Abstract
Background and objective
Omega-3 fatty acids, such as α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), are polyunsaturated fatty acids (PUFA) that have long been associated with anti-inflammatory activity and general benefit toward human health. Over the last decade, the identification of a family of cell-surface G protein-coupled receptors that bind and are activated by free-fatty acids, including omega-3 fatty acids, suggests that many effects of PUFA are receptor-mediated. One such receptor, free-fatty acid receptor-4 (FFAR4), previously described as GPR120, has been shown to modulate anti-inflammatory and insulin-sensitizing effects in response to PUFA such as ALA and DHA. Additionally, FFAR4 stimulates secretion of the insulin secretagogue glucagon like peptide-1 (GLP-1) from the GI tract and acts as a dietary sensor to regulate energy availability. The aim of the current study was to assess the effects of dietary omega-3 fatty acid supplementation on FFAR4 expression in the rat colon.
Methods
Sprague-Dawley rats were fed control soybean oil diets or alternatively, diets supplemented with either fish oil, which is enriched in DHA and EPA, or flaxseed oil, which is enriched in ALA, for seven weeks. GLP-1 and blood glucose levels were monitored weekly and at the end of the study period, expression of FFAR4 and the inflammatory marker TNF–α was assessed.
Results
Our findings indicate that GLP-1 and blood glucose levels were unaffected by omega-3 supplementation, however, animals that were fed fish or flaxseed oil-supplemented diets had significantly heightened colonic FFAR4 and actin expression, and reduced expression of the pro-inflammatory cytokine TNF-α compared to animals fed control diets.
Conclusions
These results suggest that similar to ingestion of other fats, dietary intake of omega-3 fatty acids can alter FFAR4 expression within the colon.
Keywords: Free-fatty acid receptor-4, GPR120, polyunsaturated fatty acids, fish oil, flaxseed oil
Introduction
In mammals, essential polyunsaturated fatty acids (PUFA) are mainly obtained through dietary sources and are primarily classified as omega-3 (n-3) or omega-6 (n-6), which are biochemically differentiated largely based on the position of the first double bond. Interestingly, an abundance of in vivo and in vitro studies demonstrate that n-3 and n-6 PUFA have opposing effects in regard to inflammation, whereby omega-3 fatty acids suppress inflammatory cascades, while omega-6 fatty acids promote them. Indeed, omega-3 fatty acids, including α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) have long been associated with anti-inflammatory activities beneficial toward human health, including within the gastrointestinal tract [1–5]. These benefits have led to a surge in public consumption of PUFA supplements including fish oil, which is a primary source of EPA and DHA, and vegetable-based oils, such as those derived from flaxseed, walnuts, almonds, or canola, which are enriched in ALA.
While the beneficial effects of n-3 PUFA were historically been presumed to occur via their intracellular metabolism or their incorporation into biological membranes, recently, a subfamily of cell-surface free-fatty acid receptors (FFAR) belonging to the G protein-coupled receptor (GPCR) superfamily has been discovered and shown to include 4 members: FFAR1 (originally described as GPR40), FFAR2 (GPR43), FFAR3 (GPR41), and FFAR4 (GPR120) [6], suggesting that many effects of fatty acids are receptor-mediated. While FFAR2-3 were shown to bind short-chained FFAs, FFAR1 and FFAR4 bind long chain FFAs such as palmitic and oleic acid, as well as n-3 PUFA, including ALA, EPA, and DHA [7–9]. FFAR4 first began to draw interest due to its ability to stimulate the release of the insulin secretagogue glucagon-like peptide-1 (GLP-1) from secretory L-cells of the intestinal lumen [7], suggesting that its agonism could be linked to modulation of blood glucose levels. Moreover, FFAR4 has gained considerable recent attention due to its ability to profoundly affect blood glucose levels through modulation of glucose transporters, as well as its ability to abrogate proinflammatory signals that lead to insulin resistance [8], demonstrating that FFAR4 agonism promotes insulin-sensitizing and anti-diabetic effects. Further studies that delineate a critical role of FFAR4 in metabolism are reflected by data from FFAR4 knockout (KO) mice, which show moderate insulin resistance when fed normal diets, but quickly become obese, have less energy expenditure and heightened adipose inflammation compared to wild-type animals when fed high-fat diets [10]. Taken together, these results make a convincing case that FFAR4 plays critical roles in ameliorating inflammation and mediating metabolism.
