Abstract
Background and Aims
A broadly mandated reduction of the nicotine content (RNC) of cigarettes has been proposed in the USA to reduce the addictiveness of cigarettes, to prevent new smokers from becoming addicted and to facilitate quitting in established smokers. The primary aim of this study was to determine whether following 7 months of smoking very low nicotine content cigarettes (VLNC), and then returning to their own cigarettes, smokers would demonstrate persistently reduced nicotine intake compared with baseline or quit smoking.
Methods
In a community-based clinic 135 smokers not interested in quitting were randomized to one of two groups. A research group smoked their usual brand of cigarettes followed by 5 types of research cigarettes with progressively lower nicotine content, each for one month, followed by 6 months at the lowest nicotine level (0.5 mg/cigarette) (53 subjects) and then 12 months with no intervention (30 subjects completed). A control group smoked their usual brand for the same period of time (50 subjects at 6 months, 38 completed). Smoking behavior, biomarkers of nicotine intake and smoke toxicant exposure were measured.
Results
After 7 months smoking VLNC, nicotine intake remained below baseline (plasma cotinine 149 vs 250 ng/ml, p<0.005) with no significant change in cigarettes per day or expired CO. During the 12 months follow-up, cotinine levels in RNC smokers rose to baseline levels and to those of control smokers. Quit rates among RNC smokers were very low (7.5% vs 3 % in controls, N.S.).
Conclusions
In smokers not interested in quitting, reducing the nicotine content in cigarettes over 12 months does not appear to result in extinction of nicotine dependence, assessed by persistently reduced nicotine intake or quitting smoking over the subsequent 12 months.
Introduction
In 1994 Benowitz and Henningfield proposed the idea of federal regulation of the nicotine content of cigarettes such that it would be reduced over time.(1) When nicotine levels get very low, cigarettes would be much less addictive. As a result, fewer young people would become addicted adult smokers; and currently addicted smokers would find it easier to quit smoking. The regulatory authority to promulgate such a public health intervention was provided by the Family Smoking Prevention and Tobacco Control Act, passed in 2009.(2) Although it precludes “reducing nicotine to zero”, it permits the U.S. Food and Drug Administration (FDA) to set standards for cigarette nicotine content that would prevent them from causing addiction.
We previously reported the first 6 months of a two-year study of smoking behavior and tobacco smoke toxicant exposure with the progressive reduction of nicotine content in cigarette tobacco.(3) The focus of that paper was nicotine intake and biomarkers of tobacco toxicant exposure after 6 months of progressive tapering, compared to a control group who smoked their usual brand throughout the study. Those randomized to reduced nicotine content (RNC) cigarettes, on average decreased their daily nicotine intake by 70%, without significant changes in the number of cigarettes smoked per day or in tobacco toxicant exposure biomarkers.
The present analysis focuses on the last eighteen months of that study, during which RNC smokers smoked the lowest nicotine content cigarette for 6 months, followed by a year without provision of cigarettes. We examined the hypotheses that 1) once their daily intake of nicotine had been lowered following tapering (over 6 months), the level of nicotine dependence of smokers of RNC would be decreased such that they would be satisfied to continue smoking very low nicotine content cigarettes without difficulty (for an additional 6 months) and 2) once they stopped smoking RNC cigarettes, their intake of nicotine from conventional cigarettes would remain below their baseline or they would quit smoking because they had become less nicotine dependent.
Methods
Subjects
Subjects were recruited by newspaper advertisements, radio advertisements and flyers looking for smokers interested in a reduced nicotine cigarette study, and not interested in quitting smoking in the next six months. Inclusion criteria included being between 18 and 70 years old, healthy, smoking 10 or more cigarettes per day for the past year and having screening expired carbon monoxide levels of 25 ppm or saliva cotinine levels of 100 ng/ml or more. Exclusion criteria included pregnancy or lactation, psychiatric conditions such as current major depression or a history of schizophrenia, current use of smokeless tobacco, pipes or cigars, and alcohol or drug dependence.
