- Angiogenesis
blood vessel formation from the existing vasculature, a process that exists throughout the body and is regulated by metabolic demands from surrounding tissues
- Blood–brain barrier
the interface that separates the brain from the circulatory system; it protects the CNS from potentially toxic substances and regulates transport across the barrier for molecules that are safe and essential for the CNS. Some molecules affected by exercise in periphery are known to cross the barrier (e.g., IGF-1)
- Cytokines
multifunctional proteins that mediate both the host response to infection and normal signaling between cells of non-immune tissues, including the nervous system. These proteins play roles in neuronal cell death, proliferation, migration, and differentiation
- Enriched environment (EE)
a complex combination of social, cognitive and physical stimulation. Accomplished in rodents by housing in a large cage with several animals and varying sets of toys such as balls, tunnels, and ladders. Accomplished in humans by exposure to diverse stimuli, demand for complex decisions, and social and physical stimulation
- Executive function
a heterogeneous neuropsychological construct that includes the ability to form, maintain, inhibit, and shift between mental sets and corresponds to the abilities to reason, generate, and follow through with goals and to flexibly alter goals in response to changing rules or contingencies (adapted from [269])
- Exercise
in humans, defined as a subset of physical activity that is planned, structured, and repetitive and performed for the maintenance/improvement of physical fitness [270]. In rodents, exercise is synonymous with physical activity and can be either voluntary (housing animals with a running wheel) or ‘forced’ by placing animals on a treadmill for a certain period of time
- Functional connectivity or functional coupling
terms commonly used to describe the existence of a functional relationship between two regions in fMRI data. High functional connectivity means that two (or more) brain regions have neural activity that fluctuates similarly over time. Functional connectivity is quantified by measures of association such as correlation, so no causal relationship is implied. The extent to which functional connectivity is ‘good’ depends on whether the regions are typically functionally coupled in high-performing healthy adults or the extent to which more functional coupling between regions is predictive of less risk of cognitive decline or clinical pathology [271,272]
- Functional MRI (fMRI)
commonly refers to an indirect measure of neuronal activity assessed over time. The most common type of fMRI measurement is sensitive to the amount of deoxygenated hemoglobin in the blood (T2*-weighted contrast), which is known to peak around 6–8 s following the onset of neuronal activity, based on mechanisms of neurovascular coupling: this effect is known as the blood oxygenation level-dependent (BOLD) contrast. Another common magnetic resonance technique for measuring brain function is arterial spin labeling (ASL).
- Hippocampus
a brain area in the medial temporal lobes that is essential for memory formation [24] and spatial navigation [25]. Comprises three main subfields: the DG, area CA3, and area CA1. Each of these regions has specific cell types and plasticity contributing to learning and memory processes. Only the DG subfield can generate new neurons in the adult brain, a process that is upregulated by exercise in rodents
- Long-term depression (LTD)
a reduction in synaptic efficacy induced by low-frequency afferent stimulation. LTD is theorized to be a cellular model of forgetting
- Long-term potentiation (LTP)
a persistent increase in synaptic strength between two neurons, measured by an increase in the evoked post-synaptic electrical response following afferent stimulation; there are different mechanisms for inducing LTP (for a review in the context of exercise, see [273]). LTP is a cellular model of learning and memory
- Micro- and macroscale measurements
microscale measurements are at the level of neurons and synapses, whereas macroscale measurements are at the level of anatomically distinct brain regions and inter-regional pathways [274]
- Maximal oxygen uptake (VO2max)
the maximal capacity for oxygen consumption by the body during maximal exertion. It is also known as aerobic power, maximal oxygen intake, maximal oxygen consumption, and cardiorespiratory endurance capacity. The gold-standard measurement in humans is a graded exercise test (GXT) in which the rate of work is increased gradually until fatigue or exhaustion; a gas analysis will indicate maximal oxygen uptake. When a participant does not ‘max out’ physiologically, which is common with older adults, the measurement is called VO2peak
- Neurogenesis
the formation of new neurons from neural stem and progenitor cells, in the subgranular zone of the DG (in the hippocampus) and the subventricular zone of the lateral ventricles (which migrate through the rostral migratory stream to the olfactory bulb)
- Neurotrophins
type of growth factor constituting a family of proteins that induce the survival, development, and function of neurons
- Physical activity
any bodily movement produced by the skeletal muscles that increases energy expenditure; includes both exercise and non-exercise activity thermogenesis (NEAT) [270,275]
References
- 1.Haskell WL, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 2.Erickson KI, et al. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Hertzog C, et al. Enrichment effects on adult cognitive development. Psychol Sci Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 5.Hebb DO. The effects of early experience on problem solving at maturity. Am Psychol. 1947;2:306–307. [Google Scholar]
