Abstract
Age-related increase in frailty is accompanied by a fundamental shift in cellular iron homeostasis. By promoting oxidative stress, the intracellular accumulation of non-heme iron outside of binding complexes contributes to chronic inflammation and interferes with normal brain metabolism. In the absence of direct non-invasive biomarkers of brain oxidative stress, iron accumulation estimated in vivo may serve as its proxy indicator. Hence, developing reliable in vivo measurements of brain iron content via magnetic resonance imaging (MRI) is of significant interest in human neuroscience. To date, by estimating brain iron content through various MRI methods, significant age differences and age-related increases in iron content of the basal ganglia have been revealed across multiple samples. Less consistent are the findings that pertain to the relationship between elevated brain iron content and systemic indices of vascular and metabolic dysfunction. Only a handful of cross-sectional investigations have linked high iron content in various brain regions and poor performance on assorted cognitive tests. The even fewer longitudinal studies indicate that iron accumulation may precede shrinkage of the basal ganglia and thus predict poor maintenance of cognitive functions. This rapidly developing field will benefit from introduction of higher-field MRI scanners, improvement in iron-sensitive and -specific acquisition sequences and post-processing analytic and computational methods, as well as accumulation of data from long-term longitudinal investigations. This review describes the potential advantages and promises of MRI-based assessment of brain iron, summarizes recent findings and highlights the limitations of the current methodology.
Progressive declines in brain health and cognitive function are common in human aging, with the pace of change varying across brain regions and among individuals (Kemper, 1994; Raz & Kennedy, 2009; Fjell et al., 2014). Multiple factors shape the trajectory of brain change across the lifespan, but unlike development that follows a largely programmed albeit modifiable course (Stiles & Jernigan, 2010), aging is unlikely to reflect the unfolding of a grand plan and appears more as a sum of overlapping time-dependent processes and influences that shape the fate of older organisms (Kirkwood et al., 2005). The story of brain change and its eventual demise is not acted out by a few stars but is rather narrated by many influential supporting actors. Because the brain cannot be isolated from the rest of the organism, these influences reflect changes in the basic molecular, cellular and physiological processes across virtually all organs and systems. Although the precise roster of the factors determining brain aging is yet to be completed, it is clear that fundamental energetic processes within the mitochondria deteriorate with advancing age (Boumezbeur et al., 2010), and that this deterioration is promoted by aggregate action of reactive oxygen species (ROS, Dröge & Schipper, 2007), chronic neuroinflammation (Finch and Crimmins, 2004; Finch et al., 1969; Grammas, 2011), and other contributors to a general age-related increase in frailty (Rockwood et al., 2004). The precursors of age-related frailty stem from manifold biochemical and physiological factors, yet they have at least one thing in common: their association with the action, flow and accumulation of iron.
Brain Iron: Presentation and Metabolism
Iron plays a paradoxical role in aging. On the one hand, it is a vital contributor to many aspects of normal brain functions—including synthesis of the brain’s main energetic currency, adenosine triphosphate (ATP) in mitochondria (Mills et al., 2010), as well as the brain’s main material substrate of information processing, myelin (Todorich et al., 2009). On the other hand, iron is a very active oxidizer, and its excessive accumulation promotes neurodegeneration by triggering inflammation (Haider et al., 2014) and oxidative stress (Zecca et al., 2004; Mills et al., 2010). The mammalian brain is particularly vulnerable to iron-induced oxidative damage because of the abundance of molecular oxygen, excess ROS, presence of oxidizable neurotransmitters such as dopamine (Youdim and Yehuda, 2000), and poor antioxidant defenses (Schipper, 2012).
In all vertebrates, iron appears in two distinct forms: heme and non-heme. Heme iron, the ferrous core of the hemoglobin molecule, is essential for binding oxygen and ferrying it around the circulatory system. Deoxygenation of hemoglobin exposes heme iron, thus inducing magnetic field inhomogeneity (Pauling and Coryell, 1936) that underlies the Blood Oxygen Level-Dependent (BOLD) effect—a fundamental phenomenon of functional magnetic resonance imaging (fMRI, Ogawa et al., 1990). In contrast to heme iron, which is exclusively linked to circulating or accumulating blood, non-heme iron is present in virtually all cells and is a critical contributor to many vital processes, including ATP synthesis and DNA replication (Mills et al., 2010; Ward et al., 2014).
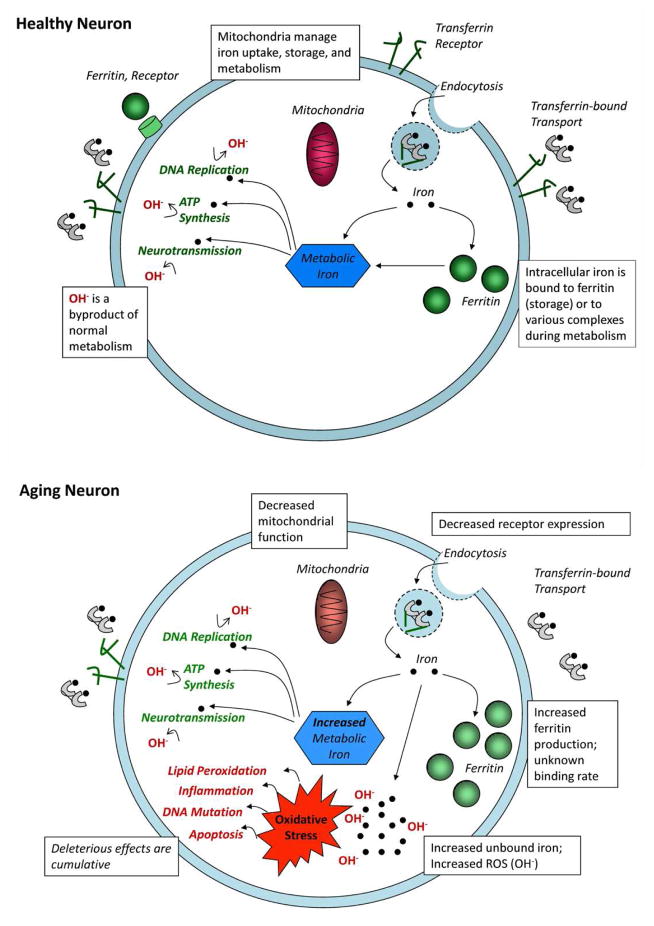
Although non-heme iron is necessary for normal cellular function, its accumulation outside of binding complexes, such as ferritin (responsible for iron sequestration) or transferrin (a means of iron transport), instigates proliferation of ROS and becomes an agent of oxidative stress (Halliwell, 1992; Mills et al., 2010; Moos and Morgan, 2004; Ward et al., 2014). Low-level oxidative stress is a by-product of normal metabolic activity (Sohal and Orr, 2011), which, in a healthy cell, is minimized through rate-limited metabolism and further curtailed by endogenous anti-oxidants (Halliwell, 1992; Mills et al., 2010). Accumulation of unbound non-heme iron upsets that equilibrium and accelerates the rate of oxidative stress that enhances degradation of macromolecular cell components, perforates cell and mitochondrial membranes, impedes mitochondrial activity, promotes DNA mutations, and accelerates the rate of apoptosis (Halliwell, 1992; Mills et al., 2010; Hare et al., 2013; Ward et al., 2014; Granold et al., 2015). Through a separate but related mechanism, iron-catalyzed oxidative stress also increases pro-inflammatory cytokines, initiating the propagating cycle of neuroinflammation (Williams et al., 2012) that has been implicated in neurodegeneration (Xu et al., 2012; see Ward et al., 2014 for a review). Non-heme iron accumulation induces a vicious cycle: responding to oxidative stress and disrupted metabolism, mitochondria increase unbound non-heme iron concentration to sustain ATP generation, thereby further accelerating oxidative stress and thus ensuring their own and the host cell’s demise through eventual apoptosis. In a seminal paper that inspired decades of experimental work, Harman (1956) proposed iron-catalyzed oxidative stress as a mechanism of tissue degeneration that characterizes aging and diseases related to it—the free-radical theory of aging.
Because of its role in ROS promotion, accumulation of non-heme iron in brain tissue may serve as an indirect marker or proxy of oxidative stress. The focus here is on non-heme iron because, although degraded hemoglobin in the form of hemosiderin can be present in cerebral hematomas or microbleeds (CMB) and can act as focal oxidative stress agents, the incidence of these phenomena in normal aging defined by the absence of age-associated neurological, endocrine, cerebrovascular or cardiovascular diseases is low and are often asymptomatic (Janaway et al., 2014; Yates et al., 2014; see Loitfelder et al. 2012 and Yates et al., 2014 for reviews). In comparison, accumulation of non-heme iron localized preferentially in certain organ systems appears to be a typical aspect of aging (Lauffer, 1992; Daugherty and Raz, 2013), and the rate of accumulation may be accelerated in age-related neurodegenerative disease (Hare et al., 2013; Schipper, 2012; Ward et al., 2014; Ward et al., 2015; Zecca et al., 2004). Thus, in this review, we will focus almost exclusively on the effects of non-heme iron on the aging brain and cognition, while acknowledging possible contributions of local heme iron foci in less-than-optimal cases of cerebrovascular disease.
Non-heme iron in the Brain
The concentration of non-heme iron in the brain is greater than in any organ system except the liver (Hallgren & Sourander, 1958; Lauffer, 1992), possibly reflecting its role in energy-intensive processes such as neurotransmission (Youdim and Yehuda, 2000; Berg et al., 2007; see Bäckman et al., 2006), and myelin production and maintenance (Bartzokis, 2011). Further, the distribution of non-heme iron varies across brain regions, showing a peculiar predilection for the circuits associated mainly with the domain of movement. In healthy individuals, the largest concentrations estimated from postmortem staining are in the basal ganglia (Thomas et al., 1993; Brass et al., 2006), with the globus pallidus having the largest concentrations at any age (Hallgren & Sourander, 1958). The red nucleus and substantia nigra also have relatively large amounts of iron, although not as much as the basal ganglia, with even smaller iron content observed in other subcortical nuclei, such as the thalamus. Cortical gray matter has less non-heme iron compared with the subcortical gray, and white matter has the least amount of iron in the brain (Thomas et al., 1993; Brass et al., 2006; Hallgren and Sourander, 1958), although the precursors of the myelinated layer, oligodendrocytes, contain significant amounts of non-heme iron (Todorich et al., 2009; Bartzokis, 2011).
Estimating Brain Iron Content in vivo
The advent of magnetic resonance imaging (MRI) techniques suitable for the in vivo estimation of brain iron content has highlighted the use of iron as a possible biomarker of neural and cognitive decline (Ward et al., 2014; Schenck and Zimmerman, 2004). To appraise this rapidly developing branch of cognitive neuroscience of aging, we review various MRI methods and current recommendations for estimating brain iron content in vivo. We summarize the MRI evidence for brain iron as a putative biomarker of metabolic dysfunction and cognitive change.
