Abstract
Huntington disease (HD) is a progressive autosomal dominant neurodegenerative disorder, characterized by abnormal movements, cognitive decline and psychiatric symptoms, caused by a CAG repeat expansion in the huntingtin (HTT) gene on chromosome 4p. A CAG/CTG repeat expansion in the junctophilin-3 (JPH3) gene on chromosome 16q24.2 causes a Huntington disease-like phenotype (HDL2). All patients to date with HDL2 have some African ancestry. The present study aimed to characterize the genetic basis of the Huntington disease phenotype in South Africans and to investigate the possible origin of the JPH3 mutation. In a sample of unrelated South African individuals referred for diagnostic HD testing, 62% (106/171) of white patients compared to only 36% (47/130) of black patients had an expansion in HTT. However, 15% (20/130) of black South African patients and no white patients (0/171) had an expansion in JPH3, confirming the diagnosis of Huntington disease like 2 (HDL2). Individuals with HDL2 share many clinical features with individuals with HD and are clinically indistinguishable in many cases, although the average age of onset and diagnosis in HDL2 is 5 years later than HD and individual clinical features may be more prominent. HDL2 mutations contribute significantly to the HD phenotype in South Africans with African ancestry. JPH3 haplotype studies in 31 families, mainly from South Africa and North America, provide evidence for a founder mutation and support a common African origin for all HDL2 patients. Molecular testing in individuals with an HD phenotype and African ancestry should include testing routinely for JPH3 mutations.
INTRODUCTION
Huntington disease (HD) is a late-onset progressive lethal neurodegenerative disorder, characterized by the triad of a movement disorder, cognitive decline and psychiatric symptoms. Clinical features of HD include progressive voluntary and involuntary motor impairment, usually with prominent chorea, mood and personality changes and dementia as a result of neuronal dysfunction and death. The onset of symptoms usually occurs in midlife, with approximately 60% of cases diagnosed between 35 and 50 years of age, and the remainder equally before and after these ages. Symptoms gradually worsen over the course of 10–20 years, with a gradual deterioration in intellectual function leading eventually to total incapacitation, dementia and death (Harper, 1996).
HD is inherited as an autosomal dominant trait and has a worldwide distribution. The HD gene, HTT, is located on chromosome 4p16.3 and contains an unstable polyglutamine-encoding CAG repeat at the 5′ end of exon 1. A CAG repeat of 40 triplets or greater is predictive of disease, while repeats of 36 to 39 triplets display reduced penetrance (Andrew et al., 1993; Duyao et al., 1993; Snell et al., 1993; Semaka et al., 2006). Mutations in this gene are considered to be the cause of HD in the great majority (>99%) of families worldwide with an autosomal dominant HD phenotype (Kremer et al., 1994).
In populations originating from Europe, HD has a minimum disease prevalence of 5 per 100 000 individuals. A similar prevalence was observed in India (Warby et al., 2011). The minimum HD prevalence in China and Japan is estimated to be 0.1–0.5 per 100 000 individuals (Chang et al., 1994; Nakashima et al., 1996). Prior to the identification of the HD mutation, a high frequency of the HD phenotype had been reported in South African White Afrikaners due to a founder effect (Hayden et al., 1980a). In South Africans of mixed ancestry comparable frequencies to those found in other European populations had been reported, but with an increased proportion of juvenile cases (Hayden et al., 1980b; Hayden et al., 1982).
The Huntington disease phenotype has been thought to be less common in black African populations, with an estimated frequency of 0.1 per million (Hayden et al., 1980b; Harper, 1992). However, many cases have now been documented, some with mutation confirmation, from countries in Africa, including South Africa (Glass and Saffer, 1979; Hayden et al., 1980b; Joubert and Botha, 1988; Silber et al., 1998; Magazi et al., 2008; Sizer et al., 2012), Zimbabwe (Samuels and Gelfand, 1978; Scrimgeour and Pfumojena, 1992), Tanzania (Scrimgeour, 1981; 1982), Kenya, Nigeria, Uganda, Togo and Sudan (Scrimgeour and Simpson, 1992; Scrimgeour et al., 1995).
Although no new disease prevalence figures have been calculated, under-ascertainment of cases seems to explain at least part of the reported low frequency. It was initially proposed that all cases could be explained by European admixture (Scrimgeour and Simpson, 1992), but different haplotypes in black South African families suggested different African origins for these mutations (Greenberg et al., 1991; Warby et al., 2009; Baine et al., 2013). However, a lower modal normal CAG repeat number at the HTT locus in the general population, with no alleles >20 repeats reported, may predict a lower disease frequency (Squitieri et al., 1994; Baine et al., 2013).
A number of families have now been reported with a Huntington disease like (HDL) phenotype and no mutations in the HD gene. An expanded octapeptide repeat in the prion protein (PRNP) gene is responsible for HDL1 (chromosome 20p12) in a Swedish family (Xiang et al., 1998; Moore et al., 2001). A possible locus on 4p15.3 (HDL3) causes a recessive form of the disease in a Saudi Arabian family (Kambouris et al., 2000). A CAA/CAG repeat expansion in the TATA binding protein gene (TBP), causing a disease termed SCA17, can also lead to an HD-like phenotype (HDL4), as observed in a few French, German/Austrian and Japanese patients (Stevanin et al., 2003; Bauer et al., 2004; Toyoshima et al., 2004). Neuroacanthocytosis syndromes (NA), including chorea acanthocytosis, McLeod syndrome, and pantothenate kinase-associated neurodegeneration (PKAN), may also present with overlapping features (Walker et al., 2003; Prohaska et al., 2012).