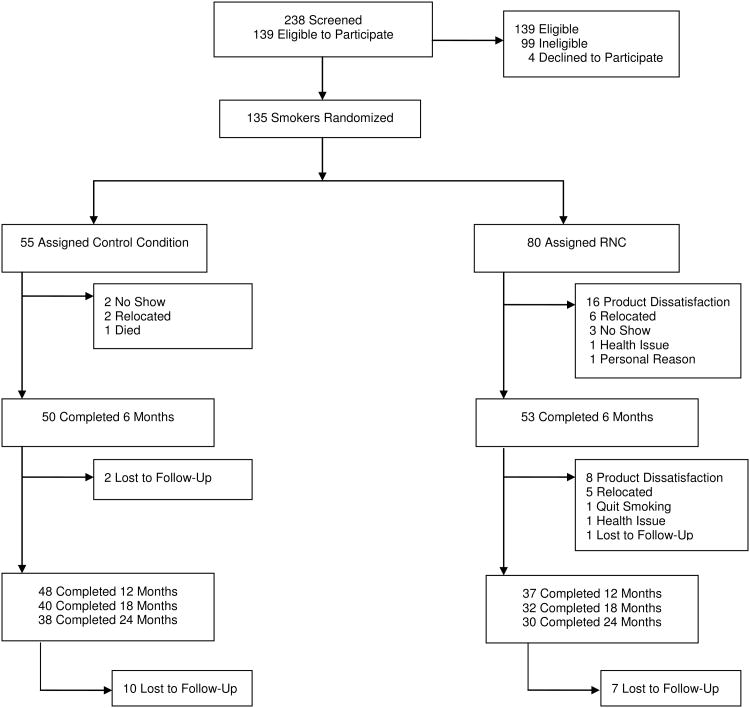
238 subjects were screened for study participation. The participant flow diagram is provided as Figure 1. Of those screened, 139 subjects met inclusion criteria and completed the baseline assessment, and 135 subjects were randomized to RNC or control groups in blocks of 10 subjects. The tapering phase of the study was completed by a 53 RNC subjects and 50 control subjects.
Fig. 1.
Participant flow diagram.
The non-completing participants in months six to twenty-four, the time interval which is the current focus, are described in the results and Figure 1. Eight RNC subjects dropped out while smoking the lowest nicotine content cigarettes due to product dissatisfaction. A total of 38 subjects in the control group and 30 subjects in the RNC group completed the twenty-four months of the study.
Study Protocol
This was a two-year, two-arm, randomized, unblinded study in which all subjects smoked their usual brand of cigarette for a baseline period of two weeks, and then were randomly assigned to a research or control arm. The research (RNC) group smoked five levels of progressively lower nicotine content cigarettes, the first four levels being smoked for four weeks each. The lowest nicotine content cigarette was smoked for 7 months. The control group smoked their usual brand of cigarettes for twelve months. Thereafter, all subjects were followed for an additional year after returning to smoking cigarettes of their own choosing (or quitting). Cigarettes were provided at no cost for the first 12 months for both RNC and control subjects. No cigarettes were provided during the 12 months of follow-up. If subjects expressed interest in quitting or had quit smoking between visits, they were given the Clearing the Air and the American Cancer Society Smart Move stop smoking manuals.
Subjects were studied in a community-based research clinic. Visits were scheduled bi-weekly for the first six months, monthly for the next six months, and then at fifteen, eighteen and twenty-four months. Subjects were instructed to smoke their cigarettes as desired, but when smoking the research cigarettes not to smoke any other type of cigarette or use other forms of tobacco or nicotine medications. RNC subjects were encouraged to report non-study cigarette use to the research staff, without penalty. At each visit expired carbon monoxide, body weight and blood pressure were measured; blood and urine samples were collected; and questionnaires were administered. Subjects were paid for participation. Written informed consent was obtained from each subject. The study was approved by the Institutional Review Board at the University of California San Francisco.
Cigarettes
Philip Morris Tobacco Company manufactured the RNCs by blending very low nicotine tobacco with tobacco containing higher amounts of nicotine. The paper and filters and weight of tobacco in the research cigarettes were similar to a Marlboro cigarette. The nicotine content per cigarette was targeted to be 12, 8, 4, 2 and 1 mg, to allow for a 50% nicotine reduction in nicotine dose at each step between 8 and 1 mg. These five levels were selected so at the end of tapering, the maximum systemic nicotine intake would be expected to be 0.2 mg per cigarette or less, based on bioavailability calculations that have been described previously.(3) The actual nicotine contents of the cigarettes, measured in our laboratory, were 10.3, 6.5, 3.9, 1.7 and 0.5 mg. The lowest level of nicotine availability was based on an estimate of the threshold level of nicotine to maintain nicotine addiction. Characteristics of research cigarettes have been published previously.(3) The research cigarettes were packaged in plain packs. Subjects were told that the research cigarettes would contain different levels of nicotine than their usual brand. Menthol-flavored RNCs were not available, so subjects who typically smoked menthol cigarettes switched to non-menthol cigarettes.