Prominent paramagnetic properties of iron make MRI a promising method for in vivo evaluation of its content in brain tissue, in which variations of iron abundance create measurable differences in local magnetic susceptibility (Haacke et al, 2005). Paramagnetic materials have a high magnetic susceptibility and therefore, a relatively long transverse relaxation rate (R2) or short relaxation time constant, T2 (= 1/R2). Thus, iron-rich regions appear hypointense on T2-weighted images, and R2 prolongation corresponds to greater iron content in several structures, including the basal ganglia (Dhenain et al., 1998; Siemonsen et al., 2008). Longer R2, however, can also be related to lower water content of the parenchyma (Haacke et al., 2005), a fact that diminishes the validity of R2 as an index of iron content, especially in injured tissue.
The field-dependent R2 increase (FDRI) method capitalizes on a strong linear association of R2* and R2 values with the strength of the external field (Bartzokis et al., 1993; Yao et al., 2009). In FDRI, images are acquired with equivalent sequence parameters (i.e., echo times, flip angle, etc.) back-to-back in scanners with two different field strengths (e.g., 0.5 T and 1.5 T). Estimates of iron content are generated from the mathematical difference in R2* measured at the two different field strengths.. The iron content estimates produced by the FDRI method are believed to be unaffected by myelin (Bartzokis et al., 1999; Pfefferbaum et al., 2009), the presence of which threatens the validity of iron estimates via R2* relaxometry (Haacke et al., 2005; Glasser and Van Essen, 2011). They do, however, share the sensitivity to tissue hydration with the estimates produced by R2 relaxometry. FDRI estimates have been validated with in vitro phantom study (Bartzokis et al., 1993), but it remains the least commonly used method, likely due to the challenging requirement of imaging without delay in two scanners of different field strengths. Whereas all iron imaging methods exploit the dependence of susceptibility effects on external field strength, only FDRI relies on the difference in relaxation rate between two field strengths for its estimation. In practice, imaging parameters are optimized for detection of iron-related susceptibility in the employed field strength. Although stronger magnetic fields can more easily accommodate higher resolution, which will improve accuracy of localized iron estimates, several studies have validated estimates via different methods at low fields (e.g., 0.5 T and 1.5T, Bartzokis et al., 1993; Thomas et al., 1993).
An additional index of local iron content, R2′ (=1/T2′), has been proposed instead of R2 as a more sensitive measure for estimating parenchymal iron (Ordidge et al., 1994; Haacke et al., 2005, review). Because differences in R2′ reflect variation in phase of the signal, the index is more specifically associated with the presence and concentration of iron, and allows distinguishing between highly paramagnetic iron and diamagnetic calcium, which can be also found in the basal ganglia (Cohen et al., 1980). However, R2′ is a relatively small quantity that is difficult to measure, particularly in the presence of multiple sources of field inhomogeneity.
A frequently used index of brain iron is R2* (=1/T2*), which is a sum of relaxation due to spin-spin interaction (R2) and local susceptibility effects (R2′). By aggregating the two effects, R2* accounts for susceptibility differences that are otherwise difficult to estimate independently. To obtain valid R2* estimates, the general relaxation component must be calculated from multiple echo times, which is commonly accomplished with spin echo (SE) or gradient-recalled echo (GRE) sequences. The latter is an example of susceptibility weighted imaging (SWI), for which several iron-imaging tools have been created. Although, in principle, two echoes suffice for calculating R2*, more echoes are preferred for a more precise estimation of the exponential decay function and improving signal-to-noise ratio. Additional considerations, such as R2* sensitivity to background field inhomogeneity that is unrelated to iron concentration, must also be kept in mind. This residual error can be minimized by sampling multiple echoes with extended echo times, and with post-acquisition filtering. Additional thresholding to eliminate artifact intensity values from the regional estimates of normal tissue is an alternative practice (e.g., Daugherty, Haacke, and Raz, 2015). An increasingly popular neuroimaging method for the study of iron, R2* produces estimates of age differences in regional iron content that are similar to those generated by FDRI and R2 (Daugherty and Raz, 2013), and correlate highly with susceptibility measures (Yao et al., 2009).
Whereas R2 measures transverse relaxation rate, precession in the transverse plane results in signal difference proportional to the particle’s magnetic susceptibility that is reflected in phase maps produced by SWI sequences (Haacke et al., 2005). Phase shift is more sensitive to differences in susceptibility than R2* estimates are, is easier to measure than R2′, is proportional to iron concentration and is highly correlated with published postmortem values (Ogg et al., 1999; Haacke et al., 2005). Although phase can be affected by blood flow, this confound is minimized with high resolution imaging that allows manual exclusion of vascular objects including small veins (Haacke et al., 2005). Like R2′ relaxometry, phase imaging can distinguish paramagnetic iron from diamagnetic calcium, which are immediately discernible by intensity values at opposite ends of the scale. However, unlike R2′, susceptibility-induced phase shifts are relatively large and therefore phase imaging may be more sensitive to even subtle differences in iron content. Thanks to these properties, SWI phase sequences are well-suited for the detecting and measuring cerebral microbleeds (Nandigam et al., 2009), which can be observed in the brains of persons with high burden of vascular risk. Phase maps can be calculated from single echo SWI sequences, and choosing an echo time between T2* and T2*/2 can improve signal-to-noise ratio while optimizing sensitivity to iron (Haacke et al., 2015). For many imaging protocols, this corresponds to approximately 13–16 ms on 3T and 4T scanners (see Haacke et al., 2015 for a review), and if a multi-echo SWI sequence is designed to include an echo within this time range, iron content can be estimated via R2* and phase from the same sequence. The relationship between R2* and phase remains, however, unclear, with widely disparate correlation values across brain regions, ranging from r = −.72 in the caudate and r = −.11 in the globus pallidus (Yan et al., 2012).
Because calculation of phase shift requires only a single echo, this method of iron imaging may be the most time-efficient for acquisition. Nonetheless, phase analysis may require substantial post-acquisition processing as the data must be read, vetted and screened for artifacts (e.g., aliasing; Haacke et al., 2010). The detectable shift in phase precession is limited by −180° and 180° (Haacke et al., 2010). If concentration of iron is sufficiently large to cause phase shift greater than 180°, the intensity value will “wrap” to the opposite scale extreme, thereby biasing against the detection of iron, and suggesting the presence of diamagnetic minerals (e.g., calcium. Normal variability during aging in regions with high iron content, such as the basal ganglia, appears to be prone to aliasing artifact (Haacke et al., 2015). Many research groups publicly distribute software (e.g., SPIN: http://www.mrc.wayne.edu/download.htm; last accessed 3/23/2015; or MEDI Toolbox for MATLAB: http://weill.cornell.edu/mri/pages/qsm.html; last accessed 3/23/2015) that is designed specifically to process SWI phase data and include post-acquisition filtering and anti-aliasing protocols.
In spite of its advantages outlined above, phase-based estimation of iron content has a unique limitation: susceptibility measures are non-local. To improve the validity of region-specific measurements, additional post-processing has been proposed to remove non-local effects from phase differences (Haacke et al., 2010; Bilgic et al., 2012). This approach to phase processing has been termed quantitative susceptibility mapping (QSM; e.g., Bilgic et al., 2012) or susceptibility weighted imaging and mapping (SWIM; Haacke et al., 2010). QSM has been validated against postmortem assay (Langkammer et al., 2012; Sun et al., 2015), and is a promising method for in vivo estimates of iron content on high-resolution images with an improved signal-to-noise ratio. Due to its specificity to local susceptibility, QSM may yields more precise estimates of iron content than R2* or traditional phase imaging do (Deistung et al., 2013). Age differences in QSM estimates of subcortical iron were larger than those from FDRI (Bilgic et al., 2012; Poynton et al., 2015), which may indicate improved sensitivity to iron-induced susceptibility as compared with relaxometry.
Across laboratories, QSM and SWIM protocols share the same four basic processing steps (see Wang and Liu, 2015 and Haacke et al., 2015 for reviews). First, phase is reconstructed from multi-channel phase-arrayed coils, with an option of initial filtering for background field inhomogeneity. Second, phase is unwrapped via spatial (for single-echo sequences) or temporal (for multi-echo sequences) algorithms that address the common aliasing artifact. Third, a brain mask is generated, which expedites the fourth step—complete removal of background field inhomogeneity and reorientation of local susceptibility. Research groups, however, vary widely in the types of filters; algorithm parameterization for phase reconstruction, aliasing and correction of field inhomogeneity; and automated brain extraction procedures (e.g., Haacke et al., 2010; Bilgic et al., 2012; Liu et al., 2011; Barbosa et al., 2015; Khabipova et al., 2015; Langkammer et al., 2015; Li et al., 2011; Li et al., 2015; Lim et al., 2014; Schwesser et al., 2012; Yablonskiy and Sukstanskii, 2015; Poynoton et al., 2015; Feng, Neelavalli, and Haacke, 2013), all of which can affect the estimates of iron content. Because of the relative novelty of these methods and the diversity of the very recent developments in QSM, there are few publications that directly compare among them (e.g., Schwesser et al., 2011; Barbosa et al., 2015; Khabipova et al., 2015; see Wang and Liu, 2015 and Haacke et al., 2015 for reviews), or evaluate sensitivity and specificity of each method and their comparative advantages for the study of iron in aging and neurodegenerative disease.
What form of iron does MRI measure?
Histological study suggests that ferritin and hemosiderin are the only paramagnetic materials of sufficient concentration to affect MR signal from brain tissue (Schenck, 1995). As about 90% of brain non-hem iron is normally bound to ferritin (Morris et al., 1992), concentrations of transferrin- and non-transferrin-bound iron and of soluble iron particles are too small to affect MR signal (Haacke et al., 2005). Other endogenous paramagnetic materials (e.g., copper and manganese) are also considered to be at insufficient concentrations in healthy individuals to affect the MRI signal (Dexter et al., 1992; Haacke et al., 2005). Therefore, MRI methods focus on the greatest localized source of non-heme iron—ferritin, which sequesters iron particles and does not directly contribute to oxidative stress. Thus, the available imaging tools cannot directly measure unbound iron that drives ROS production and thereby cannot directly test the hypothesized role of oxidative stress in brain aging.
Nonetheless, increase in ferritin-bound iron inferred from MRI is an indirect index of relative increase in soluble iron. Blood serum measures of ferritin and soluble iron are tightly yoked (Morita et al., 1981), and kinetic modeling of in vitro tissue culture demonstrates a linear dependence between ferritin-bound concentration and unbound non-heme iron (Salgado et al., 2010). Validation studies of iron-sensitive imaging sequences with postmortem staining for iron confirms the detection of susceptibility related to non-heme iron particles (e.g., Antonini et al., 1993; Vymazal et al., 1995; Thomas et al., 1993; Bartzokis et al., 1994). Finally, correlations between in vivo estimates of iron content and other indices of neurodegeneration in aging and disease (e.g., Bartzokis, 2011; Daugherty, Haacke, and Raz, 2015; Ulla et al. 2013; Walsh et al., 2013) are in accord with postmortem histology findings (e.g, Hallgren and Sourander, 1958; Hallervorden and Spatz, 1922; Dexter et al., 1992; Hossein Sadrzadeh and Saffari, 2004) and can be theoretically attributed to unbound non-heme iron that is an oxidizing agent, rather than the neutralized concentration sequestered in ferritin (see Mills et al., 2010; Ward et al., 2014 for reviews). Therefore, in vivo measure of iron content, even if primarily determined by ferritin-bound concentrations, is a plausible biomarker of metabolic dysfunction and oxidative stress (Schenck and Zimmerman, 2004).