A disorder reported to be clinically and pathologically nearly indistinguishable from HD and termed Huntington disease-like 2 (HDL2) was first described in a large African-American pedigree from the southeastern USA (Holmes et al., 2001; Margolis et al., 2001). The disease segregated completely with a CAG/CTG expansion, localized to a variably spliced exon of the junctophilin-3 (JPH3) gene on chromosome 16q24.2 (Holmes et al., 2001). The protein, which is primarily expressed in the brain, appears to help establish a junctional complex between the cytoplasmic membrane and the endoplasmic reticulum (ER) and may be involved in the functional coupling between cell surface voltage sensors and intracellular calcium channels in the ER (Kakizawa et al., 2008; Garbino et al., 2009). Normal allele sizes range from 6 to 28 triplets. Fully penetrant disease alleles reported to date have 41 or more triplets, with the longest reported repeat size being 59 triplets (Bardien et al., 2007; Margolis, 2012).
Three non-mutually exclusive mechanisms have thus far been implicated in the HDL2 pathogenesis: 1) loss of expression of full-length JPH3 protein (Seixas et al., 2012); 2) neurotoxicity of a JPH3 transcript containing an expanded CUG repeat (Rudnicki et al., 2007); and 3) toxic expression, from the strand antisense to JPH3, of a cryptic transcript containing a CAG repeat and encoding polyglutamine (Wilburn et al., 2011). The hypothesis of polyglutamine toxicity in HDL2 was initially based on the observation that HDL2 brains contain intranuclear inclusions (NIs), which are very similar to NIs observed in HD brains (Holmes et al., 2001; Margolis et al., 2001; Rudnicki et al., 2008). This hypothesis gained further support when it was shown that both NIs and a protein tract containing expanded polyglutamine are present in the brain tissue of HDL2 transgenic bacterial artificial chromosome (BAC) model mice expressing full-length JPH3 (Wilburn et al., 2011). However, there is thus far no evidence from human post-mortem HDL2 protein extracts that similar expanded polyglutamine- or expanded polyalanine/polyleucine-containing proteins, potentially encoded by the JPH3 alternative splice variants significantly contribute to HDL2 pathogenesis (Seixas et al., 2012). The role of the recently discovered repeat-associated non-ATG (RAN) translation (Zu et al., 2011), a mechanism by which multiple homopolymeric amino acid-containing protein tracts may be translated on repeat disease loci, in HDL2 pathogenesis remains to be examined.
HDL2 is rare, with fewer than 50 pedigrees described worldwide (outside of South Africa). JPH3 expansion mutations are found in as few as 1% of individuals with clinically or pathologically defined HD without an HTT mutation. It was recognized early on that most of the individuals with HDL2 have definite or likely African ancestry, including African-American individuals, a Mexican family, a Moroccan individual, and cases from Brazil and Venezuela (Holmes et al., 2001; Stevanin et al., 2002; Rodrigues et al., 2008; Paradisi et al., 2013; Castilhos et al., 2014). An early pilot study (Krause et al., 2002) and subsequent case identification suggested that the mutation may be particularly frequent in South African black or mixed ancestry patients with a Huntington disease phenotype (Bardien et al., 2007; Magazi et al., 2008). These initial findings led to the further investigation of the genetic basis of the HD phenotype in South Africans, particularly in those with African ancestry, and to investigation of the possible origin of the HDL2 mutation.
SUBJECTS AND METHODS
Subjects
This study has taken place over a number of years and thus different subsets of the overall South African patient cohort referred for diagnostic Huntington disease testing from 1992–2013 were used in different parts of the study, depending on availability of samples and clinical data. Supplementary Figure 1 shows how the cohorts are linked and derived.
Patients referred for diagnostic Huntington disease testing (1992–2010)
A total of 315 unrelated SA patients (171 white, 130 black and 14 of mixed ancestry) with clinical symptoms suggestive of Huntington disease were investigated. They had been referred from 1992 to 2010 through the same referral system to the Molecular Diagnostic Service, Division of Human Genetics, National Health Laboratory Service (NHLS) (called the South African Institute for Medical Research (SAIMR) prior to 2002), Johannesburg, South Africa. The patients originated mainly from the academic teaching hospitals in the Johannesburg and Pretoria regions, but were also referred from other State hospitals and private practitioners in the region, as well as from further afield across the northern parts of the country. All patients were tested for the CAG expansion in the HTT gene and the CTG expansion in the JPH3 gene.
Forty one individuals with HDL2 ascertained from 1992 until December 2013 and 84 with HD ascertained during the same time period were analysed further for gender, age at diagnosis and repeat lengths. Relevant information was available on 41 individuals with HDL2 and 70 with HD. Age of onset information was rarely available. Age of diagnosis is also not always provided. For 14 HD patients tested early on, repeat sizes were not available.
Patients for retrospective clinical file review on molecularly confirmed cases
Retrospective file analysis was done to obtain relevant clinical information on 39 South African Huntington disease patients from 32 HD families (27 black; 5 mixed ancestry) and 22 South African patients from 20 HDL2 families (17 black; 3 mixed ancestry). Many patients’ clinical files were not available as the patients were drawn from a wide referral base, and in many hospitals, files are retained by the patient and thus only accessible if the patient comes to a clinic. Even in available files, detailed clinical information was limited. Doctors tended to note the presence of clinical signs, but did not comment on their absence. Notes were very brief in many cases.
Unaffected controls for determining CTG allele distribution
The sizes of CTG expansion alleles in JPH3 were determined for 134 white and 176 black unaffected individuals from DNA stored in the sample bank of the Division of Human Genetics. These samples are drawn from similar areas from which the patients originated, and would be expected to represent similar genetic population groups.