Questionnaires
Questionnaires administered at each visit included a report of smoking behavior over the previous four weeks, profile of mood states (POMS)(4), the Minnesota Nicotine Withdrawal Scale (MNWS)(5), the Fagerstrom Test for Cigarette Dependence (FTCD)(6) and a cigarette acceptance questionnaire. The cigarette acceptance questionnaire uses items that cluster into 7 scales: satisfaction, similarity to usual brand, psychological reward, aversion, respiratory sensations, craving and perceived strength.(7) The cigarette acceptance scale was administered only through 12 months while subjects were smoking RNC cigarettes. A smoking-specific self-efficacy questionnaire(8), the Stages of Change questionnaire(9), and the Center for Epidemiologic Studies Depression (CES-D) scale were administered at baseline and 3, 6, 12 and 24 months. (10) We used the Stages of Change Questionnaire to assess movement toward quitting smoking, including pre contemplation (no intention to quit within the next six months), contemplation (seriously considering quitting in the next six months), and preparation. The Self-Efficacy Questionnaire (SLFE) is a fourteen item instrument, measured on a 10-point Likert scale, asking about the confidence of smokers and their ability to resist smoking in various high risks situations.(8) Quitting was assessed as 7 day point prevalence abstinence, meaning a self-report of smoking no cigarettes in the past 7 days. These reports were confirmed biochemically as plasma cotinine concentration of <14 ng/ml or, if taking nicotine replacement medication, an expired CO concentration of <5 parts per million.
Analytical Chemistry
Plasma samples were assayed for concentrations of nicotine and cotinine by gas chromatography.(11, 12) Urine samples were assayed for concentrations of 4-(methylnitrosamino)-1-(3) pyridyl-1-butanol (NNAL), the metabolite of the carcinogenic tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3)pyridyl-1-butanone (NNK) and metabolites of four polycyclic aromatic hydrocarbons (PAH) found in tobacco smoke. NNAL and PAH metabolites are biomarkers of exposure to common tobacco smoke carcinogens. Urine concentrations of NNAL (free + conjugated) and PAH metabolites, including 2-napathol, 1, 2 and 3+4 hydroxyphenathranes, 1-hydroxypyrene, and 2-hydroxyfluorene, were measured by liquid chromatography/tandem mass spectrometry(13, 14). Urine PAHs were measured through 12 months, but not beyond.
Statistical Analysis
Statistical analysis was based on the 103 subjects (53 RNC and 50 controls) who completed the first 6 months of the study. Because measurements for each individual were correlated over time, a repeated measures model was constructed for each of the major variables. A mixed effects regression analysis was conducted with Proc Mixed in SAS (version 9.3). Measurements at baseline, 6 months, 12 months, 18 months and 24 months were modeled as a function of time and study arm, using time by study arm interactions to assess intervention effects. Models were examined with and without adjustment for age, gender, race/ethnicity and baseline use of menthol cigarettes. Unadjusted analyses are presented in the tables, and the few differences that occurred between adjusted and unadjusted analyses are mentioned in the text. Differences in mean values were estimated for each pair of time points within each study arm, as well as the difference between the study arms with respect to each time point comparison; statistical significance was assessed at the 0.05 level (2-sided) using the Bonferroni adjustment to account for 10, 6, or 3 pairwise comparisons at 5, 4, or 3 time points, respectively. Variable values for urine total NNAL and PAH metabolites and time to first cigarette were log transformed to achieve approximate normality, and the analyses were conducted on the logged values. The models used observations at all available time points for participants who completed the first 6 months of the study; for those who dropped out after 6 months, data collected at all study visits during the individual's participation were included in the analyses. Means or geometric means with 95% confidence intervals were computed at each time point for control participants, RNC participants, and RNC participants who complied with the study protocol. The study arms were compared with respect to stage of change (pre-contemplation vs. all others) at each time point using chi-square tests; dropouts were assumed to be in pre-contemplation. There were relatively few menthol cigarette smokers as baseline (10-11%), but because menthol RNC were not available, we also performed the analyses excluding menthol smokers. Most of the results did not differ with and without menthol smokers; the few differences are mentioned in the results.
Results
Subject Retention
Demographic and smoking behavior characteristics are shown in Table 1. The time course and reasons for the dropouts are summarized in Fig 1 and their demographic and smoking characteristics are shown in Table 1. There was no significant difference in FTND or time to first cigarette comparing those who did and did not drop out.
Table 1. Demographic Characteristics by Group (mean, 95% C.I.).
