Abstract
Objective
While acute hypothalamic-pituitary-adrenal axis response to stress is often adaptive, prolonged responses may have detrimental effects. Many components of white matter structures are sensitive to prolonged cortisol exposure. We aimed to identify a behavioral laboratory assay for which cortisol response related to brain pathophysiology in schizophrenia. We hypothesized that an abnormally prolonged cortisol response to stress may be linked to abnormal white matter integrity in patients with schizophrenia.
Methods
Acute and prolonged salivary cortisol response was measured outside the scanner at pre-test and then at 0, 20, and 40 minutes after a psychological stress task in patients with schizophrenia (n=45) and controls (n=53). Tract-averaged white matter was measured by 64-direction diffusion tensor imaging in a subset of patients (n=30) and controls (n=33).
Results
Patients who did not tolerate and quit the psychological stress task had greater acute (t=2.52, p=0.016; t=3.51, p=0.001 at zero and 20 minutes) and prolonged (t=3.62, p=0.001 at 40 minutes) cortisol reactivity compared with patients who finished the task. Abnormally prolonged cortisol reactivity in patients was significantly associated with reduced white matter integrity (r=−0.468, p=0.009). Regardless of task completion status, acute cortisol response was not related to the white matter measures in patients or controls.
Conclusions
This paradigm was successful at identifying a subset of patients whose cortisol response was associated with brain pathophysiology. Abnormal cortisol response may adversely affect white matter integrity, partly explaining this pathology observed in schizophrenia. Prolonged stress responses may be targeted for intervention to test for protective effects against white matter damages.
Keywords: psychosis, distress, fractional anisotropy, diffusion tensor imaging
Introduction
Stress contributes to the pathophysiology of schizophrenia (SZ) at all illness stages: prenatal stress increases risk for developing schizophrenia (1); stressful life events precede psychosis onset (2); hypothalamic-pituitary-adrenal (HPA) activities are elevated in non-medicated patients (3); stress precipitates psychotic relapses (4); and is associated with worse quality of life and symptom severity (5, 6). This evidence suggests important relationships exist between stress pathophysiology and schizophrenia. Our goal here is to develop a laboratory assay that may capture the dysregulation of the stress-HPA axis response in schizophrenia that is relevant to structural brain abnormalities in SZ, in particular white matter (WM) integrity.
Diffusion tensor imaging (DTI), which measures the directional diffusivity of water in the brain, has been extensively used in schizophrenia, with most studies finding WM abnormalities in some patients (7–11). Fractional anisotropy (FA) remains the most often reported DTI measure. FA reduction, indicating decreased white matter integrity, is evident in some antipsychotic-naive first-episode patients (12, 13) and in non-ill, first-degree relatives (14, 15). The reasons for DTI deficits in SZ remain elusive. Most WM abnormalities in schizophrenia are thought to be myelin-related (16), although WM is a complex tissue comprised of axons, myelin, oligodendrocytes, interstitial neurons, astrocytes, glial cells, and an extensive capillary network, all of which are potentially vulnerable to stress. Proliferation of oligodendrocytes can be suppressed by chronic exposure to glucocorticoids (17), which can delay myelination in major WM tracts (18). Chronic stress can also render lipid membranes and myelin vulnerable to oxidative damage (19, 20). Additionally, cortisol may indirectly influence WM integrity through interaction with pro-inflammatory cytokines (21). Early adverse experiences are associated with elevated daily cortisol (22) and reduced WM FA (23). Abnormally prolonged or heightened HPA reactivity to stress may increase cumulative cortisol exposure. These mechanisms may converge to cause WM damage over time (24).
Stress activates the HPA axis, producing a release of corticosteroids that coordinate neural and physiological reactions. Successful resilience to stress is achieved by acute activation of the HPA axis, followed by timely resolution of the response (25). Negative feedback mechanisms, mostly operating through the forebrain, control resolution of stress responses. Maladaptive stress responses may result from failed suppression of stress response (25, 26). Laboratory studies have found a disrupted stress response system in some schizophrenia patients, including elevated diurnal cortisol (3), with cortisol elevations corresponding to greater symptom severity (27). Previous research has tested acute responses to various behavioral and pharmacological stress paradigms in SZ (28–30). Metabolic stress results in normal or increased response (31); physical challenge resulted in a normal response (28); and surgical stress (32), cold pressor, mental arithmetic and public speaking have been met with diminished HPA responses (28, 33). Schizophrenia is a disease marked by heterogeneity of clinical presentation and outcome, thus it is not surprising that varying patterns of stress response have been documented. Thus, we anticipated that we would observe differences in the adaptiveness of stress responses across our patient group. Our aim here is to test that stress responses to a laboratory psychological stressor may evoke increased and/or prolonged cortisol response; and that such response, if found, may be associated with impaired WM integrity, a pathology present in SZ that is potentially influenced by stress hormones.
Chronic elevations in stress and cortisol levels are associated with reduced subcortical volumes, cognitive deficits, WM lesions and reduced WM integrity (34–39). We hypothesized that the reduced WM integrity found in schizophrenia is determined in part by maladaptive cortisol responses to stress. Specifically, we tested the hypotheses that 1) a subgroup of schizophrenia patients may have an abnormally prolonged cortisol response to psychological stress; and 2) such a response may be detrimental to WM integrity.
Methods and Procedures
Sample
A total of 98 (59 males, aged 20–63, mean age=38±12) individuals completed the stress challenge task between March 2011 and March 2013. Schizophrenia patients (n=45, including 13 with schizoaffective disorder) were recruited from neighboring outpatient clinics. Healthy controls (n=53) were recruited through media advertisements. Of these participants, 30 patients and 33 controls also completed DTI. The Structured Clinical Interview for DSM-IV (40) was utilized to obtain diagnoses, which were based on consensus agreement from two psychiatrists. Controls had no current DSM-IV Axis I diagnoses and no family history of psychosis in the prior two generations. Except three medication-free participants, all patients were on antipsychotic medications. Major medical and neurological illnesses, history of head injury with cognitive sequelae, mental retardation (as per DSM-IV), substance dependence within the past 6 months, or current substance abuse (except nicotine) were exclusionary. Participants gave written informed consent as approved by local IRB. Overall clinical symptoms were assessed by the 20 item Brief Psychiatric Rating Scale. Processing speed was measured using the Digit Symbol Coding subtest of the Wechsler Adult Intelligence Scale-3.