Families for JPH3 haplotype analysis
DNA samples from members of 19 South African families, 16 black and 3 of mixed ancestry, with 22 individuals affected with HDL2, were studied for haplotype analysis. Population allele frequencies for the markers used were determined in DNA samples from black individuals from the sample bank of the Division of Human Genetics (25 individuals for SNP analysis and 32 for micro-satellite analysis).
In addition, haplotype analysis was also performed on samples from 31 individuals (23 affected) from 10 North American families (Margolis et al., 2004) a family of French West Indian origin (2 members) (Stevanin et al., 2003) and an individual of Moroccan origin (Stevanin et al., 2002; Stevanin et al., 2003).
Methods
DNA extraction
Genomic DNA was extracted from whole blood using a salting out method (Miller et al., 1988) or a commercial DNA extraction kit (High Pure PCR Template Preparation Kit, Roche Diagnostics) for blood samples <1ml.
Detection of the HD and HDL2 associated triplet repeat expansions
The HTT CAG expansion and the JPH3 CTG expansion were amplified together in a multiplex PCR reaction, using the HTT primers HU4 (HEX labeled) and HDC2 (Yu et al., 2000) and the JPH3 primers L237-1 (FAM labeled) and L237-2 (Holmes et al., 2001) respectively. Products were amplified as follows: 1 cycle at 94°C for 5 minutes, 30 cycles of 94°C for 1 minute/69°C for 1 minute/72°C for 2 minutes and a final cycle at 72°C for 10 minutes. Products were detected on the ABI PRISM™ 377 or ABI 3130xl Genetic Analyzer (Applied Biosystems).
Haplotype analysis
Six polymorphisms in and around JPH3 were screened to determine the JPH3-associated haplotypes. There were 3 SNP (rs2544635, rs3849254, rs918368) and 3 microsatellite (D16S3074, D16S3048, D16S413) markers. Primer sequences and reaction conditions are available on request. SNPs rs2544635, rs3849254, rs918368 were typed by RFLP analysis using the enzymes AciI, SmlI and BsrBI respectively. Microsatellite genotypes were analysed using an ABI PRISM™ 377 genetic analyzer. Haplotypes were constructed in families according to Mendelian principles, or through homozygosity. In affected individuals where haplotypes could not be unequivocally determined, they were inferred if the data were consistent with the common disease haplotype being present on one chromosome.
RESULTS
Genetic basis for Huntington disease phenotype (HD/HDL2) - HTT/JPH3 triplet expansion analysis
Table 1 shows the HTT/JPH3 analysis for 315 unrelated South African patients referred for Huntington disease diagnostic testing from 1992 until 2010. Significantly fewer black patients (36%, 47/130) than white patients (62%, 106/171) had an expansion in the HTT gene (causing HD) (χ2 =19.7, p<0.001). An additional 15% (20/130) of black patients and 21% (3/14) of mixed ancestry patients, but no white patients, had an expansion in JPH3 (causing HDL2). Of those patients in whom a genetic diagnosis was confirmed, 100% (106) of white patients had HD, while approximately 70% (53/76) of black and mixed ancestry patients had HD and 30% (23/76) had HDL2.
Table 1.
Frequency of HD and HDL2 (HTT and JPH3 mutations) in South African patients referred for diagnostic testing for Huntington disease phenotype
| Patients | N | HD (HTT Expansion) | HDL2 (JPH3 expansion) | Non-HD/HDL2 | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| White | 171 | 106 | 62 | 0 | 0 | 65 | 38 |
| Black | 130 | 47 | 36 | 20 | 15 | 63 | 48 |
| Mixed Ancestry | 14 | 6 | 43 | 3 | 21 | 5 | 36 |
|
| |||||||
| Total | 315 | 159 | 51 | 23 | 7 | 133 | 42 |
N, Number of individuals
A large number of patients (42%) referred for diagnostic HD testing tested negative for both loci, with the proportion in black patients (48%) somewhat, but not significantly higher (χ2=3.3, p=0.07), than that in white (38%) and mixed ancestry patients (36%). As these patients were referred through a broad diagnostic base, the majority are likely to have other clinical diagnoses. Available clinical data were assessed in a subset of 22 black patients without a genetic diagnosis and their features were inconsistent with an HD phenotype in all but three cases.
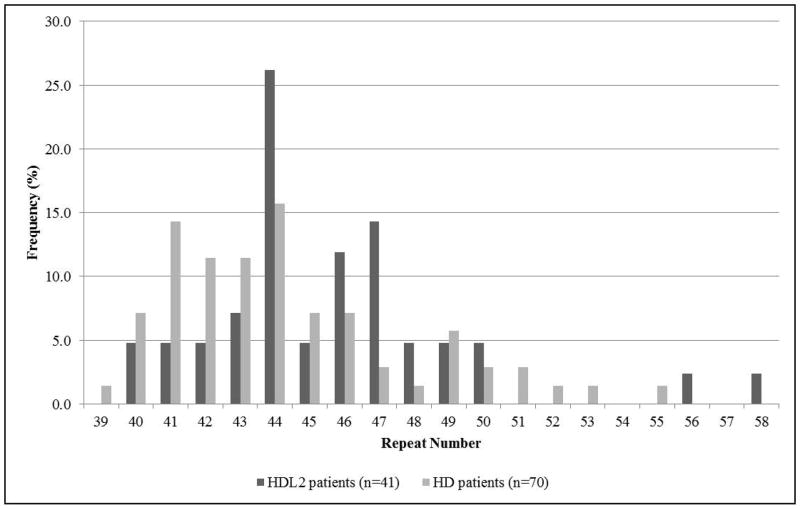
As of December 2013, 41 individuals with JPH3 CTG expansions have been ascertained from 34 families referred to the Molecular Genetic Diagnostic Laboratory, Division of Human Genetics, NHLS, in Johannesburg with the expansion sizes ranging from 40–58 triplets, 44 triplets (11/41, 26%) being the most frequent. Of these, 32 are black patients from 28 families and 9 mixed ancestry patients from 6 families. The youngest age of diagnosis was 31 years with 58 repeats and the oldest 68 years with 45 repeats. HTT repeat sizes ranged from 39–73 repeats. The distribution of the repeats for the HDL2 patients and the 70 ethnically matched HD patients ascertained over the same time period are shown in Figure 1.