- 6.Greenough WT, et al. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp Neurol. 1973;41:371–378. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- 7.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 8.van Praag H, et al. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 9.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 10.Schooler C. Cognitive effects of complex environments during the lifespan: a review and theory. In: Schooler C, Schaie KW, editors. Cognitive Functioning and Social Structure Over the Life Course. Ablex Publishing; 1987. pp. 24–49. [Google Scholar]
- 11.Diamond A, et al. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson MC, et al. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J Gerontol A: Biol Sci Med Sci. 2009;64:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karp A, et al. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 14.Hultsch DF, et al. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- 15.Carlson MC, et al. Lifestyle activities and memory: variety may be the spice of life. The Women’s Health and Aging Study II. J Int Neuropsychol Soc. 2011;18:286–294. doi: 10.1017/S135561771100169X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson-Hanley C, et al. Exergaming and older adult cognition. Am J Prev Med. 2012;42:109–119. doi: 10.1016/j.amepre.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 17.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 18.Kobilo T, et al. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mustroph ML, et al. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchman AS, et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson KI, et al. Physical Activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson EB, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 23.Middleton LE, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171:1251–1257. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Keefe J, et al. Fornix lesions selectively abolish place learning in the rat. Exp Neurol. 1975;48:152–166. doi: 10.1016/0014-4886(75)90230-7. [DOI] [PubMed] [Google Scholar]
- 26.Fox NC, et al. Atrophy of the hippocampal formation in early familial Alzheimer’s disease. A longitudinal MRI study of at-risk members of a family with an amyloid precursor protein 717Val-Gly mutation. Ann N Y Acad Sci. 1996;777:226–232. doi: 10.1111/j.1749-6632.1996.tb34423.x. [DOI] [PubMed] [Google Scholar]
- 27.Neeper SA, et al. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 28.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46:123–133. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- 30.O’Callaghan R, et al. The effects of forced exercise on hippocampal plasticity in the rat: a comparison of LTP, spatial-and non-spatial learning. Behav Brain Res. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Falls WA, et al. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behav Brain Res. 2010;207:321–331. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Liu YF, et al. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90:81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Mello PB, et al. Physical exercise can reverse the deficit in fear memory induced by maternal deprivation. Neurobiol Learn Mem. 2009;92:364–369. doi: 10.1016/j.nlm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Creer DJ, et al. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Intlekofer KA, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38:2027–2034. doi: 10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaddock L, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaddock L, et al. Aerobic fitness and executive control of relational memory in preadolescent children. Med Sci Sports Exerc. 2011;43:344–349. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- 38.Herting MM, Nagel BJ. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav Brain Res. 2012;233:517–525. doi: 10.1016/j.bbr.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monti JM, et al. Aerobic fitness enhances relational memory in preadolescent children: the FITKids randomized control trial. Hippocampus. 2012;22:1876–1882. doi: 10.1002/hipo.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stroth S, et al. Aerobic endurance exercise benefits memory and affect in young adults. Neuropsychol Rehabil. 2009;19:223–243. doi: 10.1080/09602010802091183. [DOI] [PubMed] [Google Scholar]
- 41.Nakazawa K, et al. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- 42.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15:808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- 44.Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- 45.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 46.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 47.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Praag H, et al. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 49.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B: Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 50.Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morton JA, et al. Measuring cognitive deficits in disabled mice using an automated interactive touchscreen system. Nat Methods. 2006;3:767. doi: 10.1038/nmeth1006-767. [DOI] [PubMed] [Google Scholar]