FFAR4 expression is dense in the mammalian intestinal tract, particularly the colon [7], and as such, alterations to the expression of FFAR4 have been linked to dietary intake of fats and inflammation in mammals. For example, mice that were fed high-fat diets for 11 weeks showed an increase in adipocyte FFAR4 expression compared to those fed control diets [11]. Meanwhile, epididymal adipose of mice fed high-fat diets with variable n-6:n-3 ratios ranging from 1:1 to 20:1 showed significantly lower levels of FFAR4 expression, and also had significantly higher levels of inflammatory mediators such as TNF-α and IL-10 compared to control-diet fed mice, suggesting that the n-6 and n-3 components of fats make important contributions toward both inflammation and expression of FFAR4 [12]. Similarly evidence exists in rats, where energy-dense, high-fat diets were found to increase expression of intestinal FFAR4 in a diet-induced obese (DIO) rat model as early as 6 months of age [13]. Interestingly, others have showed that the effects of high-fat diet on FFAR4 expression are tissue specific, as rats fed a high-fat diet for 12 weeks showed upregulation in FFAR4 expression in cardiac tissue and extensor digitorum longus (EDL) skeletal muscle, but not in liver or soleus skeletal muscle [14]. Nonetheless, diet-induced alteration to FFAR4 in rodents seems to be a physiologically relevant effect in line with the role of the receptor as sensor for dietary nutrients [8,10], and these results are consistent with the finding that human FFAR4 expression is higher in obese compared to lean subjects [10]. Given the dense expression of FFAR4 in the mammalian colon, its role as a dietary sensor, and the noted effects of dietary fats on FFAR4 expression, the aim of the current study was to assess the effects of dietary n-3 supplementation on FFAR4 expression in the rat colon. Specifically, our goal was to gauge whether diets enriched in omega-3 PUFA from fish oil or flax seed oil would contribute to alterations in expression of FFAR4 in the colon.
Materials and Methods
Animals and diets
Male Sprague-Dawley rats (ca. 150 g; ca. 40 days old) were purchased from Harlan Laboratories (Madison, WI). Animals were housed singly to account for food intake and were maintained on a 12:12-hr light/dark cycle (lights on at 7 am) in a humidity (50–60%) and temperature (22–25 °C) controlled room. Experiments were conducted with approval of and in compliance with the Mercer University Institutional Animal Care and Use Committee and the National Institutes of Health Guide for Care and Use of Laboratory Animals. Animals were divided into cohorts of four separated by diet type and given ad libitum access to food and water, which was removed for 6 hr under fasting conditions. The control diet (AIN-93M) was formulated for the maintenance of non-reproducing adult rats and consisted of 12.4% fat, 68.4% carbohydrate, and 12% protein and also included 40 g/kg soybean oil to meet basic unsaturated and saturated fatty acid needs [15]. Importantly, while soybean oil does contain basic n-3 fats, it is more highly enriched in n-6 fatty acids at approximately 1:6–1:8 ratios [16]. On the contrary, the experimental diets consisted of the 93M diet supplemented with 100 g/kg (10%) of either purified fish oil (FO) or flaxseed oil (FSO) (Harlan Laboratories). Animal weight, food intake, fasting and non-fasting blood glucose, and plasma GLP-1 levels were assessed at the same time for each cohort weekly. Animals were sacrificed at the beginning of the eighth week and a section of the transverse colon was removed and frozen at −80°C for assessment of FFAR4 protein expression.
Immunoblotting
Immunoblotting was performed as we have previously described [17]. Briefly, 20–30 mg of tissue was lysed in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM NaF, 10 mM Na2HPO4, pH 7.4) plus protease inhibitor cocktail and homogenized with an electric homogenizer at maximum speed for 10–15 sec. The lysate was cleared for insoluble debris by centrifugation at 14,000 × g for 15 minutes at 4°C, and protein concentrations were standardized using DC Protein Assay (Bio-Rad, Hercules, CA). An aliquot of the lysate was denatured in 2X SDS-sample buffer, resolved by SDS-PAGE, followed by transfer to PVDF membranes, and immunoblotted with the appropriate antibody (FFAR4: Abcam, 75315; total actin: Santa Cruz Biotechnology, sc-1615; ERK1/2: Cell Signaling, 9102). Blots were visualized with HRP-conjugated secondary antibody followed by ECL. Blots were stripped of IgG and reprobed to verify equal protein loading as described.