Myelin biases in vivo estimates of iron
Although the reviewed imaging methods capitalize on the magnetic susceptibility of iron, they do not yield specific measures of iron content. Among several properties of brain tissue that contribute to differences in susceptibility, one of the most influential is myelin (Fukunaga et al., 2010; Liu et al., 2011; Lodygensky et al., 2012; Langkammer et al., 2012). Thus, confounding effects of myelin and iron on MRI signal poses the greatest threat to validity of in vivo estimates of iron content. Presence of myelin inflates the estimates of iron content that are derived from R2* (Glasser and Van Essen, 2011; Haacke et al., 2005), phase and QSM/SWIM (Bilgic et al., 2012; Deistung et al., 2013; Langkammer et al., 2012; Wisnieff et al., 2014) and the bias is understandably the strongest in myelin-rich regions. In addition, phase values of cerebral white matter can be affected by fiber orientation within each voxel with respect to the main magnetic field (B0; Lee et al., 2010; Schafer et al., 2009; Duyn et al., 2007). In comparison, R2 and FDRI are considered to be robust against this particular source of bias but have their own problematic aspects, such as sensitivity to the influence of interstitial fluid (Bilgic et al., 2012; Pfefferbaum et al., 2009). Indeed, relative decrease in R2 (e.g., House et al., 2006) or phase shift (e.g., Li et al., 2011; Liu et al., 2011) in the white matter are often interpreted as evidence of breached myelin integrity rather than lesser iron content. Whereas in regions with a relatively low myelin content and few myelinated fibers, such as the basal ganglia, this bias may be small or negligible (Daugherty and Raz, 2013), the validity of iron estimates via relaxometry and phase shift in deep white matter may be significantly compromised. Although increase in unbound iron content in oligodendrocytes has been proposed as a mechanism of demyelination in normal aging and neurodegenerative disease (Todorich et al., 2009; Bartzokis, 2011), current neuroimaging methods cannot test these hypotheses without substantial confounds (also see Pfefferbaum et al., 2010). In any case, whereas oligodendrocytes are indeed the main repository of iron in the brain and determine normal levels of iron measured in the brain, age-related increase in iron content of the basal ganglia is apparently driven mainly by its accumulation in the astrocytes (Conner et al., 1990).
Common limitations of in vivo iron estimation methods
Independent of method, all in vivo measures of iron have common limitations. For instance, none can readily distinguish between heme and non-heme iron sources, and in vivo measures of the two sources may be highly correlated (Anderson et al., 2005). Although this confound can be minimized by using SE instead of GRE sequences (Yamada et al., 2002), in vivo methods underestimate iron quantities due to low spatial resolution relative to particle size and the necessary filtering aimed to minimize confounding field inhomogeneity. The lower the resolution, the more palpable is the confounding. These methods, like postmortem measures, can detect and approximate differences in relative iron content but cannot quantify absolute concentration. The proposed use of phase shifts and iron concentration values reported in the postmortem literature for deriving a conversion value to quantify iron concentration in vivo (Haacke et al., 2005; 2007) is limited by the lack of a standardized conversion factor that is independent of MRI acquisition parameters. Finally, susceptibility-induced differences in intensity contrast can bias segmentation of regional areas on these images (Lorio et al., 2014), which should be considered in developing measurement protocols for iron estimation or volumetry.
Estimates of regional iron content vary widely across studies, even when the same method of estimation is employed (see Haacke et al., 2005; Daugherty and Raz, 2013 for reviews). However, on a gross anatomical level, the rank-order of in vivo estimates of regional differences in iron content is similar to that from postmortem studies (e.g., Hallgren & Sourander, 1958). As a rule, the globus pallidus and putamen have the highest iron content corresponding to the shortest T2 values (median T2 = 60 ms and 69 ms, respectively) compared with the caudate nucleus (median T2=77 ms) and cortical gray (median T2 = 75 ms) and white (median T2= 73 ms) matter (Haacke et al., 2005). Despite the listed limitations (Gomori & Grossman, 1993; Schenck, 1995; Schenker et al., 1993; Vymazal et al., 1995), in vivo measures have been validated against postmortem data across several brain regions and in phantom studies (Antonini et al., 1993; Bizzi et al., 1990; Vymazal et al., 1995; Péran et al., 2009; Pujol et al., 1992; Brass et al., 2006; Thomas et al., 1993; Bartzokis et al., 1994; Pfefferbaum et al., 2009; Bilgic et al., 2012).
Age-Related Differences in Iron Content Estimated in vivo
MRI studies have largely replicated the regional differences in iron content reported in postmortem investigations, and have expanded the results of age differences therein. The striatal nuclei have relatively large concentrations of iron beginning in early life (Hallgren and Sourander, 1958; Thomas et al., 1993; Aquino et al., 2009), and the adult age differences in these regions are greater than that in the red nucleus (Daugherty and Raz, 2013). In normal aging, cross-sectional age differences in the globus pallidus are relatively small, which may be due to the large amount of iron already present in this region beginning at a relatively early age (Li et al., 2014). Moreover, based on a meta-analysis of 20 MRI studies of healthy aging that used various methods, the magnitude of age differences reported in the globus pallidus (d = 0.61, 95% CI: 0.50–0.72; Daugherty and Raz, 2013) was very similar to the estimate reported by Hallgren and Sourander (1958) in a postmortem sample (d = 0.62). In contrast, the average age difference in iron content estimated in vivo in the caudate (d = 0.74; 95% CI: 0.64/0.84) and putamen (d = 0.88; 95% CI: 0.77/0.98) were larger than in the postmortem study (caudate: d = 0.45; putamen: d = 0.41). Despite adult age-related increase in iron content in all subcortical nuclei, the globus pallidus still has the largest iron content in later life, followed by the neostriatum, red nucleus and substantia nigra (Daugherty and Raz, 2013; Morris et al., 1992; Brass et al., 2006). Cross-sectional imaging studies also suggest a smaller, but significant, positive correlation between age and iron content in the thalamus and hippocampus, with small or negligible age differences in cortical gray (with exception of the motor cortex) and subcortical white matter (Thomas et al., 1993; Hirai et al., 1996; Brass et al., 2006; Rodrigue et al., 2013; Callaghan et al., 2014).
Until recently, the age trajectories of change in regional brain iron in healthy adults were inferred exclusively from cross-sectional comparisons. Judging by research on aging of other aspects of the brain, cross-sectional evidence is unsuitable for understanding change, because longitudinal measures of change frequently diverge from cross-sectional predictions (e.g., Raz et al., 2005; 2010; Pfefferbaum et al., 2014; Bender and Raz, 2015). Moreover, cross-sectional models aimed at gauging individual differences and evaluating the role of mediators in age-related associations between the brain and behavior cannot capture the dynamics of the aging process (Lindenberger et al., 2011; Maxwell and Cole, 2007; Raz and Lindenberger, 2011). For example, in a 2-year longitudinal study of healthy adults spanning a broad age range, the striatum but not the hippocampus evidenced longitudinal increase in iron (Daugherty, Haacke, and Raz, 2015), in contrast to cross-sectional correlations between advanced age and greater iron in the latter region (Rodrigue et al., 2013). Two-year increase in iron in the putamen and globus pallidus, but not the caudate, was reported in a sample of younger and middle-aged adults (Walsh et al., 2013), whereas another small study of middle-aged and older adults found no evidence for striatal iron increase (Ulla et al., 2013). The pattern of longitudinal change may indicate that the rate of iron accumulation in the basal ganglia slows in later life (also see Li et al., 2014; Hallgren and Sourander, 1958).
Critically, the age-related regional differences in iron content cannot be explained by dietary iron intake (Beard et al., 2005) and are instead believed to reflect a fundamental shift in iron homeostasis, either at the blood brain barrier (BBB) or inside the cell (see Ward et al., 2014; Mills et al., 2010; Burdo and Connor, 2003 for reviews). Disruption of binding complexes expressed at the BBB and in the cell during metabolism, decreased rate of ferritin sequestration, or increased rate of cellular uptake may occur in aging following mitochondrial dysfunction (Hare et al., 2013; Ward et al., 2014; Zecca et al., 2004) or a shift in the relative trans-membrane concentration gradient (see Singh et al., 2009). The causes for such changes are unclear, but are likely diverse and interactive.
In addition to gauging age-related change in regional iron content, longitudinal studies allow for evaluating the role of iron accumulation in the age-related change in other indicators of brain health and function. In a longitudinal study of an adult lifespan sample of healthy volunteers, greater iron content in the striatum predicted greater shrinkage of these regions after two years (Daugherty, Haacke, and Raz, 2015), a finding that was replicated in an independent sample of older adults with multiple measurements spanning seven years (Daugherty, 2014; Daugherty and Raz, 2015). These two studies provide the first longitudinal evidence of a role of iron accumulation in structural brain aging, which had been suggested by cross-sectional correlations (e.g., Cherubini et al., 2009; Rodrigue et al., 2013). In addition, these longitudinal studies explicitly tested the hypothesis that the detected increase in iron stems from a relative shift in concentration due to regional brain shrinkage. Indeed, iron accumulation preceded shrinkage and greater baseline iron predicted greater shrinkage up to 7 years later, whereas the reverse proposition stating that smaller baseline volumes predict greater iron accumulation was not supported (Daugherty, Haacke, and Raz, 2015; Daugherty, 2014; Daugherty and Raz, 2015). Taken together, brain iron content may be a meaningful biomarker of impending decline in normal aging (Daugherty, Haacke, and Raz, 2015) and disease (Walsh et al., 2013). However, significant individual differences in age-related changes in iron content, their contribution to cognitive declines and the assortment of factors that shape individual trajectories of change remain to be elucidated.