- 52.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vivar C, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nature Comm. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakker A, et al. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dery N, et al. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front Neurosci. 2013;7:66. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobs BL, et al. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 57.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer TD, et al. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 59.Ho NF, et al. In vivo imaging of adult human hippocampal neurogenesis: progress, pitfalls and promise. Mol Psychiatry. 2013;18:404–416. doi: 10.1038/mp.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erickson KI, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voss MW, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colcombe SJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burdette JH, et al. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- 65.Cowansage KK, et al. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 66.Neeper SA, et al. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 67.Molteni R, et al. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 68.Berchtold NC, et al. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 69.Ding Q, et al. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–780. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sartori CR, et al. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 71.Vaynman S, et al. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Abel JL, Rissman EF. Running-induced epigenetic and gene expression changes in the adolescent brain. Int J Dev Neurosci. 2013;31:382–390. doi: 10.1016/j.ijdevneu.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farmer J, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 74.Berchtold NC, et al. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hopkins ME, et al. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marlatt MW, et al. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol. 2012;72:943–952. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu YF, et al. Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. J Physiol. 2009;587:3221–3231. doi: 10.1113/jphysiol.2009.173088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaynman S, et al. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- 79.Griffin EW, et al. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- 80.Wu CW, et al. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- 81.O’Callaghan RM, et al. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- 82.Gómez-Pinilla F, et al. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 83.Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiol Learn Mem. 2010;94:278–284. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myhrer T. The role of medial and lateral hippocampal perforant path lesions and object distinctiveness in rats’ reaction to novelty. Physiol Behav. 1988;42:371–377. doi: 10.1016/0031-9384(88)90279-x. [DOI] [PubMed] [Google Scholar]
- 85.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 86.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38:61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 89.Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis. 2012;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 90.von Bohlen und Halbach O. Involvement of BDNF in age-dependent alterations in the hippocampus. Front Aging Neurosci. 2010;2:36. doi: 10.3389/fnagi.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adlard PA, et al. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 92.Barrientos RM, et al. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aguiar JAS, et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132:560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 94.Gold SM, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol. 2003;138:99–105. doi: 10.1016/s0165-5728(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 95.Rojas Vega S, et al. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 96.Ferris LT, et al. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 97.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–541. [PubMed] [Google Scholar]
- 98.Knaepen K, et al. Neuroplasticity – exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010;40:765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 99.Rasmussen P, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 100.Matthews VB, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 101.Ogborn DI, Gardiner PF. Effects of exercise and muscle type on BDNF, NT-4/5, and TrKB expression in skeletal muscle. Muscle Nerve. 2010;41:385–391. doi: 10.1002/mus.21503. [DOI] [PubMed] [Google Scholar]
- 102.Fujimura H, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- 103.Lommatzsch M, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Griffin ÉW, et al. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 105.Gómez-Pinilla F, et al. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- 106.Carro E, et al. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trejo JL, et al. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakajima S, et al. Regular voluntary exercise cures stress-induced impairment of cognitive function and cell proliferation accompanied by increases in cerebral IGF-1 and GST activity in mice. Behav Brain Res. 2010;211:178–184. doi: 10.1016/j.bbr.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 109.Eliakim A, et al. Increase in muscle IGF-I protein but not IGF-I mRNA after 5 days of endurance training in young rats. Am J Physiol. 1997;273:R1557–R1561. doi: 10.1152/ajpregu.1997.273.4.R1557. [DOI] [PubMed] [Google Scholar]
- 110.Tang K, et al. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir Physiol Neurobiol. 2010;170:16–22. doi: 10.1016/j.resp.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aberg MA, et al. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding Q, et al. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 113.Krum JM, et al. Angiogenic and astroglial responses to vascular endothelial growth factor administration in adult rat brain. Neuroscience. 2002;110:589–604. doi: 10.1016/s0306-4522(01)00615-7. [DOI] [PubMed] [Google Scholar]
