Abstract
The progressive depletion of CD4 T cells underlies clinical progression to AIDS in untreated HIV-infected subjects. Most dying CD4 T cells correspond to resting nonpermissive cells residing in lymphoid tissues. Death is due to an innate immune response against the incomplete cytosolic viral DNA intermediates accumulating in these cells. The viral DNA is detected by the IFI16 sensor leading to inflammasome assembly, caspase 1 activation, and the induction of pyroptosis, a highly inflammatory form of programmed cell death. We now show that cell-to-cell transmission of HIV is obligatorily required for activation of this death pathway. Cell-free HIV-1 virions, even when added in large quantities, fail to activate pyroptosis. These findings underscore the infected CD4 T cells as the major killing units promoting progression to AIDS and highlight a previously unappreciated role for the virological synapse in HIV pathogenesis.
INTRODUCTION
The primary cause of AIDS in subjects is the progressive loss of CD4 T cells due to HIV infection (Thomas, 2009). The depletion of these cells has often been studied using cell-free virions infections of activated blood-derived CD4 T cells because of their ready availability and capacity to support productive viral infection (Cooper et al., 2013). However, the cytopathic response to HIV is not restricted to productively infected cells. Indeed, most dying CD4 T-cells in lymphoid tissues are resting cells that cannot support productive infection, and instead become abortively infected (Doitsh et al., 2010). We have used an ex vivo human lymphoid aggregate culture (HLAC) system formed with fresh human tonsil tissues to study CD4 T cell death during HIV infection (Glushakova et al., 1995). HLACs can be infected with a small number of viral particles in the absence of exogenous mitogens, allowing analysis of HIV-1 cytopathicity in a natural and preserved lymphoid microenvironment (Eckstein et al., 2001). Infection of HLACs with HIV-1 produces extensive loss of CD4 T cells — less than 5% of the cells die as a result of productive viral infection while >95% of them die as a consequence of abortive infection (Doitsh et al., 2010). Due to the nonpermissive nature of these quiescent cells, the viral lifecycle attenuates during chain elongation phase of reverse transcription, giving rise to incomplete transcripts of cytosolic viral DNA. These intermediates are sensed by interferon gamma inducible protein 16 (IFI16) (Monroe et al., 2014), which activates caspase 1 in inflammasomes leading in turn to pyroptosis, a highly inflammatory form of programmed cell death (Doitsh et al., 2014).
Retroviruses disseminate between susceptible cells either by cell-free infection or by direct cell-to-cell spread (Sattentau, 2010). The advantage of cell-to-cell spread on viral infectivity has been recognized for two decades (Jolly and Sattentau, 2004; Lehmann et al., 2011; Phillips, 1994; Sato et al., 1992; Sourisseau et al., 2007). For HIV-1, the infectivity of virus-producing cells, as measured in co-culture systems, is approximately 102 to 103 times higher than the infectivity of cell-free particles from the same infected cells (Jolly, 2011). However, in the context of pathogenesis, it was unclear whether transfer of HIV-1 through cell-to-cell contact triggers the same innate immune responses as cell-free particles in resting CD4 T cells, the predominant target cells depleted by HIV in lymphoid tissues.
RESULTS
The mode of HIV-1 transfer markedly affects the death response in target lymphoid CD4 T cells
Most studies examining innate immune recognition of HIV-1 have utilized cell-free particles and characterized responses occurring in dendritic cells or macrophages (Gao et al., 2013; Hayashi et al., 2010; Jakobsen et al., 2013; Lahaye et al., 2013; Manel et al., 2010; Sun et al., 2013; Yan et al., 2010). More recently, attention has focused on resting CD4 T cells in lymphoid tissue, which are mostly non-permissive for productive HIV infection. We previously have shown that the massive death of lymphoid CD4 T cells that are abortively infected with HIV-1 requires close interaction between uninfected target and HIV-producing cells (Doitsh et al., 2010). These findings were consistent with in vitro (Garg et al., 2007; Holm and Gabuzda, 2005) and in vivo (Finkel et al., 1995) studies showing that dying non-productively infected cells in human lymph nodes often cluster near productively infected cells (Finkel et al., 1995). In contrast, we found that cell-free virions accumulating in the supernatants of HIV-infected HLACs, even at high concentrations, were much less efficient at inducing killing of resting target cells by abortive infection. One potential explanation for these differences was that transfer of cell-free particles may not generate sufficient incomplete reverse DNA transcripts to induce a cytopathic response in target CD4 T cells. Cell-to-cell spread increases infection kinetics by two to three orders of magnitude by directing virus assembly and obviating the rate-limiting step of extracellular diffusion required for cell-free virus to find and engage a susceptible target cell (Jolly, 2011; Martin and Sattentau, 2009; Sato et al., 1992; Sourisseau et al., 2007).