Modifiers of Age-Related Differences in Non-heme Iron Content
Several risk factors, age-related and independent of age, have been proposed to explain individual differences in regional iron content. Genetic predisposition for decreased expression of iron-binding proteins is an example of the latter. A decreased binding rate to ferritin or transferrin is associated with an increased rate of iron-catalyzed oxidative stress that is observed in disease, such as hemochromatosis (Jahanshad et al., 2013), and mutations in the human hemochromatosis protein gene (HFE) and in a gene that encodes for transferrin (TF) have been implicated in diseases characterized by abnormally high iron content (Jahanshad et al., 2013; Nandar & Connor, 2011). The common variants of HFE gene, H63D and C282Y, increase iron absorption by altering membrane expression of HFE protein that regulate binding affinity of the transferrin receptor (Lehmann et al., 2006; Nandar & Connor, 2011). Each variant potentially increases risk for iron accumulation, in a dose-related fashion (Lehmann et al., 2006; Nandar & Connor, 2011). The P589S polymorphism of the TF gene may also increase risk for iron accumulation by altering transferrin expression (Bartzokis et al., 2010). To date, only a single study of healthy men linked elevated genetic risk for increased brain iron load with cognitive deficits (Bartzokis et al., 2010). Therefore, genetic predisposition to less efficient iron sequestration and decreased efflux of excess iron may explain some of the individual differences in the rate of iron accumulation in normal aging.
In addition to genetic risk for inefficient binding of non-heme iron, inflammatory, metabolic and cardiovascular risk factors may act as age-related modifiers of individual differences in neural health (Franklin et al., 1997; Grundy et al., 2005; Finch and Morgan, 2007), including iron accumulation. Iron-related oxidative stress promotes release of pro-inflammatory cytokines that trigger a cascade of neuroinflammation that culminates in apoptosis (Zecca et al., 2004). Either genetic or phenotypic risk factors for inflammation have been proposed to explain individual differences in iron accumulation (Zecca et al., 2004). In vitro studies and animal models provide a wealth of evidence for the mutual influence of iron homeostasis and inflammation (see Wessling-Resnick, 2010; Urrutia, Mena & Núñez, 2014 for reviews). Nonetheless, the direct tests of the association between brain iron and inflammatory markers in humans are rare. In a longitudinal study of healthy older adults, subclinical elevations in circulating blood serum markers of inflammation were unrelated to individual differences in the rate of subcortical iron accumulation over seven years (Daugherty, 2014; Daugherty and Raz, 2015). It is unclear if the lack of effect was due to the sample selection for optimal health, or if more sensitive biomarkers may better detect variability in normal aging. In rheumatic disease, characterized by chronic inflammation, patients often present with increased non-heme iron content, and reducing iron stores may bring some symptomatic relief (see Baker and Ghio, 2009 for a review). To the best of our knowledge, at the time of this writing, there are no studies of genetic inflammatory risk on brain iron content.
Cardiovascular and metabolic risk factors: hypertension, elevated fasting blood glucose, elevated low-density lipoprotein (“bad” cholesterol), low high-density lipoprotein (“good” cholesterol), high total triglycerides, and high body mass index—a constellation of symptoms termed metabolic syndrome (Grundy et al., 2005)—are related to inflammation. Elevations in one or more of these factors have been linked to greater brain iron content. Adults with uncomplicated arterial hypertension have greater subcortical iron content as compared with normotensive counterparts (Raz, Rodrigue, and Haacke, 2007; Rodrigue, Haacke, and Raz, 2011; also see Berry et al., 2001), and obese persons have more than their non-obese counterparts do (Blasco et al., 2014). In a longitudinal study, subclinical elevations in a compound index of metabolic syndrome risk predicted greater iron content in the putamen, but not in the caudate or hippocampus (Daugherty, Haacke, and Raz, 2015). The mechanisms explaining the association between cardiovascular health and regional brain iron accumulation are unclear. They may plausibly reflect reduced cerebral blood flow (Grundy et al., 2005) that would slow the delivery of iron-binding complexes to the brain (Hare et al., 2013). Global decrease in cerebral blood flow may also decrease the function of the brain vascular endothelium in regulating iron uptake and clearance at the BBB (Deane et al., 2004).
Brain Iron and Cognitive Performance
Although description of the age-related dynamics of non-heme iron accumulation is important, a crucial question is whether increase in iron content has significant cognitive consequences. Given the role of excessive unbound iron in induction of mitochondrial dysfunction, energetic decline and oxidative stress, its accumulation is likely to have detrimental cognitive consequences. In extant cross-sectional studies in healthy adults, greater iron content in subcortical structures was associated with poorer memory performance (Bartzokis et al., 2011; Rodrigue et al., 2013; Ghadery et al., 2015), lower general cognitive aptitude (Penke et al., 2012), mental slowing (Pujol et al., 1992; Sullivan et al., 2009), and poorer cognitive and motor control (Adamo, Daugherty, Raz, 2014). Greater iron content and small hippocampal volume conjointly contributed to cross-sectional age differences in memory (Rodrigue et al., 2013).
Unfortunately, cross-sectional mediation models of age-related change have limited validity (Maxwell & Cole, 2007; Lindenberger et al., 2011) and to date, understanding the role of iron in cognitive declines has been hampered by the dearth of longitudinal studies. A sole longitudinal study that examined two-year changes in iron content and cognition (Daugherty, Haacke, and Raz, 2015) failed to confirm the role of hippocampal iron accumulation in cognitive performance, while using a similar population and methods as the original investigation (Rodrigue et al., 2013). In that longitudinal study, greater iron content in the caudate nucleus estimated from R2* predicted lesser repeated-testing gains in verbal working memory after two years, while no association between individual differences in hippocampal iron content and change in memory were observed (Daugherty, Haacke, and Raz, 2015). In the same adult lifespan sample, greater iron content in the caudate, but not the hippocampus, accounted for lesser repeated-testing gains in spatial navigation in a virtual Morris water maze task (Daugherty, 2014). See Table 1 for a summary of published studies of cognitive correlates toin vivo estimates of iron content in healthy aging.
Table 1.
A summary of published studies of cognitive correlates to in vivo estimates of iron content in healthy aging.
| Publication | Sample Characteristics | MRI Index | Regions of Interest | Result |
|---|---|---|---|---|
| Daugherty et al. 2015 | N = 125, age 19–77 years; longitudinal study | R2* | Striatum, Hippocampus | Longer R2* in caudate: lesser repeated testing gains in working memory; No correlation with episodic memory |
| Ghadery et al. 2015 | N = 336, age 38–86 years | R2* | Basal ganglia, Thalamus, Hippocampus, Cortex | Longer R2* in putamen/pallidum: worse global cognition, executive function, and psychomotor speed; No correlation with memory |
| Adamo et al. 2014 | N = 25, age 51–78 years | T2* | Basal ganglia | Shorter T2* in basal ganglia: worse psychomotor control |
| Rodrigue et al. 2013 | N = 113, age 19–83 years | T2* | Caudate, Hippocampus, Visual cortex | Shorter T2* in hippocampus: worse episodic memory |
| Penke et al. 2012 | N = 143, age 71–72 years | T2* | Gross iron deposition | Shorter T2*: worse general cognitive ability |
| Bartzokis et al. 2011 | N = 63, age 55–76 years | FDRI | Caudate, Thalamus, Hippocampus, Frontal white matter, Corpus callosum | Greater FDRI in hippocampus: worse delayed memory (men only); Greater FDRI in the basal ganglia: worse working memory (HFE non-carrier group only); No correlation with psychomotor speed |
| Sullivan et al. 2009 | N = 10, age 65–79 years | FDRI | Striatum, Thalamus | Greater FDRI in striatum: worse dementia rating and longer reaction time; Lesser FDRI in thalamus: worse MMSE and worse psychomotor control/speed |
| Pujol et al. 1992 | N = 25, age 65–76 years | T2 | Globus pallidus, Red nucleus | Shorter T2 in globus pallidus: slower psychomotor speed, worse executive function, worse incidental learning; Shorter T2 in red nucleus: worse executive function |
Note: Studies are listed chronologically; all studies are cross-sectional, except where indicated otherwise. Longer R2* and R2 (or shorter T2* and T2) and greater FDRI indicate more iron. FDRI, field dependent rate increase; HFE, human hemochromatosis protein gene; MMSE, mini-mental state exam
Iron accumulation can be a harbinger of neural and cognitive declines that typify aging, and it can affect global brain health and function through several interdependent effects: promotion of inflammation and apoptosis, interruption of metabolic function and neurotransmission, and demyelination. These iron-related changes are plausible mechanisms of aging that warrant additional attention.
Brain Iron in Neurodegenerative Disorders
Age is a major risk factor for neurodegenerative diseases (Reeve et al., 2014), and the observed age-related increase in brain iron content in conjunction with other risk factors, such as neuroinflammation and metabolic dysfunction, make iron a potential biomarker for Alzheimer’s and Parkinson’s diseases. Indeed, abnormally high brain iron content is found in several neurodegenerative diseases (see Ward et al., 2015; Zecca et al., 2004; Langkammer et al., 2014 for reviews). A whole class of diseases termed neurodegeneration with brain iron accumulation (NBIA), for example, are exclusively diagnosed by abnormal high concentrations, typically in the basal ganglia and many with psychomotor symptoms beginning in earlier life (Schipper, 2012; Gregory and Hayflick, 2014). Other iron-dependent diseases, such as hematic encephalopathy associated with cirrhosis of the liver, also present with abnormal brain iron content and associated cognitive deficits (Liu et al., 2013; Xia et al., 2015).
The role of iron in more common neurodegenerative disease is less clear. Elevated concentrations of brain iron have also been observed in Parkinson’s disease (PD; Antonini et al., 1993; Gorell et al., 1995; Ulla et al., 2013; Kosta et al., 2006; Kienzel et al., 1995), Alzheimer’s disease (AD; Qin et al., 2011; Ding et al., 2009; Wang et al., 2014; Bartzokis et al., 2000; 2004; Quintana et al., 2006; Raven et al., 2013; Langkammer et al., 2014; Acosta-Cabronero et al., 2013), and multiple sclerosis (Brass et al., 2006; Ceccarelli et al., 2009; Rudko et al., 2014; Walsh et al., 2014; Pinter et al., 2015; Khalil et al., 2015). When, however, the totality of published evidence was evaluated by a meta-analysis, no significant differences between AD and normal aging were noted (Schrag et al., 2011).
Nonetheless, with the disease groups, increased iron content in the brain may be associated with cognitive declines. For instance, in PD, greater iron content in the basal ganglia and substantia nigra correlates with severity of cognitive and motor impairment (Atasoy et al., 2004; Gorell et al., 1995), as well as progressive increase in symptoms’ severity (Ulla et al., 2013). Greater regional iron deposition has also been associated with cognitive impairment in AD (Zhu et al., 2009; Ding et al., 2009; Qin et al., 2011; Luo et al., 2013; van Rooden et al., 2014). Altered iron homeostasis may explain the pathology of iron in Lewy bodies in PD (Berg et al., 2007), as well as tangles (House et al., 2004), plaques (Quintana et al., 2006; Smith and Perry, 1995) and amyloid burden (El Tannir El Tayara et al., 2006; Rival et al., 2009; Becerril-Ortega et al., 2014) in AD. Thus, in absence of a significant difference between age-matched controls and a disease group, iron may still be a marker of severity of cognitive deterioration within the diagnostic groups.