- 114.Heine VM, et al. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 115.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 116.van Praag H, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van der Borght K, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19:928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- 118.Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- 119.Yang JP, et al. Direct transport of VEGF from the nasal cavity to brain. Neurosci Lett. 2009;449:108–111. doi: 10.1016/j.neulet.2008.10.090. [DOI] [PubMed] [Google Scholar]
- 120.Kohman RA, et al. Voluntary wheel running reverses age-induced changes in hippocampal gene expression. PLoS ONE. 2011;6:e22654. doi: 10.1371/journal.pone.0022654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Latimer CS, et al. Reversal of glial and neurovascular markers of unhealthy brain aging by exercise in middle-aged female mice. PLoS ONE. 2011;6:e26812. doi: 10.1371/journal.pone.0026812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Voss MW, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gomez-Pinilla F, et al. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Voss MW, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22119. http://dx.doi.org/10.1002/hbm.22119. [DOI] [PMC free article] [PubMed]
- 125.Driscoll I, et al. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS ONE. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shimizu E, et al. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet. 2004;126B:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- 127.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 128.Chen ZY. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sen S, et al. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- 130.Verhagen M, et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2008;15:260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- 131.Soliman F, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bath KG, et al. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology. 2012;37:1297–1304. doi: 10.1038/npp.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tong L, et al. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- 134.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 135.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 136.Vasuta C, et al. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus. 2007;17:1201–1208. doi: 10.1002/hipo.20349. [DOI] [PubMed] [Google Scholar]
- 137.Wang S, et al. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- 138.Schmidt-Hieber C, et al. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 139.Ge S, et al. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang YP, et al. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 141.Tang YP, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 142.Snyder JS, et al. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 143.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kheirbek MA, et al. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012;32:8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 146.Nägerl UV, et al. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 147.Stranahan AM, et al. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eadie BD, et al. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 149.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 150.Zhao C. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Clark PJ, et al. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2011;184:16–27. doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morgenstern NA, et al. Newborn granule cells in the ageing dentate gyrus. J Physiol. 2008;586:3751–3757. doi: 10.1113/jphysiol.2008.154807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cirillo J, et al. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J Physiol. 2009;587:5831–5842. doi: 10.1113/jphysiol.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fathi D, et al. Effects of aging on the human motor cortical plasticity studied by paired associative stimulation. Clin Neurophysiol. 2010;121:90–93. doi: 10.1016/j.clinph.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 156.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 157.Vivar C, van Praag H. Functional circuits of new neurons in the dentate gyrus. Front Neural Circuits. 2013;7:15. doi: 10.3389/fncir.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 159.Deng W, et al. New neurons and new memories:how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Van der Borght K, et al. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121:324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- 161.Bednarczyk MR, et al. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus. 2009;19:913–927. doi: 10.1002/hipo.20621. [DOI] [PubMed] [Google Scholar]
- 162.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brown J, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 164.Clark PJ, et al. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 165.Speisman RB, et al. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kronenberg G, et al. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 167.Ming G-l, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 168.Blackmore DG, et al. GH mediates exercise-dependent activation of SVZ neural precursor cells in aged mice. PLoS ONE. 2012;7:e49912. doi: 10.1371/journal.pone.0049912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Trejo JL, et al. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 171.Moon HY, et al. Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise. Proc Natl Acad Sci USA. 2012;109:13094–13099. doi: 10.1073/pnas.1205535109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walton NM, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 173.Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 174.Vukovic J, et al. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 2012;32:6435–6443. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brandt MD, et al. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2010;32:1256–1264. doi: 10.1111/j.1460-9568.2010.07410.x. [DOI] [PubMed] [Google Scholar]