Psychological stress tasks
Participants completed an automated testing session, consisting of two computerized psychological distress-inducing tasks; the Paced Auditory Serial Addition Task (PASAT) and the Mirror-Tracing Persistence Task (MTPT). Task order was randomized. During the PASAT participants performed continuous mental arithmetic by selecting the sum of consecutive numbers presented briefly on a computer screen [e.g. 3+5 (correct response=8) +7 (=12) +10 (=17)] (41). Incorrect or delayed responses were met with a loud (90 decibel) aversive explosion sound. The task consisted of two learning sessions followed by an experimental session. Speed of response and accuracy were measured and the speed of the task presentation was titrated automatically to account for some individual differences in cognitive capacity but not to secure equal performance. The titration changed only to some extent and the boundaries of the titration were calibrated initially to achieve correct response in every other trial (or 50% error rate) on average in healthy participants. During the MTPT, participants traced a red dot along the outline of a star image on the computer screen, using the mouse (42). Tracing was challenging because the cursor movement was opposite to the mouse movement. When the participant traced outside the line or kept the mouse stationary the loud aversive sound played. The MTPT task consisted of three learning sessions followed by an experimental session. The width of the star outline was also partially titrated automatically depending on performance. For both tasks, participants could quit the experimental session at any time, but were informed that the better they performed the greater monetary bonus they would receive. The PASAT and MTPT could last up to 12 and 13 minutes each, respectively. Participants were not told the maximum amount of time allowed for either task. Task training and administration was computer automated. Staff were trained to exert minimal interference or instruction.
Both the PASAT and the MTPT have been widely used as measures of distress intolerance (DI) (43, 44). Participants were defined as “distress intolerant” if they quit both tasks before completion. They were considered “distress tolerant” if they tolerated one or both tasks. Combining the tasks allows for a more rigorous definition of DI by reducing the potential skill-dependent bias that would result if some participants were particularly skilled at arithmetic or eye-hand coordination.
Measurement of salivary cortisol
Participants provided saliva at four time points during the testing session: prior to task initiation, immediately following completion of the last task or quitting (post 0 minutes), 20 and 40 minutes post task completion. After the post 0 minute collection participants sat quietly, and read magazines until the remaining samples were collected. All testing sessions were held between 12:00 and 4:00 pm, and participants were asked to refrain from eating, drinking or smoking for one hour before testing. Saliva samples were immediately stored at −80° Celsius until assay. Prior to assay, samples were thawed and centrifuged at 10,000 × g for 10 minutes. Cortisol was assayed using a commercial enzyme immunoassay kit (Salimetrics), following the manufacturer protocol recommended. Intra-assay coefficient of variance (CV) was 7.77% and inter-assay CV was 4.18% in our lab. Salivary cortisol levels (ug/dL) are highly correlated with serum levels and typically peak at 30 minutes following the initiation of stressor tasks (45).
Imaging
Imaging was performed at the University of Maryland Center for Brain Imaging Research by using a Siemens 3T TRIO scanner (Erlangen, Germany) and 32 channel head coil. The high-angular resolution diffusion imaging (HARDI) protocol was used to assess WM integrity as measured by FA (46). Diffusion tensor data were collected using a single-shot, echo-planar, single refocusing spin-echo, T2-weighted sequence with a spatial resolution of 1.7×1.7×3.0 mm. The sequence parameters were: TE/TR=87/8000ms, FOV=200mm, axial slice orientation with 50 slices and no gaps, 64 isotropically distributed diffusion weighted directions, two diffusion weighting values (b=0 and 700 s/mm2) and five b=0 images (47). The total scan time was about 9 minutes.
All data passed QA control of <3mm accumulated motion during the scan. There were no differences in the average motion per TR between patients and controls (0.42±0.21 vs. 0.43±0.20, respectively). The HARDI data was processed by using a tract-based spatial statistics method in FSL (46). The population-based, 3D, DTI WM tract atlas developed in John Hopkins University and distributed with the FSL package (48) was used to calculate population average FA values along the spatial course of major WM tracts (49). Tract-averaged FA was the primary measure. In addition, the relationships of cortisol responses with 12 major WM tracts were explored to examine the tract-specific effects.
Statistical analyses
Cortisol reactivity in response to the stress challenge was the primary measure. It was measured at three time points as the difference between: 1) post 0 minute and pre-test cortisol, 2) post 20 minute and pre-test cortisol, and 3) post 40 minute and pre-test cortisol. The cortisol reactivity within 0–20 minutes was considered an acute response to stress and the cortisol reactivity at 40 minutes a prolonged response to stress. Pre-test cortisol level was not examined independently due to diurnal curves that vary across individuals and because sleep-wake cycles and activity level were not controlled. Only the cortisol reactivity to the stress challenge, which is a subtraction from the pre-test value, was considered. A repeated measures ANOVA was utilized, where the three time points were the repeated measures, and diagnosis (SZ vs. control) and distress intolerance (DI vs. non-DI) were the between-person factors. Significant findings were followed up, using simple effect two tailed t-tests to assess specific group differences. Similarly, post-stress cortisol change was measured at two time points as the difference between: 1) post-test 20 minute and post-test 0 minute cortisol, and 2) post 40 minute and post 0 minute cortisol. Here, repeated measures ANOVA was performed where the two time points were the repeated measures, and diagnosis and DI were the between-person factors. We investigated differences in tract-averaged FA by diagnostic group, using a two tailed t-test, and then examined the associations between tract-averaged FA and acute vs. prolonged cortisol reactivity, using linear regression and Pearson’s correlations. We calculated the averaged error rate on both stressor tasks, and controlled for errors in investigations of cortisol response and FA. Equality of group variances, outlying values, residuals, and leverage of specific data points were examined and no datapoints were found to violate the ANOVA assumptions or exert excessive influence on the cortisol-FA association. Lastly, we investigated the influence of medications effects on cortisol reactivity to stress and tract-averaged FA.