Figure 1.
Triplet repeat distributions in patients with HD and HDL2. (Three HTT alleles with repeat size greater than 58 not shown).
The mean reported age of diagnosis for HDL2 patients is 51.3 years, about 5 years older than for ethnically matched HD patients (46.8 years), although the differences between the two means do not reach statistical significance (t=1.92, p=0.057). The mean age of onset, was only available for a very small number of patients, and was based on patient-reported information. It was thus not calculated. The gender ratios for HD and HDL2 patients do not differ, both 0.9 (M: F). These data are presented in Table 2A.
Table 2.
(A) Demographic features of black and mixed ancestry HD and HDL2 cohorts ascertained until December 2013. (B) Clinical Features of HD and HDL2 individuals obtained from retrospective file-based analysis
| Characteristic | n | HD | n | HDL2 | |
|---|---|---|---|---|---|
| A | Sex ratio (M:F) | 70 | 33:37 (0.9) | 41 | 19:22 (0.9) |
| Number of repeats | 70 | 39–73 | 41 | 40–58 | |
| Age at diagnosis (years) | 60 | 46.8±12.4 | 40 | 51.3±9.9 | |
|
| |||||
| B | Positive family history | 32 | 27 (84%) | 20 | 10 (50%)** |
| Chorea1 | 39 | 31 (79%) | 22 | 19 (86%) | |
| Parkinsonian features1 | 39 | 1 (3%) | 22 | 5 (23%)* | |
| Dementia1 | 39 | 26 (67%) | 22 | 15 (68%) | |
| Affective disorder1 | 39 | 16 (41%) | 22 | 11 (50%) | |
| Abnormal CT | 11 | 9 (82%) | 9 | 9 (100%) | |
n, number of individuals for whom data available
Reported as positive feature, clinicians rarely commented on negative findings
p<0.01
p<0.05
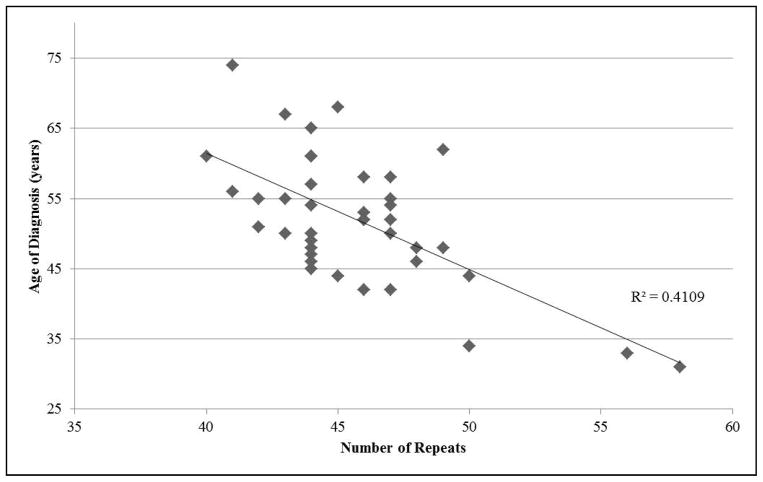
A broad inverse correlation was observed between the repeat length size and the age of diagnosis (correlation = −0.641). The distribution is shown in Figure 2. Greater variation was observed in the lower disease repeat range, possibly because patients present at different disease stages.
Figure 2.
Age of diagnosis vs number of repeats in patients with HDL2
Unfortunately, to date, few meiotic transmissions have been observed. Thus it is difficult to comment on gender-specific transmissions, or the level of anticipation occurring in JPH3 expansions. Only three families have been ascertained thus far, in which parent to child transmissions of an expanded JPH3 repeat was documented. In two maternal transmissions, expansion occurred from 41 to 42 repeats and 43 to 47 repeats respectively. In a paternal transmission, contraction from 45 to 44 repeats occurred. In one family 4 siblings have 43, 47, 49 and 44 repeats, after probable paternal transmission. Their ages of diagnosis were 55 years, 52 years, 48 years and 61 years respectively. The father was reported to have died in his sixties of a neurodegenerative condition. In a second family, siblings had 56 and 58 repeats, with ages of diagnosis in their thirties, after paternal transmission. No information is available on their father. In a third family, two first cousins had 46 and 47 repeats.
Family History and Clinical Features of South African Patients with HDL2 expansions
Clinical features for the 39 patients with HTT expansions and 22 with JPH3 expansions on whom some data from patient files were accessible are summarized in Table 2B.
A positive family history was recorded in significantly more HD families (84%) compared to HDL2 families (50%) analysed (χ2=7.09 p=0.008).
The classical triad of features of Huntington disease namely chorea, dementia and emotional disturbance were reported in 12/39 (31%) of the HD and 10/22 (45%) of the HDL2 patients. Parkinsonian features were noted in significantly more HDL2 5/22 (23%) than HD 1/39 (3%) patients (χ2=6.45 p=0.011).