- 176.Hill MN, et al. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. Eur Arch Psychiatry Clin Neurosci. 2007;257:271–280. doi: 10.1007/s00406-007-0731-5. [DOI] [PubMed] [Google Scholar]
- 178.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 179.Marlatt MW, et al. Comparison of neurogenic effects of fluoxetine, duloxetine and running in mice. Brain Res. 2010;1341:93–99. doi: 10.1016/j.brainres.2010.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hanson ND, et al. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36:2589–2602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kuhn HG, et al. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rodríguez JJ, et al. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS ONE. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rodriguez JJ, et al. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2011;8:707–717. doi: 10.2174/156720511797633214. [DOI] [PubMed] [Google Scholar]
- 185.Llorens-Martin MV, et al. Effects of voluntary physical exercise on adult hippocampal neurogenesis and behavior of Ts65Dn mice, a model of Down syndrome. Neuroscience. 2010;171:1228–1240. doi: 10.1016/j.neuroscience.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 186.Heyn P, et al. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 187.Lautenschlager NT, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 188.Baker LD, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Colcombe SJ, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A: Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 190.Zatorre RJ, et al. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lövdén M, et al. Structural brain plasticity in adult learning and development. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.02.014. http://dx.doi.org/10.1016/j.neubiorev.2013.02.014. [DOI] [PubMed]
- 192.Biedermann S, et al. In vivo voxel based morphometry: detection of increased hippocampal volume and decreased glutamate levels in exercising mice. Neuroimage. 2012;61:1206–1212. doi: 10.1016/j.neuroimage.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 193.Thomas AG, et al. The effects of aerobic activity on brain structure. Front Psychol. 2012;3:86. doi: 10.3389/fpsyg.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Marks BL, et al. Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci. 2007;1097:171–174. doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- 195.Johnson NF, et al. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Erickson KI, et al. Beyond vascularization: aerobic fitness is associated with N-acetylaspartate and working memory. Brain Behav. 2012;2:32–41. doi: 10.1002/brb3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gonzales MM, et al. Aerobic fitness and the brain: increased N-acetyl-aspartate and choline concentrations in endurance-trained middle-aged adults. Brain Topogr. 2012;26:126–134. doi: 10.1007/s10548-012-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bloor CM. Angiogenesis during exercise and training. Angiogenesis. 2005;8:263–271. doi: 10.1007/s10456-005-9013-x. [DOI] [PubMed] [Google Scholar]
- 199.Lopez-Lopez C, et al. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci USA. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kleim JA, et al. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- 201.Swain RA, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 202.Ding Q, et al. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 203.Ding YH, et al. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3:15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- 204.Bullitt E, et al. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. AJNR Am J Neuroradiol. 2009;30:1857–1863. doi: 10.3174/ajnr.A1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bullitt E, et al. The effects of healthy aging on intracerebral blood vessels visualized by magnetic resonance angiography. Neurobiol Aging. 2010;31:290–300. doi: 10.1016/j.neurobiolaging.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gräff J, Tsai LH. The potential of HDAC inhibitors as cognitive enhancers. Annu Rev Pharmacol Toxicol. 2013;53:311–330. doi: 10.1146/annurev-pharmtox-011112-140216. [DOI] [PubMed] [Google Scholar]
- 207.Elsner VR, et al. Exercise induces age-dependent changes on epigenetic parameters in rat hippocampus: a preliminary study. Exp Gerontol. 2013;48:136–139. doi: 10.1016/j.exger.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lovatel GA, et al. Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol Learn Mem. 2013;101:94–102. doi: 10.1016/j.nlm.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 209.Feng W, et al. The chromatin remodeler CHD7 regulates adult neurogenesis via activation of SoxC transcription factors. Cell Stem Cell. 2013;13:62–72. doi: 10.1016/j.stem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 210.Farrer LA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 211.Greenwood PM, et al. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zehnder AE, et al. Impact of APOE status on cognitive maintenance in healthy elderly persons. Int J Geriatr Psychiatry. 2009;24:132–141. doi: 10.1002/gps.2080. [DOI] [PubMed] [Google Scholar]
- 213.Schuit AJ, et al. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 214.Rovio S, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 215.Abbott RD, et al. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 216.Scarmeas N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ravaglia G, et al. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology. 2008;70:1786–1794. doi: 10.1212/01.wnl.0000296276.50595.86. [DOI] [PubMed] [Google Scholar]