Results
Cortisol response
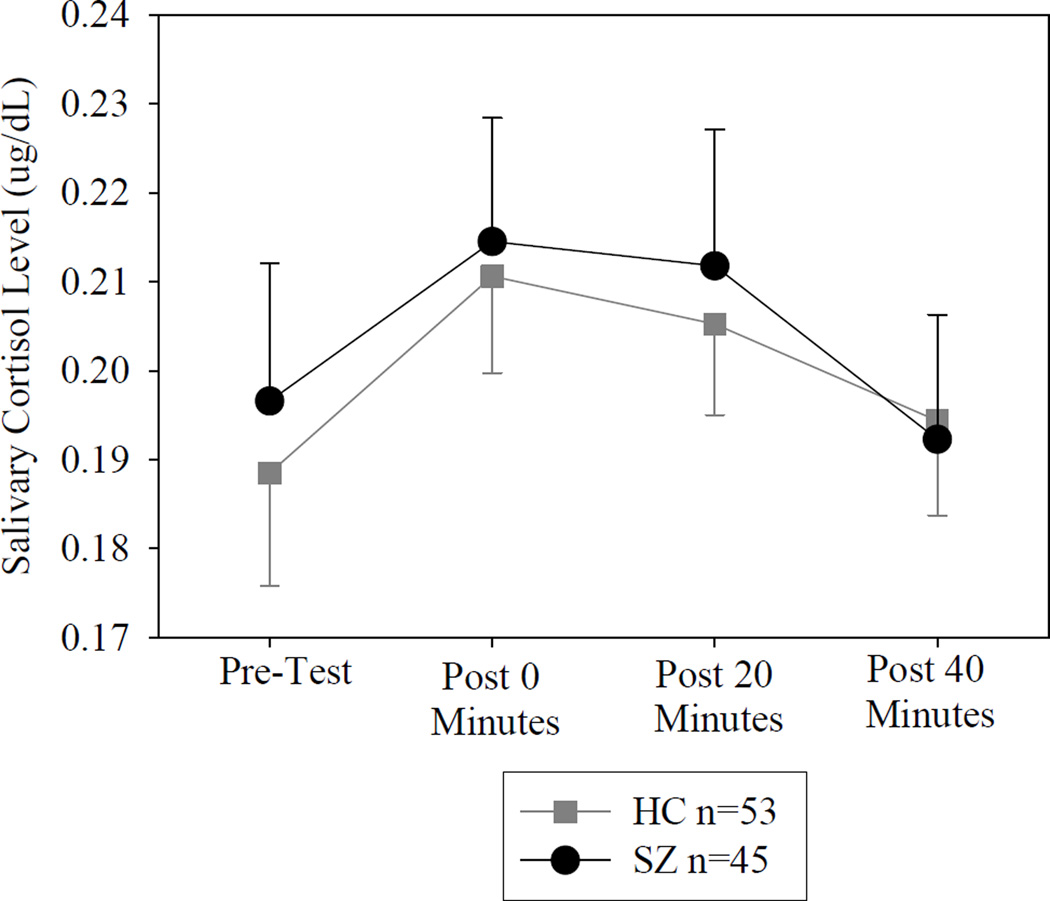
Socio-demographic and clinical characteristics are presented in Table 1. There were no significant differences in age, sex, BMI or smoking status between patients and controls. Controls began the laboratory stress at a mean(SD) time of 13:40 (00:55), while SZ began at 13:26(01:04), with no significant difference between groups (t=1.56, p=0.12). To investigate the overall stress response, a repeated-measures ANOVA of cortisol across all four time points showed a significant effect of time (Greenhouse-Geisser corrected F=4.19, p=0.019), with no significant differences by diagnosis or diagnosis × time interaction (Figure 1). There was no significant difference between patients and controls on their pre-test cortisol level (t=0.406, p=0.69). These findings suggest that this task evoked a significant salivary cortisol response that was not significantly different by diagnosis when their behavioral response of distress intolerance was not considered.
Table 1.
Sociodemographic and Clinical Characteristics of the Study Sample
| Stress Challenge Sample | DTI Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Controls (n=53) |
Schizophrenia (n=45) |
Test Statistic |
p value |
Healthy Controls (n=33) |
Schizophrenia (n=30) |
Test Statistic |
p value |
|
| Male, % | 57% | 64% | X2=0.625 | 0.43 | 52% | 73% | X2=3.172 | 0.075 |
| Age, mean (SD) | 38.4 (12.8) | 37.6 (11.6) | t=0.318 | 0.75 | 38.9 (13.1) | 39.4 (12.3) | t=−0.142 | 0.89 |
| Smoking status (% smokers) | 38% | 49% | X2=1.236 | 0.27 | 36% | 47% | X2=0.688 | 0.41 |
| Education (less than HS vs. HS vs. above HS) | 2:26:72% | 13:35:52% | X2=6.785 | 0.034 | 0:31:69% | 17:23:60% | X2=6.010 | 0.050 |
| BMI, mean (SD) | 26.8 (4.1) | 29.1 (6.0) | t=−1.824 | 0.073 | 26.8 (4.1) | 29.4 (6.5) | t=−1.845 | 0.071 |
| BPRS Total, mean (SD) | 23.5 (3.0) | 23.6 (3.1) | ||||||
| PASAT Error Rate, mean (SD) | 0.52(0.2) | 0.63(0.2) | t=−3.645 | <0.001 | 0.47(0.1) | 0.65(0.2) | t=−4.448 | <0.001 |
| MTPT Error Rate, mean(SD) | 0.62(0.4) | 0.81(0.4 | t=−2.215 | 0.029 | 0.60(0.4) | 0.79(0.4) | t=−1.772 | 0.081 |
HS=High School, BMI = Body Mass Index, BPRS = Brief Psychiatric Rating Scale, PASAT = Paced Auditory Serial Addition Task, MTPT = Mirror-Tracing Persistence Task.
Figure 1.
Cortisol response in healthy controls and schizophrenia patients (ug/dL, mean and standard error). HC=Healthy control, SZ=Schizophrenia patient.
