Abstract
Purpose
The objective of this review is to summarize the literature (and to the extent possible, report the magnitude and direction of the association) concerning history of CSA and depression or depressive symptoms among pregnant and postpartum women.
Methods
Publications were identified through literature searches of seven databases (PubMed, EMBASE, PyscINFO, CINAHL, Web of Science, BIOSIS, and Science Direct) using keywords including “child abuse,” “depression,” “pregnancy,” “prenatal,” “pregnancy,” and “postpartum”.
Results
The literature search yielded seven eligible studies on the prenatal period and another seven studies on the postpartum period. All, but one prenatal study observed statistically significant positive associations of CSA with depression or depressive symptoms during pregnancy. Findings on the association of CSA with postpartum depression or depressive symptoms were inconsistent; pooled unadjusted and adjusted odds ratios were 1.82 (95% CI 0.92, 3.60) and 1.20 (95% CI 0.81, 1.76).
Conclusions
In sum, findings suggest a positive association of history of CSA with depression and depressive symptoms in the prenatal period. Findings on the postpartum period were inconsistent. Clinical and public health implications of evidence from the available literature are discussed, as are desirable study design characteristics of future research.
Keywords: Childhood sexual abuse, childhood trauma, depression, pregnancy, postpartum
INTRODUCTION
Definitions, prevalence and correlates of CSA
The immediate and long-term adverse health consequences of early life stress are well documented in the epidemiologic and psychiatric literature. One example of early life stress is childhood sexual abuse (CSA). Defined as developmentally inappropriate sexual activity between a child and an individual who is in a relationship of power, trust, or responsibility to the child (World Health Organization 2003), CSA is a serious public health problem with an estimated global prevalence of 7.6% among boys and 18% among girls (Stoltenborgh et al. 2011). The World Health Organization reports a CSA prevalence range of 3.8–35% for boys and 8.4–67.7% for girls (Andrews et al. 2004). Available research documents strong associations of CSA with adverse psychiatric and physical health conditions in adulthood (Finestone et al. 2000; Kendler et al. 2000; Molnar et al. 2001). In pregnant women, exposure to CSA has been associated with psychiatric disorders (Benedict et al. 1999; Robertson-Blackmore et al. 2013), health risk behaviors (Grimstad and Schei 1999) and unfavorable pregnancy outcomes (Noll et al. 2007). For example, in a prospective cohort study, Noll and colleagues observed that preterm delivery rates were significantly higher among women with CSA history compared to their counterparts without CSA history (odds: 2.80 ± 1.44, p <0.05) (Noll et al. 2007).
Definitions, prevalence and correlates of depression
Depression, the most prevalent mental disorder and a leading cause of disability globally, is perhaps the most extensively studied psychological sequelae of CSA. Over 350 million individuals worldwide experience depression, and it is thought that the majority of depression cases are undiagnosed and untreated (World Health Organization 2012). The fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM), designates an individual as having major depressive disorder if he or she experiences at least five of nine symptoms that persist for two weeks or longer, for most of the day or nearly every day (American Psychiatric Association 2000). These symptoms include depressed mood, anhedonia, fatigue or loss of energy, clinically significant weight gain/loss or appetite disturbance, insomnia or hypersomnia, feelings of excessive guilt or worthlessness, psychomotor agitation or retardation, diminished ability to concentrate or think, and recurrent thoughts of death or suicide. The severity of symptoms determines the categorization of the disorder as mild, moderate or severe (American Psychiatric Association 2000). In the US, lifetime prevalence of major depressive disorder is estimated to be 16.6% (Kessler et al. 2005) with approximately 13% among men and 21% among women (Kessler et al. 1994).
Depression in pregnancy and postpartum
The fifth edition of the DSM defines postpartum depression as depression having peripartum onset, i.e., during pregnancy and within the first four weeks after delivery (American Psychiatric Association 2013). However, the definition is usually extended to include depression with onset within the first year following delivery (Skalkidou et al. 2012).
A large meta-analysis estimated that the prevalence (and 95% CI) of prenatal depression was 7.4% (2.2, 12.6%) in the first, 12.8% (10.7, 14.8%) second and 12.0% (7.4, 16.7%) third trimesters (Bennett et al. 2004). The prevalence of postpartum depression has been reported to be as high as 19.2% in the first three months following delivery (Gavin et al. 2005). Investigators have noted that the high prevalence of postpartum depression is driven by the high rates of relapse of prenatal depression after delivery (Cohen et al. 2006). Notably, investigators have reported rates of relapse in pregnant women with a history of recurrent mood disorder to be as high as 43% (Cohen et al. 2006).
Psychiatric disorders during the prenatal and postpartum periods are of high clinical and public health significance because of their strong associations with adverse pregnancy, infant and parenting outcomes. For instance, a mature and diverse literature has documented associations of maternal psychiatric symptoms and disorders with adverse outcomes including shortened gestational length or preterm delivery (Seng et al. 2011; Sanchez et al. 2013; Yonkers et al. 2014), placental abruption (de Paz et al. 2011), preeclampsia (Qiu et al. 2007; Kim et al. 2013), and maternal health risk behaviors such as suicidality (Farber et al. 1996), cigarette smoking (Lopez et al. 2011) and substance use (Massey et al. 2011). Furthermore, maternal postpartum depression has been linked to emotional and behavioral difficulties in offspring (Cicchetti et al. 1998; Verbeek et al. 2012; Walker et al. 2013).
Although there have been several reviews on prenatal and postpartum depression (Varkukla et al. 2009; Patel et al. 2012; Sockol et al. 2013), and some reviews on the relationship between a maternal history of abuse and risk of prenatal or postpartum depression (Beydoun et al. 2012; Alvarez-Segura et al. 2014), to the best of our knowledge, no detailed systematic review has summarized or quantified the relationship between maternal history of CSA and depression or depressive symptoms during the prenatal and postpartum periods. Given this gap in the literature, and the high prevalence of history of early life sexual abuse (Andrews et al. 2004) and depression among women (Kessler et al. 1994), we undertook this systematic review and meta-analysis so as to summarize the literature concerning history of CSA with depression and depressive symptoms in pregnant and postpartum women.
MATERIALS AND METHODS
Search protocol
Published research papers were retrieved and included in this meta-analysis according to guidelines for Meta-analysis of Observational Studies in Epidemiology (MOOSE) (Stroup et al. 2000). The following seven online databases were searched from inception until August 2014: PubMed, EMBASE, PyscINFO, CINAHL, Web of Science, BIOSIS, and Science Direct. Key search terms included “childhood sexual abuse” “childhood trauma” “pregnancy” “postpartum” and “perinatal” (see Online Resource 1 for full list of search terms). The titles of all retrieved articles were screened to exclude non-pertinent papers and duplicates, after which study abstracts were read. Subsequently, relevant papers were identified for full reading. We also examined the bibliographies of retrieved papers to identify other potentially relevant publications.