- 218.Podewils LJ, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 219.Deeny SP, et al. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008;78:179–187. doi: 10.1016/j.biopsycho.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Etnier JL, et al. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39:199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 221.Smith JC, et al. Interactive effects of physical activity and APOE-epsilon4 on BOLD semantic memory activation in healthy elders. Neuroimage. 2011;54:635–644. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Head D, et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Honea RA, et al. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liang KY, et al. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nichol K, et al. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kohl Z, et al. Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington’s disease. Brain Res. 2007;1155:24–33. doi: 10.1016/j.brainres.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 227.Potter MC, et al. Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington’s disease. PLoS Curr. 2010;2:RRN1201. doi: 10.1371/currents.RRN1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pang TY, et al. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience. 2006;141:569–584. doi: 10.1016/j.neuroscience.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 229.Renoir T, et al. Treatment of depressive-like behaviour in Huntington’s disease mice by chronic sertraline and exercise. Br J Pharmacol. 2012;165:1375–1389. doi: 10.1111/j.1476-5381.2011.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Altschuler EL. Strenuous, intensive, long-term exercise does not prevent or delay the onset of Huntington’s disease. Med Hypotheses. 2006;67:1429–1430. doi: 10.1016/j.mehy.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 231.Kosinski CM, et al. Myopathy as a first symptom of Huntington’s disease in a marathon runner. Mov Disord. 2007;22:1637–1640. doi: 10.1002/mds.21550. [DOI] [PubMed] [Google Scholar]
- 232.Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 233.Duman R, Monteggia L. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 234.Figueira P, et al. An association study between the Val66Met polymorphism of the BDNF gene and postpartum depression. Arch Womens Ment Health. 2010;13:285–289. doi: 10.1007/s00737-010-0146-6. [DOI] [PubMed] [Google Scholar]
- 235.Mata J, et al. BDNF genotype moderates the relation between physical activity and depressive symptoms. Health Psychol. 2010;29:130–133. doi: 10.1037/a0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bryan A, et al. A transdisciplinary model integrating genetic, physiological, and psychological correlates of voluntary exercise. Health Psychol. 2007;26:30–39. doi: 10.1037/0278-6133.26.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Matzel LD, Kolata S. Selective attention, working memory, and animal intelligence. Neurosci Biobehav Rev. 2010;34:23–30. doi: 10.1016/j.neubiorev.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Smith PJ. Physical activity, vascular health, and cognitive impairment. Arch Intern Med. 2012;172:83. doi: 10.1001/archinternmed.2011.615. author reply 84. [DOI] [PubMed] [Google Scholar]
- 239.Lindsay J, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 240.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 241.Adlard PA, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Belarbi K, et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol Dis. 2011;43:486–494. doi: 10.1016/j.nbd.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 243.Garcia-Mesa Y, et al. Physical exercise protects against Alzheimer’s disease in 3×Tg-AD mice. J Alzheimers Dis. 2011;24:421–454. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- 244.Liu H-l, et al. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav Brain Res. 2011;218:308–314. doi: 10.1016/j.bbr.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 245.Gimenez-Llort L, et al. Gender-specific neuroimmunoendocrine response to treadmill exercise in 3×Tg-AD mice. Int J Alzheimers Dis. 2010;2010:128354. doi: 10.4061/2010/128354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yuede CM, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Um HS, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–539. [PubMed] [Google Scholar]
- 248.Um HS, et al. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci Res. 2011;69:161–173. doi: 10.1016/j.neures.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 249.Leem YH, et al. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J Neurosci Res. 2009;87:2561–2570. doi: 10.1002/jnr.22075. [DOI] [PubMed] [Google Scholar]
- 250.Castelli DM, et al. Physical fitness and academic achievement in third- and fifth-grade students. J Sport Exerc Psychol. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- 251.Roberts CK, et al. Low aerobic fitness and obesity are associated with lower standardized test scores in children. J Pediatr. 2010;156:711–718. doi: 10.1016/j.jpeds.2009.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Donnelly JE, et al. Physical Activity Across the Curriculum (PAAC): a randomized controlled trial to promote physical activity and diminish overweight and obesity in elementary school children. Prev Med. 2009;49:336–341. doi: 10.1016/j.ypmed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chaddock L, et al. The role of childhood aerobic fitness in successful street crossing. Med Sci Sports Exerc. 2012;44:749–753. doi: 10.1249/MSS.0b013e31823a90cb. [DOI] [PubMed] [Google Scholar]
- 254.Guiney H, Machado L. Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon Bull Rev. 2012;20:73–86. doi: 10.3758/s13423-012-0345-4. [DOI] [PubMed] [Google Scholar]