| Characteristic | Control group (n = 50) | Research group (n = 53) | Drop outs Post 6-Months (n=35) |
|---|---|---|---|
|
|
|
|
|
| Age, yrs | 37.4 (34.4,41.0) | 36.6 (33.4,39.2) | 34.1 (33.4,34.8) |
| Gender | |||
| Male | 31 | 25 | 20 |
| Female | 19 | 28 | 15 |
| Race/Ethnicity (%) | |||
| Caucasian | 70 | 70 | 71 |
| AA | 8 | 8 | 6 |
| Asian | 10 | 6 | 9 |
| Other/mixed | 12 | 16 | 14 |
| BMI | 24.8 (24.5,25.0) | 26.3 (26.1,26.6) | 25.0 (24.0,26.1) |
| Education, yrs | 15.7 (14.9,16.1) | 15.1 (14.6,15.8) | 15.7 (14.1, 17.2) |
| CPD | 19.9 (17.9,22.0) | 23.4 (21.5.25.4) | 23.0 (22.0, 24.0) |
| Years smoked | 21.4 (17.9,24.8) | 20.5 (17.5,23.5) | 18.2 (18.0,19.0) |
| Menthol n (%) | 5 (10%) | 6 (11%) | 4(11%) |
| FTC nicotine (mg) | 1.0 (0.9,1.0) | 1.0 (0.9,1.0) | 1.0 (0.9,1.0) |
| FTC tar (mg) | 11.6 (10.8,12.3) | 11.4 (10.6,12.1) | 10.7 (10.0, 12.0) |
| FTND score | 5.5 (4.9,6.2) | 5.6 (5.2,6.1) | 5.4 (4.0,7.0) |
AA: African American; BMI: Body Mass Index; CPD: Cigarettes Per Day; FTC: Federal Trade Commission
Cigarette Consumption and Carbon Monoxide Exposure (Table 2, Figs 2 and 3)
Table 2. Smoking behavior and biomarkers of exposure while smoking reduced nicotine cigarettes means.
| Characteristic | Baseline (usual) | 6 Months (1 mg) | 12 Months (1mg) | 18 Months | 24 Months | Significant Interactions |
|---|---|---|---|---|---|---|
| Control (n=55) | Control (n=50) | Control (n=48) | Control (n=40) | Control (n=38) | ||
| Research (n=80) | Research (n=53) | Research (n=37) | Research (n=32) | Research (n=30) | ||
| Cigarettes per day† | 20 (18, 22) | 22 (19, 25) | 21 (18, 23) | 17 (14, 20) a,b,c | 16 (14, 19) a,b,c | 6M v. BL, 24M v. BL |
| 23 (21, 25) | 20 (17, 23) | 22 (18, 25) | 17 (14, 21) a,b,c | 13 (9, 17) a,c | ||
|
|
||||||
| Plasma cotinine ng/mL† | 268 (233, 302) | 239 (202, 278) a | 267 (223, 310) | 238 (192, 284) | 209 (174, 244) a,c | 6M v. BL, 12M v. BL |
| 250 (222, 277) | 113 (81, 145) a | 149 (110, 188) a | 210 (170, 250) b,c | 173 (135, 211) c | 18M v. 6M, 24M v. 6M | |
| 18M v. 12 M, 24M v. 12M | ||||||
|
|
||||||
| Expired CO (ppm) † | 22 (19, 2) | 20 (17, 23) | 23 (20, 26) | 20 (18, 24) | 19 (15, 22) | NS |
| 25 (22, 28) | 23 (19, 26) | 23 (19, 27) | 22 (17, 27) | 20 (15, 24) | ||
|
| ||||||
| Urine (pmol/mg creatinine) | ||||||
| Total NNAL* | 1.0 (0.7, 1.3) | 0.9 (0.6, 1.2) | 0.8 (0.7, 0.9) | 0.6 (0.4, 0.7) | 6M v. BL | |
| 1.4 (1.1, 1.7) | 0.8 (0.5, 1.1)a | 0.8 (0.7, 0.9) a | 0.7 (0.6, .09) | |||
|
|
||||||
| Sum of phenanthrenes* | 3.5 (2.8, 4.4) | 4.0 (3.3, 4.7) | 4.0 (1.1, 7.0) | NS | ||
| 4.0 (3.3, 4.7) | 3.9 (3.1, 4.8) | 3.8 (2.0, 6.1) | ||||
|
|
||||||
| 2-naphthol* | 97 (73, 129) | 112 (92, 136) | 105 (102, 108) | NS | ||
| 161 (123, 210) | 137 (107, 174) | 115 (113, 117) | ||||
|
|
||||||
| 2-hydroxyfluorene* | 13 (10, 17) | 15 (12, 18) | 14 (12, 16) | NS | ||
| 17 (14, 22) | 17 (13, 23) | 16 (14, 18) | ||||
|
|
||||||
| 1-hydroxypyrene* | 1.1 (0.9, 1.5) | 1.4 (1.1, 1.6) | 1.5 (0.6, 3.0) | NS | ||
| 1.4 (1.1, 1.7) | 1.5 (1.2, 1.9) | 1.4 (0.4, 3.0) | ||||
geometric means (95% C.I.);
arithmetic means (95% C.I.)
significantly different from Baseline, p<0.005
significantly different from 6 Months, p<0.005
significantly different from 12 Months, p<0.005
Fig. 2. Cigarettes Per Day by Group.