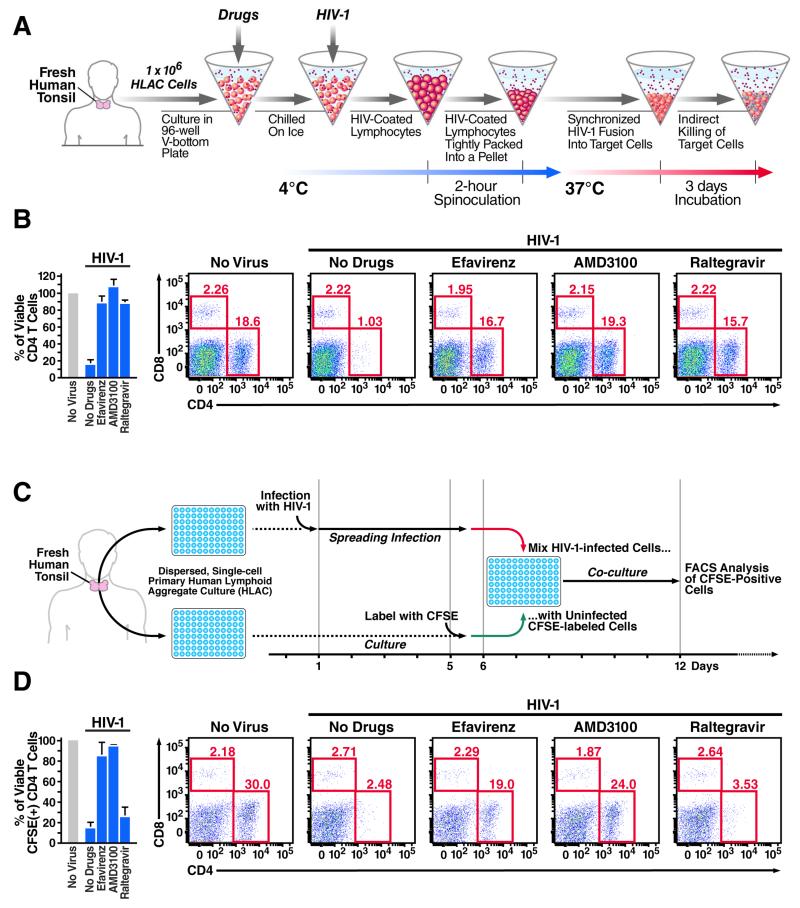
To test this hypothesis, we used spinoculation to emulate efficient cell-to-cell spread of virus (Geng et al., 2014). Spinoculation accelerates the binding of cell-free virions to target cells, facilitates synchronized delivery of a large number of particles into the cells (O’Doherty et al., 2000; Saphire et al., 2002), and enhances accumulation of cytoplasmic reverse DNA transcripts (Figure 1A) (O’Doherty et al., 2000; Pace et al., 2012). As expected, spinoculation of HLACs with free HIV-1 promoted high levels of HIV-1 fusion into target lymphoid CD4 T cells (Figure S1). Spinoculation also caused extensive and selective depletion of target CD4 T cells (Figure 1B). The relative proportion of CD8 T cells was unaltered. CD3+/CD8− T cells were similarly depleted, indicating that cell loss was not an artifact of down-regulated surface expression of CD4 following direct infection (not shown). Consistent with our previous reports (Doitsh et al., 2010; Doitsh et al., 2014; Monroe et al., 2014), loss of CD4 T cells was prevented by addition of efavirenz, an NNRTI that allosterically inhibits HIV-1 reverse transcriptase, and by AMD3100, an entry inhibitor that blocks gp120 engagement of the CXCR4 coreceptor. However, unexpectedly and not in keeping with our previous reports, addition of raltegravir, an integrase inhibitor also blocked CD4 T-cell death (Figure 1B and S5). Because cell death involves viral life cycle events occurring prior to viral integration, raltegravir should act too late to affect the abortive infection process that triggers the pyroptotic response.
Figure 1. The mode of HIV-1 transfer generates disparate death responses of target lymphoid CD4 T cells.
The death of lymphoid CD4 T cells was examined by spinoculation of target cells with large quantities of cell-free virions to target cells (A, B), as previously described (Doitsh et al., 2014; Geng et al., 2014; O’Doherty et al., 2000), or in co-cultures of CFSE-labeled target CD4 T with productively infected HLACs (Doitsh et al., 2010; Jekle et al., 2003) (C, D). All samples were infected with a multiple-round X4-tropic NL4-3 strain of HIV-1. NL4-3 was selected because tonsillar tissue contains a high percentage of resting CD4 T cells that express CXCR4 (90–100%). Target cells were treated with the same concentrations of drugs prior to co-culture with productively infected HLACs or spinoculation with free virions. Inhibitors blocking HIV entry (AMD3100) or early steps of reverse transcription (efavirenz) prevented death of target CD4 T cells. In sharp contrast, inhibiting later events in the viral life cycle (raltegravir) did not prevent cell death in co-cultures with productively infected cells (B), but abrogated the death response of target cells spinoculated with cell-free HIV-particles (D). Bar graph represents summary of flow data presented. Error bars represent SD/√n (SEM) of three independent donors. FACS plots are representative of six independent experiments performed with tonsils from different donors. See also Figure S1.
To further investigate this surprising result, CFSE-labeled target CD4 T cells were co-cultured with productively infected HLACs and raltegravir was added at the time of mixing of productively infected and target CD4 T cells (Figure 1C). Under these conditions, raltegravir had no effect on target CD4 T-cell death while efavirenz and AMD3100 blocked the response (Figure 1D).