CT scan reports were available for a limited number of patients. Of the 11 scans in HD patients, 9 reported positive findings, 6 with caudate atrophy and 3 with generalised atrophy. In the HDL2 patients, all 9 scans reported positive findings, 5 with caudate atrophy and 4 with generalised cerebral atrophy.
Origin of the HDL2 mutation
In order to understand the reasons for the relatively high frequency of JPH3 expansions, in black HD phenotype patients, we examined JPH3 repeat distributions in unaffected controls and the haplotypes on which JPH3 expansions occurred in HDL2 families.
JPH3 CTG allele sizes in white and black controls
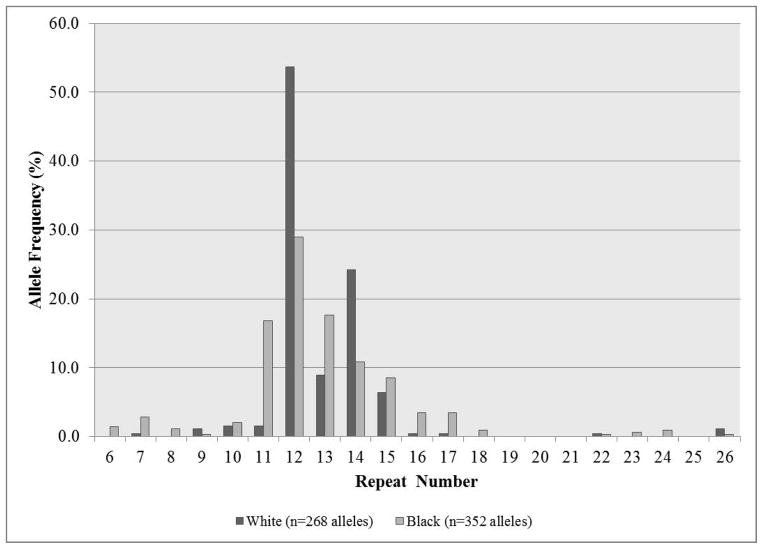
The distributions of normal JPH3 CTG repeat lengths were determined in black (176) and white (134) individuals and are shown in Figure 3. Allele sizes ranged from 6 to 26 repeats, with no alleles observed between 19 and 21 repeats in either population. In whites, 54% (144/268) of alleles had 12 repeats, 24% (65/268) had 14 repeats and 93% (250/268) of alleles had between 12 and 15 repeats, with 1.5% (4/268) of alleles having greater than 21 repeats. In blacks, a larger number of different-sized alleles were present, with 29% (102/352) of alleles having 12 repeats, 18% (62/352) having 13 repeats, 17% (59/352) having 11 repeats and 83% (291/352) of alleles being between 11 and 15 repeats, with 2% (7/352) of alleles having greater than 21 repeats. The general distributions are not significantly different statistically, neither are the numbers of alleles with greater than 21 repeats (χ2=0.245, p=0.643).
Figure 3.
JPH3 CTG repeat distributions in 134 white and 176 black unaffected individuals
Haplotype analysis
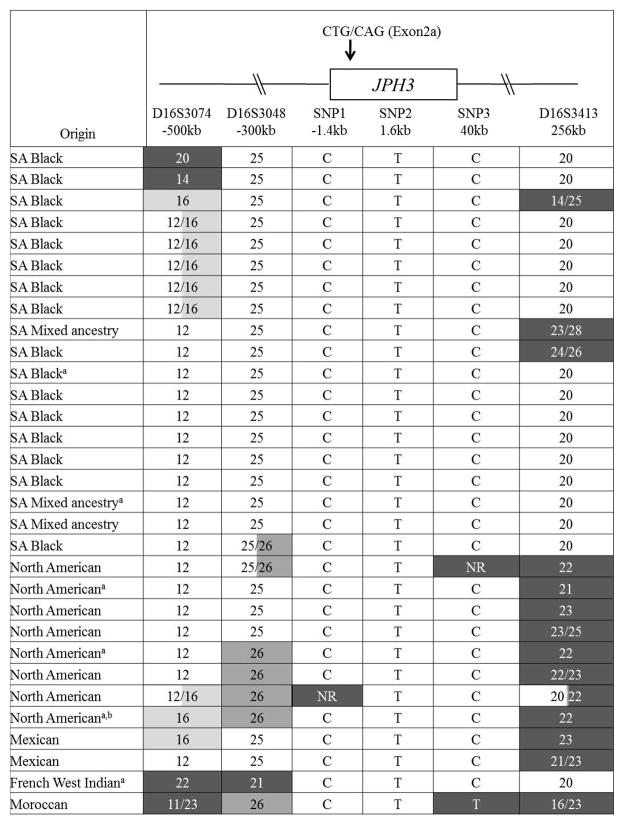
All cases of HDL2 described to date have definite or probable African ancestry, leading us to postulate that the HDL2 mutation arose in Africa. Six markers (3 SNPs and 3 micro-satellites), tightly linked to JPH3 were analyzed. Their positions relative to the CTG/CAG repeat in JPH3 are shown in Figure 4.
Figure 4.