Study eligibility criteria
For studies to be included in this review, they had to be: (a) full length papers published in peer-reviewed journals; (b) observational studies (prospective cohort, retrospective cohort, case-control and cross-sectional; i.e., not case series, case reports, letters to the editor, abstracts or review articles); (c) report quantitative summaries on the relationship between CSA and depression or depressive symptoms, e.g. counts, prevalence, odds ratios, correlation coefficients; (d) relevant to the prenatal and/or postpartum period(s); (e) CSA had to have occurred at < 18 years of age or before adulthood; (f) have comparison groups who did not have the exposure and/or outcome; (g) study participants had to be ≥ 18 years at time of outcome assessment; and (h) studies in English. A summary of the inclusion and exclusion criteria is provided in Table 1.
Table 1.
Study inclusion and exclusion criteria
| Characteristics | Inclusion | Exclusion |
|---|---|---|
| Publication type | Full length articles published in peer-reviewed journals | Conference abstracts/presentations, dissertations, gray literature |
| Study design | Prospective cohort, retrospective cohort, case-control, cross-sectional | Reviews, case reports, case series |
| Language | English language articles, without restriction to country | Non-English language articles |
| Sample | Pregnant women and women within a few months or years postpartum; women reporting information relevant to the pregnancy or postpartum periods | Men, non-pregnant or non-postpartum women, participants < 18 years at time of depression data collection |
| Exposure and outcome evaluation | Sexual abuse occurring at < 18 years of age or before adulthood; outcome assessment at ≥ 18 years; Use of medical records, questionnaire or interviews for collecting information on CSA exposure and depression or depressive symptoms | No comparison group (i.e., non-CSA exposed or non-depressed group) |
| Measure of association | Studies reporting quantitative information on CSA and depression (including counts, prevalence, odds ratios, r coefficients, etc) | Qualitative studies; no quantitative information on CSA-depression association |
Data synthesis, quality assessment and statistical analyses
Information from each study was compiled using a data extraction sheet that included the first name of the author, publication year, country of sample origin, study design, recruitment procedures, description of the final sample size, and summary of findings on the relationship of CSA with depression or depressive symptoms (see Tables 2 and 3). The quality of the studies included in this review was assessed using The Newcastle-Ottawa Scale (NOS) for cohort studies (Wells et al. 1999) and a modified Newcastle-Ottawa Scale for cross-sectional studies (Herzog et al. 2013). The NOS assesses studies by taking into account the selection of study groups, the comparability of the groups, and exposure and outcome assessment. For the meta-analysis, odds ratio (OR) and 95% confidence interval (95% CI) were used as a measure of association. If a study did not report OR, but reported the prevalence of depression or depressive symptoms according to CSA groups, the OR was calculated, and the corresponding author was contacted for unpublished data whenever possible. We used the software, Comprehensive Meta-analysis (CMA) to generate summary ORs and 95% CIs (Borenstein et al. 2005). CMA has been reliably used in meta-analytic studies (Martinussen et al. 2005; Segerstrom et al. 2006; Bax et al. 2007). Given that the studies included in this review differed with regard to population samples, methods of exposure and outcome ascertainment, and potential confounders adjusted for, the ORs were pooled using the random effects model that included between study heterogeneity (i.e., the DerSimonian and Laird method) (DerSimonian and Laird 1986; Bax et al. 2007; IntHout et al. 2014). We report summary ORs and 95% CI for the relationship of maternal history of CSA with postpartum depression on forest plots. We estimated the I2 statistics, where values of 25%, 50%, and 75% were considered to represent low, medium, and high heterogeneity, respectively. Due to variations in level of adjustment for confounding across studies, we conducted separate analyses for adjusted and unadjusted studies.
Table 2.
Summary of findings on the relationship between CSA and prenatal depression
| Author (year) | Country | Study design | Recruitment | Sample size | CSA assessment (data collection) | Depression or depressive symptom assessment (data collection) | Main findings |
|---|---|---|---|---|---|---|---|
| (Benedict et al. 1999) | USA | CS | Prenatal clinics in large university-based hospital (at 28–32 weeks gestation) | 357 pregnant women | ≥1 non-consensual and non-experimental contact or noncontact sexual episode (< 18 years) by a perpetrator who was ≥5 years older than victim (interviews & self-administered questionnaires) | > 30 on the CES-D scale (via interviews and self-administered questionnaires) | CSA associated with severe depressive symptomatology (aOR 2.44; 95% CI = 1.12, 5.31). Confounders adjusted for: payment source; past and current physical abuse/violence; past and current verbal abuse; negative life events; and alcohol and illegal drug use |
| (Bonacquisti et al. 2014) | USA | CS | Ob/gyn clinic affiliated with an urban university hospital (at ≥ 24 weeks gestation) | 50 HIV positive, 113 HIV-negative pregnant women | A positive answer to the question: “have you ever experienced childhood sexual abuse?” (via interviews) | ≥16 on the CES-D and high SCID scores indicated MDD (via interviews) | CSA associated with depressive symptoms; β = 0.17, p = 0.03. Confounders controlled for: HIV status, history of depression, and social support |
| (Bublitz and Stroud 2012) | USA | CS | Women were recruited throughout pregnancy | 135 pregnant women | Sexual abuse prior to age 18 (4 sexual abuse specific questions on the Adverse Childhood Experiences Scale) | Inventory for Depressive Symptomatology (IDS) | Mean IDS scores were not significantly different among women exposed to CSA (mean ± SD: 14±12) compared to women exposed to other forms of child abuse (mean ± SD: 14±8) and women with no child abuse (mean ± SD: 12 ± 10, p = 0.80) |