- 255.Chaddock-Heyman L, et al. The effects of physical activity on functional MRI activation associated with cognitive control in children: a randomized controlled intervention. Front Hum Neurosci. 2013;7:72. doi: 10.3389/fnhum.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 257.Smith PJ, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kramer AF, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 259.Aberg MAI, et al. Cardiovascular fitness is associated with cognition in young adulthood. Proc Natl Acad Sci USA. 2009;106:20906–20911. doi: 10.1073/pnas.0905307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Prakash RS, et al. Cardiorespiratory fitness and attentional control in the aging brain. Front Hum Neurosci. 2011;4:229. doi: 10.3389/fnhum.2010.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rosano C, et al. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A: Biol Sci Med Sci. 2010;65:639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pontifex MB, et al. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J Cogn Neurosci. 2011;23:1332–1345. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- 263.Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Voss MW, et al. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Herting MM, Nagel BJ. Differences in brain activity during a verbal associative memory encoding task in high- and low-fit adolescents. J Cogn Neurosci. 2013;25:595–612. doi: 10.1162/jocn_a_00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 268.Lu H, et al. Rat brains also have a default mode network. Proc Natl Acad Sci USA. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Suchy Y. Executive functioning: overview, assessment, and research issues for non-neuropsychologists. Ann Behav Med. 2009;37:106–116. doi: 10.1007/s12160-009-9097-4. [DOI] [PubMed] [Google Scholar]
- 270.Caspersen CJ, et al. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 271.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Christie BR, et al. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Med. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- 274.Sporns O, et al. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Levine JA. Non-exercise activity thermogenesis (NEAT) Best Pract Res Clin Endocrinol Metab. 2002;16:679–702. doi: 10.1053/beem.2002.0227. [DOI] [PubMed] [Google Scholar]
- 276.Ernst C, et al. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31:84–92. [PMC free article] [PubMed] [Google Scholar]
- 277.Babyak M, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 278.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hanson ND, et al. Lithium, but not fluoxetine or the corticotropin-releasing factor receptor 1 receptor antagonist R121919, increases cell proliferation in the adult dentate gyrus. J Pharmacol Exp Ther. 2011;337:180–186. doi: 10.1124/jpet.110.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- 281.Wang YX, et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Narkar VA, et al. AMPK and PPARδ agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- 284.Kobilo T, et al. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.McOmish CE, Hannan AJ. Enviromimetics: exploring gene environment interactions to identify therapeutic targets for brain disorders. Expert Opin Ther Targets. 2007;11:899–913. doi: 10.1517/14728222.11.7.899. [DOI] [PubMed] [Google Scholar]
- 286.Massa SM, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Knowles JK, et al. A small molecule p75NTR ligand prevents cognitive deficits and neurite degeneration in an Alzheimer’s mouse model. Neurobiol Aging. 2013;34:2052–2063. doi: 10.1016/j.neurobiolaging.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tucker JM, et al. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40:454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 289.Owen N, et al. Understanding environmental influences on walking; review and research agenda. Am J Prev Med. 2004;27:67–76. doi: 10.1016/j.amepre.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 290.Hinkley T, et al. Correlates of preschool children’s physical activity. Am J Prev Med. 2012;43:159–167. doi: 10.1016/j.amepre.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 291.Corder K, et al. Parent awareness of young children’s physical activity. Prev Med. 2012;55:201–205. doi: 10.1016/j.ypmed.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Dlugonski D, et al. Meanings, motivations, and strategies for engaging in physical activity among women with multiple sclerosis. Disabil Rehabil. 2012;34:2148–2157. doi: 10.3109/09638288.2012.677935. [DOI] [PubMed] [Google Scholar]
- 293.Kalinowski S, et al. Physical activity in nursing homes–barriers and facilitators: a cross-sectional study. J Aging Phys Act. 2012;20:421–441. doi: 10.1123/japa.20.4.421. [DOI] [PubMed] [Google Scholar]
- 294.Thorpe O, et al. Barriers and enablers to physical activity participation in patients with COPD: a systematic review. J Cardiopulm Rehabil Prev. 2012;32:359–369. doi: 10.1097/HCR.0b013e318262d7df. [DOI] [PubMed] [Google Scholar]
- 295.McAuley E, et al. Self-regulatory processes and exercise adherence in older adults: executive function and self-efficacy effects. Am J Prev Med. 2011;41:284–290. doi: 10.1016/j.amepre.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mullen SP, et al. Physical activity and functional limitations in older adults: the influence of self-efficacy and functional performance. J Gerontol B: Psychol Sci Soc Sci. 2012;67:354–361. doi: 10.1093/geronb/gbs036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Etnier JL, et al. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 298.Nagamatsu LS, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172:666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liu-Ambrose T, et al. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33:1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 300.Reynolds G. New York Times. 2012 May 9 [Google Scholar]
- 301.Stroth S, et al. Impact of aerobic exercise training on cognitive functions and affect associated to the COMT polymorphism in young adults. Neurobiol Learn Mem. 2010;94:364–372. doi: 10.1016/j.nlm.2010.08.003. [DOI] [PubMed] [Google Scholar]