Mean cigarette consumption over 24 months of the study in smokers smoking their usual brand of cigarettes (C) or during progressive reduction of nicotine content of cigarettes (R). The bars represent SEM.
Fig. 3. Expired CO by Group.
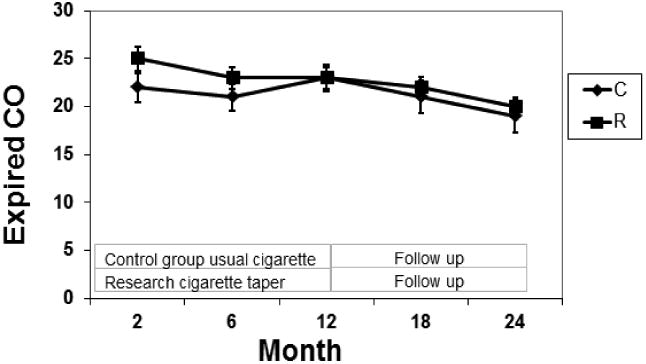
Mean expired CO concentrations over 24 months of the study in smokers smoking their usual brand of cigarettes (C) or during progressive reduction of nicotine content of cigarettes (R). The bars represent SEM.
Cigarettes per day remained unchanged from baseline to twelve months, then decreased significantly in both the RNC and control smokers at 18 and 24 months. Among control subjects, the mean CPD was significantly lower at 18 and 24 months compared to baseline, 6 and 12 months. Among RNC subjects the mean CPD was significantly lower at 18 months than at baseline and 12 months, and significantly lower at 24 months compared to baseline, 6 and 12 months. After adjustment, the difference between baseline and 18 months was no longer statistically significant. The RNC group had a significantly greater drop in CPD than the control group between baseline vs 6 months and baseline vs. 24 months. With exclusion of menthol smokers, the decrease in CPD between 12 and 18 months and the research-control group difference in CPD between baseline and 24 months were no longer significant. Expired CO levels remained stable with no significant change from baseline in either treatment group.
Plasma Cotinine Concentrations (Fig 4)
Fig. 4. Plasma Cotinine by Group.
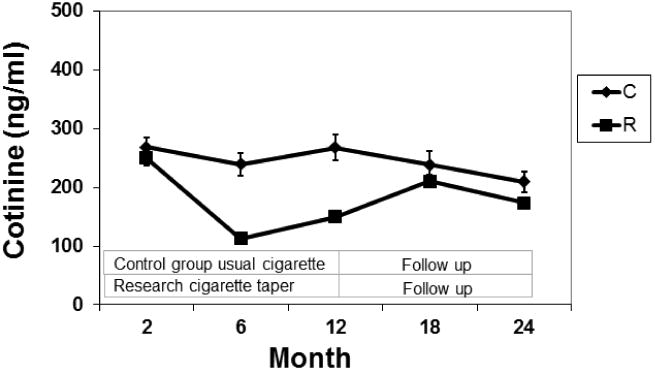
Mean plasma cotinine concentration over 24 months of the study in smokers smoking their usual brand of cigarettes (C) or during progressive reduction of nicotine content of cigarettes (R). The bars represent SEM.
In the RNC group, cotinine concentrations decreased significantly from an average of 250 ng/ml at baseline to 113 ng/ml at six months, but increased to 149 ng/ml in those subjects continuing to smoke the lowest nicotine level cigarettes for an additional 6 months. Cotinine levels were significantly lower in RNC vs control smokers at 6 and 12 months. Cotinine concentrations in RNC smokers were significantly higher at 18 months vs 6 and 12 months. With exclusion of menthol smokers, the cotinine changes between 6 and 12 months and between 6 and 24 months in RNC were significant.
Total NNAL and PAH metabolites (Table 2)
Total urine NNAL decreased significantly compared to baseline at months 6 and 12 in RNC subjects, but was not significantly different from baseline values at 24 months. There were no significant changes in NNAL in the control group. Urine PAH levels were similar in RNC and control groups and not significantly different over time.