| (Lang et al. 2006) | USA | CS | University-affiliated obstetric clinic (average of 17.5±7.3 gestational weeks) | 44 pregnant women | Total score on sexual abuse sub-scale of CTQ (via mailed self-administered questionnaires) | Total score on the 21-item BDI-II (via mailed self-administered questionnaires) | CSA associated with depressive symptoms during pregnancy (β = 0.35, p = 0.03). No information given about control of confounding |
| (Leeners et al. 2014) | Germany | CS | Centers offering support to women who have experienced sexual abuse; and local kindergartens | 85 women with history of CSA, 170 women without CSA | Contact and non-contact sexual abuse occurring at < 18 years old (8 specific questions via interviews) | Self-reported depression during index-pregnancy (via interviews) | Depression during index-pregnancy endorsed by 24.7% of CSA exposed women compared to 1.8% among unexposed women (P < 0.001) |
| (Lev-Wiesel and Daphna-Tekoah 2010) | Israel | CS | Prenatal care visit referrals | 1003 women | Penetrative and non-penetrative sexual abuse at < 14 years old (via self-administered questionnaires) | Center for Epidemiologic Studies - Depression scale (CES-D) indicates major depression (via self-administered questionnaires) | Mean and (SD) depression score in the second trimester was 1.60 (0.38) for women with no trauma, 1.77 (0.47) for CSA-exposed women and 1.59 (0.41) for women with other trauma (p < 0.001) |
| (Robertson-Blackmore et al. 2013) | USA | CS | Hospital-based obstetrics practice (< 18 weeks gestation) | 374 women | SCID for DSM-IV-TR (via interviews) | Self -reported diagnosis of MDD, minor depression, or depressive disorder not otherwise specified | CSA was associated with depression at 18 or 32 weeks (aOR = 2.47; 95% CI, 1.27 - 4.78). Confounders controlled for: age, education, ethnicity and marital status |
ALPHA = Antenatal Psychosocial Health Assessment; aOR = adjusted odds ratio; aRR = adjusted risk ratio; BDI-II = Beck Depression Inventory II; CES-D = Center for Epidemiologic Studies - Depression; CI = Confidence Interval; CIDI = Composite International Diagnostic Interview; CTQ = Childhood Trauma Questionnaire; EPDS = Edinburgh Postnatal Depression Scale; ETI-SR = Early Trauma Inventory Self-Report; MDD = Major Depressive Disorder; Ob/gyn = Obstetrics and gynecology; PPD = Postpartum Depression; SCID = Structured Clinical Interview for DSM-IV; uOR = unadjusted odds ratio; uRR = unadjusted risk ratio; Study designs; CS = Cross Sectional; P = Prospective; R = Retrospective
Table 3.
Summary of findings on the relationship between CSA and postpartum depression
| Author (year) | Country | Study design | Recruitment | Sample size description | CSA assessment (data collection) | Depression or depressive symptom assessment (data collection) | Main findings |
|---|---|---|---|---|---|---|---|
| (Bonacquisti et al. 2014) (personal communication) | USA | P | Ob/gyn clinic affiliated with an urban university hospital (at ≥24 weeks gestation) | 50 HIV positive, 113 HIV-negative pregnant women | A positive answer to the question: “have you ever experienced childhood sexual abuse?” (via interviews) | ≥16 on the CES-D (via interviews) | 46.7% of postpartum women with CSA history had high CES-D scores compared with 20.9% of women without CSA history (uOR = 3.32, 95% CI: 1.45, 7.59) in the period between 6 weeks to 6 months postpartum |
| (Cohen et al. 2002) | Canada | P | Hospital labor and delivery ward | 200 postpartum women | Unwanted sexual experiences at < 14 years old assessed using questions modified from (Briere and Runtz 1988) (via telephone interviews) | Score of ≥ 12 on the 10-item EPDS indicative of probable PPD at 8–10 weeks postpartum (via telephone interviews) | Prevalence of CSA in women with EPDS score of 0–8 was 16% and 15% in women with EPDS score of 9–11; prevalence of CSA was 0% in women with EPDS score ≥12. No significant association between CSA and EPDS score ≥9 (uOR = 0.39, 95 CI 0.11, 1.38) |
| (Dennis and Vigod 2013) | Canada | P | Healthcare provider offices | 497 women | ALPHA questionnaire was used to assess experience of sexual abuse as a child (via standardized questionnaires) | > 9 on the 10-item EPDS indicates depressive symptomatology (via standardized questionnaires) | CSA significantly associated with 8-week postpartum depression scores (uOR = 1.93, 95% CI 1.12, 3.35) |
| (Garabedian et al. 2011) | USA | CS | Kentucky Women’s Health Registry (KWHR) | 5380 women | Yes to single question: “when you were a child, did any parent, step-parent, or guardian or any other person make you have sex (any sex act, not just intercourse) by using force or threatening to harm you or someone close to you?” (via questionnaires) | Yes to single yes/no item related to “significant symptoms of depression which women experience within a few days of giving birth and may continue to experience for weeks or months following delivery” Symptoms were not linked to a specific pregnancy (via questionnaires) | Relationship between CSA and PPD symptoms was: (uRR 1.48, 95% CI 1.26–1.74); (aRR 1.16, 95% CI 0.75–1.78). Confounders adjusted for: age, current marital status, smoking status, history of alcohol abuse, history of drug abuse, history of complicated pregnancy, and history of breastfeeding, and any adult violence exposure |
| (Plaza et al. 2012) | Spain | CS | Obstetrics units (24–48 hours postpartum) | 236 women | Sexual abuse subscale of the ETI-SR (via semi-structured interviews) Score of ≥2 of 15 on sexual abuse questions was used as the criteria for CSA exposure (via semi-structured interviews) | ≥10 on the EPDS indicated depressive symptomatology (via semi-structured interviews) | CSA associated with depressive symptomatology within 2 days of childbirth (uOR = 2.58, 95% CI 1.12–5.96) |