Cortisol reactivity as related to distress intolerance and diagnosis
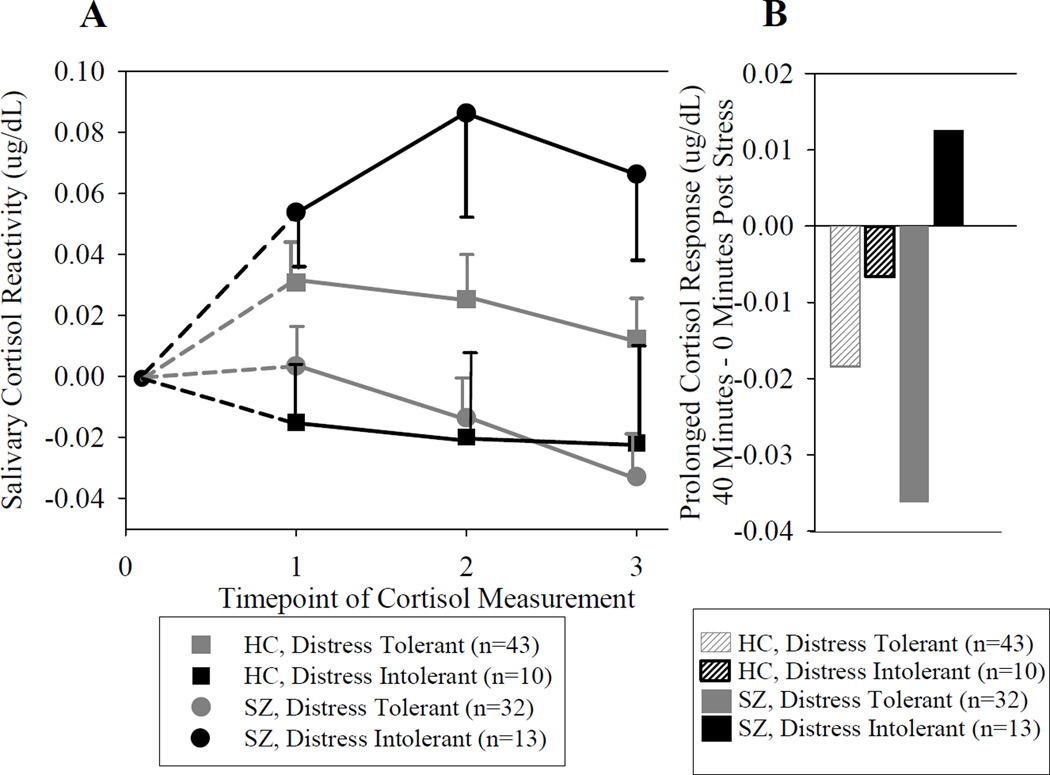
Distress intolerance (DI) was found in 19% of controls and 29% of SZ (X2=1.36, p=0.24). Distress tolerant SZ and controls did not differ on their in-tasks times (t=1.02, p=0.31) while DI patients had a significantly shorter total task time as compared to DI controls [mean(SD) minutes in SZ=14.0(2.9) vs. 17.6(2.8) in controls; t=2.27, p=0.034]. For cortisol reactivity, there was a significant diagnosis by DI interaction [F(1, 94)=12.40, p=0.001] and time × DI interaction [F(1.8, 168.6)=3.35, p=0.042] (Figure 2). The diagnosis by DI interaction was due to patients with DI responding with heightened cortisol reactivity, such that they had higher acute cortisol reactivity, and also that their cortisol continued to rise after the stress test was completed, and did not fall below their pre-test value by 40 minutes post task. The significant DI by time interaction was limited to patients [F(1.9, 80.8)=6.23, p=0.004], where DI SZ had a pattern of significantly higher and prolonged cortisol reactivity compared with distress tolerant patients (t=3.51, p=0.001 at 20 minutes and t=3.62, p=0.001 at 40 minutes) (Figure 2).
Figure 2.
A. Cortisol reactivity over time grouped by diagnosis and distress intolerance. Data represent mean (SE) cortisol response for each subgroup after subtracting the group’s mean pre-test cortisol level (ug/dL), HC=Healthy control, SZ=Schizophrenia patient. 0=pre-test; 1= immediately after stress test; 2=20 minute; and 3= 40 minute after stress test. B. Prolonged post-stress test cortisol change grouped by diagnosis and distress intolerance. Data represent mean change in cortisol level from 0 to 40 minutes post stress task. Note that all subgroups showed reduced cortisol levels at 40 minutes compared to cortisol levels measured immediately after stress test, with the only exception in the subgroup of schizophrenia patients with distress intolerance, who had increased cortisol level at 40 minutes compared with 0 minutes after the stress test, indicating a further rise of cortisol level after the stress test was ended.
The DI by time interaction was not significant in controls [F(1.6, 83.6)=0.24, p=0.74]. Controls with DI displayed an opposite pattern, such that their cortisol level remained at or even fell below pre-testing levels, which suggested little or no reactivity as diurnal cortisol levels normally decline during the afternoon testing time period (noon to 4:00 pm). There was no significant differences in cortisol reactivity by DI group in controls any time point (all p>0.05).
The post-stress cortisol response calculation represents the change of cortisol level occurring after the stress test, when participants were at rest and no longer exposed to direct stress. A repeated measures ANOVA showed that there was a significant effect of DI [F(1, 94)=5.62, p=0.020] and a non-significant trend of a diagnosis × DI interaction [F(1,94)=3.36, p=0.070]. In HC, exploratory post-hoc tests showed no significant differences in post-stress cortisol change in DI vs. non-DI [F(1,51)=0.11, p=0.75]. However, in patients, cortisol changes post task compared to 0 minutes post task were significantly different in DI vs. non-DI patients [F(1,43)=13.48, p<0.001]. This difference was due to a remarkable increase in post-stress cortisol in schizophrenia with DI (see Figure 2), demonstrating that cortisol levels continued to rise even “at rest”. In comparison, other participants’ cortisol levels tended to gradually decline after the stress test, representing either a return to pre-stress baseline or a part of the normal cortisol diurnal decline during the afternoon.
Task titration was partially successful, as the mean(SD) PASAT error rate in controls was 0.50(0.2), and was 0.63(0.2) in SZ; (t=−3.65, p<0.001). Increased errors were significantly associated with task termination in patients (t=−2.6, p=0.013) but not controls (t=−1.45, p=0.153). For the MTPT, the error rate was 0.62(0.4) for controls vs. 0.81(0.4) for SZ (t=−2.21, p=0.029). Increased errors were significantly associated with terminating the task early in patients (t=−2.83, p=0.007) but not controls (t=−1.44, p=0.156). Errors for PASAT were significantly correlated with prolonged cortisol response in patients only (r=0.56, p<0.001 in SZ; r=0.07, p=0.60 in controls). Errors for MTPT were not significantly associated with prolonged cortisol response in either group.