Summary of the likely disease-associated haplotypes for all HDL2 families studied. The relative positions of the SNP and microsatellite markers used for haplotype analysis are also shown.
aUnequivocal haplotype determined from family study
bAncestry from Europe, American Indian, Africa
NR, no result
SNP analysis
Table 3 shows the allele frequencies for the 3 SNPs analysed in 16 South African unrelated black HDL2 patients, and 25 random South African black unaffected controls. Mixed ancestry and North American patients were excluded from the statistical analysis. Full genotyping data for all patients and controls are provided in Supplementary Tables 1 and 2 respectively. For the 3 SNPs analysed, there were no statistically significant differences in allele frequencies between patients and controls, using Chi-squared analysis. However, it should be noted that that the C allele for SNP 1, the T allele for SNP 2 and the C allele for SNP 3 appeared to be disease-associated. For SNP 1 all HDL2 patients had at least one C allele and for SNP 2 and SNP 3 no CC or TT homozygotes respectively were observed in the patient cohort, but were in the healthy controls.
Table 3.
Allele frequencies for 3 SNPs linked to the JPH3 gene in South African black HDL2 patients and unaffected individuals
| Unrelated South African black HDL2 patients | Unrelated South African black unaffected individuals | Statistical Comparison | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| N | Allele 1 | n | Freq | Allele 2 | n | Freq | N | Allele 1 | n | Freq | Allele 2 | n | Freq | Χ2 | P Value | |
| SNP1 rs254463 | 16 | C | 32 | 1.00 | A | 0 | 0.00 | 25 | C | 48 | 0.96 | A | 2 | 0.04 | 0.169 | 0.680 |
| SNP2 rs384925 | 16 | C | 9 | 0.28 | T | 23 | 0.72 | 24 | C | 21 | 0.44 | T | 27 | 0.56 | 1.389 | 0.239 |
| SNP3 rs918368 | 16 | C | 27 | 0.84 | T | 5 | 0.16 | 25 | C | 33 | 0.66 | T | 17 | 0.34 | 2.485 | 0.115 |
N, Number of individuals
n, number of alleles
Underlined alleles are HDL2 associated
In 15/16 black HDL2 patients, haplotypes for the 3 SNPs could be unambiguously assigned, although their disease association could not be directly determined. Three different haplotypes were present, with a CTC haplotype present in all individuals with HDL2. Further, in 17/25 unaffected black controls, haplotypes could be assigned unequivocally, and four different haplotypes were present (Table 4). The CTC haplotype is significantly more frequent in patients compared to unaffected controls (χ2 = 3.864, p = 0.049), suggestive of a founder SNP haplotype in HDL2 patients.
Table 4.
Haplotype frequencies for 3 SNPs linked to JPH3 gene in South African black HDL2 patients and unaffected individuals
| Haplotypea | Unrelated South African black HDL2 patients (N=16) | Unrelated South African black unaffected individuals (N=25) | ||
|---|---|---|---|---|
| n | Freq | n | Freq | |
| CTT | 4 | 0.13 | 9 | 0.18 |
| CCC | 8 | 0.25 | 12 | 0.24 |
| CTC | 18 | 0.56 | 11 | 0.22 |
| CCT | 0 | 0.00 | 2 | 0.04 |
| Unassigned | 2 | 0.06 | 16 | 0.32 |
| Total | 32 | 1.00 | 50 | 1.00 |
SNP order: SNP1 – rs254463, SNP2 – rs3849254, SNP3 – rs918368
n, number of unambiguous haplotypes
Underlined haplotype is HDL2 associated
In the cohort of HDL2 patients (including mixed ancestry and North American patients), 9 families shared a common 3 SNP (CTC) disease-associated haplotype (unequivocal assignment by family study or homozygosity). In 21 of the remaining 22 families, the SNP data were consistent with the presence of the same disease haplotype. The Moroccan patient has a T allele at SNP 3.
Micro-satellite analysis
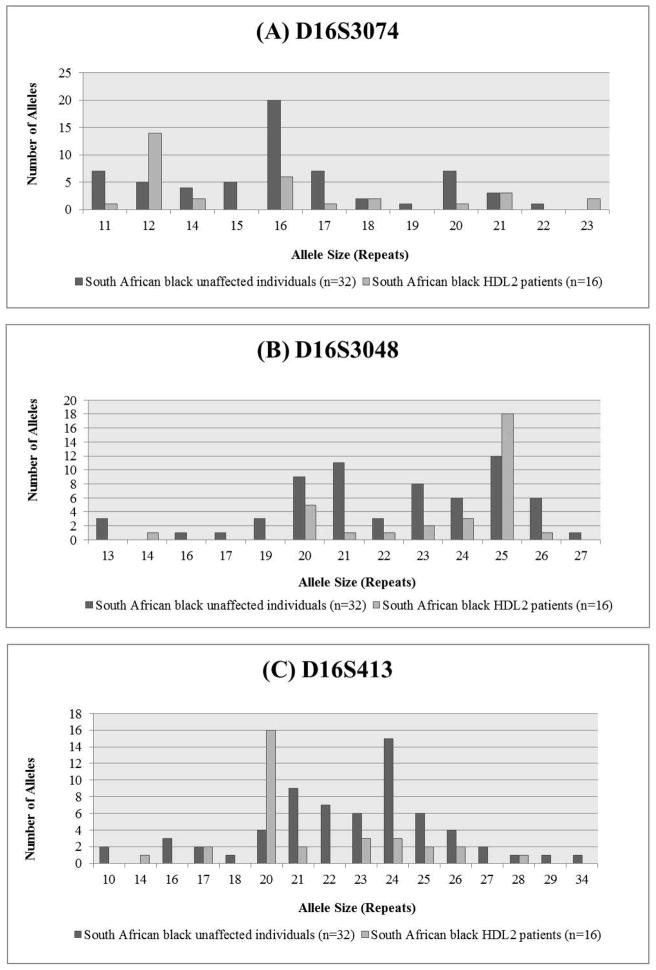
For each microsatellite marker, there was a single common allele in the South African black HDL2 patients, and the frequency of the common allele was significantly different in the patients compared to the unaffected black South African controls (Fisher Exact tests: D16S3074, p=0.0001; D16S413 p<0.0001; D16S3048 p< 0.0005). The distributions are shown in Figure 5A–C respectively and the genotyping data in Supplementary Tables 1 and 2.