| (Roberts et al. 2004) | UK | P | Residents of Avon, England recruited through midwives, local publicity, and direct contact | 8292 women | Self-report questions about if the respondent had ever been sexually abused, and at what age (CSA defined as sexual assault before the teenage years) | Total continuous EPDS score (via questionnaire) | CSA associated with higher levels of depressive symptoms within 3 years after childbirth (7.89 vs. 6.11, p = 0.0001; β = 0.04) |
| (Robertson-Blackmore et al. 2013) | USA | P | Hospital-based obstetrics practice (< 18 weeks gestation) | 374 women | SCID for DSM-IV-TR (via interviews) | Diagnosis of MDD, minor depression, or depressive disorder not otherwise specified within 6 weeks to 6 months postpartum (according to SCID for DSM-IV-TR) | CSA was not significantly associated with 6 – 8 week or 6-month postpartum depression (aOR = 1.10, 95% CI 0.44 – 2.7). Confounders controlled for: age, education, ethnicity, marital status, and antepartum depression |
ALPHA = Antenatal Psychosocial Health Assessment; aOR = adjusted odds ratio; aRR = adjusted risk ratio; BDI-II = Beck Depression Inventory II; CES-D = Center for Epidemiologic Studies - Depression; CI = Confidence Interval; CIDI = Composite International Diagnostic Interview; CTQ = Childhood Trauma Questionnaire; EPDS = Edinburgh Postnatal Depression Scale; ETI-SR = Early Trauma Inventory Self-Report; MDD = Major Depressive Disorder; Ob/gyn = Obstetrics and gynecology; PPD = Postpartum Depression; SCID = Structured Clinical Interview for DSM-IV; uOR = unadjusted odds ratio; uRR = unadjusted risk ratio; Study designs; CS = Cross Sectional; P = Prospective; R = Retrospective
RESULTS
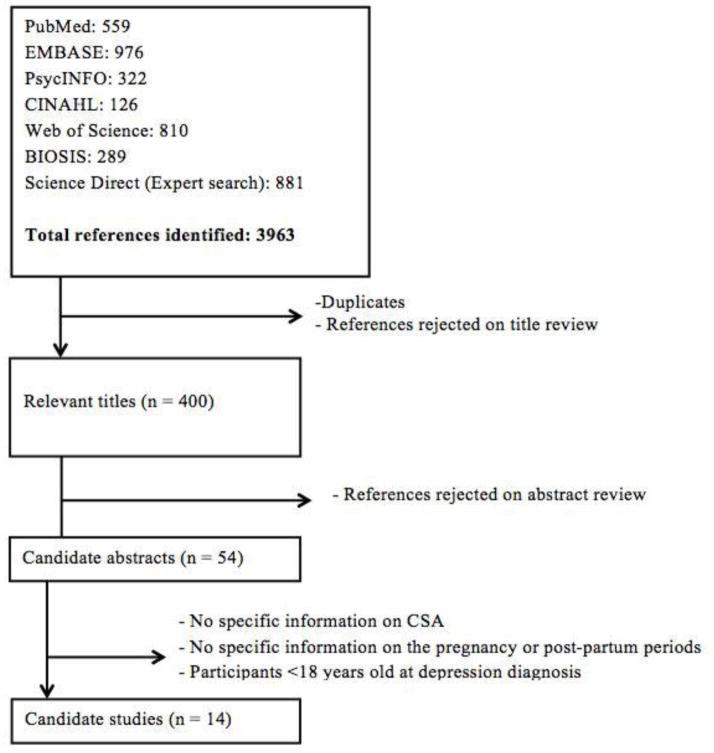
Figure 1 details the process by which relevant publications were selected. The literature search yielded 3,963 papers, of which 3,563 were duplicates or rejected on title review. The abstracts of the remaining 400 papers were read. After exclusion of non-pertinent abstracts, 54 publications were selected for full reading and data abstraction. Seven studies on prenatal depression and seven studies on postpartum depression met the eligibility criteria for inclusion in our systematic review (Tables 2 and 3). We provide a narrative review of all 14 studies. Only three prenatal studies provided information suitable and sufficient for meta-analysis. Due to this small number, we are unable to provide a quantitative summary for the relationship between CSA history and prenatal depression or depressive symptoms. Six postpartum depression studies had information suitable and sufficient for meta-analysis and thus were meta-analyzed. Two studies (Robertson-Blackmore et al. 2013; Bonacquisti et al. 2014) were counted twice as the authors observed women in both the prenatal and postpartum periods.
Fig. 1.
Selection of articles reporting on the relationship between CSA and depression in pregnant or postpartum women
Prenatal Depression
The seven original articles reporting on the association between maternal history of CSA and prenatal depression or depressive symptoms (Benedict et al. 1999; Lang et al. 2006; Lev-Wiesel and Daphna-Tekoah 2010; Bublitz and Stroud 2012; Robertson-Blackmore et al. 2013; Bonacquisti et al. 2014; Leeners et al. 2014) were based on cross-sectional data. Five studies were from the USA, one from Germany, and one from Israel. For the majority of studies, women were recruited at hospital or clinic facilities. Two studies assessed depression (Robertson-Blackmore et al. 2013; Leeners et al. 2014) while the remaining five assessed depressive symptoms. Findings from all but one study (Bublitz and Stroud 2012) yielded results that were consistent with positive and statistically significant associations between maternal history of CSA and prenatal depression or depressive symptoms.
In a sample of 357 pregnant US women, Benedict and colleagues observed that maternal history of CSA was positively associated with prenatal depressive symptoms. Approximately 59% of CSA-exposed women compared with 42% of non-exposed women had a score of >15 on the Center for Epidemiologic Studies–Depression (CES-D) Scale (p < 0.002); and approximately 20% of CSA-exposed women and 7% of non-exposed women had CES-D score >30 (p <0.0004) (Benedict et al. 1999). After adjustment for confounding by medical care payment source, past and current physical abuse/violence, past and current verbal abuse, negative life events, and alcohol and illegal drug use, the association between CSA history and CES-D score >30 was OR = 2.44 (95% CI 1.12, 5.31). Similarly, Bonacquisti and colleagues observed a statistically significant positive association between CSA and CES-D scores (β = 0.17, p = 0.03) after adjusting for HIV status, history of depression, and social support (Bonacquisti et al. 2014). In a sample of 44 pregnant women attending prenatal care clinics, Lang et al. observed a significant association between the Childhood Trauma Questionnaire score and Beck Depression Inventory score (β = 0.35, p = 0.03) (Lang et al. 2006). Leeners et al. observed a 24.7% prevalence of self-reported depression in women with CSA history compared to 1.8% in women without CSA history (p < 0.001) (Leeners et al. 2014). Lev-Wiesel et al. observed higher mean CES-D scale scores among pregnant women with CSA history (mean ± SD: 1.77 ± 0.47) as compared to women with no trauma history (mean ± SD: 1.60 ± 0.38) and women with trauma other than CSA (mean ± SD: 1.59 ± 0.41, p < 0.05) (Lev-Wiesel and Daphna-Tekoah 2010). Robertson-Blackmore and colleagues reported that a history of CSA was associated with prenatal depression assessed using the Structured Clinical Interview for DSM-IV (SCID) (OR = 2.47, 95% CI 1.27, 4.78, after adjusting for maternal age, educational attainment, ethnicity, and marital status) (Robertson-Blackmore et al. 2013). Finally, Bublitz et al. observed no statistically significant difference in mean scores on the Inventory for Depressive Symptoms among pregnant women with CSA history (mean ± SD: 14±12) compared to counterparts exposed to other forms of child abuse (mean ± SD: 14 ± 8) or no child abuse (mean ± SD: 12 ± 10), p = 0.80 (Bublitz and Stroud 2012).