Relationship of cortisol response and white matter integrity
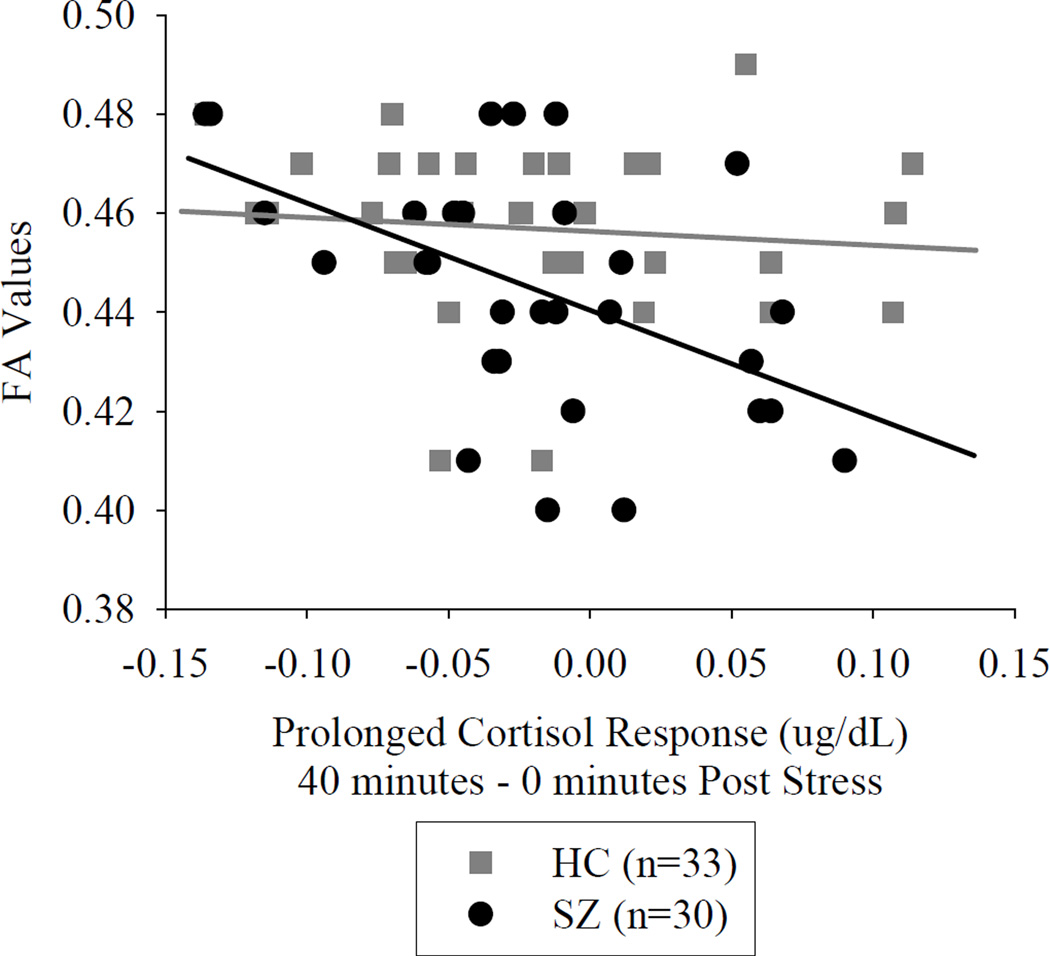
The tract-averaged FA value was significantly reduced in patients (p=0.016) (Table 2). Acute cortisol reactivity measured at 0 and 20 minutes post task were not significantly associated with tract-averaged FA in either group. Prolonged cortisol reactivity, measured at 40 minutes post task, was inversely associated with tract-averaged FA in schizophrenia (r=−0.468, p=0.009, significant after Bonferroni correction for three comparisons) but not in controls (r=−0.122, p=0.50). When we used the “post-stress cortisol change” measure, we found that this measure at 40 minutes was also associated with FA in schizophrenia (r=−0.525, p=0.003, significant after Bonferroni correction) but not in controls (r=−0.105, p=0.56) (Figure 3).
Table 2.
Fractional Anisotropy of the Tract Averaged and 12 Separate White Matter Tracts: Mean Values and Associations with Prolonged Cortisol Reactivity
| Mean FA Values (SE) | Association Between Prolonged Cortisol Reactivity Value (40 minutes – Pre-Test) and Tract FA |
|||||||
|---|---|---|---|---|---|---|---|---|
| White matter tract | Healthy Controls (n=33) |
Schizophrenia (n=30) |
Test Statistic |
p Value | Healthy Controls (n=33) | Schizophrenia (n=30) | ||
| Coef. | p Value | Coef. | p Value | |||||
| Total Tracts (Averaged) | 0.458 (0.003) | 0.445 (0.004) | t=2.489 | 0.016 | β= −222.8 | p=0.50 | β= −1211.4 | p=0.009 |
| Genu of CC | 0.738 (0.006) | 0.704 (0.001) | t=2.927 | 0.005* | β= −315.8 | p=0.650 | β= −2110.9 | p=0.051 |
| Body of CC | 0.659 (0.007) | 0.621 (0.001) | t=2.764 | 0.008 | β= −240.5 | p=0.752 | β= −3321.6 | p=0.010 |
| Splenium of CC | 0.761 (0.004) | 0.737 (0.007) | t=3.029 | 0.004* | β= −161.6 | p=0.680 | β= −294.4 | p=0.712 |
| Fornix | 0.456 (0.007) | 0.433 (0.001) | t=1.811 | 0.075 | β= 297.2 | p=0.700 | β= −2834.4 | p=0.009 |
| Corticospinal | 0.567 (0.005) | 0.560 (0.006) | t=0.968 | 0.337 | β= −859.0 | p=0.096 | β= −192.2 | p=0.754 |
| Internal capsule | 0.622 (0.004) | 0.610 (0.005) | t=1.963 | 0.054 | β= −749.5 | p=0.112 | β= −898.7 | p=0.082 |
| External capsule | 0.495 (0.004) | 0.478 (0.005) | t=2.745 | 0.008 | β= −404.6 | p=0.356 | β= −1078.1 | p=0.034 |
| Corona radiata | 0.493 (0.004) | 0.478 (0.005) | t=2.283 | 0.026 | β= 106.3 | p=0.810 | β= −1505.4 | p=0.007 |
| Thalamic radiation | 0.611 (0.005) | 0.588 (0.007) | t=2.557 | 0.013 | β= −410.5 | p=0.462 | β= −1365.5 | p=0.074 |
| Sagittal striatum | 0.563 (0.004) | 0.534 (0.007) | t=3.735 | <0.001* | β= −615.7 | p=0.192 | β= −1685.0 | p=0.015 |
| Cingulum | 0.622 (0.006) | 0.608 (0.009) | t=1.353 | 0.181 | β= −386.1 | p=0.543 | β= −2121.9 | p=0.018 |
| SLF | 0.507 (0.005) | 0.492 (0.006) | t=2.043 | 0.045 | β= −261.8 | p=0.595 | β=−1649.7 | p=0.009 |
CC=corpus callosum; SLF = superior longitudinal fasciculus;
Statisticaly significant after Šidák-Holm correction for multiple comparison (adjusted p-value < 0.05, controlling for 12 comparisons).
Figure 3.
Prolonged cortisol change after stress test is associated with tract averaged average fractional anisotrophy (FA) in schizophrenia patients. HC=Healthy control, SZ=Schizophrenia patient.