Free HIV-1 particles do not induce cell death of target lymphoid CD4 T cells
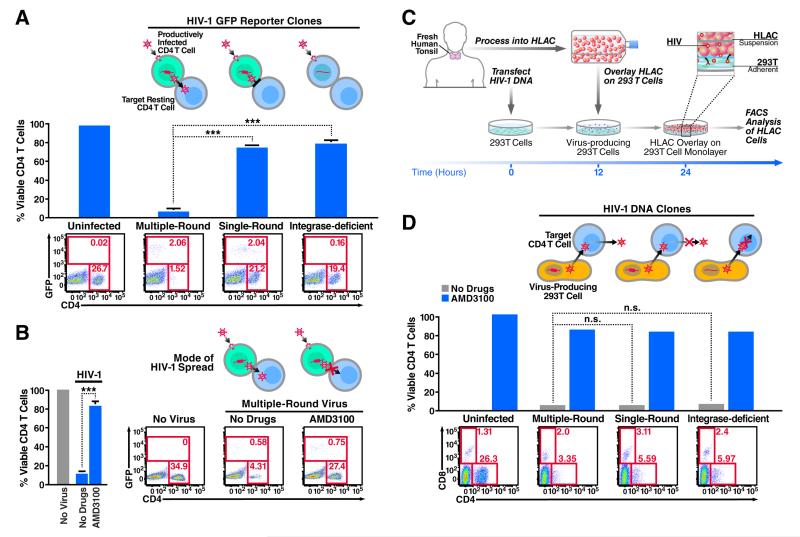
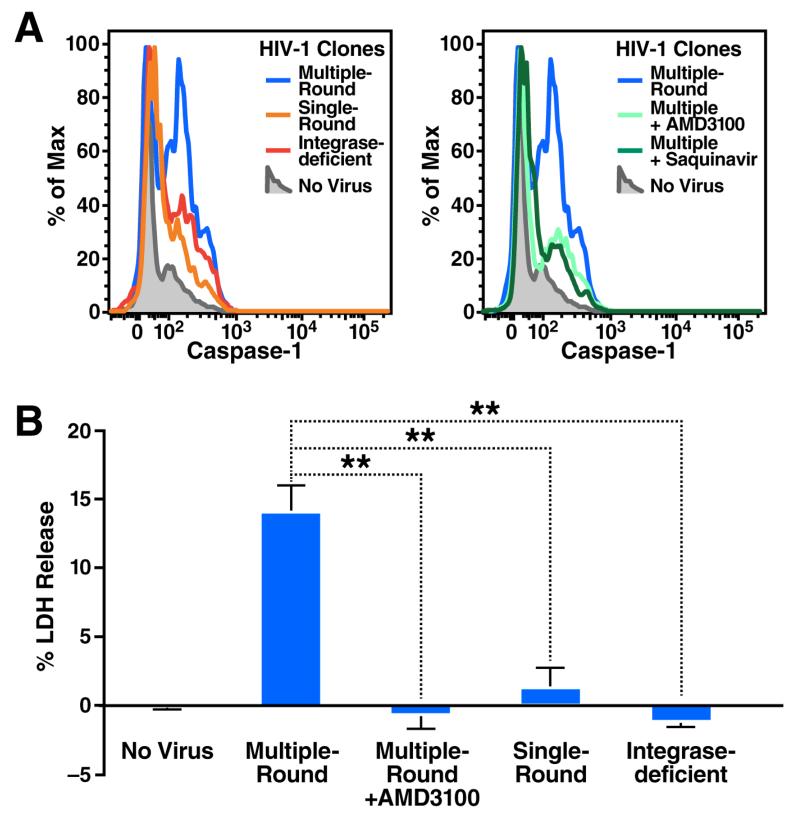
Based on these contrasting effects of raltegravir, we hypothesized that in the co-culture experiments involving mixing of HIV-infected HLACs with target cells, raltegravir had no effect on the ensuing death of target CD4 T cells that became abortively infected because the culture already contained productively infected cells. Conversely, in the spinoculation experiments, raltegravir blocked cell death because it prevented the establishment of a productively infected subset of cells needed for cell-to-cell spread the virus to target CD4 T cells. To test this hypothesis, we spinoculated HLACs with either single-round or multiple-round viruses containing a GFP reporter (NLENG1I) (Levy et al., 2004). These viruses permit the dynamics of HIV-1 infection and T-cell depletion to be simultaneously monitored in the spinoculated cultures. Four days after spinoculation, we observed a similar number of GFP-positive, productively infected cells with both the single-round and multiple round viruses, indicating that viral spread was not required to establish an initial population of productively infected cells. However, we observed a massive loss of CD4 T cells only in cultures spinoculated with the multiple-round virus. Notably, spinoculation with an integrase-deficient GFP HIV-1 (NLENG1I-D116N) (Gelderblom et al., 2008) resulted in no productive infection and no CD4 T-cell death (Figure 2A). These results suggested that viral spread from productively infected cells, but not spinoculation of cell-free virions, is promoting the death of non-permissive lymphoid CD4 T cells. In agreement with this conclusion, addition of the AMD3100 entry inhibitor four hours after spinoculation efficiently blocked the ensuing death response while not affecting the number of GFP-positive productively infected cells (Figure 2B). Moreover, treatment with the viral protease inhibitor saquinavir, which acts during the budding stage of HIV-1 replication, did not inhibit productive infection but prevented CD4 T-cell death by newly released HIV-1 virions (Figure S2). These findings further indicated that CD4 T-cell death occurs after establishment of productive infection, but not during infection with cell-free viruses.
Figure 2. Free HIV-1 particles do not induce cell death of abortively infected lymphoid CD4 T cells.
(A) HLACs were spinoculated with a multiple-round (NLENG1I), a single-round (NLENG1-ES-IRES, pseudotyped with HIV-1 Env), or integrase deficient (NLENG1I-D116N) viral clones containing a GFP reporter. An IRES upstream of the nef gene preserves Nef expression and supports LTR-driven GFP expression in productively infected target cells (Levy et al., 2004). No drugs were added to the spinoculated cultures. The levels of productive infection and CD4 T-cell depletion in the cultures were analyzed by flow cytometry four days after spinoculation. (B) Treatment with the entry inhibitor AMD3100 four hours after spinoculation with multiple-round NLENG1I HIV-1 particles does not prevent productive infection, but efficiently blocks the killing of target resting CD4 T cells. Thus, death of CD4 T cells occurs after establishment of productive infection, but not during initial spinoculation of cell-free viruses. Of note, spinoculation of supernatant from infected HLACs failed to induce death of target CD4 T cells (See Figure S3). (C) A method to assess death of CD4 T cells with non-infectious HIV-1 clones. The single-round and integrase-deficient HIV-1 clones are not competent for multiple rounds of viral replication. Instead, we modified the experimental system by overlaying HLAC cells on a monolayer of 293T cells transfected with these proviral clones, as previously described (Doitsh et al., 2014). As illustrated, fresh human tonsil is processed into HLAC and cells are cultured in suspension. After 12 hours, transfected 293T cells in a 24-well plate are washed and overlaid with 4×106 HLAC cells in RPMI. Virus-producing 293T cells directly interact with target overlaying HLAC cells. After 24–72 hours, HLAC suspensions were collected from wells and analyzed by flow cytometry. (D) Single-round and integrase-deficient HIV-1 clones kill target CD4 T cells as efficiently as multiple-round HIV-1 clones when transmitted via virus-producing cells. FACS plots are representative of three independent experiments performed with tonsils from different donors. Error bars, SEM; *** P < 0.001 (Student’s t test); n.s., not significant, P > 0.05. See also Figure S2.