Postpartum Depression
Seven original research papers reported on the association of maternal history of CSA with postpartum depression (Robertson-Blackmore et al. 2013) or depressive symptoms (Cohen et al. 2002; Roberts et al. 2004; Garabedian et al. 2011; Plaza et al. 2012; Dennis and Vigod 2013). For one study, postpartum information was obtained over email (Bonacquisti et al. 2014; personal communication). Two studies were from Canada, three from the USA, one from Spain and one from the UK. Five studies were prospective analyses, and two were cross-sectional (Table 3). Findings were inconsistent across these seven studies. Statistically significant positive associations were observed in four studies (Roberts et al. 2004; Plaza et al. 2012; Dennis and Vigod 2013; Bonacquisti et al. 2014); however, these studies only reported unadjusted ORs of depressive symptoms in relation to history of CSA. The ORs from the two studies (Garabedian et al. 2011; Robertson-Blackmore et al. 2013) that reported adjusted measures of association were not statistically significant. One study did not report adjusted OR and observed no significant association between CSA and depressive symptoms (Cohen et al. 2002).
In a prospective cohort study of 497 women, Dennis et al. observed a statistically significant association between history of CSA and depressive symptoms (EPDS score > 9) (unadjusted OR = 1.93, 95% CI 1.12, 3.35) (Dennis and Vigod 2013). Similarly, Plaza et al. observed increased odds of depressive symptoms using EPDS among women with history of CSA compared to those without such history (unadjusted OR = 2.58, 95% CI 1.12, 5.95) (Plaza et al. 2012). In a study of women residing in Avon, England, Roberts and colleagues observed higher mean EPDS scores within three years of childbirth for women with CSA history compared to women without CSA history (7.89 vs. 6.11, p < 0.01) (Roberts et al. 2004). In a cross-sectional study, 46.7% of postpartum women with CSA history had CES-D scores ≥ 16 compared with 20.9% of women without CSA history (unadjusted OR = 3.32, 95% CI: 1.45, 7.59) (Bonacquisti et al. 2014) (personal communication).
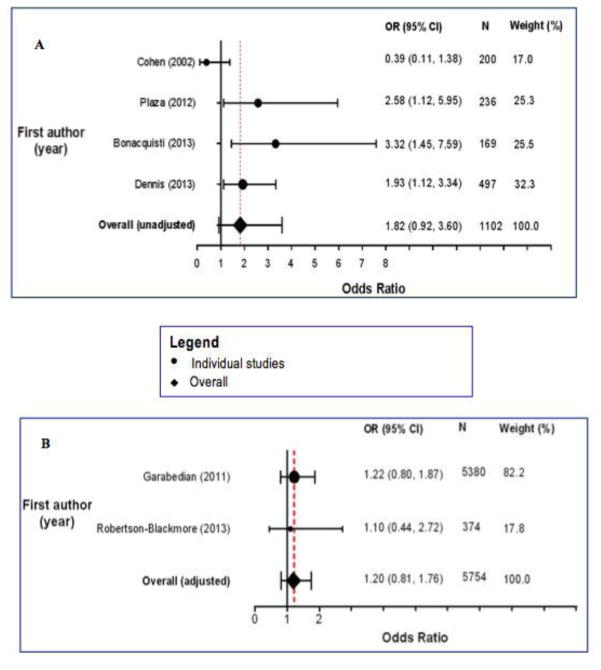
Conversely, three other studies documented no statistically significant associations of CSA with postpartum depression or depressive symptoms (Cohen et al. 2002; Garabedian et al. 2011; Robertson-Blackmore et al. 2013). In a prospective cohort study, CSA was not associated with postpartum depression (OR = 1.10, 95% CI 0.44, 2.77) after adjustment for maternal age, educational attainment, ethnicity, marital status, and prenatal depression) (Robertson-Blackmore et al. 2013). Garabedian and colleagues did not observe a significant association between CSA and postpartum depressive symptoms (OR = 1.22, 95% CI 0.80, 1.87) after they accounted for confounding by maternal age, current marital status, smoking status, history of alcohol and drug abuse, history of complicated pregnancy, and history of breastfeeding (Garabedian et al. 2011). Finally, in a study of 198 postpartum women, Cohen et al. observed that only 14.2% of women with CSA history had EPDS scores ≥ 9 as compared with 23.4% of women with no CSA history (unadjusted OR = 0.39, 95% CI 0.11, 1.38) (Cohen et al. 2002). As shown in Figure 2A, the pooled, summary unadjusted OR for postpartum depression given maternal history of CSA was 1.82 (95% CI 0.92, 3.60) for the four studies reporting unadjusted measures of association (Cohen et al. 2002; Plaza et al. 2012; Dennis and Vigod 2013; Bonacquisti et al. 2014) (Figure 2A). Notably, there was evidence of heterogeneity (I2 = 63.7%, p = 0.04) so this pooled OR should be interpreted with caution. The pooled OR for the two studies reporting adjusted measures of association (Garabedian et al. 2011; Robertson-Blackmore et al. 2013) was 1.20 (95% CI 0.81, 1.76), with no evidence of heterogeneity (I2 = 0.0%, p = 0.84) (Figure 2B). On balance, available evidence on the association of maternal history of CSA and risk of postpartum depression or depressive symptoms is equivocal at best.
Fig. 2.
Fig. 2a Forest plot showing studies on the relationship between CSA and postpartum depression (unadjusted estimates)
Fig. 2b Forest plot showing studies on the relationship between CSA and postpartum depression (adjusted estimates)
DISCUSSION
In this review, we have summarized findings from 14 studies that examined the association of maternal history of CSA with prenatal and postpartum depression or depressive symptoms. To the best of our knowledge, this is the first review presenting both a narrative and quantitative summary on this topic. Six of seven prenatal studies observed a statistically significant positive association; however, this interpretation warrants some caution as only three (Benedict et al. 1999; Robertson-Blackmore et al. 2013; Bonacquisti et al. 2014) of the seven prenatal studies adjusted for potential confounders. The findings from studies focused on the relationship of maternal history of CSA and risk of postpartum depression were inconsistent, with four of five unadjusted studies reporting statistically significant associations. Of importance, the two studies that controlled for potential confounders found no evidence of statistically significant associations of CSA with postpartum depression or depressive symptoms. Thus the findings suggest that CSA history may be more strongly associated with prenatal depression or depressive symptoms than with postpartum depression or depressive symptoms. Some hypothesized mechanisms that may underlie the relationship between CSA and depression are discussed below.