DI controls had significantly reduced tract-averaged FA as compared to distress tolerant controls (t=−2.27, p=0.030). In contrast, DI patients did not have reduced FA as compared to distress tolerant patients (mean=0.437 vs. 0.448, t=1.016, p=0.32).
Finally, we explored the effect of prolonged cortisol reactivity on twelve major WM tracts within SZ. Prolonged cortisol reactivity (40 – 0 minutes post task) was negatively associated with FA in the body of corpus callosum, fornix, corona radiate, sagittal striatum, cingulum and the superior longitudinal fasciculus (SLF; see Table 2), although none were below the corrected significance threshold of p<0.003.
Clinical correlates
Age, sex, education, BMI or smoking status were not significantly associated with acute or prolonged cortisol reactivity (all p>0.05). Sex, smoking status, education and BMI were not significantly associated with tract-averaged FA in either group. Age was significantly associated with reduced FA in controls (r=−0.438, p=0.011) and in patients (r=−0.502, p=0.005). Comparing schizophrenia and schizoaffective patients, pre-test cortisol was significantly higher in schizoaffective patients (t=2.03, p=0.048). However, cortisol reactivity did not significantly differ between patient subgroups (t=1.16, p=0.25 at 0 minutes; t=0.08, p=0.93 at 20 minutes; t=0.25, p=0.81 at 40 minutes). Schizoaffective patients were not more likely than schizophrenia to be distress intolerant (X2=0.30, p=0.58), and were not significantly different in tract-averaged FA (t=0.95, p=0.35).
Reduced processing speed was associated with greater acute (r=−0.37, p=0.018 at 0 minutes; r=−0.39, p=0.012 at 20 minutes) and prolonged (r=−0.46, p=0.003) cortisol reactivity in patients but not controls (r=0.05, p=0.76 at 0 minutes; r=−0.006, p=0.97 at 20 minutes; r=−0.03, p=0.834 at 40 minutes).
There were no significant correlations between chlorpromazine dose equivalent of antipsychotic medication and acute (r=.029, p=.85), prolonged (r=.065, p=.70), or post-stress cortisol response (r=.048, p=.78). Chlorpromazine dose equivalent was also not significantly associated with tract-averaged FA (p=0.32).
Discussion
In this investigation, schizophrenia patients as a group experience similar increases in cortisol after participating in a psychological stressor task when compared with healthy controls. However, using behaviorally defined distress intolerance as a means to reduce heterogeneity, we found that patients with this marker experienced prolonged cortisol reactivity. Similar to many prior investigations, SZ had significantly reduced tract-averaged FA as compared to controls (50, 51). In regards to our primary aim, this behavioral stress paradigm was successful such that cortisol response significantly related to brain pathophysiology in patients. Prolonged cortisol reactivity, but not acute cortisol reactivity, was associated with reduced tract-averaged FA in schizophrenia. The post-stress, “at rest” cortisol rise in patients may be particularly interesting as this change in cortisol level was strongly associated with FA, such that 25% of the tract-averaged FA variance was explained by the measure of cortisol change occurring after the stress challenge. The automated titration of the stressor tasks was partially successful, yet patients experienced a higher error rate than controls.
While acute cortisol response to stress is adaptive due to its effect at mobilizing energy resources to respond to demands (52), it typically peaks 20–30 minutes after initiation of the stressor and then declines (45). Elevated levels of stress hormones are expected when encountering a stressor; yet continued elevation beyond stressor termination is conceptualized as maladaptive as it could be due to rumination, anger, or increased sensitivity for future stress (53, 54). Indeed, we found no evidence that acute cortisol reactivity corresponded to WM integrity in either controls or patients. However, prolonged cortisol reactivity and/or inadequate recovery, especially after a period of 40 minutes of rest, is likely not an adaptive response. Continued stress hormone secretion may undermine protective mechanisms and increase allostatic load (54, 55). Additionally, quick recovery from stress-induced arousal may reflect effective coping and is a core component of theories of stress and disease (56). Laboratory stressors are hampered in their limited generalizability to real world challenges; however the stressor task utilized in this study evokes frustration and cognitive challenge, which are similar to many stressful experiences SZ would experience in everyday life. Overall, our finding of a correlation between reduced FA with prolonged cortisol reactivity is consistent with previous reports that chronic stress is associated with WM lesions (34) and reduced FA (57), and supports the a priori hypothesis that poor resolution of cortisol responses could cumulatively lead to WM alterations.
One should also consider the alternative hypothesis that reduced WM integrity may contribute to abnormal stress responses, for example by disrupting the negative feedback loop responsible for ending the cortisol response. Regulation of the cortisol response depends on the integrated activity of prefrontal regions, the amygdala, and hippocampus (58). Prolonged cortisol reactivity could represent a failure of the hippocampus and/or the hypothalamic-pituitary-adrenal axis to provide negative feedback to inhibit the stress response (59). However, this hypothesis would suggest that low FA would be associated with prolonged cortisol reactivity in controls as well as patients, which was not the case.
In contrast to the patients, we were puzzled by the finding that controls with DI exhibited no significant increase in their cortisol response, suggesting different mechanisms or effects of early task termination between patients and controls. The lack of a cortisol response in DI controls is perhaps consistent with the theory that cortisol systems are only activated when an individual is motivated, and their goals are threatened (Lazarus, 1999). A subset of our controls who have quit early may not have felt motivated to perform the tasks and perhaps it was only the distress tolerant controls who fully engaged themselves in the task, thus explaining why they displayed an acute cortisol response followed by recovery.
There were a number of limitations. The neurobiological specificity of measures of diffusivity and fractional anisotropy obtained from DTI remains unclear, which is a limitation of the study and limits our ability to fully explain the correlational finding between FA and prolonged cortisol response. We limited our analysis to use of FA, as this is the most commonly used marker for ‘white matter integrity’ using DTI techniques, and best serves our purpose as a more global measure of relative differences in WM microstructure. However, there are limits to how far we can interpret FA values at the cellular level. The stress paradigm is also limited in its inability for separating the causal relationships among cognitive capacity, errors, performance and motivation level; nor can it identify specific effects from arithmetic vs. manual challenges. However, the task was successful in eliciting prolonged cortisol reactivity to the task in some patients, and the abnormal response was related to WM.