Single-round and integrase-deficient HIV-1 clones are not competent for cell-to-cell dissemination following spinoculation with HLACs. To confirm that the mode of viral transfer influenced the death response of target CD4 T cells, we modified the infection system by overlaying HLACs on a monolayer of 293T cells that had been transfected with these single-round proviral clones (Figure 2C). Interestingly, when these single-round viruses were transferred to HLACs by direct interaction with virus-producing 293T cells, a massive killing of target lymphoid CD4 T cells was observed (Figure 2D). These results demonstrate that recapitulating the cell-to-cell mode of viral transfer is sufficient to restore the killing capacity of these single-round clones.
The death response involves cell adhesion molecules required for virological synapse formation
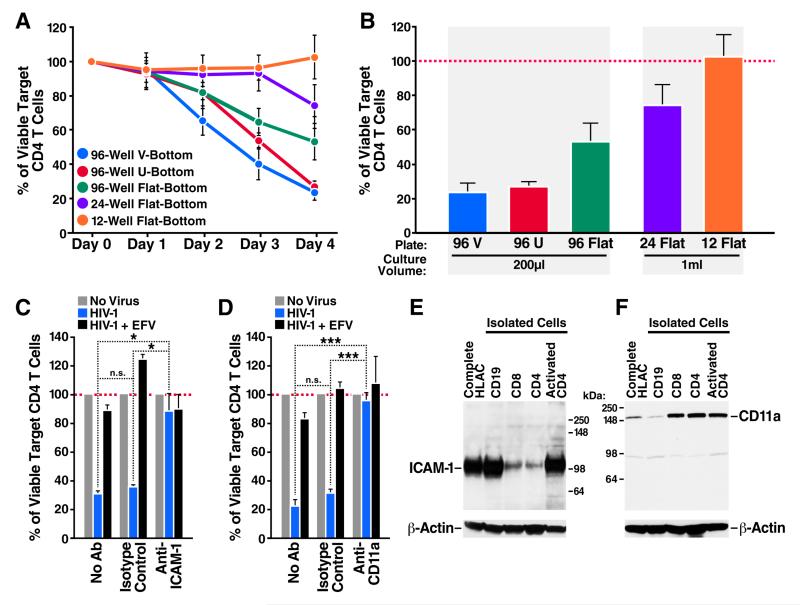
To further explore whether cell-cell contact was needed to induce death of CD4 T cells, we repeated the co-culture assay using productively infected and target CFSE-labeled HLACs. However, in this experiment the cells were co-cultured under conditions of increasing surface area thereby reducing the likelihood of cell-cell interactions. Using flow cytometry, we analyzed the levels of viable target CD4 T cells in the plates every 24 hours during four days of co-culture. The death of target CD4 T cells decreased as the surface area of the culture increased (Figure 3A), even in samples where the volume of culture medium remained constant (Figure 3B). These data suggest that the physical distance between HIV-producing and target cells directly affects the kinetics of CD4 T-cell depletion, and argue further against a role for free virions released into the medium in the death response.
Figure 3. Death of lymphoid CD4 T cells requires close interaction with productively infected cells.
(A) Increasing the cell culture surface area in HIV-infected cultures decreases cell-to-cell interactions, and reduces the kinetics of CD4 T-cell depletion. (B) Inverse correlation between culture surface area and CD4 T-cell death. Death of target CD4 T cells in each vessel was examined after four days of co-culturing with HIV-infected cells. Note that cell death decreases even in vessels where the volume of culture medium remained constant. (C, D) Blocking antibodies against either ICAM-1 or CD11a prevent death of target CD4 T cells (E) Exclusive expression of ICAM-1 on activated (permissive) lymphoid CD4 T cells. ICAM-1 expression is also high in antigen presenting B cells, but not CD8 T cells. (F) High expression of LFA-1 on lymphoid CD4 and CD8 T cells, but not on B cells. In contrast to ICAM-1, LFA-1 expression is not increased on activated CD4 T cells. Error bars, SEM; *P < 0.05, ***P < 0.001 (Student’s t test). EFV, Efavirenz. See also Figures S3, S4, and S5.
Cell-to-cell spread of HIV-1 predominantly takes place across specialized contact-induced structures known as virological synapses (Agosto et al., 2015; Jolly et al., 2004; Jolly et al., 2007; Jolly and Sattentau, 2004). These synapses facilitate efficient transmission of virus toward the uninfected and engaged target cell. The synapse gains stability through a rapid actin-mediated recruitment of adhesion molecules, such as the integrin leukocyte function-association antigen 1 (LFA-1) and its cognate ligand ICAM-1 to the junction point of cellular interaction (Jolly et al., 2007). To examine whether virological synapse formation between HIV-infected and target cells is required to promote CD4 T-cell death, productively infected and target CFSE-labeled HLACs were co-cultured in the presence of blocking antibodies against ICAM-1 or CD11a, the α-subunit of the LFA-1 heterodimer. Addition of either the anti-ICAM-1 (Figure 3C) or anti-CD11a (Figure 3D) antibodies, but not isotype matched control antibodies, effectively blocked depletion of target CD4 T cells in the mixed cultures as efficiently as the antiviral drug efavirenz. These findings suggest that the death response involves adhesion molecules that are required for virological synapse formation, indicative of a requirement for close cell-cell contact in mediating pyroptotic cell death. Because cell-free virions also express LFA-1 and ICAM-1 (Bounou et al., 2002; Fortin et al., 1997), we cannot completely rule out an additional effect on HIV virions. However, when combined with all of the data (Figure S2), we conclude that cell-to-cell transmission is critical for the induction of pyroptosis.