Active GLP-1 and Blood Glucose Assessment
Blood was collected from the tail vein in EDTA-containing tubes and plasma was separated by centrifugation at 2500 × g for 10 min at 4°C. Glucose was measured using Accutrend Glucose (Roche Diagnostics, Indianapolis, IN) from fresh plasma. Active GLP-1 was measured using the Glucagon-Like Peptide-1 (Active) ELISA kit (Merck KGaA, Darmstadt, Germany), according to the manufacturer's instructions.
Data Analysis
Resulting data was quantified by densitometric analysis using Image J (National Institutes of Health, Bethesda, MD) and Prism 3.0 (GraphPad, San Diego, CA). Animal metric data are expressed as mean ± S.E.M for representative experiments performed in triplicate. Western blot experiments were repeated at least three independent times. Where not visible, error bars fall within the symbol size. Statistical analysis was performed, as appropriate, either by one-way analysis of variance and post-hoc Tukey's test or by Student's t-test using Graphpad Instat or Prism, and are represented as * p < 0.05 or *** p < 0.001.
Results
Animal Metrics
Animals fed control (CON), fish oil enriched (FO), or flaxseed oil enriched (FSO) diets were monitored for food intake, weight gain, and adjusted nutrient intake for seven weeks. Animals that were fed FO or FSO diets consumed similar amounts of the respective diet compared to those fed control diets over the period of observation (Fig 1A). Animals that were fed the n-3 fatty acid enriched diets demonstrated a statistically significant gain in weight by week 5, and this effect continued through week seven of the study (Fig 1B). However, differences in caloric content of the control (3.6 kcal/g) and n-3 enriched (4.1 kcal/g) diets lead to significant differences in caloric intake amongst the cohorts. As such, animals fed the CON diet consumed fewer calories than those fed FO (p < 0.01 versus CON) or FSO (p < 0.001 versus CON) enriched diets, which most-likely explains the elevated weight gain in the n-3 fed groups (Fig 1C). Since acute FFAR4 agonism by n-3 PUFA has been shown to increase GLP-1 secretion from intestinal L-cells, we also assessed whether chronic intake of n-3 enriched diets would mimic this effect. Our results show no significant alterations in GLP-1 secretion over the study period in FSO and FO fed animals, compared to control (Fig 1D). Moreover, since FFAR4 agonism has been shown to decrease blood glucose levels, we assessed this effect in n-3 enriched diets and found no significant effect of the n-3-supplemented diets on fasting (Fig 1E) or non-fasted (data not shown) blood glucose.
Figure 1. Study animal metrics.
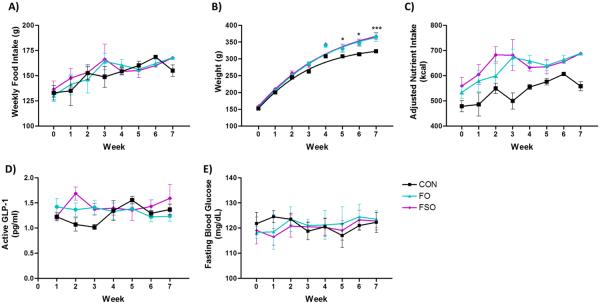
Animals fed control (CON), fish oil enriched (FO), or flaxseed oil enriched (FSO) diets had no differences in weekly food intake (A). Animals fed the enriched diets began to demonstrate heightened weight gain compared to control at week 5, an effect that continue through the study (p < 0.05 versus CON at week 5–6, p < 0.001 versus CON at week 7) (B). Animals fed FO and FSO enriched diets consumed a greater caloric load compared to CON (p < 0.01 and 0.001, respectively) (C). There were no notable or significant differences in plasma GLP-1 levels (D) or fasting blood glucose (E) in animals fed the n-3 enriched diets compared to control. * denotes p < 0.05, *** denotes p < 0.001 versus CON.