There have been several attempts to establish laboratory based paradigms to examine stress response in schizophrenia. Direct pharmacological stimulation of the HPA axis using serotonin agonists was found to induce normal increases in pituitary-adrenal hormones, thus the biological path of the axis is presumed to be intact (60). Blunted or normal cortisol responses have been reported in SZ exposed to stress induced by surgical procedures (61) and public speaking tasks (28, 62). None of these paradigms provided a choice of “quitting” from the stressor; this design produces a behavioral phenotype that corresponds with the pattern of cortisol reactivity, and thus may be useful for parsing the clinical heterogeneity of schizophrenia. Variability in neuroendocrine responses to stress across paradigms could also be introduced by confounds inherent to the disorder such as motivational deficits or paranoia; for example the presence of a research staff member(s) or tester(s) during stress challenges could heighten or reduce stress in different individuals. In our distress task, we minimize the role of staff by automating the entire testing sequence, hopefully providing a simpler but more consistent laboratory paradigm for examining stress biology in schizophrenia.
In conclusion, acute cortisol response to a laboratory psychological stress task was not associated with abnormal neurobiology as investigated by WM integrity. It was only a prolonged cortisol elevation to the stress task that was associated with reduced WM integrity in SZ. We propose that this cross-sectional laboratory marker may mimic responses to stress challenges experienced outside of the laboratory in these patients, such that they may have prolonged exposure to cortisol resulting from poor resolution of stress responses in everyday life, resulting in altered WM and likely other neuropathophysiology observed in schizophrenia. This observation, if replicated, may guide our efforts aiming to identifying more effective interventions. For example, SZ with abnormally heightened cortisol responses to this simple stress test can be identified as a less heterogeneous subgroup for whom targeted management for reducing their abnormal stress responses could extend a neuroprotective effect.
Acknowledgements
None.
Source of Funding: This research was supported by National Institute of Health grants R01DA027680, P50MH103222, R01MH085646, T32MH067533 and R01EB01561, and a NARSAD Young Investigator Award.
Glossary
- SZ
schizophrenia
- HC
healthy control
- DI
distress intolerance
- HPA
hypothalamic-pituitary-adrenal
- FA
fractional anisotropy
- DTI
diffusion tensor imaging
- WM
white matter
- PASAT
Paced Auditory Serial Addition Task
- MTPT
Mirror-Tracing Persistence Task
- CV
coefficient of variance
- HARDI
high-angular resolution diffusion imaging
- FSL
FMRIB Software Library
- BMI
body mass index
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.van Os J, Selten J. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. The British Journal of Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 2.Norman RM, Malla AK. Stressful life events and schizophrenia. I: A review of the research. The British Journal of Psychiatry. 1993;162:161. doi: 10.1192/bjp.162.2.161. [DOI] [PubMed] [Google Scholar]
- 3.Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic–pituitary–adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Tessner KD, Mittal V, Walker EF. Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophr. Bull. 2011;37:432–441. doi: 10.1093/schbul/sbp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garner B, Phassouliotis C, Phillips LJ, Markulev C, Butselaar F, Bendall S, Yun Y, McGorry PD. Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. J. Psychiatr. Res. 2011;45:249–255. doi: 10.1016/j.jpsychires.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Belvederi Murri M, Pariante CM, Dazzan P, Hepgul N, Papadopoulos AS, Zunszain P, Di Forti M, Murray RM, Mondelli V. Hypothalamic–pituitary–adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocrinology. 2012;37:629–644. doi: 10.1016/j.psyneuen.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Kanaan RA, Kim J, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol. Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Kubicki M, McCarley R, Westin C, Park H, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatr. Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, Hall J, Bastin ME, Johnstone EC, Lawrie SM. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 10.Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2007;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 11.Kochunov P, Glahn DC, Rowland LM, Olvera RL, Winkler A, Yang Y, Sampath H, Carpenter WT, Duggirala R, Curran J. Testing the Hypothesis of Accelerated Cerebral White Matter Aging in Schizophrenia and Major Depression. Biol. Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung V, Cheung C, McAlonan G, Deng Y, Wong J, Yip L, Tai K, Khong P, Sham P, Chua S. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol. Med. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 13.Gasparotti R, Valsecchi P, Carletti F, Galluzzo A, Liserre R, Cesana B, Sacchetti E. Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophr. Res. 2009;108:41–48. doi: 10.1016/j.schres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Camchong J, Lim KO, Sponheim SR, Macdonald AW. Frontal white matter integrity as an endophenotype for schizophrenia: diffusion tensor imaging in monozygotic twins and patients' nonpsychotic relatives. Front. Hum. Neurosci. 2009;3:35. doi: 10.3389/neuro.09.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz Maniega S, Lymer G, Bastin M, Marjoram D, Job D, Moorhead T, Owens D, Johnstone E, McIntosh A, Lawrie S. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophr. Res. 2008;106:132–139. doi: 10.1016/j.schres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch. Gen. Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 17.Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Harper C, Evans S, Newnham J, Dunlop S. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. International Journal of Developmental Neuroscience. 2001;19:415–425. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 19.Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology. 2001;24:420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 20.Schiavone S, Jaquet V, Sorce S, Dubois-Dauphin M, Hultqvist M, Bäckdahl L, Holmdahl R, Colaianna M, Cuomo V, Trabace L. NADPH oxidase elevations in pyramidal neurons drive psychosocial stress-induced neuropathology. Translational psychiatry. 2012;2:e111. doi: 10.1038/tp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions 1. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 22.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev. Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 23.Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. Journal of psychiatry & neuroscience: JPN. 2012;37:37. doi: 10.1503/jpn.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Translational psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman J. Neural Control of Chronic Stress Adaptation. Frontiers in Behavioral Neuroscience. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 27.Ritsner M, Maayan R, Gibel A, Strous RD, Modai I, Weizman A. Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients. European Neuropsychopharmacology. 2004;14:267–273. doi: 10.1016/j.euroneuro.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Jansen LM, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology (Berl.) 2000;149:319–325. doi: 10.1007/s002130000381. [DOI] [PubMed] [Google Scholar]