Body weight and Cardiovascular Measures (Table 3)
Table 3. Questionnaire and Cardiovascular Data.
| Questionnaires | Baseline (usual) | 6 Months (1 mg) | 12 Months (1mg) | 18 Months | 24 Months |
|---|---|---|---|---|---|
| Control (n=55) | Control (n=50) | Control (n=48) | Control (n=40) | Control (n=38) | |
| Research (n=80) | Research (n=53) | Research (n=37) | Research (n=32) | Research (n=30) | |
| FTND† | 5.5 (4.9, 6.1) | 5.4 (4.6, 6.1) | 5.4 (4.7, 6.2) | 5.1 (4.4, 5.9) | 4.9 (4.1, 5.7) |
| 5.9 (5.5, 6.4) | 5.2 (4.7, 6.0) | 5.3 (4.6, 6.0) | 4.8 (3.9, 5.7) | 5.2 (4.3, 6.0) | |
|
|
|||||
| TFC† | 21 (12, 29) | 33 (9.0, 57) | 34 (12, 56) | 51 (1.2, 100) | 28 (5.1, 51) |
| 24 (8.4, 39) | 19 (3.2, 35) | 26 (10, 40) | 21 (10, 31) | 19 (7.7, 30) | |
|
|
|||||
| MNWS† | 11 (8.2, 12) | 7.6 (6.0, 9.2) | 9.1 (7.3, 10) | 8.2 (6.5, 9.9) | 7.6 (6.0, 9.2) |
| 13 (11, 14) | 6.9 (5.2, 8.6) | 10 (8.5, 12) | 9.4 (7.0, 11) | 9.4 (6.6, 12) | |
|
|
|||||
| CESD† | 11 (8.5, 12) | 11 (8.2, 13) | 13 (10, 15) | 12 (8.4, 15) | |
| 13 (10, 15) | 8.9 (6.6, 11) | 14 (10, 17) | 11 (7.6, 14) | ||
| POMS† | 10 (4.7, 16) | 6.0 (1.8, 10) | 9.2 (4.1, 14) | 6.8 (1.7, 11) | 6.8 (0.9, 12) |
| 13 (9.1, 17) | 13 (7.4, 17) | 10 (4.5, 15) | 10 (2.8, 16) | 7.3 (0.9, 13) | |
| SLFE† | 3.5 (3.1, 3.9) | 4.0 (3.5, 4.4) | 4.2 (3.6, 4.7) | 4.6 (3.9, 5.3) | |
| 3.6 (3.2, 3.9) | 4.1 (3.5, 4.7) | 5.0 (4.2, 5.7) a | 5.6 (4.5, 6.7) a | ||
|
| |||||
| Body weight (kg) * | 76 (72, 80) | 74 (70, 78) | 75 (71, 79) | 75 (69, 81) | 76 (69, 83) |
| 80 (75, 85) | 81 (75, 87) | 82 (76, 88) | 84 (78, 90) | 89 (80, 98) a | |
|
|
|||||
| Systolic blood pressure (mm Hg) * | 123 (119, 127) | 120 (115, 125) | 122 (118, 126) | 117 (113, 121) | 121 (116, 126) |
| 122 (118, 126) | 119 (114, 124) | 122 (116, 128) | 120 (115, 125) | 120 (113, 127) | |
|
|
|||||
| Heart rate* | 83 (78, 86) | 79 (75, 83) | 81 (77, 85) | 80 (76, 84) | 79 (75, 83) |
| 81 (78, 84) | 79 (76, 82) | 83 (80, 86) | 83 (79, 87) | 79 (74, 84) | |
|
|
|||||
| WBC count (1,000) * | 7.5 (6.9, 8.0) | 6.8 (6.0, 7.5) | 7.3 (6.7, 7.8) | 7.5 (6.7, 8.2) | |
| 6.9 (6.3, 7.4) | 7.3 (6.8, 7.7) | 8.0 (7.2, 8.7) | 7.1 (6.3, 7.8) | ||
|
|
|||||
| Hemoglobin (%)* | 14.7 (14.3, 15.0) | 14.4 (14.0, 14.7) | 14.6 (14.2, 14.9) | 14.4 (13.8, 14.9) | |
| 14.5 (14.2, 14.7) | 14.3 (14.0, 14.6) | 14.2 (13.8, 14.5) a | 14.0 (13.4, 14.5) a | ||
|
|
|||||
| HDL cholesterol (ng/dL) * | 53 (48, 58) | 54 (49, 59) | 52 (47, 57) | 53 (46, 60) | |
| 54 (51, 58) | 53 (49, 57) | 53 (49, 57) | 52 (46, 58) | ||
|
|
|||||
| Fibrinogen* | 278 (252, 304) | 281 (256, 306) | 291 (267, 315) | 288 (258, 318) | |
| 281 (264, 298) | 301 (279, 323) | 338 (307, 369) a | 320 (289, 351) | ||
FTND: Fagerstrom Test for Nicotine Dependence; TFC: Time to First Cigarette; MNWS: Minnesota Nicotine Withdrawal Scale; CESD: Center for Epidemiological Studies Depression Scale; POMS: Profile of Mood States; SLFE: Self-Efficacy
geometric means (95% C.I.);
arithmetic means (95% C.I.)
significantly different from Baseline, p<0.005
significantly different from 6 Months, p<0.005
significantly different from 12 Months, p<0.005
No significant interactions were observed.