FFAR4 Expression
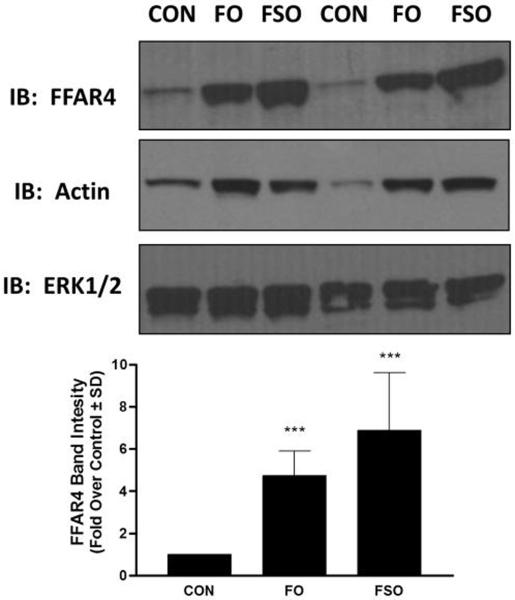
Given the previously noted alterations in FFAR4 expression based on dietary fat components, the major aim of this study was to assess the effects of FO and FSO enriched diets on FFAR4 expression in the rat colon. At the conclusion of the study, rat colon was removed and the levels of FFAR4 protein were assessed by immunoblotting. Our results demonstrate that FO and FSO enriched diets significantly upregulate FFAR4 protein expression in the rat colon compared to animals fed CON diets (Fig 2). Densitometric quantification of the data reveals approximately 4.7 ± 1.1 and 6.9 ± 2.7 –fold increases in FFAR protein expression in FO and FSO fed animals compared to control, respectively (Fig 2) (p < 0.001 versus CON for both). Upon stripping of IgG from blots and reprobing with anti-actin IgG to demonstrate equivalent protein loading, to our surprise, the protein lysates from FO and FSO fed animals also expressed significantly heightened actin levels compared to lysates from animals fed CON diets (Fig 2). However, when blots were probed with ERK1/2, a protein that is regulated by phosphorylation rather than by alterations to expression, there was no difference between the respective conditions, demonstrating equivalent protein loading in each lane of the gel.
Figure 2. FO and FSO enriched diets increase FFAR4 expression.
Animals fed fish oil enriched (FO) or flaxseed oil enriched (FSO) diets demonstrate heightened FFAR4 protein expression compared to those fed CON diets, as demonstrated by immunoblotting. FO and FSO diets were also correlated with increased actin expression compared to animals fed CON diets. ERK1/2 was used as a housekeeping protein to demonstrate equivalent protein loading in each lane (upper). Samples from two of the four representative cohorts are shown. Densitometric quantification of FFAR4 expression shows 4.7 ± 1.1 and 6.9 ± 2.7 –fold increases in FFAR protein expression in FO and FSO fed animals compared to control, respectively. *** denotes p < 0.001 versus CON.
TNF-α expression
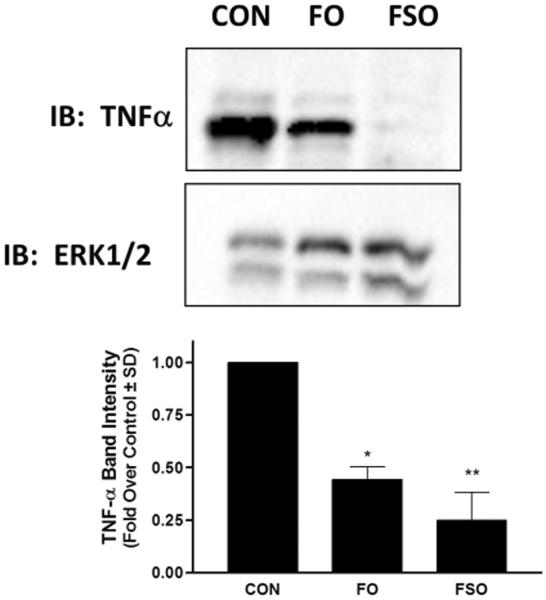
Since n-3 PUFA have been shown to reduce pro-inflammatory mediators through activation of FFAR4 [8], we also wished to assess whether FO or FSO enriched diets would affect expression of TNF-α protein in the rat colon. Immunoblotting of rat colon lysates revealed a decrease in TNF-α protein expression in animals fed FO and FSO enriched diets compared to those fed CON diets (Fig 3), consistent with those of others showing a decrease in proinflammatory mediators, including TNF-α, upon PUFA treatment and FFAR4 activation [8, 10–12].
Figure 3. FO and FSO enriched diets decrease TNF-α expression.