- 29.Marcelis M, Cavalier E, Gielen J, Delespaul P, Van Os J. Abnormal response to metabolic stress in schizophrenia: marker of vulnerability or acquired sensitization? Psychol. Med. 2004;34:1103–1111. doi: 10.1017/s0033291703001715. [DOI] [PubMed] [Google Scholar]
- 30.Breier A, Wolkowitz OM, Doran AR, Bellar S, Pickar D. Neurobiological effects of lumbar puncture stress in psychiatric patients and healthy volunteers. Psychiatry Res. 1988;25:187–194. doi: 10.1016/0165-1781(88)90050-9. [DOI] [PubMed] [Google Scholar]
- 31.Elman I, Adler CM, Malhotra AK, Bir C, Pickar D, Breier A. Effect of acute metabolic stress on pituitary-adrenal axis activation in patients with schizophrenia. Am. J. Psychiatry. 1998;155:979–981. doi: 10.1176/ajp.155.7.979. [DOI] [PubMed] [Google Scholar]
- 32.Kudoh A, Kudo T, Ishihara H, Matsuki A. Depressed pituitary-adrenal response to surgical stress in chronic schizophrenic patients. Neuropsychobiology. 1997;36:112–116. doi: 10.1159/000119372. [DOI] [PubMed] [Google Scholar]
- 33.Albus M, Ackenheil M, Engel RR, Müller F. Situational reactivity of autonomic functions in schizophrenic patients. Psychiatry Res. 1982;6:361–370. doi: 10.1016/0165-1781(82)90026-9. [DOI] [PubMed] [Google Scholar]
- 34.Johansson L, Skoog I, Gustafson DR, Olesen PJ, Waern M, Bengtsson C, Björkelund C, Pantoni L, Simoni M, Lissner L. Midlife Psychological Distress Associated With Late-Life Brain Atrophy and White Matter Lesions: A 32-Year Population Study of Women. Psychosom. Med. 2012;74:120–125. doi: 10.1097/PSY.0b013e318246eb10. [DOI] [PubMed] [Google Scholar]
- 35.Howell BR, McCormack KM, Grand AP, Sawyer NT, Zhang X, Maestripieri D, Hu X, Sanchez MM. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biology of mood & anxiety disorders. 2013;3:21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lupien SJ, de Leon M, De Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh HI, Joanisse MF, Mackrell SM, Kryski KR, Smith HJ, Singh SM, Hayden EP. Links between white matter microstructure and cortisol reactivity to stress in early childhood: Evidence for moderation by parenting. NeuroImage: Clinical. 2014;6:77–85. doi: 10.1016/j.nicl.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macritchie KA, Gallagher P, Lloyd AJ, Bastin ME, Vasudev K, Marshall I, Wardlaw JM, Ferrier IN, Moore PB, Young AH. Periventricular white matter integrity and cortisol levels in healthy controls and in euthymic patients with bipolar disorder: An exploratory analysis. J. Affect. Disord. 2013;148:249–255. doi: 10.1016/j.jad.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Cox SR, Bastin ME, Ferguson KJ, Maniega SM, MacPherson SE, Deary IJ, Wardlaw JM, MacLullich AM. Brain white matter integrity and cortisol in older men: the Lothian Birth Cohort 1936. Neurobiol. Aging. 2015;36:257–264. doi: 10.1016/j.neurobiolaging.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV-patient edition (SCID-P) New York: Biometric Research; 1995. [Google Scholar]
- 41.Lejuez C, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor. The Behavior Therapist. 2003 [Google Scholar]
- 42.Strong D, Lejuez C, Daughters S, Marinello M, Kahler C, Brown R. The computerized mirror tracing task version 1. 2003 Unpublished manual. [Google Scholar]
- 43.McHugh RK, Daughters SB, Lejuez CW, Murray HW, Hearon BA, Gorka SM, Otto MW. Shared variance among self-report and behavioral measures of distress intolerance. Cognitive therapy and research. 2011;35:266–275. doi: 10.1007/s10608-010-9295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daughters SB, Lejuez C, Kahler CW, Strong DR, Brown RA. Psychological distress tolerance and duration of most recent abstinence attempt among residential treatment-seeking substance abusers. Psychology of Addictive Behaviors. 2005;19:208. doi: 10.1037/0893-164X.19.2.208. [DOI] [PubMed] [Google Scholar]
- 45.Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Kochunov P, Glahn DC, Lancaster J, Thompson PM, Kochunov V, Rogers B, Fox P, Blangero J, Williamson D. Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage. 2011;58:41–49. doi: 10.1016/j.neuroimage.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones D, Horsfield M, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 1999;42 [PubMed] [Google Scholar]
- 48.Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PC, Mori S. Fiber Tract–based Atlas of Human White Matter Anatomy1. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 49.Kochunov P, Williamson D, Lancaster J, Fox P, Cornell J, Blangero J, Glahn D. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol. Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, Noguchi H, Nakabayashi T, Hori H, Harada S, Saitoh O. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Research: Neuroimaging. 2007;154:133–145. doi: 10.1016/j.pscychresns.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Friedman J, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof P. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am. J. Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- 52.Weiner H. Perturbing the organism: The biology of stressful experience. University of Chicago Press; 1992. [Google Scholar]
- 53.Anisman H, Zalcman S, Shanks N, Zacharko RM. Anonymous. Springer; 1992. Multisystem regulation of performance deficits induced by stressors; pp. 1–59. [Google Scholar]
- 54.Linden W, Earle T, Gerin W, Christenfeld N. Physiological stress reactivity and recovery: Conceptual siblings separated at birth? J. Psychosom. Res. 1997;42:117–135. doi: 10.1016/s0022-3999(96)00240-1. [DOI] [PubMed] [Google Scholar]
- 55.McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 56.Johansson G, Frankenhaeuser M. Temporal factors in sympatho-adrenomedullary activity following acute behavioral activation. Biol. Psychol. 1973;1:63–73. doi: 10.1016/0301-0511(73)90014-8. [DOI] [PubMed] [Google Scholar]
- 57.Sun Y, Wang Z, Ding W, Wan J, Zhuang Z, Zhang Y, Liu Y, Zhou Y, Xu J. Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident-induced PTSD. PloS one. 2013;8:e83473. doi: 10.1371/journal.pone.0083473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 59.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheepers FE, Gispen de Wied Christine C, Kahn RS. The effect of olanzapine treatment on m-chlorophenylpiperazine-induced hormone release in schizophrenia. J. Clin. Psychopharmacol. 2001;21:575–582. doi: 10.1097/00004714-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Kudoh A, Ishihara H, Matsuki A. Pituitary-adrenal and parasympathetic function in chronic schizophrenic patients with postoperative ileus or hypotension. Neuropsychobiology. 1999;39:125–130. doi: 10.1159/000026572. [DOI] [PubMed] [Google Scholar]
- 62.Brenner K, Liu A, Laplante D, Lupien S, Pruessner J, Ciampi A, Joober R, King S. Cortisol response to a psychosocial stressor in schizophrenia: Blunted, delayed, or normal? Psychoneuroendocrinology. 2009;34:859–868. doi: 10.1016/j.psyneuen.2009.01.002. [DOI] [PubMed] [Google Scholar]