Hypothesized mechanisms
In early life, a critical period of development, the brain and its neural systems are highly sensitive to transient and permanent alterations in morphology and function secondary to environmental, behavioral, and genetic influences (Penza et al. 2003; Pascual-Leone et al. 2011; Davidson and McEwen 2012; Oberman and Pascual-Leone 2013). Brain imaging studies have observed significant structural changes in individuals at risk of depression, particularly those exposed to childhood abuse (Rao et al. 2009; Frodl et al. 2011; Carballedo et al. 2012). Human and non-human primate studies have noted long-term aberrations in concentrations of corticotopin releasing hormone (CRH) and cortisol, two gluccorticoid hormones released by the hypothalamic-pituitary-adrenal (HPA) axis, after exposure to early life adversity (Bublitz and Stroud 2012; Dettmer et al. 2012; Feng et al. 2012; Yehuda et al. 2010; Hulme 2011). For example, Bublitz et al. observed higher cortisol awakening response at 35 weeks gestation among women with a history of CSA (mean ± SD: 6.58 ± 1.20), as compared with women who had no such history (mean ± SD: 1.80 ± 1.13), p = 0.009 (Bublitz and Stroud 2012). Although pregnancy is accompanied by profound changes in the neuroendocrine system, characterized by pituitary gland enlargement, and increases in pituitary peptide output (Glynn et al. 2013) and CRH production (Horan et al. 2000; Glynn et al. 2013), chronic overproduction of glucocorticoid hormones, particularly CRH, before and during pregnancy in women with early life stress, may play a role in higher rates of adverse pregnancy outcomes among these women (Horan et al. 2000). This hypothesis is supported by research linking elevated CRH concentrations with increased risk of depression in postpartum (Yim et al. 2009; Hahn-Holbrook et al. 2013; Glynn and Sandman 2014).
Investigators have also observed inflammatory and epigenetic pathway similarities between individuals who experienced childhood adversity and individuals with depression (Kiecolt-Glaser et al. 2011; Bertone-Johnson et al. 2012; Slopen et al. 2012; Drury et al. 2014). Research links shortened telomere length, a molecular marker of premature aging, with exposure to psychosocial stressors including childhood adversity (Kiecolt-Glaser et al. 2011; Drury et al. 2014). Shortened telomere lengths have also been documented in depressed individuals compared to non-depressed controls (Hartmann et al. 2010; Karabatsiakis et al. 2014; Verhoeven et al. 2014). Of note, Karabatsiakis et al. observed that women with depression had a telomere length shortening of CD8+ cytotoxic T lymphocytes that was equivalent to a mean age difference of 27.9 (SD: 25.3) years compared to women without depression (Karabatsiakis et al. 2014).
Finally, some investigators have noted that pregnant women with a history of sexual abuse may re-experience memories of their abuse during procedures of routine pregnancy care (Leeners et al. 2006) and labor (Courtois and Courtois Riley 1992; Waymire 1997). The reactivation of such memories may increase the risk of depression. To the best of our knowledge, no study has prospectively examined the relationship between a history of CSA and incident prenatal or postpartum depression. Of the few studies on postpartum depression, none have been able to establish that the women did not have depression and depressive symptoms during the prenatal period. Although exact mechanisms are not fully understood, noted similarities in biological aberrations in individuals with adverse childhood events and depression may yield important clues about the relationships and these clues may give way to identification and development of strategies and protocols that may be implemented during prenatal care to help mitigate the risk of prenatal and postpartum depression.
Strengths and Limitations
Strengths of this review include an extensive systematic search of available literature on seven online databases, use of established MOOSE guidelines, a narrative summary of the findings, and to the extent possible, a presentation of summary quantitative estimates of the relationship of CSA with depression and depressive symptoms in postpartum women. Despite these strengths, some limitations should be considered in the interpretation of findings from this study. First, instruments used to assess CSA and depressive characteristics varied across the 14 studies and could have accounted for heterogeneity in research findings. Second, the primary objective of the majority of the studies was not to examine the association between CSA and depression. Hence, important potential confounders, mediators and moderators of the CSA-depression relationship (e.g., adult exposure to abuse, frequency of abuse, age at time of abuse, relationship of victim to perpetuator) were unaccounted for, limiting causal inference from most available studies. Third, most studies lacked information on maternal pre-pregnancy depression status, and so were unable to establish the incidence of depression or depressive symptoms during the prenatal or postpartum periods. Fourth, all the studies were conducted in high-income countries, which limits generalizability of findings to middle- and low- income countries, which may differ with regards to the burden of CSA among pregnant and postpartum women.
Implications
CSA and depression are two public health problems with major impact on women’s health. Globally, approximately 1 in 5 girls are exposed to CSA (Stoltenborgh et al. 2011); and depression is the most common mental disorder among women, with up to 20% of women experiencing major depression during their lifetime (Kessler et al. 1994). Prenatal and postpartum mental disorders, such as depression, are associated with adverse pregnancy outcomes (de Paz et al. 2011; Kim et al. 2013; Sanchez et al. 2013) and with emotional disturbance in offspring (Verbeek et al. 2012; Walker et al. 2013), thus the burden of maternal depression has implications on the physical and cognitive development of the next generation.
Findings from studies included in this review suggest that women with a history of CSA may be significantly more likely to experience depression or depressive symptoms during pregnancy whilst the association with postpartum depression may be somewhat more equivocal. Given the significance of the topic, noted gaps and limitations of the available evidence and the high prevalence of CSA and depression among women globally, we maintain that large multi-national longitudinal studies with systematic exposure and outcome assessment are needed to provide higher quality information about the incidence and progression of depressive symptoms and depression in prenatal and postpartum women given history of early life stressors, access to social support and other buffers of stress across the life course. In addition, there is need for studies that incorporate biochemical and molecular risk markers that will facilitate exploration of mechanistic hypotheses and prognostic indicators of morbidity and response to treatment. Finally, given that all available studies were conducted in high-income countries, studies examining the implications of the CSA-depression relationship in middle- and low-income countries are needed.
CONCLUSION
In conclusion, our systematic review and meta-analysis contribute to the existing literature on the effects of CSA on the mental health of women of reproductive age by summarizing current knowledge on the relationship of CSA with depression and depressive symptoms in pregnant and postpartum women, and providing suggestions for improving literature on this topic.
Supplementary Material
Search terms used to identify relevant publications
Quality assessment of CSA-depression relationship in studies included in this review (n=14), using the Newcastle-Ottawa Scale
Acknowledgments
This research was supported by an award from the National Institutes of Health (NIH), the Eunice Kennedy Shriver Institute of Child Health and Human Development (R01-HD-059835).