The reports of iron accumulation as a major feature of neurodegenerative conditions spurred the search for clinical therapy to alleviate iron-induced oxidative stress (Ward et al., 2015). Due to the shared pathology, slowing the rate of iron accumulation or decreasing the concentration of ROS in the brain may be a highly desirable therapeutic target for many neurodegenerative diseases. To this end, several pharmaceutical interventions aimed at promoting iron chelation and anti-oxidant activity have been developed (Ward et al., 2015; Stankiewicz et al., 2007). Many of the proposed compounds, however, do not pass the BBB (Recalcati et al., 2010), and evidence of their efficacy in humans is mixed (Ward et al., 2015; Stankiewicz et al., 2007). Although in rodents, introducing anti-oxidant rich foods or restricting dietary iron may partially alleviate the behavioral consequences of iron accumulation, it remains unclear whether the intervention has an effect on concentrations of iron, endogenous anti-oxidants, or ROS (Cook and Yu, 1998; Joseph et al., 1999; Joseph et al., 2005). Further, brain regions with normally large amounts of iron do not consistently show differences in response to dietary intervention (e.g., Erikson et al., 1997). The failure to translate many of these therapies into human treatment may be in part due to discrepancies in relevant oxidative mechanisms and action sites between animal models and the human cerebrum, which was only recently demonstrated (Granold et al., 2015). Together, this underscores the importance of continuing improvement in thein vivo methods of iron estimation and attention to individual differences in regional brain iron deposition.
The role of iron accumulation as a possible biomarker of neurodegeneration makes developing clinical criteria for early detection of neurodegenerative diseases a useful undertaking. At this stage, however, designing such criteria and enabling normative judgment based on the brain iron content observedin vivo, will require a truly normative, population-based study with a large representative sample and cross-validation with independent samples and multiple methods. Samples of convenience used in the reviewed studies cannot produce trustworthy norms.
Conclusions
Age-related disruption of iron homeostasis in the brain may be gauged via fast and reliable MRI methods. However, the utility of brain iron content estimated via MRI as a biomarker of metabolic dysfunction and its role in age-related cognitive declines remain to be demonstrated. The few longitudinal studies of healthy aging (Daugherty, Haacke, and Raz, 2015; Daugherty, 2014; Daugherty and Raz, 2015) and of neurodegenerative disease (Ulla et al., 2013; Walsh et al., 2014) suggest that regional brain iron accumulation partially accounts for neurodegeneration and related cognitive deficits. MRI holds great promise as a tool for evaluating accumulation of brain iron and examining its role in cognitive aging. Advancing towards this goal will require increase in resolution and signal-to-noise ratio of existing MRI protocols geared to evaluating brain iron, further developments in the post-processing methods, and accumulation of data from longitudinal studies conducted with attention to individual differences in brain iron homeostasis.
Figure 1.
Iron homeostasis, and its contributions to normal metabolism and oxidative stress in a healthy neuron and in aging. The figures are based on Ward et al. (2014); Hare et al. (2013); Sohal and Orr (2012); Mills et al. (2010); Zecca et al. (2004). OH−, reactive oxygen species (ROS) that induce oxidative stress.
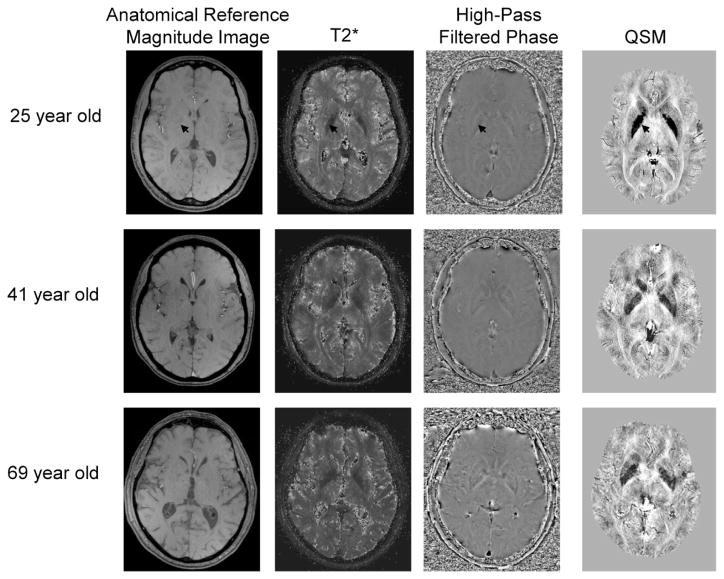
Figure 2.
Example MR images for healthy young, middle-aged, and older adults; a similar midbrain slice was chosen for each person to showcase the basal ganglia structures that have large concentrations of iron (an arrow points to the globus pallidus, a region with the greatest iron content in the brain across all ages). On T2*-weighted images, iron appears hypointense (dark intensity values). The high-pass filtered phase and quantitative susceptibility mapping (QSM) images were inverted so that iron also corresponds to hypointensity. All images were collected in a 3 Tesla scanner. The anatomical reference magnitude image (0.5 × 0.5 mm in plane; echo time = 5.68 ms) has similar alignment as the other images, although phase may not correspond identically due to its non-local nature. The T2* images were calculated from an 11 echo SWI sequence (0.5 × 0.5 mm in plane; echo times = 5.68 – 31.38 ms; similar processing procedure as Daugherty et al., 2015) with no additional filtering, and the phase images from one of the SWI echoes collected for T2* (0.5 × 0.5 mm in plane; echo time = 15.96 ms) applying a high-pass filter (64 × 64, Hanning). The QSM image (0.5 × 0.5 mm in plane) was calculated from a field variation map obtained through a pixel-wise least squares fitting of the first 10 echoes of the T2* sequence via a catalytic multiecho phase unwrapping scheme (CAMPUS, see Feng et al., 2013 for method details). Abbreviation: SWI, susceptibility weighted imaging.
References
- Acosta-Cabronero J, Williams GB, Cardenas-Blanco A, Arnold RJ, Lupson V, Nestor PJ. In vivo quantitative susceptibility mapping (QSM) in Alzheimer’s disease. PLoS One. 2013;8(11):e81093. doi: 10.1371/journal.pone.0081093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo DE, Daugherty AM, Raz N. Brain iron content and grasp force-matching ability in older women. Brain Imag Behav. 2014;8(4):579–87. doi: 10.1007/s11682-013-9284-6. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Kaufman MJ, Lowen SB, Rohan M, Renshaw PF, Teicher MH. Brain T2 relaxation times correlate with regional cerebral blood volume. MGMA. 2005;181:3–6. doi: 10.1007/s10334-004-0076-2. [DOI] [PubMed] [Google Scholar]
- Antonini A, Leenders KL, Meier D, Oertel MD, Boesiger P, Anliker M. T2 relaxation time in patients with Parkinson’s disease. Neurology. 1993;43:697–700. doi: 10.1212/wnl.43.4.697. [DOI] [PubMed] [Google Scholar]
- Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini L. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252(1):165–172. doi: 10.1148/radiol.2522081399. [DOI] [PubMed] [Google Scholar]
- Atasoy HT, Nuyan O, Tunc T, Yorubulut M, Unal AE, Inan LE. T2-weighted MRI in Parkinson’s disease; substantia nigra pars compacta hypointensity correlates with the clinical scores. Neurology India. 2004;52(3):332–337. [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S-C, Larde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav rv. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baker JF, Ghio AJ. Iron homeostasis in rheumatic disease. Rheumatology. 2009;48:1339–1344. doi: 10.1093/rheumatology/kep221. [DOI] [PubMed] [Google Scholar]
- Barbosa JHO, Santos AC, Tumas V, Liu M, Zheng W, Haacke EM, Salmon CEG. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2*. Mag Res Imag. 2015 doi: 10.1016/j.mri.2015.02.021. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiology of Aging. 2011;32(8):1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Aravagiri M, Oldendorf WH, Mintz J, Marder SR. Field dependent transverse relaxation rate increase may be a specific measure of tissue iron stores. Magn Reson Med. 1993;29(4):459–464. doi: 10.1002/mrm.1910290406. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Markham CH, Marmarelis PZ, Treciokas LJ, Tishler TA, Marder SR, Mintz J. MRI evaluation of brain iron in earlier- and later-onset Parkinson’s disease and normal subjects. Magnetic Resonance Imaging. 1999;17(2):213–222. doi: 10.1016/s0730-725x(98)00155-6. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Mintz J, Sultzer D, Marx P, Herzberg JS, Phelan CK, Marder SR. In vivo MR evaluation of age-related increases in brain iron. American Journal of Neuroradiology. 1994;15(6):1129–1138. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu P, Tingus K, Peters DG, Amar CP, Tishler TA, Finn JP, Willablanca P, Altshuler LL, Mintz J, Neely E, Connor JR. Gender and iron genes may modify associations between brain iron and memory in healthy aging. Neuropsychopharmacology. 2011;36:1375–1384. doi: 10.1038/npp.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler T, Peters D, et al. Prevalent iron metabolism gene variants associated with increased brain ferritin iron in healthy older men. Journal of Alzheimer’s Disease. 2010;20:333–341. doi: 10.3233/JAD-2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Cummings J, Holt LE, Hance DB, Henderson VW, Mintz J. In vivo evaluation of brin iron in Alzheimer disease using magnetic resonance imaging. Arch Gen Psych. 2000;57(1):47–53. doi: 10.1001/archpsyc.57.1.47. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Shin IS, Lu PH, Cummings JL. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann N Y Acad Sci. 2004;1012:224–36. doi: 10.1196/annals.1306.019. [DOI] [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Li N, Connor JR. Brain iron uptake in hypotransferrinemic mice: influence of systemic iron status. J Neurosci Res. 2005;79(1–2):254–261. doi: 10.1002/jnr.20324. [DOI] [PubMed] [Google Scholar]
- Becerril-Ortega J, Bordji K, Fréret T, Rush T, Buisson A. Iron overload accelerates neuronal amyloid-β production and cognitive impairment in transgenic mice model of Alzheimer’s disease. Neurobiol Aging. 2014;35(10):2288–2301. doi: 10.1016/j.neurobiolaging.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Bender AR, Raz N. Normal-appearing cerebral white matter in healthy adults: mean change over 2 years and individual differences in change. Neurobiol Aging. 2015 doi: 10.1016/j.neurobiolaging.2015.02.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, Kruger R, Rie BR, Riederer P. Parkinson’s disease. In: Youdim M, Riederer P, Mandel S, Battistin L, editors. Handbook of Neurochemistry and Molecular Neurobiology: Degenerative Diseases of the Nervous System. 3. New York, NY: Springer; 2007. pp. 1–20. [Google Scholar]
- Berry C, Brosnan MJ, Fennel J, Hamilton CA, Dominiczak AF. Oxidative stress and vascular damage in hypertension. Current opinion in Nephrology and Hypertension. 2001;10(2):247–255. doi: 10.1097/00041552-200103000-00014. [DOI] [PubMed] [Google Scholar]
- Bilgic B, Pfefferbuam A, Rohlfing T, Sullivan E, Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. NeuroImage. 2012;59(3):2625–2635. doi: 10.1016/j.neuroimage.2011.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi A, Brooks RA, Brunetti A, et al. Role of iron and ferritin in MR imaging of the brain: A study in primates at different field strengths. Radiology. 1990;177:59–65. doi: 10.1148/radiology.177.1.2399339. [DOI] [PubMed] [Google Scholar]