Western blotting analysis of HLAC revealed high expression levels of ICAM-1 in B cells, but not in CD4 or CD8 T lymphocytes. However, activated CD4 T cells, which correspond to those that become productively infected with HIV-1, express high levels of this adhesion molecule (Figure 3E). In contrast to ICAM-1, CD11a expression levels were high in both resting and activated CD4 T cells (Figure 3F). Thus, synapse formation between activated CD4 T cells expressing ICAM-1 and target CD4 T cells (either activated or resting) expressing LFA-1 may occur regularly in lymphoid tissues.
Caspase 1 activation in abortively infected cells requires cell-to-cell spread of HIV-1
Most CD4 T cells in lymphoid tissues infected with HIV die by caspase 1-mediated pyroptosis triggered by abortive viral infection (Doitsh et al., 2014). To test whether caspase 1 is induced by cell-free HIV-1 particles or by cell-to-cell spread of HIV-1, we spinoculated HLACs with single-round or multiple-round clones of a DsRedExpress reporter virus (NLRX-IRES) (Gelderblom et al., 2008), and analyzed intracellular caspase 1 activity using cell permeable fluorogenic caspase 1 specific substrates (CaspaLux1) (Komoriya et al., 2000; Packard and Komoriya, 2008). Consistent with our previous reports (Doitsh et al., 2014; Monroe et al., 2014), spinoculation with multiple-round HIV-1 particles triggered high levels of intracellular caspase 1 activity in target CD4 T cells. In contrast, spinoculation with single-round or integrase-deficient HIV-1 particles produced only background levels of caspase 1 activity (Figure 4A). Inhibition of cell-to-cell spread using the viral protease inhibitor saquinavir, or by treatment with AMD3100 four hours after spinoculation, also markedly inhibited caspase 1 activation induced by multiple-round HIV-1 particles.
Figure 4. Cell-to-Cell transmission of HIV-1 is required to trigger innate recognition and caspase 1-dependent pyroptosis of lymphoid CD4 T Cells.
(A) HLACs were spinoculated with multiple- or single-round NLRX-IRES reporter clones as indicated. Saquinavir was added to the culture before spinoculation. AMD3100 was added to the culture four hours after spinoculation. Cells were analyzed by flow cytometry using cell permeable fluorogenic substrates that contain amino acids sequences specifically cleaved by active caspase 1 (CaspaLux1). Abundant caspase 1 activity is exclusively observed in cultures spinoculated with multiple-round HIV-1 clones. Essentially no caspase 1 activity is observed in target CD4 T cells where cell-to-cell spread of HIV-1 is blocked or does not occur. Histograms show one experiment, a representative of three independent experiments performed with tonsils from different donors (B) Supernatants from spinoculated cultures were analyzed for levels of released cytoplasmic LDH enzyme, an indicator of pyroptosis (Decker and Lohmann-Matthes, 1988) FACS plots are representative of three independent experiments performed with tonsils from different donors. Error bars, SEM; **P < 0.01 (Student’s t test). See also Figure S5.
Consistent with pyroptosis as the pathway of programmed cell death, spinoculation with multiple-round HIV particles resulted in the release of the intracellular enzyme lactate dehydrogenase (LDH) (Fink and Cookson, 2005) (Figure 4B). Further, the release of LDH was completely blocked when AMD3100 was added four hours after spinoculation or when single-round or integrase-deficient HIV-1 particles were used for initial infection. Together, these findings indicate that infection with cell-free HIV-1 particles does not lead to caspase 1 activation despite apparent abortive infection of lymphoid CD4 T cells. Rather, capsase-1 activation and the induction of pyroptosis require the generation of productively infected cells and successful cell-to-cell spread of HIV-1 to quiescent bystander lymphoid CD4 T cells.
DISCUSSION
The life cycle of HIV-1 involves the release of particles into the extracellular space, followed by spread to distant susceptible cellular hosts. HIV-1 can also spread directly from productively infected cells to neighboring cells through virological synapses, a process that is 102 to 103 fold more efficient than infection with cell free virions. Despite the high efficiency of this mode of viral transmission, most HIV-1 pathogenesis research has involved the study of cell-free viruses (Cummins and Badley, 2010; Fevrier et al., 2011) in part because highly permissive cells, such as activated peripheral blood lymphocytes, have been used as cellular targets. These cells are fully permissive to HIV infection and give rise to new virions but then die primarily by caspase 3-mediated apoptosis (Cooper et al., 2013; Gougeon et al., 1996). However, in human lymphoid tissues such as tonsil and spleen, activated and permissive cells represent only 5% of the total CD4 T cell population. Far more commonly, HIV enters resting non-permissive cells that represent >95% of the CD4 T cell population (Doitsh et al., 2010; Eckstein et al., 2001; Moore et al., 2004). These non-permissive cells undergo abortive infection and ultimately die due to an innate immune response launched by the host against cytosolic viral DNA culminating in caspase 1-dependent pyroptosis, a highly inflammatory form of programmed cell death (Doitsh et al., 2014; Monroe et al., 2014).