Significant time-related changes occurred in the RNC group with significantly higher body weight at 24 months versus baseline, but this did not differ significantly from controls. The change in body weight in RNC subjects was no longer significant after exclusion of menthol smokers. Subjects in the RNC group had significantly lower hemoglobin concentration at 12 months compared to baseline and at 24 months compared to baseline. After adjustment or exclusion of menthol smokers, changes in hemoglobin concentration were no longer statistically significant. Fibrinogen was significantly higher fibrinogen at 12 months compared to baseline. No significant changes in body weight, hemoglobin or fibrinogen were seen in the control group over time. No significant changes were seen in systolic blood pressure, heart rate, white blood cell count and HDL cholesterol in either group.
Questionnaires (Table 3)
No significant time or group-related changes in total MNWS, total POMS or CESD were observed. With exclusion of menthol smokers, MNWS significantly decreased between baseline and 18 months. Responses on the cigarette acceptance questionnaire indicated that RNC were milder, less satisfying, had lower nicotine effect and of lesser quality compared to their usual cigarettes. In response to the Self-Efficacy Questionnaire, the RNC group reported significantly higher scores at 24 and 12 months compared to their baseline assessment. No significant group-related differences were found.
Regarding Stages of Change, percentages of RNC participants in pre-contemplation throughout the study were : 83% at baseline, 49% at 6 months, 53% at 12 months, 68% at 18 months and 66% at 24 months. For the control group, percentages in the pre-contemplation stage were : 92% at baseline, 86% at 6 months, 60% at 12 months, 60% at 18 months and 70% at 24 months. Significant group differences were found at 6 months during which more subjects in the RNC group were beyond pre-contemplation compared to control smokers.
No significant changes were observed in FTCD or in Time to First Cigarette over time or between groups.
Quitting Smoking
Quit rates were low in both RNC and control groups, and not significantly different between groups any time. Point prevalence quitting based on self-reported cigarettes per day in the RNC group was 5.6% at 6 months, 3.8% at 12 months, 7.5% at 18 months and 11.3% at 24 months. For the control group these rates were 2%, 0%, 6% and 6% for the same time points. Biochemically-verified quit rates for RNC subjects were 5.6% at 6 months, 3.8% at 12 months, 3.8% at 18 months, and 7.5% at 24 months, and 2%, 2%, 6% and 2% for the control group at the same time points. Only one subject in the RNC group had continued verified abstinence from 6 months to study completion.
Compliance
At the 6 and 12 month visits RNC participants were asked if they had used conventional cigarettes. At 6 months 30% and at 12 months 43% of subjects admitted to smoking other cigarettes in addition to the RNCs. When asked why, participants' reasons included the following: “just to compare flavor and strength”, “ran out or didn't have research cigarettes” and “for nicotine”. 25% of those who reported non-compliance stated it was in the second 6 months of the study.
Discussion
Previously we observed that in smokers who are not planning to quit, gradual reduction of the nicotine content of cigarettes results in reduced intake of nicotine without compensatory over-smoking of cigarettes.(3, 15) We hypothesized that with continued smoking of very low nicotine cigarettes, smokers would become less dependent, have less desire for nicotine and would quit smoking. We found that after 7 months of smoking very low RNC, cotinine levels remained significantly lower than baseline. However from the first to the seventh month of smoking the same very low RNC, cotinine levels increased significantly. There was no change in CPD or exposure to combustion products (carbon monoxide and PAHs), and no increase in nicotine withdrawal symptoms. Very few subjects quit smoking while receiving very low RNCs.
During the 12 month follow-up when subjects were free to smoke their own cigarettes, cotinine levels in RNC subjects rose to levels similar to control smokers who had never received RNCs. Quitting remained low over the 12 month follow-up period. At 24 months the percent of smokers in the RNC group who quit smoking was higher than that of the control group, but not significantly so.
Our data suggest that lengthy (6 months) exposure to RNC does not result in the extinction of nicotine dependence, as might be seen in loss of smoking urges, reduction in CPD or increased quitting. One explanation may have been that subjects were able to obtain adequate levels of nicotine to sustain addiction during the period of nicotine reduction. This could be due to subjects getting more nicotine than expected by intensive smoking of very low RNCs, or to supplementing the RNCs with conventional cigarettes.
Cotinine levels for many subjects were higher than expected given that the nicotine content of the very low nicotine cigarettes was only 5% of that of conventional cigarettes. Based on lack of change in self-reported cigarettes per day, expired carbon monoxide and PAH metabolite levels, it is unlikely that smokers smoked their research cigarettes exceptionally intensively, as would be necessary to achieve the observed cotinine levels.