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Alvarez-Segura M, Garcia-Esteve L, Torres A, Plaza A, Imaz ML, Hermida-Barros L, San L, Burtchen N. Are women with a history of abuse more vulnerable to perinatal depressive symptoms? A systematic review. Arch Womens Ment Health. 2014;17:343–357. doi: 10.1007/s00737-014-0440-9. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington D.C: 2000. text revision. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Association; Washington D.C: 2013. [Google Scholar]
- Andrews G, Corry J, Slade T, Issakidis C, Swanston H. Child sexual abuse. In: Ezzati M, Lopez A, Rodgers A, Murray C, editors. Comparative quantification of health risks : global and regional burden of disease attributable to selected major risk factors. Vol. 2. World Health Organization; Geneva: 2004. pp. 1851–1940. [Google Scholar]
- Bax L, Yu L-M, Ikeda N, Moons K. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med Res Methodol. 2007;7:40. doi: 10.1186/1471-2288-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MI, Paine LL, Paine LA, Brandt D, Stallings R. The association of childhood sexual abuse with depressive symptoms during pregnancy, and selected pregnancy outcomes. Child Abuse Negl. 1999;23:659–670. doi: 10.1016/s0145-2134(99)00040-x. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: Systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med. 2012;43:611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun HA, Beydoun MA, Kaufman JS, Lo B, Zonderman AB. Intimate partner violence against adult women and its association with major depressive disorder, depressive symptoms and postpartum depression: A systematic review and meta-analysis. Soc Sci Med. 2012;75:959–975. doi: 10.1016/j.socscimed.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacquisti A, Geller PA, Aaron E. Rates and predictors of prenatal depression in women living with and without HIV. AIDS Care. 2014;26:100–106. doi: 10.1080/09540121.2013.802277. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis Version 2. Biostat; Englewood, NJ: 2005. [Google Scholar]
- Briere J, Runtz M. Symptomatology associated with childhood sexual victimization in a non-clinical sample. Child Abuse Negl. 1988;12:51–59. doi: 10.1016/0145-2134(88)90007-5. [DOI] [PubMed] [Google Scholar]
- Bublitz MH, Stroud LR. Childhood sexual abuse is associated with cortisol awakening response over pregnancy: Preliminary findings. Psychoneuroendocrinology. 2012;37:1425–1430. doi: 10.1016/j.psyneuen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballedo A, Lisiecka D, Fagan A, Saleh K, Ferguson Y, Connolly G, Meaney J, Frodl T. Early life adversity is associated with brain changes in subjects at family risk for depression. World J Biol Psychiatry. 2012;13:569–578. doi: 10.3109/15622975.2012.661079. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: Contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol. 1998;10:283–300. doi: 10.1017/s0954579498001618. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Schei B, Ansara D, Gallop R, Stuckless N, Stewart DE. A history of personal violence and postpartum depression: Is there a link? Arch Womens Ment Health. 2002;4:83–92. [Google Scholar]
- Courtois CA, Courtois RC. Pregnancy and childbirth as triggers for abuse memories: Implications for care. Birth. 1992;19:222–223. doi: 10.1111/j.1523-536x.1992.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paz NC, Sanchez SE, Huaman LE, Chang GD, Pacora PN, Garcia PJ, Ananth CV, Qiu C, Williams MA. Risk of placental abruption in relation to maternal depressive, anxiety and stress symptoms. J Affect Disord. 2011;130:280–284. doi: 10.1016/j.jad.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, Vigod S. The relationship between postpartum depression, domestic violence, childhood violence, and substance use: Epidemiologic study of a large community sample. Violence Against Wom. 2013;19:503–517. doi: 10.1177/1077801213487057. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: Hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37:191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, Theall KP. The association of telomere length with family violence and disruption. Pediatrics. 2014;134:e128–137. doi: 10.1542/peds.2013-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber EW, Herbert SE, Reviere SL. Childhood abuse and suicidality in obstetrics patients in a hospital-based urban prenatal clinic. Gen Hosp Psychiatry. 1996;18:56–60. doi: 10.1016/0163-8343(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Feng X, Wang L, Yang S, Qin D, Wang J, Li C, Lv L, Ma Y, Hu X. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci USA. 2012;108:14312–14317. doi: 10.1073/pnas.1010943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finestone HM, Stenn P, Davies F, Stalker C, Fry R, Koumanis J. Chronic pain and health care utilization in women with a history of childhood sexual abuse. Child Abuse Negl. 2000;24:547–556. doi: 10.1016/s0145-2134(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. J Psychiatry Neurosci. 2012;37:37–45. doi: 10.1503/jpn.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian MJ, Lain KY, Hansen WF, Garcia LS, Williams CM, Crofford LJ. Violence against women and postpartum depression. J Womens Health (Larchmt) 2011;20:447–453. doi: 10.1089/jwh.2010.1960. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: A systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47:363–370. doi: 10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. 2014;76:355–362. doi: 10.1097/PSY.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Grimstad H, Schei B. Pregnancy and delivery for women with a history of child sexual abuse. Child Abuse Negl. 1999;23:81–90. doi: 10.1016/s0145-2134(98)00113-6. [DOI] [PubMed] [Google Scholar]
- Hahn-Holbrook J, Schetter CD, Arora C, Hobel CJ. Placental corticotropin-releasing hormone mediates the association between prenatal social support and postpartum depression. Clin Psychol Sci. 2013;1:253–264. doi: 10.1177/2167702612470646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27:1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Angel G. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan DL, Hill LD, Schulkin J. Childhood sexual abuse and preterm labor in adulthood: An endocrinological hypothesis. Womens Health Issues. 2000;10:27–33. doi: 10.1016/s1049-3867(99)00038-9. [DOI] [PubMed] [Google Scholar]
- Hulme PA. Childhood sexual abuse, HPA axis regulation, and mental health: an integrative review. West J Nurs Res. 2011;33:1069–1097. doi: 10.1177/0193945910388949. [DOI] [PubMed] [Google Scholar]
- IntHout J, Ioannidis J, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;18:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE. Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry. 2014;14:192. doi: 10.1186/1471-244X-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DR, Sockol LE, Sammel MD, Kelly C, Moseley M, Epperson CN. Elevated risk of adverse obstetric outcomes in pregnant women with depression. Arch Womens Ment Health. 2013;16:475–482. doi: 10.1007/s00737-013-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AJ, Rodgers CS, Lebeck MM. Associations between maternal childhood maltreatment and psychopathology and aggression during pregnancy and postpartum. Child Abuse Negl. 2006;30:17–25. doi: 10.1016/j.chiabu.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Leeners B, Rath W, Block E, Gorres G, Tschudin S. Risk factors for unfavorable pregnancy outcome in women with adverse childhood experiences. J Perinat Med. 2014;42:171–178. doi: 10.1515/jpm-2013-0003. [DOI] [PubMed] [Google Scholar]