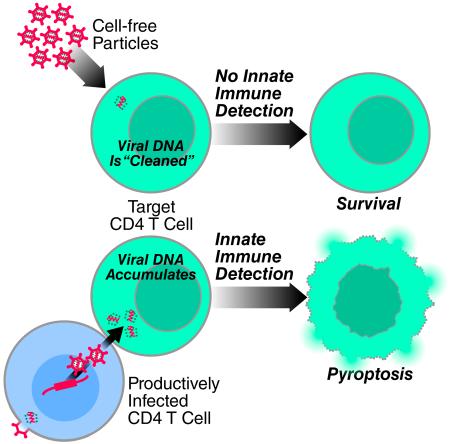
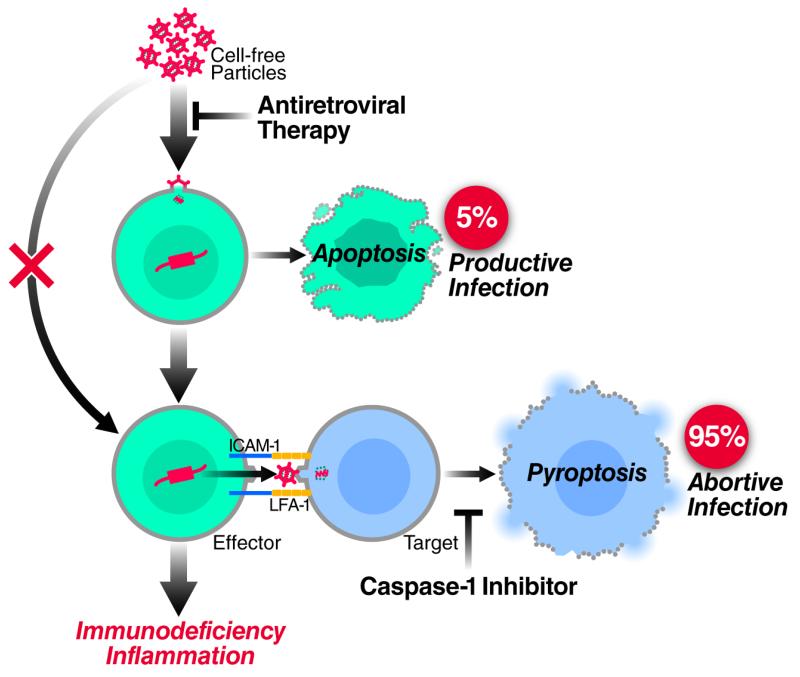
Here, we explored the death of lymphoid CD4 T cells in HLACs using experimental strategies that unambiguously distinguish between cell-free and cell-to-cell modes of HIV-1 transmission. Using this system we now demonstrate that the mode of HIV-1 spread determines the outcome form of cell death. Specifically, cell-to-cell spread of HIV-1 is required to deplete non-permissive lymphoid CD4 T cells via caspase 1-dependent pyroptosis. Free HIV-1 particles, even when added in large quantities, are unable to trigger innate immune recognition leading to pyroptosis. Conversely, infection with free HIV-1 particles does cause a small fraction of permissive cells in HLACs to become productively infected. It is these cells that mediate cell-to-cell spread culminating in the pyroptotic death on nonpermissive CD4 T cells. These findings suggest a radical change in the prevailing view of HIV pathogenesis where most of the pathogenic effects of HIV-1 are attributed to killing of CD4 T cells by circulating free virions. We propose that the fundamental “killing units” of CD4 T cells leading to CD4 T-cell depletion and ultimately progression to AIDS are predominantly infected cells residing in lymphoid tissues that mediate cell-to-cell spread of the virus. Productive (“direct”) and abortive (“bystander”) infections are often viewed as independent pathways underlying the progressive depletion of CD4 T cells (Cooper et al., 2013; Doitsh et al., 2010; Doitsh et al., 2014). Our findings now show that productive and abortive infections are not independent cytopathic events, but rather are linked in a single pathogenic cascade (Figure 5). Productively infected cells are obligatorily required to transmit the virus across the virological synapse formed with resting CD4 T cells. The productively infected cell ultimately dies by apoptosis, while the bystander resting cell dies by pyroptosis.
Figure 5. The mode of HIV-1 spread determines the outcome form of cell death.
Infection of free HIV-1 particles produces productive infection and caspase 3-apoptosis in a small fraction of permissive lymphoid CD4 T cells. Next, cell-to-cell spread of HIV-1 is required to deplete the non-permissive lymphoid CD4 T cells, which represent 95% or more of the target cells in lymphoid tissues, via caspase 1-dependent pyroptosis. Free HIV-1 particles, even at high quantities, cannot trigger innate immune recognition and produce this form of cell death. Thus, in contrast to the previous view indicating productive and abortive HIV-1 infection as independent cytopathic events causing CD4 T-cell death (Cummins and Badley, 2010; Doitsh et al., 2010; Fevrier et al., 2011; Garg et al., 2012), these events are essentially linked in a single pathogenic cascade. Therefore, antiretroviral drugs such as AZT or raltergravir that do not directly prevent the death of abortively infected CD4 T cells (Doitsh et al., 2010), effectively inhibit HIV pathogenesis in vivo by blocking upstream events of productive HIV-1 infection. Caspase 1 inhibitors do not inhibit productive HIV-1 infection but block pyroptosis of abortively infected CD4 T cells (Doitsh et al., 2014). See also Supplemental Experimental Procedures.
The interaction of the cognate adhesion molecules ICAM-1 and LFA-1 at the virological synapse is critically important for efficient HIV-1 spread between permissive effector and target CD4 T cells. Our findings in HLACs demonstrate the role of the virological synapse in viral infection and depletion of non-permissive CD4 T-cell targets. Human lymphoid tissues predominantly consist of non-permissive CD4 T cells (Doitsh et al., 2010; Doitsh et al., 2014; Eckstein et al., 2001; Glushakova et al., 1995). Therefore, the interaction and formation of virological synapses between productively infected cells expressing ICAM-1 and non-permissive targets expressing LFA-1 likely occur at high frequency in the T cell zone found in HIV-infected lymphoid tissues and centrally contribute to the immunopathogenic effects of HIV-1. The interaction of LFA-1 on T cells with ICAM-1 also mediates the arrest and migration of T cells on surfaces of postcapillary venules at sites of infection or injury, as well as the ability of these cells to crawl out of the blood stream between high endothelial venules and into lymph nodes (Girard et al., 2012). Importantly, interleukin (IL)-1β increases the expression of adhesion molecules such as ICAM-1 on endothelial cells (Dinarello, 2009; Dustin et al., 2011; Hubbard and Rothlein, 2000). The release of IL-1β by dying pyroptotic CD4 T cells in HIV-infected lymphoid tissues likely attracts more cells from the blood into the infected lymph nodes to die and produce more inflammation. Thus, the interaction of LFA-1 with ICAM-1 contributes to a pathogenic cycle occurring during HIV infection by both promoting the depletion of CD4 T cells and facilitating a state of chronic inflammation, two key processes that propel clinical progression of disease ultimately culminating in AIDS (Deeks, 2011).