Animals fed fish oil enriched (FO) or flaxseed oil enriched (FSO) diets demonstrate decrease TNF-α protein expression compared to those fed CON diets, as demonstrated by immunoblotting. ERK1/2 was used as a housekeeping protein to demonstrate equivalent protein loading in each lane. Densitometric quantification of TNF-α expression shows relative density of 0.42 ± 0.06 and 0.23 ± 0.1, in FO and FSO fed animals compared to control (1.0), respectively. * denotes p < 0.05 versus CON, ** denotes p < 0.01 versus CON.
Discussion
In this study, we have assessed the effects of dietary fish oil and flaxseed oil supplementation on FFAR4 expression in the rat colon. Additionally, we have gauged the effects of dietary FO and FSO supplementation on GLP-1 secretion and blood glucose levels. Interestingly, while agonism of FFAR4 with n-3 PUFA has clearly been shown to increase GLP-1 secretion from rodent and human intestinal L-cell lines [7–8], the in vivo effects of FFAR4 on GLP-1 secretion have been less poorly resolved. While some studies have shown that n-3 PUFA such as ALA and DHA increase plasma GLP-1 levels in vivo [7, 18], the physiological relevance of FFAR4 in this role have been challenged by other studies that have shown that FFAR4 agonism by ALA does not play a major role in increasing plasma GLP-1 levels in vivo in rats [13], as well as those that point to a contribution of the related FFAR1 in modulating the GLP-1 secretagogue effect of PUFA in vivo [19]. Here, we did not observe any alterations in GLP-1 concentrations in animals fed FO or FSO diets compared to those fed CON diets. However, since GLP-1 secretion is glucose-dependent, this lack of response could be attributed to the fact that we did not augment diets with exogenous glucose. More importantly, since elevations in physiological levels of GLP-1 are short-lived due to its rapid inactivation by DPP-IV, the chronic dietary-intake modality of n-3 PUFA based on free access to chow and weekly, as opposed to post-prandial, measurement of GLP-1 used within this study could have neglected to capture rapidly occurring increases in GLP-1 following ingestion of supplemented chow. Similarly, this affect could have prevented us from capturing alterations to blood glucose. Additionally, the soybean oil control diet used in this study contains a basal level of n-3 fats, albeit at a much lower level and at roughly 1:7 ratio to n-6 fats, which could also have masked some of the effects that may otherwise be apparent in diets that are completely lacking in n-3 fatty acids. Nevertheless, chronic supplementation of FO or FSO in animals given free access to these diets had no effect on either GLP-1 or blood glucose throughout the time course of our study.
Our finding that FO and FSO enriched diets significantly increase FFAR4 expression in the colon are in line with results described for FFAR4 expression in other tissues. This effect was initially reported in adipose tissue of mice fed high-fat diets [11], and has subsequently been demonstrated in other tissues. For example, both FFAR4 transcript and protein expression in the intestines was increased in diet-induced obese (DIO) rats compared to the diet-resistant (DR) genotype [21]. While Paulsen and colleagues also showed that expression of FFAR4 is upregulated in DIO rats compared to the DR rats, they also found that in this case, there were no differences in FFAR4 transcript expression induced by the fat composition in the diets [13]. Meanwhile, Cornall and colleagues describe the tissue-specific upregulation of FFAR4 expression in rats, such that high-fat diets increased FFAR4 expression in cardiac muscle and EDL skeletal muscle, but not in liver or soleus skeletal muscle [14]. A recent report by Widmayer and colleagues demonstrates that both FFAR4+ cells and FFAR4 transcript expression in the mouse gastric corpus increased 1.5-fold in as little as three weeks' time on a high-fat diet, while interestingly, in the antral compartment of the stomach, high-fat diets had no effect on FFAR4 expression and actually reduced the number of FFAR4+ cells [20], suggesting that the effects of fats on expression of FFAR4 extend past tissue specific and are likely cell-type specific.