- Leeners B, Richter-Appelt H, Imthurn B, Rath W. Influence of childhood sexual abuse on pregnancy, delivery, and the early postpartum period in adult women. J Psychosom Res. 2006;61:139–151. doi: 10.1016/j.jpsychores.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Lev-Wiesel R, Daphna-Tekoah S. The role of peripartum dissociation as a predictor of posttraumatic stress symptoms following childbirth in Israeli Jewish women. J Trauma Dissociation. 2010;11:266–283. doi: 10.1080/15299731003780887. [DOI] [PubMed] [Google Scholar]
- Lopez WD, Konrath SH, Seng JS. Abuse-related post-traumatic stress, coping, and tobacco use in pregnancy. Obstet Gynecol. 2011;40:422–431. doi: 10.1111/j.1552-6909.2011.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Massey SH, Lieberman DZ, Reiss D, Leve LD, Shaw DS, Neiderhiser JM. Association of clinical characteristics and cessation of tobacco, alcohol, and illicit drug use during pregnancy. Am J Addict. 2011;20:143–150. doi: 10.1111/j.1521-0391.2010.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar BE, Berkman LF, Buka SL. Psychopathology, childhood sexual abuse and other childhood adversities: Relative links to subsequent suicidal behaviour in the US. Psychol Med. 2001;31:965–977. doi: 10.1017/s0033291701004329. [DOI] [PubMed] [Google Scholar]
- Noll JG, Schulkin J, Trickett PK, Susman EJ, Breech L, Putnam FW. Differential pathways to preterm delivery for sexually abused and comparison women. J Pediatr Psychol. 2007;32:1238–1248. doi: 10.1093/jpepsy/jsm046. [DOI] [PubMed] [Google Scholar]
- Oberman L, Pascual-Leone A. Changes in plasticity across the lifespan: Cause of disease and target for intervention. Prog Brain Res. 2013;207:91–120. doi: 10.1016/B978-0-444-63327-9.00016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, Rotenberg A. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24:302–315. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Bailey RK, Jabeen S, Ali S, Barker NC, Osiezagha K. Postpartum depression: A review. J Health Care Poor Underserved. 2012;23:534–542. doi: 10.1353/hpu.2012.0037. [DOI] [PubMed] [Google Scholar]
- Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: Implications for the pathophysiology of depression and anxiety. Arch Womens Ment Health. 2003;6:15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- Plaza A, Garcia-Esteve L, Torres A, Ascaso C, Gelabert E, Luisa Imaz M, Navarro P, Valdes M, Martin-Santos R. Childhood physical abuse as a common risk factor for depression and thyroid dysfunction in the earlier postpartum. Psychiatry Res. 2012;200:329–335. doi: 10.1016/j.psychres.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Qiu C, Sanchez SE, Lam N, Garcia P, Williams MA. Associations of depression and depressive symptoms with preeclampsia: Results from a Peruvian case-control study. BMC Womens Health. 2007;7:15. doi: 10.1186/1472-6874-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, O'Connor T, Dunn J, Golding J ALSPAC Study Team. The effects of child sexual abuse in later family life; mental health, parenting and adjustment of offspring. Child Abuse Negl. 2004;28:525–545. doi: 10.1016/j.chiabu.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Robertson-Blackmore E, Putnam FW, Rubinow DR, Matthieu M, Hunn JE, Putnam KT, Moynihan JA, O'Connor TG. Antecedent trauma exposure and risk of depression in the perinatal period. J Clin Psychiatry. 2013;74:e942–948. doi: 10.4088/JCP.13m08364. [DOI] [PubMed] [Google Scholar]
- Sanchez SE, Puente GC, Atencio G, Qiu C, Yanez D, Gelaye B, Williams MA. Risk of spontaneous preterm birth in relation to maternal depressive, anxiety, and stress symptoms. J Reprod Med. 2013;58:25–33. [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2006;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng JS, Low LK, Sperlich M, Ronis DL, Liberzon I. Post-traumatic stress disorder, child abuse history, birthweight and gestational age: a prospective cohort study. Brit J Obset Gynaec. 2011;118:1329–1339. doi: 10.1111/j.1471-0528.2011.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalkidou A, Hellgren C, Comasco E, Sylven S, Sundstrom Poromaa I. Biological aspects of postpartum depression. Womens Health (Lond Engl) 2012;8:659–672. doi: 10.2217/whe.12.55. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2012;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockol LE, Epperson CN, Barber JP. Preventing postpartum depression: A meta-analytic review. Clin Psychol Rev. 2013;33:1205–1217. doi: 10.1016/j.cpr.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenborgh M, van Ijzendoorn MH, Euser EM, Bakermans-Kranenburg MJ. A global perspective on child sexual abuse: Meta-analysis of prevalence around the world. Child Maltreat. 2011;16:79–101. doi: 10.1177/1077559511403920. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Varkukla M, Viguera AC, Gonsalves L. Depression and pregnancy. Compr Ther. 2009;35:44–49. [PubMed] [Google Scholar]
- Verbeek T, Bockting CL, van Pampus MG, Ormel J, Meijer JL, Hartman CA, Burger H. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. J Affect Disord. 2012;136:948–954. doi: 10.1016/j.jad.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: Results from a large psychiatric cohort study. Mol Psychiatry. 2014;19:895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- Walker MJ, Davis C, Al-Sahab B, Tamim H. Reported maternal postpartum depression and risk of childhood psychopathology. Matern Child Health J. 2013;17:907–917. doi: 10.1007/s10995-012-1071-2. [DOI] [PubMed] [Google Scholar]
- Waymire V. A triggering time. Childbirth may recall sexual abuse memories. AWHONN Lifelines. 1997;1:47–50. doi: 10.1111/j.1552-6356.1997.tb00931.x. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Health Research Institute; 1999. [Accessed November 6, 2014]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- World Health Organization. Guidelines for medico-legal care for victims of sexual violence. Geneva, Switzerland: 2003. pp. 1–144. [Google Scholar]
- World Health Organization. [Accessed November 5, 2014];Depression: Fact Sheet. 2012 http://www.who.int/mediacentre/factsheets/fs369/en.
- Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berl) 2010;212:405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Smith MV, Forray A, Epperson CN, Costello D, Lin H, Belanger K. Pregnant women with posttraumatic stress disorder and risk of preterm birth. JAMA Psychiatry. 2014;71:897–904. doi: 10.1001/jamapsychiatry.2014.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search terms used to identify relevant publications
Quality assessment of CSA-depression relationship in studies included in this review (n=14), using the Newcastle-Ottawa Scale