- Blasco G, Puig J, Daunis-I-Estadella J, Molina XL, Xifra G, Fernández-Aranda F, Pedraza S, Ricart W, Portero-Otín M, Fernández-Real J. Brain iron overload, insulin resistance and cognitive performance in obese subjects: A preliminary MRI case-control study. Diabetes Care. 2014;37(11):3076–83. doi: 10.2337/dc14-0664. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. Journal of Cerebral Blood Flow & Metabolism. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass SD, Chen N, Mulkern R, Baksni R. Magnetic resonance imaging of iron deposition in neurological disorders. Topics in Magnetic Resonance Imaging. 2006;17(1):31–40. doi: 10.1097/01.rmr.0000245459.82782.e4. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Connor JR. Brain iron uptake and homeostatic mechanisms: an overview. Biometals. 2003;16:63–75. doi: 10.1023/a:1020718718550. [DOI] [PubMed] [Google Scholar]
- Callaghan MF, Freund P, Draganski B, Anderson E, Cappelletti M, Chowdhury R, Diedrichsen J, Fitzgerald TH, Smittenaar P, Helms G, Lutti A, Weiskopf N. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiology of Aging. 2014;35:1862–1872. doi: 10.1016/j.neurobiolaging.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli A, Flippi M, Neema M, Arora A, Valsasina P, Rocca MA, Healy BC, Bakshi R. T2 hypintensity in the deep gray matter of patients with benign multiple sclerosis. Multiple Sclerosis. 2009;15:678–686. doi: 10.1177/1352458509103611. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Péran P, Caltagirone C, Sabatini U, Spalletta G. Aging of subcortical nuclei: Microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage. 2009;48:29–36. doi: 10.1016/j.neuroimage.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Cohen CR, Duchesneau PM, Weinstein MA. Calcification of the basal ganglia as visualized by computed tomography. Radiology. 1980;134:97–99. doi: 10.1148/radiology.134.1.7350641. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL, St Martin SM, Mufson EJ. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res. 1990;27:595–611. doi: 10.1002/jnr.490270421. [DOI] [PubMed] [Google Scholar]
- Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech Ageing Dev. 1998;102(1):1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Raz N. Age-related differences in iron content of subcortical nuclei observed in vivo: A meta-analysis. NeuroImage. 2013;70:113–121. doi: 10.1016/j.neuroimage.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM. Doctoral dissertation. ETD Collection; 2014. Accumulation of subcortical iron as a modifier of volumetric and cognitive decline in healthy aging: Two longitudinal studies; p. AAI3640105. [Google Scholar]
- Daugherty AM, Haacke EM, Raz N. Striatal iron content predicts its shrinkage and changes in working memory after two years in healthy adults. J Neurosci. 2015;35(17):6731–6743. doi: 10.1523/JNEUROSCI.4717-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Raz N. Iron accumulation over 7 years in the striatum predicts its shrinkage in healthy adults. Conference abstract, Society for Neuroscience Annual Meeting.2015. [Google Scholar]
- Deane R, Zheng W, Zlokovic BV. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. Journal of Neurochemistry. 2004;88:813–820. doi: 10.1046/j.1471-4159.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR. Towardin vivo histology: A comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase- and R2*-imaging at ulta-high magnetic field strength. NeuroImage. 2013;65:299–314. doi: 10.1016/j.neuroimage.2012.09.055. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Jenner P, Schapira AHV, Marsden CD. Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. Ann Neurol. 1992;32:S94–S100. doi: 10.1002/ana.410320716. [DOI] [PubMed] [Google Scholar]
- Dhenain M, Duyckaerts C, Michot J-L, Volk A, Picq J-L, Boller F. Cerebral T2-weighted signal decrease during aging in the mouse lemur primate reflects iron accumulation. Neurobiology of Aging. 1998;19(1):65–69. doi: 10.1016/s0197-4580(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Ding B, Chen KM, LJW, Sun F, et al. Correlation of iron in the hippocampus with MMSE in patients with Alzheimer’s disease. Journal of Magnetic Resonance Imaging. 2009;29:793–798. doi: 10.1002/jmri.21730. [DOI] [PubMed] [Google Scholar]
- Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007;104:11796–11801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Tannir El Tayara N, Delatour B, Le Cudennec C, Guegan M, Volk A, Dhenain M. Age-related evolution of amyloid burden, iron load, and MR relaxation times in a transgenic mouse model of Alzheimer’s disease. Neurobiology of Disease. 2006;22:199–208. doi: 10.1016/j.nbd.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Pinero DJ, Connor JR, Beard JL. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr. 1997;127(10):2030–2038. doi: 10.1093/jn/127.10.2030. [DOI] [PubMed] [Google Scholar]
- Feng W, Neelavalli J, Haacke EM. Catalytic multiecho phase unwrapping scheme (CAMPUS) in multiecho gradient echo imaging: Removing phase wraps on a voxel-by-voxel basis. Magn Reson Med. 2013;70(1):117–126. doi: 10.1002/mrm.24457. [DOI] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Finch CE, Foster JR, Mirsky AE. Ageing and the regulation of cell activities during exposure to cold. J Gen Physiol. 1969;54:690–712. doi: 10.1085/jgp.54.6.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Morgan TE. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res. 2007;4(2):185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB Alzheimer’s Disease Neuroimaging Initiative. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. Epub 2014 Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS, Gustin W, 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Li TQ, van Gelderen P, de Zwart JA, Shmueli K, Yao B, Lee J, Maric D, Aronova MA, Zhang G, Leapman RD, Schenck JF, Merkle H, Duyn JH. Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci U S A. 2010;107:3834–3839. doi: 10.1073/pnas.0911177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadery C, Pirpamer L, Hofer E, Langkammer C, Petrovic K, Loitfelder M, Schwingenschuh P, Seiler S, Duering M, Jouvent E, Schmidt H, Fazekas F, Mangin JF, Chabriat H, Dichgans M, Ropele S, Schmidt R. R2* mapping for brain iron: associations with cognition in normal aging. Neurobiology of Aging. 2015;36:925–932. doi: 10.1016/j.neurobiolaging.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 2011;31(32):11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomori J, Grossman R. The relation between regional brain iron and T2 shortening. American Journal of Neuroradiology. 1993;14:1049–1050. [PMC free article] [PubMed] [Google Scholar]
- Gorell JM, Ordidge RJ, Brown GG, Deniau J-C, Muderer NM, Helpern JA. Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology. 1995;45(6):1138–1143. doi: 10.1212/wnl.45.6.1138. [DOI] [PubMed] [Google Scholar]
- Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflamm. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granold M, Moosmann B, Staib-Lasarzik I, Arendt T, del Rey A, Engelhard K, Behl C, Hajieva P. High membrane protein oxidation in the human cerebral cortex. Redox Bio. 2015;4:200–207. doi: 10.1016/j.redox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Hayflick S. Neurodegeneration with brain iron accumulation disorders overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews. Seattle, WA: University of Washington, Seattle; 2014. pp. 1–22. [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circ. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Ayaz M, Khan A, Manova ES, Krishnamurthy B, Gollapalli L, Ciulla C, Kim I, Petersen F, Kirsch W. Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. Journal of Magnetic Resonance Imaging. 2007;26(2):256–264. doi: 10.1002/jmri.22987. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Cheng NYC, House MJ, Liu Q, Neelaavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magnetic Resonance Imaging. 2005;23(1):1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Liu S, Buch S, Zheng W, Wu D, Ye Y. Quantitative susceptibility mapping: current status and future directions. Mag Res Imag. 2015;33:1–25. doi: 10.1016/j.mri.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Tang J, Neelavalli J, Cheng YCN. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. Journal of Magnetic Resonance Imaging. 2010;32:663–676. doi: 10.1002/jmri.22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L, Simeonidou C, Steinberger G, Hametner S, Grigoriadis N, Deretzi G, Kovacs GG, Kutzeinigg A, Lassmann H, Frischer JM. Multiple sclerosis deep gray matter: the relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry. 2014;85(12):1386–95. doi: 10.1136/jnnp-2014-307712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallervorden J, Spatz H. Peculiar disease of the extrapyramidal system with particular affection of the globus pallidus and the substantia nigra. (Translation) Z Ges Neurol Psychiat. 1922;79:254–302. [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. Journal of Neurochemistry. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Iron and damage to biomolecules. In: Lauffer, editor. Iron and Human Disease. Roca Baton, FL: CRC Press; 1992. pp. 209–236. [Google Scholar]
- Hare D, Ayton S, Bush A, Lei P. A delicate balance: Iron metabolism and diseases of the brain. Frontiers in Aging Neuroscience. 2013;5:34. doi: 10.3389/fnagi.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: A theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hirai W, Korogi Y, Sakamoto Y, Hamatake S, Ikushima I, Takahashi M. T2 shortening in the motor cortex: Effect of aging and cerebrovascular diseases. Radiology. 1996;199:799–803. doi: 10.1148/radiology.199.3.8638008. [DOI] [PubMed] [Google Scholar]
- House MJ, St Pierre TG, Foster JK, Martins RN, Clarnette R. Quantitative MR imaging R2 relaxometry in elderly participants reporting memory loss. AJNR. 2006;27:430–39. [PMC free article] [PubMed] [Google Scholar]
- House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminum, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2004;6(3):291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- Hossein Sadrzadeh SM, Saffari Y. Iron and brain disorders. Am J Clin Pathol. 2004;121(Suppl 1):S64–S70. doi: 10.1309/EW0121LG9N3N1YL4. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Rajagopalan P, Thompson PM. Neuroimaging, nutrition, and iron-related genes. Cell Mol Life Sci. 2013;70:4449–4461. doi: 10.1007/s00018-013-1369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janaway BM, Simpson JE, Hoggard N, Highley JR, Forster G, Drew D, Gebril OH, Matthews FE, Bryane C, Wharton SB, Ince PG MRC Cognitive Function, Ageing Neuropathology Study. Brain haemosiderin in older people: pathological evidence for an ischemic origin of magnetic resonance imaging (MRI) microbleeds. Neuropathol Appl Neurobiol. 2014;40(3):258–69. doi: 10.111/nan.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Casadesus G, Fisher D. Oxidative stress and inflammation in brain aging: Nutritional considerations. Neurochemical Research. 2005;30(6/7):927–935. doi: 10.1007/s11064-005-6967-4. [DOI] [PubMed] [Google Scholar]