The molecular mechanisms that limit pyroptosis to virus transmission occurring via the cell-to-cell route are unknown. One possibility relates to TREX1, a cellular 3′ DNA exonuclease, and SLX4-associated MUS81-EME1 endonucleases that function as “cytoplasmic cleaners” that degrade single- and double-stranded DNA, respectively (Laguette et al., 2014; Stetson et al., 2008). Indeed, the intrinsic action of the TREX1 and SLX4-associated endonucleases in the cytoplasm may set a threshold level for reverse-transcribed DNA products needed for either productive infection in permissive cells, or, alternatively, pyroptosis in abortively infected non-permissive cells (Laguette et al., 2014; Yan et al., 2010). Cell-to-cell spread across the virological synapse may overcome TREX1/SLX4-mediated restriction by rapidly transferring large quantities of viral nucleic acid to the opposing target cell. Ironically, while this mechanism likely evolved for efficient viral spread between permissive cells, it acts against HIV-1 in non-permissive targets where it triggers abortive infection and pyroptosis that drives inflammation and disease in the host.
EXPERIMENTAL PROCEDURES
Preparation of HIV-1 Virions
Replication-competent HIV-1 virions were produced using the X4-tropic reporter NL4-3 clones of HIV-1. For replication-competent reporter viruses we use the NLENG1-IRES (NLENG1I), and a DsRedExpress reporter NLRX-IRES (NLRXI). All virus stocks were quantitated by measuring p24gag levels by Elisa (Perkin Elmer, Cat # NEK050B001KT). Please see our Supplemental Experimental Procedures for a detailed experimental protocol.
FACS Analysis and Gating Strategy
Cell death was determined in co-culture systems by gating on live cells with forward and side scatter, followed by gating and counting of CD4 and CD8 T cells. The survival percentage of CD4 T cells was calculated by dividing the number of CD4 T cells by that of CD8 T cells. All conditions were normalized to uninfected conditions. For a detailed description to our method of measuring and calculating the levels of CD4 T-cell death in HIV-infected HLACs see Figure S4. Viable cells were also detected using Zombie Red (BioLegend, Cat # 423105), a fluorescent dye that is non-permeant to live cells, but permeant to the cells with compromised membranes. The results showed a close concordance to the experimental approach using gating on forward scatter versus side scatter (Figure S5). Please see our Supplemental Experimental Procedures for a complete experimental description.
Supplementary Material
Highlights.
The mode of HIV-1 spread determines the outcome form of cell death
Cell-to-cell spread is required to deplete non-permissive CD4 T cells via pyroptosis
Free HIV-1 particles, even in large quantities, are unable to trigger pyroptosis
The fundamental “killing units” of CD4 T cells are infected cells, not the virus.
ACKNOWLEDGMENTS
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: AMD3100, Efavirenz, and Raltegravir. We thank Jason Neidleman for assistance with HIV-1–based virion fusion assays, stimulating discussions and technical advice. We thank Dr. Marielle Cavrois, Marianne Gesner and Mekhala Maiti for assistance with flow cytometry. We also thank Gary Howard, Crystal Herron, Celeste Brennecka and Anna Lisa Lucido for editorial assistance, John C.W. Carroll, Giovanni Maki, and Teresa Roberts for graphic arts, and Robin Givens and Sue Cammack for administrative assistance. This work was supported by NIH/NIAID grants R21 AI102782, DP1 DA036502, and U19 AI096113 (W.C.G), the UCSF/Robert John Sabo Trust Award (Doitsh), and A.P. Giannini Foundation Postdoctoral Research Fellowship (Monroe). We also acknowledge support from NIH P30 AI027763 (UCSF-GIVI Center for AIDS Research) to Dr. Doitsh, Dr. Yang, and for the Gladstone Flow Cytometry Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
N.LK.G. performed most of the studies and participated in writing the manuscript; G.D. identified the absolute requirement for cell-to-cell transmission in promoting pyroptosis of lymphoid CD4 T cells abortively infected with HIV-1, developed and designed most of the studies, collected the data, and wrote the manuscript; K.M.M. examined the effect of ICAM-1 and LFA-1 antibodies on CD4 T-cell death; Z.Y. analyzed expression of cellular ICAM-1 and LFA-1; I.M-A. explored the effect of integrase inhibitors on CD4 T-cell depletion during spinoculation, provided reagents and tissues; D.N.L. developed and provided the reporter HIV-1 clones used in this study, W.C.G supervised the studies and participated in the preparation of the final manuscript.
The authors have no conflicting financial interests.