Along with an increase in intestinal FFAR4 expression, intestinal tissue of animals fed FO and FSO supplemented diets had significantly elevated levels of actin, a finding that was surprisingly exposed as a consequence of using actin as a loading-control housekeeping protein for FFAR4 immunoblots. Since our immunoblots demonstrated uniform levels of ERK1/2, which is regulated by phosphorylation, between the experimental conditions, we interpret the actin results as representing a genuine increase in expression of actin in animals fed n-3 PUFA supplemented diets. The actin protein family is an essential component of the cell cytoskeleton and has important roles in cell proliferation and migration. Interestingly, it is noteworthy that other studies have demonstrated that cdc42-dependent actin polymerization occurs in rodent intestinal L-cell lines, and that such actin remodeling is a requirement prior to insulin-induced GLP-1 secretion from these enteroendocrine cells [22]. Given that we did not observe absolute increases in GLP-1 secretion given our study paradigm, the link between increases in actin polymerization and GLP-1 secretion from the intestines in vivo will require more elaborate study.
Omega-3 PUFA, have been historically linked to anti-inflammatory effects, and recently, FFAR4 has been noted to, at least in part, modulate many of these effects. In a landmark study that thrust FFAR4 to the forefront of drug discovery efforts, Oh and colleagues described broad anti-inflammatory effects that are mediated by FFAR4 upon its agonism with either n-3 PUFA or synthetic agonists [8]. Since, other studies have linked FFAR4 agonism with anti-inflammatory effects, specifically, decreases in synthesis of proinflammatory mediators such as TNF-α, IL-1β, IL-10, and COX-2, amongst others [8, 23–27]. Consistent with these effects, we found that protein levels of TNF-α were significantly decreased in intestinal tissue of animals fed FO or FSO diets compared to those fed control diets, suggesting decreased inflammatory role of TNF-α in the colons of n-3 supplemented animals. FFAR4 has been shown to be overexpressed in ileal mucosa from patients with Crohn's Disease, an effect that was correlated with increased mucosal inflammation, which was dependent on increased TNF-α, suggesting that TNF-α regulates expression of FFAR4, at least in the upper gastric tissue [28]. Interestingly, our data demonstrate elevations in FFAR4 expression in the absence of TNF-α increases, suggesting differential regulation of FFAR4 expression in the distal intestines (e.g., colon). The effects of n-6:n-3 PUFA ratio also likely plays a role in modulation of FFAR4 expression as diets rich in n-6 PUFA have been shown to greatly increase inflammatory mediators, including TNF-α, in adipocytes [12], however interestingly in this tissue, the increased TNF-α was associated with significant decreases in FFAR4 expression. Future studies will be required to bear out the relationship between dietary PUFA intake and FFAR4 regulation in the context of TNF-α alterations.
In conclusion, chronic supplementation of diets with 10% fish oil or flaxseed oil for a period of seven weeks facilitates upregulation of FFAR4 and decreases in TNF-α in the rat colon. At the same time, dietary supplementation with fish oil or flaxseed oil had no effect on plasma GLP-1 or blood glucose levels.
Acknowledgments
This work was supported fully or in part by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant [DK098730] to N.H.M and a Diabetes Action Research and Educational Foundation grant to N.H.M. The authors wish to thank Mrs. Vivienne Brown for secretarial support.
References
- [1].Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–82. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- [2].Hokari R, Matsunaga H, Miura S. Effect of dietary fat on intestinal inflammatory diseases. J Gastroenterol Hepatol. 2013;S4:33–6. doi: 10.1111/jgh.12252. [DOI] [PubMed] [Google Scholar]
- [3].Cabré E, Mañosa M, Gassull MA. Omega-3 fatty acids and inflammatory bowel diseases - a systematic review. Br J Nutr. 2012;107:240–52. doi: 10.1017/S0007114512001626. [DOI] [PubMed] [Google Scholar]
- [4].Kantha SS. Dietary effects of fish oils on human health: a review of recent studies. Yale J Biol Med. 1987;60:37–44. [PMC free article] [PubMed] [Google Scholar]
- [5].Hawkey CJ, Mahida YR, Hawthorne AB. Agents Actions. 1992. Therapeutic interventions in gastrointestinal disease based on an understanding of inflammatory mediators; pp. C22–6. [DOI] [PubMed] [Google Scholar]
- [6].Brown AJ, Jupe S, Briscoe CP. A family of fatty acid binding receptors. DNA Cell Biol. 2005;24:54–61. doi: 10.1089/dna.2005.24.54. [DOI] [PubMed] [Google Scholar]