A number of subjects (30% at 6 months and 43% at 12 months) reported that they supplemented their reduced nicotine content cigarettes with some conventional cigarettes. We suspect that the higher cotinine levels at 12 compared to 6 months in the RNC smokers was due to non-compliance, that is, smoking some conventional cigarettes in addition to the research cigarettes.
Our study has some important limitations that impact the extrapolation of results to what might be expected with a national nicotine reduction intervention. Our study was conducted in the context of freely available conventional cigarettes. If subjects were not getting adequate nicotine from RNCs, they could easily supplement them with conventional cigarettes. In addition, the RNCs were rated as poor quality. Presumably, if major tobacco manufacturers were making RNC cigarettes in a competitive marketplace, the cigarettes would be more consumer acceptable. There was a high non-completion rate, particularly among RNC smokers, primarily related to dissatisfaction with the research cigarettes. Cigarettes were provided at no cost, which may have affected the number of cigarettes smoked. The termination of free cigarettes may explain the decrease in CPD in both groups in the second year of the study. Our subjects were volunteers who were compensated for participation in the study. They understood that this was a time-limited study, and at the end they would be free to return to their usual brand. We excluded subjects with major mental health or substance abuse disorders, who might respond differently to RNC compared to healthy smokers. Finally, while 11% of smokers preferred menthol cigarettes, menthol research cigarettes were not available. We controlled for menthol preference in our data analysis and performed a separate analysis excluding menthol smokers, with few changes in results.
Despite the observation that nicotine reduction did not increase quitting, we did see that RNC smokers expressed greater interest in quitting, as measured using the Stages of Change Questionnaire. Our study, as well as others, found no evidence of safety concerns in smokers of RNCs.(3, 15-17) Specifically there was no evidence of withdrawal distress or increased depression, and no increases in tobacco smoke toxicants and no adverse changes in selected cardiovascular biomarkers.
The implications of our findings for a possible federally- mandated reduction in the nicotine content of cigarettes are as follows. The level of reduction of the nicotine content of cigarettes needed to extinguish nicotine dependence is as yet unknown. We did not observe extinction of dependence in our study, but it likely that many of our subjects supplemented their nicotine intake from conventional cigarettes. Simply reducing the nicotine content of cigarettes alone may be insufficient to extinguish smoking behavior. A nicotine reduction intervention combined with public education about the reasons for reduction, behavioral support for quitting and/or the easy access to alternative sources of nicotine (such as nicotine medications or electronic cigarettes) may be needed to achieve cessation of cigarette smoking.
Acknowledgments
We thank Dr. Faith Allen for data management, Lita Ramos for performing the nicotine and cotinine analyses, Olivia Yturralde for the PAH metabolite analyses and Christopher Havel for the NNAL analyses, and Scott Rostler for editorial assistance. We thank Philip Morris for providing research cigarettes. (Philip Morris had no involvement in any aspect of the design of the study or analysis or interpretation of the data).
Supported by US Public Health Service grants CA78603 from the National Cancer Institute, DA02277, DA12393 and DA016752 from the National Institute on Drug Abuse, and FDA Center for Tobacco Products grant U54031659. Clinical Trials No: NCT00264342.
Footnotes
Disclosure of Potential Conflicts of Interest: Dr. Benowitz is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. Dr. Hall has received material support for a clinical trial from Pfizer. The other authors have no conflicts to declare.
References
- 1.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 2.Family Smoking Prevention and Tobacco Control Act - Public Law 111-31. 2009;2012(5/14) [Google Scholar]
- 3.Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNair DM, Lorr M, Doppleman LF. Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 5.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of general psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 6.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 7.Rose JE, Westman EC, Behm FM, Johnson MP, Goldberg JS. Blockade of Smoking Satisfaction Using the Peripheral Nicotinic Antagonist Trimethaphan. Pharmacology Biochemistry and Behavior. 1999;62(1):165–72. doi: 10.1016/s0091-3057(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 8.Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: an exploration of individual differences. J Consult Clin Psychol. 1988;56(1):104–10. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- 9.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 10.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 11.Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222(1):61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- 12.Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Spectrom. 1991;20(5):247–52. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 13.Jacob P, 3rd, Wilson M, Benowitz NL. Determination of Phenolic Metabolites of Polycyclic Aromatic Hydrocarbons in Human Urine as Their Pentafluorobenzyl Ether Derivatives Using Liquid Chromatography-Tandem Mass Spectrometry. Anal Chem. 2007;79(2):587–98. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 14.Jacob P, 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80(21):8115–21. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., 3rd Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 16.Hitchman SC, Fong GT, Borland R, Hyland A. Predictors of smoking in cars with nonsmokers: findings from the 2007 Wave of the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2010;12(4):374–80. doi: 10.1093/ntr/ntq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1015–24. doi: 10.1158/1055-9965.EPI-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]