REFERENCES
- Agosto LM, Uchil PD, Mothes W. HIV cell-to-cell transmission: effects on pathogenesis and antiretroviral therapy. Trends in microbiology. 2015;23:289–295. doi: 10.1016/j.tim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounou S, Leclerc JE, Tremblay MJ. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J Virol. 2002;76:1004–1014. doi: 10.1128/JVI.76.3.1004-1014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Garcia M, Petrovas C, Yamamoto T, Koup RA, Nabel GJ. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature. 2013 doi: 10.1038/nature12274. [DOI] [PubMed] [Google Scholar]
- Cummins NW, Badley AD. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell death & disease. 2010;1:e99. doi: 10.1038/cddis.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. Journal of immunological methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annual review of medicine. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV Infection Mediates CD4 T Cell Depletion and Inflammation in Human Lymphoid Tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J. Immunol. 2011;137:245–254. 1986. [PubMed] [Google Scholar]; J Immunol. 186:5024–5033. [Google Scholar]
- Eckstein DA, Penn ML, Korin YD, Scripture-Adams DD, Zack JA, Kreisberg JF, Roederer M, Sherman MP, Chin PS, Goldsmith MA. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity. 2001;15:671–682. doi: 10.1016/s1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- Fevrier M, Dorgham K, Rebollo A. CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses. 2011;3:586–612. doi: 10.3390/v3050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- Fortin JF, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg H, Joshi A, Freed EO, Blumenthal R. Site-specific mutations in HIV-1 gp41 reveal a correlation between HIV-1-mediated bystander apoptosis and fusion/hemifusion. J Biol Chem. 2007;282:16899–16906. doi: 10.1074/jbc.M701701200. [DOI] [PubMed] [Google Scholar]
- Garg H, Mohl J, Joshi A. HIV-1 induced bystander apoptosis. Viruses. 2012;4:3020–3043. doi: 10.3390/v4113020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom HC, Vatakis DN, Burke SA, Lawrie SD, Bristol GC, Levy DN. Viral complementation allows HIV-1 replication without integration. Retrovirology. 2008;5:60. doi: 10.1186/1742-4690-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Doitsh G, Yang Z, Galloway NL, Greene WC. Efficient delivery of lentiviral vectors into resting human CD4 T cells. Gene therapy. 2014 doi: 10.1038/gt.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nature reviews Immunology. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- Hayashi T, Nishitsuji H, Takamori A, Hasegawa A, Masuda T, Kannagi M. DNA-dependent activator of IFN-regulatory factors enhances the transcription of HIV-1 through NF-kappaB. Microbes and infection / Institut Pasteur. 2010;12:937–947. doi: 10.1016/j.micinf.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Holm GH, Gabuzda D. Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions. J Virol. 2005;79:6299–6311. doi: 10.1128/JVI.79.10.6299-6311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free radical biology & medicine. 2000;28:1379–1386. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- Jakobsen MR, Bak RO, Andersen A, Berg RK, Jensen SB, Tengchuan J, Laustsen A, Hansen K, Ostergaard L, Fitzgerald KA, et al. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci U S A. 2013;110:E4571–4580. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekle A, Keppler OT, De Clercq E, Schols D, Weinstein M, Goldsmith MA. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol. 2003;77:5846–5854. doi: 10.1128/JVI.77.10.5846-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C. Cell-to-cell transmission of retroviruses: Innate immunity and interferon-induced restriction factors. Virology. 2011;411:251–259. doi: 10.1016/j.virol.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Mitar I, Sattentau QJ. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol. 2007;81:13916–13921. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Sattentau QJ. Retroviral spread by induction of virological synapses. Traffic. 2004;5:643–650. doi: 10.1111/j.1600-0854.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- Komoriya A, Packard BZ, Brown MJ, Wu ML, Henkart PA. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates. J Exp Med. 2000;191:1819–1828. doi: 10.1084/jem.191.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguette N, Bregnard C, Hue P, Basbous J, Yatim A, Larroque M, Kirchhoff F, Constantinou A, Sobhian B, Benkirane M. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell. 2014;156:134–145. doi: 10.1016/j.cell.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, El Marjou A, Lacabaratz C, Lelievre JD, Manel N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity. 2013;39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Nikolic DS, Piguet V. How HIV-1 takes advantage of the cytoskeleton during replication and cell-to-cell transmission. Viruses. 2011;3:1757–1776. doi: 10.3390/v3091757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DN, Aldrovandi GM, Kutsch O, Shaw GM. Dynamics of HIV-1 recombination in its natural target cells. Proc Natl Acad Sci U S A. 2004;101:4204–4209. doi: 10.1073/pnas.0306764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Sattentau Q. Cell-to-cell HIV-1 spread and its implications for immune evasion. Curr Opin HIV AIDS. 2009;4:143–149. doi: 10.1097/COH.0b013e328322f94a. [DOI] [PubMed] [Google Scholar]
- Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors--central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS research and human retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace MJ, Graf EH, Agosto LM, Mexas AM, Male F, Brady T, Bushman FD, O’Doherty U. Directly infected resting CD4+T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog. 2012;8:e1002818. doi: 10.1371/journal.ppat.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard BZ, Komoriya A. A method in enzymology for measuring hydrolytic activities in live cell environments. Methods in enzymology. 2008;450:1–19. doi: 10.1016/S0076-6879(08)03401-0. [DOI] [PubMed] [Google Scholar]
- Phillips DM. The role of cell-to-cell transmission in HIV infection. AIDS. 1994;8:719–731. doi: 10.1097/00002030-199406000-00001. [DOI] [PubMed] [Google Scholar]
- Saphire AC, Bobardt MD, Gallay PA. Cyclophilin a plays distinct roles in human immunodeficiency virus type 1 entry and postentry events, as revealed by spinoculation. J Virol. 2002;76:4671–4677. doi: 10.1128/JVI.76.9.4671-4677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Orenstein J, Dimitrov D, Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992;186:712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Sattentau QJ. Cell-to-Cell Spread of Retroviruses. Viruses. 2010;2:1306–1321. doi: 10.3390/v2061306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007;81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. Roadblocks in HIV research: five questions. Nat Med. 2009;15:855–859. doi: 10.1038/nm0809-855. [DOI] [PubMed] [Google Scholar]
- Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11:1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.