- [7].Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–4. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- [8].Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burns RN, Moniri NH. Agonism with the omega-3 fatty acids alpha-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem Biophys Res Commun. 2010;396:1030–5. doi: 10.1016/j.bbrc.2010.05.057. [DOI] [PubMed] [Google Scholar]
- [10].Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–4. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- [11].Gotoh C, Hong YH, Iga T, Hishikawa D, Suzuki Y, Song SH, Choi KC, Adachi T, Hirasawa A, Tsujimoto G, Sasaki S, Roh SG. The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun. 2007;9:591–7. doi: 10.1016/j.bbrc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- [12].Enos RT, Velázquez KT, McClellan JL, Cranford TL, Walla MD, Murphy EA. Reducing the dietary omega-6:omega-3 utilizing α-linolenic acid; not a sufficient therapy for attenuating high-fat-diet-induced obesity development nor related detrimental metabolic and adipose tissue inflammatory outcomes. PLoS One. 2014;14:e94897. doi: 10.1371/journal.pone.0094897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paulsen SJ, Larsen LK, Hansen G, Chelur S, Larsen PJ, Vrang N. Expression of the fatty acid receptor GPR120 in the gut of diet-induced-obese rats and its role in GLP-1 secretion. PLoS One. 2014;10:e88227. doi: 10.1371/journal.pone.0088227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cornall LM, Mathai ML, Hryciw DH, McAinch AJ. Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol Biochem. 2011;28:949–58. doi: 10.1159/000335820. [DOI] [PubMed] [Google Scholar]
- [15].Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- [16].Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–88S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- [17].Burns RN, Singh M, Senatorov IS, Moniri NH. Mechanisms of homologous and heterologous phosphorylation of FFA receptor 4 (GPR120): GRK6 and PKC mediate phosphorylation of Thr347, Ser350, and Ser357 in the C-terminal tail. Biochem Pharmacol. 2014;87:650–9. doi: 10.1016/j.bcp.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tanaka T, Yano T, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:515–22. doi: 10.1007/s00210-007-0250-y. [DOI] [PubMed] [Google Scholar]
- [19].Xiong Y, Swaminath G, Cao Q, Yang L, Guo Q, Salomonis H, et al. Activation of FFA1 mediates GLP-1 secretion in mice. Evidence for allosterism at FFA1. Mol Cell Endocrinol. 2013;369:119–29. doi: 10.1016/j.mce.2013.01.009. [DOI] [PubMed] [Google Scholar]
- [20].Widmayer P, Goldschmid H, Henkel H, Küper M, Königsrainer A, Breer H. High-fat feeding affects the number of GPR120 cells and enteroendocrine cells in the mouse stomach. Front Physiol. 2015;6(53):1–6. doi: 10.3389/fphys.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duca FA, Swartz TD, Sakar Y, Covasa M. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes (Lond) 2013;37:375–81. doi: 10.1038/ijo.2012.45. [DOI] [PubMed] [Google Scholar]
- [22].Lim GE, Xu M, Sun J, Jin T, Brubaker PL. The Rho guanosine 5'-triphosphatase, cell division cycle 42, is required for insulin-induced actin remodeling and glucogan-like peptide-1 secretion in the intestinal endocrine L cell. Endocrinology. 2009;150:5249–61. doi: 10.1210/en.2009-0508. [DOI] [PubMed] [Google Scholar]
- [23].Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–63. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- [24].Mobraten K, Haug TM, Kleiveland CR, Lea T. Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Health Dis. 2013;12:101. doi: 10.1186/1476-511X-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li X, Yu Y, Funk CD. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4) FASEB J. 2013;27:4987–97. doi: 10.1096/fj.13-235333. [DOI] [PubMed] [Google Scholar]
- [26].Liu Y, Chen LY, Sokolowska M, Eberlein M, Alsaaty S, Martinez-Anton A, Logun C, Qi HY, Shelhamer JH. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A2 via GPR120 receptor to produce prostaglandin E2 and plays an anti-inflammatory role in macrophages. Immunology. 2014;143:81–95. doi: 10.1111/imm.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wellhauser L, Belsham DD. Activation of the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J Neuroinflammation. 2014;11:60. doi: 10.1186/1742-2094-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsukahara T, Watanabe K, Watanabe T, Yamagami H, Sogawa M, Tanigawa T, et al. Tumor necrosis factor α decreases glucagon-like peptide-2 expression by up-regulating G-protein-coupled receptor 120 in Crohn disease. Am J Pathol. 2015;185:185–196. doi: 10.1016/j.ajpath.2014.09.010. [DOI] [PubMed] [Google Scholar]