- Joseph JC, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19(18):8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabipova D, Wiaux Y, Gruetter R, Marques JP. A modulated closed form solution for quantitative susceptibility mapping—A thorough evaluation and comparison to iterative methods based on edge prior knowledge. NeuroImage. 2015;107:163–174. doi: 10.1016/j.nueorimage.2014.11.038. [DOI] [PubMed] [Google Scholar]
- Khalil M, Langkammer C, Pichler A, Pinter D, Gattringer T, Bachmaier G, Ropele S, Fuchs S, Enzinger C, Fazekas F. Dynamics of brain iron levels in multiple sclerosis: A longitudinal 3T MRI study. Neurol. 2015;84(24):1–7. doi: 10.1212/WNL.0000000000001679. [DOI] [PubMed] [Google Scholar]
- Kienzel E, Pychinger L, Jellinger K, Linert W, Stachelberger H, Jameson R. The role of transition metals in the pathogenesis of Parkinson’s disease. Journal of the Neurological Sciences. 1995;134(Suppl):69–78. doi: 10.1016/0022-510x(95)00210-s. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Feder M, Finch CE, Franceschi C, Globerson A, Klingenberg CP, LaMarco K, Omholt S, Westendorp RG. What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mech Age Dev. 2005;126:439–443. doi: 10.1016/j.mad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Kosta P, Argyropoulou MI, Markoula S, Konitsiotis S. MRI evaluation of the basal ganglia size and iron content in patients with Parkinson’s disease. J Neurol. 2006;253:26–32. doi: 10.1007/s00415-005-0914-9. [DOI] [PubMed] [Google Scholar]
- Langkammer C, Bredies K, Poser BA, Barth M, Reishofer G, Fan AP, Bilgic B, Fazekas F, Mainero C, Ropele S. Fast quantitative susceptibility mapping using 3D EPI and total generalizaed variation. NeuroImage. 2015;111:622–630. doi: 10.1016/j.neuroimage.2015.02.041. [DOI] [PubMed] [Google Scholar]
- Langkammer C, Krebs N, Goessler W, Scheurer E, Yen K, Fazekas F, Ropele S. Susceptibility induced gray-white matter MRI contrast in the human brain. NeuroImage. 2012;59:1413–1419. doi: 10.1016/j.neuroimage.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkammer C, Ropele S, Pirpamer L, Faezekas F, Schmidt R. MRI for iron mapping in Alzheimer’s disease. Neurodegener Dis. 2014;13(2–3):189–91. doi: 10.1159/000353756. [DOI] [PubMed] [Google Scholar]
- Langkammer C, Schweser F, Krebs N, Deistung A, Goessler W, Scheurer E, Sommer K, Reishofer G, Yen K, Fazekas F, Ropele S, Reichenbach JR. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage. 2012;62:1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer R, editor. Iron and Human Disease. Boca Raton, FL: CRC Press; 1992. Introduction. Iron, aging, and human disease: Historical background and new hypotheses; pp. 1–22. [Google Scholar]
- Lee J, Shmueli K, Fukunaga M, van Gelderen P, Merkle H, Silva AC, Duyn JH. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proc Natl Acad Sci U S A. 2010;107:5130–5135. doi: 10.1073/pnas.0910222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann DJ, Worwood M, Ellis R, Wimhurst VLJ, Merryweather-Clarke AT, Warden DR, Smith AD, Robson KJH. Iron genes, iron load and risk of Alzheimer’s disease. J Med Genet. 2006;43:e52. doi: 10.1136/jmg.2006.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu B, Batrachenko A, Bancroft-Wu V, Morey RA, Shashi V, Langkammer C, De Bellis MD, Ropele S, Song AW, Liu C. Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Human Brain Mapping. 2014;35:2698–2713. doi: 10.1002/hbm.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang N, Yu F, Han H, Cao W, Romero R, Tantiwongkosi B, Duong TQ, Liu C. A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. NeuroImage. 2015;108:111–122. doi: 10.1016/j.neuroimage.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. NeuroImage. 2011;55:1645–1656. doi: 10.1016/j.neuroimage.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim IA, Li X, Jones CK, Farrell JAD, Vikram DS, van Zijl PCM. Quantitative magnetic susceptibility mapping without phase unwrapping using WASSR. NeuroImage. 86:265–279. doi: 10.1016/j.neuroimage.2013.09.072. doi:10,1016/j.neuroimage.2013.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: What’s change got to do with it? Psychol Aging. 2011;26(1):34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Liu C, Li W, Johnson A, Wu B. High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. NeuroImage. 2011;56:930–938. doi: 10.1016/j.neuroimage.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Ding J, Dong L, He YF, Dai Z, Chen CZ, Cheng WZ, Wang H, Zhou J, Wang X. T2* MRI of minimal hepatic encephalopathy and cognitive correlates in vivo. J Mag Res Imag. 2013;37:179–186. doi: 10.1002/jmri.23811. [DOI] [PubMed] [Google Scholar]
- Lodygensky GA, Marques JP, Maddage R, Perroud E, Sizonenko SV, Hüppi PS, Gruetter R. In vivo assessment of myelination by phase imaging at high magnetic field. NeuroImage. 2012;59:1979–1987. doi: 10.1016/j.neuroimage.2011.09.057. [DOI] [PubMed] [Google Scholar]
- Loitfelder M, Seiler S, Schwingenschuh P, Schmidt R. Cerebral microbleeds: a review. Panimerva Med. 2012;54(3):149–60. [PubMed] [Google Scholar]
- Lorio S, Lutti A, Kherif F, Ruef A, Dukart J, Chowdhury R, Frackowiak RS, Ashburner J, Helms G, Weiskopf N, Draganski B. Disentangling in vivo the effects of iron content and atrophy on the ageing human brain. NeuroImage. 2014;103:280–289. doi: 10.1016/j.neuroimage.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Zhuang X, Kumar D, Wu X, Yue C, Han C, Lv J. The correlation of hippocampal T2-mapping with neuropsychology test in patients with Alzheimer’s disease. PLOSOne. 2013;8(9):e76203. doi: 10.1371/journal.pone.0076203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- Mills E, Dong X, Wang F, Xu H. Mechanisms of brain iron transport: Insight into neurodegeneration and CNS disorders. Future Med Chem. 2010;2(1):51–72. doi: 10.4155/fmc.09.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos T, Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurologic disease: review. Ann N Y Acad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- Morita R, Yoshii M, Nakajima K, Kohsaka T, Miki M, Torizuka K. Clinical evaluation of serum ferritin to iron ratio in malignant diseases. European J of Nuc Med. 1981;6(7):331–336. doi: 10.1007/BF00262528. [DOI] [PubMed] [Google Scholar]
- Morris CM, Candy JM, Keith AB, Oakley AE, Taylor GA, Pullen RG, Bloxham CA, Gocht A, Edwardson JA. Brain iron homeostasis. Journal of Inorganic Biochemistry. 1992;47:257–265. doi: 10.1016/0162-0134(92)84071-t. [DOI] [PubMed] [Google Scholar]
- Nandar W, Connor JR. HFE gene variants affect iron in the brain. Journal of Nutrition. 2011;141:729S–739S. doi: 10.3945/jn.110.130351. Epub 2011 Feb 23. [DOI] [PubMed] [Google Scholar]
- Nandigam RNK, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, Greenberg SM, Dickerson BC. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR. 2009;30(2):338–343. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg RJ, Langston JW, Haacke EM, Steen RG, Taylor JS. The correlation between phase shifts in gradient-echo MR images and regional brain iron concentration. Magn Reson Imaging. 1999;17(8):1141–1148. doi: 10.1016/s0730-725x(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Ordidge RJ, Gorell JM, Deniau JC, Knight RA, Helpern JA. Assessment of relative brain iron concentrations using T2-weighted and T2*-weighted MRI at 3 tesla. Magnetic Resonance in Medicine. 1994;32:335–341. doi: 10.1002/mrm.1910320309. [DOI] [PubMed] [Google Scholar]
- Pauling L, Coryell CD. The magnetic properties and structure of the hemochromogens and related substances. Proc Natl Acad Sci USA. 1936;22(3):159–63. doi: 10.1073/pnas.22.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L, Hernandéz MCV, Maniega SM, Gow AJ, Murray C, Starr JM, Bastin ME, Deary IJ, Wardlaw JM. Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiol Aging. 2012;33:510–517. doi: 10.1016/j.neurobiolaging.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Péran P, Cherubini A, Luccichenti G, Hagberg G, Démonet JF, Rascol O, Celsis P, Caltagirone C, Spalletta G, Sabatini U. Volume and iron content in the basal ganglia and thalamus. Human Brain Mapping. 2009;30:2667–2675. doi: 10.1002/hbm.20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohfling T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: Comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage. 2009;47(2):493–500. doi: 10.1016/j.neuroimage.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: Effects of age and iron concentration. Neurobiology of Aging. 2010;31(3):482–500. doi: 10.1016/j.neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Zahr NM, Sullivan EV. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiology of Aging. 2014;35(7):1755–68. doi: 10.1016/j.neurobiolaging.2014.01.008. Epub 2014 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter D, Khali M, Pichler A, Langkammer C, Ropele S, Marschik PB, Fuchs S, Fazekas F, Enzinger C. Predictive value of different conventional and non-conventional MRI-parameters for specific domains of cognitive function in multiple sclerosis. Neuroimage: Clinical. 2015;7:715–720. doi: 10.1016/j.nicl.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynton CB, Jenkinson M, Adalsteinsson E, Sullivan EV, Pfefferbaum A, Wells W., III Quantitative susceptibility mapping by inversion of a perturbation field model: Correlation with brain iron in normal aging. IEEE Trans Med Imag. 2015;34(1):339–353. doi: 10.1109/TMI.2014.2358552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Junque C, Vendrell P, Grau JM, Marti-Vilalta JL, Olivé C, Gili J. Biological significance of iron-related magnetic resonance imaging changes in the brain. Archives of Neurology. 1992;49(7):711–717. doi: 10.1001/archneur.1992.00530310053012. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhu W, Zhan C, Zhao L, Wang J, Tian Q, Wang W. Investigation on positive correlation of increased brain iron deposition with cognitive impairment in Alzheimer disease by using quantitative MR R2′ mapping. J Huazhong Univ Sci Technol [Med Sci] 2011;31(4):578–585. doi: 10.1007/s11596-011-0493-1. [DOI] [PubMed] [Google Scholar]
- Quintana C, Bellefqih S, Laval JY, Guerquin-Kern JL, Wu TD, Avila J, Ferrer I, Aranz R, Patiño C. Study of the localization of iron, ferritin, and hemosedrin in Alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. Journal of Structural Biology. 2006;153:42–54. doi: 10.1016/j.jsb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J Alzheimers Dis. 2013;37(1):127–36. doi: 10.3233/JAD-130209. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. News of cognitive cure for age-related brain shrinkage is premature: a comment on Burgmans et al., (2009) Neuropsychol. 2011;24(2):255–257. doi: 10.1037/a0018828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue K, Kennedy K, Lindenberger U. Trajectories of brain aging in middle-age and older adults: Regional and individual differences. NeuroImage. 2010;51(2):501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dhale C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults : General trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: Insights from in vivo neuromophometry and susceptibility weighted imaging. Annals of the New York Academy of Sciences. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S, Minotti G, Cairo G. Iron regulatory proteins: from molecular mechanisms to drug development. Antioxid Redox Signal. 2010;13(10):1593–616. doi: 10.1089/ars.2009.2983. [DOI] [PubMed] [Google Scholar]
- Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. Epub 2014 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival T, Page RM, Chandraratna DS, Sendall TJ, Ryder E, Liu B, Lewis H, Rosahl T, Hider R, Camargo LM, Shearman MS, Crowther DC, Lomas DA. Fenton chemistry and oxidative stress mediate the toxicity of the B-amyloid peptide in a Drosophila model of Alzheimer’s disease. European Journal of Neuroscience. 2009;29:1335–1347. doi: 10.1111/j.1460-9568.2009.06701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Daugherty AM, Haacke EM, Raz N. The role of hippocampal iron content and hippocampal volume in age-related differences in memory. Cer Cor. 2012;23(7):1533–41. doi: 10.1093/cercor/bhs139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Haacke EM, Raz N. Differential effects of age and history of hypertension of regional brain volumes and iron. Neuroimage. 2011;54:750–759. doi: 10.1016/j.neuroimage.2010.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Mogilner A, Mitnitski A. Changes with age in the distribution of a frailty index. Mech Age Dev. 2004;125:517–519. doi: 10.1016/j.mad.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rudko DA, Solovey I, Gati JS, Kremenchutzky M, Menon RS. Multiple sclerosis: improved identification ofdisease-relevant changes in gray and white matter by using susceptibility-based MR imaging. Radiol. 2014;272(3):851–64. doi: 10.1148/radiol.14132475. [DOI] [PubMed] [Google Scholar]
- Salgado JC, Olivera-Nappa A, Gerdtzen ZP, Tapia V, Theil EC, Conca C, Nuñez MT. Mathematical modeling of the dynamic storage of iron in ferritin. BMC Sys Bio. 2010;4:147. doi: 10.1186/1752-0509-4-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer A, Wharton S, Gowland P, Bowtell R. Using magnetic field simulation to study susceptibility-related phase contrast in gradient echo MRI. NeuroImage. 2009;48:126–137. doi: 10.1016/j.neuroimage.2009.05.093. [DOI] [PubMed] [Google Scholar]
- Schenck J. Imaging of brain iron by magnetic resonance: T2 relaxation at different field strengths. Journal of the Neurological Sciences. 1995;134(Suppl):10–18. doi: 10.1016/0022-510x(95)00203-e. [DOI] [PubMed] [Google Scholar]
- Schenck JF, Zimmerman EA. High-field magnetic resonance imaging of brain iron: birth of a biomarker? NMR Biomed. 2004;17:433–445. doi: 10.1002/nbm.922. [DOI] [PubMed] [Google Scholar]
- Schenker C, Meier D, Wichmann W, Boesiger P, Valavanis A. Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology. 1993;35:119–124. doi: 10.1007/BF00593967. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Neurodegeneration with brain iron accumulation - clinical syndromes and neuroimaging. Biochimica et Biophysica Acta. 2012;1822:350–360. doi: 10.1016/j.bbadis.2011.06.016. Epub 2011 Jul 13. [DOI] [PubMed] [Google Scholar]
- Schrag M, Mueller C, Oyoyo U, Kirsch WM. Iron, zinc, and copper in the Alzheimer’s disease brain: A quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Progress in Neurobiology. 2011;94(3):296–306. doi: 10.1016/j.pneurobio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwesser F, Sommer K, Deistung A, Reichenbach JR. Quantitative susceptibility mapping for investigating subtle susceptibility variation in the human brain. NeuroImage. 2012;62:2083–2100. doi: 10.1016/j.neuroimage.2012.05.067. [DOI] [PubMed] [Google Scholar]
- Schwesser F, Deistung A, Lehr BW, Reichenbach JR. Quantiative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? NeuroImage. 2011;54:2789–2807. doi: 10.1016/j.neuroimage.2010.10.070. [DOI] [PubMed] [Google Scholar]
- Siemonsen S, Finsterbusch J, Matschke J, Loernzen A, Ding XQ, Fiehler J. Age-dependent normal values of T2and T2’ in brain parenchyma. American Journal of Neuroradiology. 2008;29:950–955. doi: 10.3174/ajnr.A0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Isaac AO, Luo X, Mohan ML, Cohen ML, Chen F, Kong Q, Bartz J, Singh N. Abnormal brain iron homeostasis in human and animal prion disorders. PLoS Pathog. 2009;5:e1000336. doi: 10.1371/journal.ppat.1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Perry G. Free radical damage, iron, and Alzheimer’s disease. Journal of the Neurological Sciences. 1995;134(Suppl):92–94. doi: 10.1016/0022-510x(95)00213-l. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radical Biology and Medicine. 2012;52(3):539–555. doi: 10.1016/j.freeradbiomed.2011.10.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz J, Panter SS, Neema M, Arora A, Batt C, Bakshi R. Iron in chronic brain disorders: Imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–386. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The Basics of brain development. Neuropsychological Review. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Rohlfing T, Pfefferbaum A. Relevance of iron deposition in deep gray matter brain structures of cognitive and motor performance in healthy elderly men and women: exploratory findings. Brain Imag Behav. 2009;3:167–175. doi: 10.1007/s11682-008-9059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Walsh AJ, Lebel RM, Belvins G, Catz I, Lu JQ, Johnson ES, Emery DJ, Warren KG, Wilman AH. Validation of quantitative susceptibility mapping with Perls’ iron staining for subcortical gray matter. NeurroImage. 2015;105:486–492. doi: 10.1016/j.neuroimage.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Thomas LO, Boyko OB, Anthony DC, Burger PC. MR detection of brain iron. American Journal of Neuroradiology. 1993;14(5):1043–1048. [PMC free article] [PubMed] [Google Scholar]
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- Ulla M, Bonny JM, Ouchchane L, Rieu I, Claise B, Durif F. Is R2* a new MRI biomarker for the progression of Parkinson’s disease? A longitudinal follow-up. PLoS One. 2013;8(3):e57904. doi: 10.1371/journal.pone.0057904. Epub 2013 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia PJ, Mena NP, Núñez MT. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front Pharmacol. 2014;5:article 38. doi: 10.3389/fphar.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooden S, Buijs M, van Vilet ME, Versluis MJ, Webb AG, Oleksik AM, van de Wiel L, Middlekoop HAM, Jan Baluw G, Weverling-Rynsburger AWE, Goos JDC, van der Flier WM, Koene T, Scheltens P, Barkhof F, van de Rest O, Slagboom PE, van Buchem MA, van der Grond J. Cortical phase changes measured using 7-T MRI in subjects with subjective cognitive impairment, and their association with cognitive function. NMR Biomed. 2014 doi: 10.1002/nbm.3248. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Brooks RA, Patronas N, Hajek M, Bulte JWM, Di Chiro G. Magnetic resonance imaging of brain iron in health and disease. Journal of the Neurological Sciences. 1995;134(Suppl):19–26. doi: 10.1016/0022-510x(95)00204-f. [DOI] [PubMed] [Google Scholar]
- Walsh AJ, Belvins G, Lebel RM, Seres P, Emery DJ, Wilman AH. Longitudinal MR imaging of iron in multiple sclerosis: An imaging marker of disease. Radiol. 2014;270(1):186–196. doi: 10.1148/radiol.13130474. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu T. Qualitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Mag Res Med. 2015;73:82–101. doi: 10.1002/mrm.25358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Li YY, Luo JH, Li YH. Age-related iron deposition in the basal ganglia of controls and Alzheimer disease patients quantified using susceptibility weighted imaging. Archives of Gerontology and Geriatrics. 2014;59:439–449. doi: 10.1016/j.archger.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Dexter DT, Crichton RR. Neurodegenerative diseases and therapeutic strategies using iron chelators. J Trace Elem Med Biol. 2015 doi: 10.1016/j.jtemb.2014.12.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–60. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M. Iron homeostasis and the inflammatory response. Ann Rev Nutr. 2010;30:105–122. doi: 10.1146/annurev.nutr.012809.104804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Buchheit CL, Berman NE, LeVine SM. Pathogenic implications of iron accumulation in multiple sclerosis. Journal of Neurochemistry. 2012;120:7–25. doi: 10.1111/j.1471-4159.2011.07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieff C, Ramanan S, Olesik J, Gauthier S, Wang Y, Pitt D. Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: interpreting positive susceptibility and the presence of iron. Mag Res Med. 2014 doi: 10.1002/mrm.25420. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Zheng G, Shen W, Liu S, Zhang LJ, Haacke EM, Lu GM. Quantitative measurements of brain iron deposition in cirrhotic patients using susceptibility mapping. Acta Radiol. 2015;56(3):339–346. doi: 10.1177/0284185114525374. [DOI] [PubMed] [Google Scholar]
- Xu J, Jia Z, Knutson MD, Leeuwenburgh C. Impaired iron status in aging research. Int J Mol Sci. 2012;13:2368–86. doi: 10.3390/ijms13022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonskiy DA, Sukstanskii AL. Generalized lorentzian tensor approach (GLTA) as a biophysical background for quantitative susceptibility mapping. Mag Res Med. 2015;73:757–764. doi: 10.1002/mrm.25538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Gonzalez RG, ØStergaard L, Komili S, Weisskoff RM, Rosen BR, Koroshetz WJ, Nishimura T, Sorensen AG. Iron-induced susceptibility effect at the globus pallidus causes underestimation of flow and volume on dynamic susceptibility contrast-enhanced MR perfusion images. Am J Neuroradiol. 2002;23:1022–1029. [PMC free article] [PubMed] [Google Scholar]
- Yan SQ, Sun JZ, Yan YQ, Wang H, Lou M. Evaluation of brain iron content based on magnetic resonance imaging (MRI): comparison among phase value, R2* and magnitude signal intensity. PLoS ONE. 2012;7:e31748. doi: 10.1371/journal.pone.0031748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, Li TQ, Gelderen Pv, Shmueli K, de Zwart JA, Duyn JH. Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. Neuroimage. 2009;44:1259–1266. doi: 10.1016/j.neuroimage.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurol. 2014;82:1266–73. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurosci. 2014;4:205. doi: 10.3389/fneuro.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MBH, Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: involvement of dopamine-opiate system. Cell Mol Biol. 2000;46(3):491–500. [PubMed] [Google Scholar]
- Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Zhong WD, Wang W, Zhan CJ, Wang CY, Qi JP, Wang JZ, Lei T. Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiol. 2009;253(2):497–504. doi: 10.1148/radiol.2532082324. [DOI] [PubMed] [Google Scholar]