Figure 5.
Microsatellite allele distribution for markers (A) D16S3074, (B) D16S3048, and (C) D16S413 around the JPH3 locus
Haplotype analysis
Figure 4 shows a summary of the likely disease-associated haplotypes for all the HDL2 families studied. In 19 South African families, unequivocal haplotype assignment was only possible in the 2 South African families, in which more than one individual was available. Both had the same disease-associated haplotype. In 12 other individuals with HDL2, the same disease-associated haplotype could be inferred (based on the presence of the common disease-associated alleles). An additional 2 patients differed only at the 5′ microsatellite marker (D16S3074), 2 only at the 3′ marker (D16S413), and an additional one differing both 5′ and 3′. There were no common haplotypes (if microsatellites data were included) in the unaffected individuals.
All 8 North American families, the 2 Mexican families and the French West Indian family tested share the core SNP haplotype with the South African families, but with some overlap and some divergence of the microsatellite markers. The Moroccan family shares a partial 5′ haloptype, including the 2 SNPs closest to and flanking the JPH3 repeat, but the most 3′ SNP is divergent. This could be explained by a single cross-over event.
DISCUSSION
Genetic basis for Huntington disease phenotype (HD/HDL2) - HTT/JPH3 triplet expansion analysis
In large studies of patients tested for HD, who have a clear HD phenotype and autosomal dominant inheritance, very few, typically <3%, test negative for HTT mutations. Those that do are normally ascribed to misdiagnosis, sample mix-up, or clerical error (Andrew et al., 1994). True phenocopies are thought to account for 1–7% of cases in most European and North American series (Andrew et al., 1994; Kremer et al., 1994; Rosenblatt et al., 1998; Vuillaume et al., 2000; Stevanin et al., 2002; Margolis et al., 2004).
On the other hand, our findings demonstrate that in South Africa, 15% of individuals with black or mixed ancestry referred for HD testing have HDL2, by far the highest known rate in the world. JPH3 mutations are the cause of a Huntington disease phenotype in South Africa, in about 30% of cases in which a genetic diagnosis can be established.
This necessitates a different diagnostic approach in which routine analysis should include simultaneous testing for mutations at both the HTT and JPH3 loci. This approach has been adopted in the two major academic laboratories in South Africa that perform clinical diagnostic tests for HD (Krause and Greenberg, 2008). An analogous situation exists for a number of other inherited disorders, in the South African black population, like cystic fibrosis (Goldman et al., 2001), spinal muscular atrophy (Stevens et al., 1999), and Fanconi anaemia (Morgan et al., 2005) where either allelic or genetic heterogeneity is reported, necessitating a population specific diagnostic approach.
All HDL2 cases in our series and others, identified through diagnostic tests done in South Africa, are black or of mixed ancestry, and thus of full or partial African ancestry. A JPH3 expansion has yet to be detected in a white South African patient. To date, 34 HDL2 families, comprising a total of 40 genetically confirmed affected individuals have been identified in Johannesburg, South Africa (this study). An additional seven HDL2 families have been identified through genetic diagnostic testing services in the Western Cape region. Some of these may overlap with the families identified in Johannesburg, as the cases identified in the Western Cape did not necessarily reside there (Professor Jonathan Carr, personal communication 2012; Professor J Greenberg personal communication 2013). This number of HDL2 families is larger than that published worldwide to date (Margolis, 2012). Patients originate from many regions of South Africa, and one case originated in Angola (Jonathan Carr, personal communication 2012) though, interestingly, no cases of HDL2 (or HD) have been reported in black individuals residing in the Western Cape, for reasons that remain unclear. They would not be expected to represent a genetically distinct group.
In the present series, the higher proportion of black compared to white patients referred for diagnostic HD testing who tested negative for both loci, raises some interesting questions. While most of these cases do not have a clear HD phenotype, the possibility remains that the few cases with an HD-like phenotype have another genetic disorder, either known (eg. SCA17, neuroacanthocytoses) or unknown. The possibility of a novel HD-like disorder, even if rare, is possible, particularly noting the genetic heterogeneity in Africa.
Disease frequencies for HD and HDL2 remain uncertain. Fewer black patients with a HD phenotype are diagnosed than would be expected if the HD prevalence was similar to that in populations of European ancestry (Sizer et al., 2012). With increased awareness of HDL2, more access to appropriate predictive and diagnostic genetic testing, and active follow-up of families, disease prevalence will hopefully become clearer in the future. The traditional medical teaching that the HD phenotype is rare in blacks may mean that clinicians are more reluctant to entertain the diagnosis, particularly where the presentation is atypical. Further affected patients, particularly with psychiatric symptoms, may seek treatment from traditional healers in the community, rather than Western medical professionals.
The repeat size range in HDL2 is similar to that previously described. Two patients with an HD phenotype and 40 repeats were identified in this series, thus shifting the lower end of the disease repeat range to 40 from 41 (Margolis, 2012). Interestingly, the repeat range also mirrors the repeat range reported in HD. The broad inverse correlation between age of onset and number of repeats is similar to that reported in North American patients (Margolis et al., 2004).
Family History and Clinical Features of South African Patients with HDL2 expansions
Fewer patients with HDL2 are documented to have a positive family history by the referring clinicians than HD patients. There is little evidence, at present, to suggest that this is due to anything more than poor history taking or poor ascertainment. Reduced lifespan, later age of onset and variable phenotype may also contribute. The role of new mutations is also unknown, although no confirmed new mutations have been documented to date.
Margolis (Margolis, 2012) has suggested that there are two clinical phenotypes in patients with HDL2 mutations, which may reflect two ends of a spectrum. The first is similar to juvenile HD usually presenting in the fourth decade with diminished coordination and weight loss, with Parkinsonian rigidity, bradykinesia, tremor, dysarthria and hyperreflexia, possibly associated with longer repeat length. The second subtype is more variable, but corresponds in general to typical HD, with onset in the fifth decade and beyond, with chorea and prominent abnormal eye movements. Further, (Bardien et al., 2007) described three individuals in one South African mixed ancestry family, one with typical Huntington disease features, a second with predominantly Parkinsonian features and cognitive impairment and a third with myoclonus, cognitive impairment, and atypical imaging with relative sparing of the caudate nucleus. A patient from the French West Indies with prominent ataxia has also been reported (Stevanin et al., 2003).
Individuals in the South Africa cohort with HDL2 and HD ascertained to date share many clinical features, and in many cases individuals with HDL2 are clinically indistinguishable from those with the classic triad presentation of HD. However, from review of the limited South African clinical data available, patients with HDL2 may present with dementia earlier, may have less marked chorea and may retain mobility longer. HDL2 is considerably more likely to include Parkinsonian features, and myoclonus similar to previous reports, particularly of the index family (Margolis et al., 2001). This is particularly interesting, considering the significantly later age of diagnosis in HDL2.
The individuals studied may represent a biased cohort as they were referred through a Huntington disease diagnostic referral system and thus presumably manifested features typical of HD. It is possible that patients with HDL2 present with a wider spectrum of neuropsychiatric and movement disorders than do those with HD. Cases with earlier ages of diagnosis may be missed because of a symptom profile that differs from typical HD. Longitudinal and prospective studies on cases diagnosed after predictive testing or from a wider referral base are planned to characterize the phenotype more comprehensively, including subtle differences in phenotype, the course of disease, the order in which symptoms develop, and the primary presenting features.
Origin of the HDL2 mutation
Two different, but not mutually exclusive, hypotheses have been proposed to account for differences in population frequencies of repeat expansion disorders. Firstly, the presence of larger alleles in the unaffected population may represent a pool of potentially unstable alleles which can occasionally expand into the repeat range. Secondly, disease alleles have been associated with particular predisposing haplotypes, where cis-elements may have a predisposing influence on trinucleotide instability (Warby et al., 2009).
Our results demonstrate that at the JPH3 locus, the distribution of normal alleles is similar in white and black South Africans, with no apparent skewing or increase in large normal (greater than 20) repeats in black individuals. The distribution is similar to that described in a North American cohort (Holmes et al., 2001), French and North African cohort (Stevanin et al., 2002) and a small South African study (Bardien et al., 2007). Thus normal allele length in the population is not correlated with HDL2 prevalence, at least in the population investigated to date. This is in contrast to HD (Squitieri et al., 1994; Baine et al., 2013) and myotonic dystrophy type 1 (Goldman et al., 1996) in which repeat length distribution is correlated with population disease frequency.
The haplotype analysis performed as part of this study is highly suggestive of a single founder origin for the JPH3 expansion mutation, with all patients typed in this study, except one the patient from Morocco), sharing a common three-SNP core JPH3 haplotype, and the South African patients also sharing the flanking microsatellite alleles in most cases. It is unknown whether the haplotype in some way predisposes to JPH3 expansion due to cis-acting elements or whether only large normal alleles and/or expanded alleles occur on that haplotype. The JPH3 CTG repeat size distribution on the expansion associated haplotype has not been determined in the general population. The haplotype is not common in unaffected individuals.
The presence of a shared haplotype in African Americans, who originated mainly from the west coast of Africa approximately 300 years ago and also in black South Africans who migrated south from central and west Africa at least 2000 years ago, with the Bantu expansion, suggests that the mutation or predisposing haplotype originated in sub-Saharan Africa at least 2000 years ago. The distribution of the JPH3 mutation across Africa remains to be defined, but these data would predict that HDL2 should occur widely across sub-Saharan Africa.
CONCLUSIONS
The HD phenotype displays locus heterogeneity in patients of African origin, with at least two common loci implicated. The novel HTT-associated haplotypes in African patients with HD (Baine et al., 2013), as well as the presence of JPH3 mutations on a founder haplotype, in a significant number of African patients, contribute to this genetic heterogeneity. This heterogeneity necessitates a different diagnostic approach for patients with African ancestry and a phenotype suggestive of Huntington disease. The distribution of HD genocopies in Africa and the full clinical spectrum of HDL2 disease remain to be determined.
Supplementary Material
Definitions of terms used in this paper.
Mixed ancestry individuals, in South Africa, are those whose gene pool is derived from one or more of the indigenous African populations (San, Khoikhoi or Bantu speaking), European immigrants from western Europe and/or slaves and indentured labourers from Madagascar, the Malaysian archipelago and India. Black individuals are those whose gene pool is derived from one or more of the indigenous Bantu-speaking African populations.
HD and HDL2 represent the two diseases with a Huntington disease phenotype, caused by expansion mutations in the HTT and JPH3 genes, respectively
Acknowledgments
Funding was obtained from the National Health Laboratory Service (South Africa) and the Hereditary Disease Foundation, NIH NS16375, NIH NS078643, NS075825, and NS064138. The following scientists contributed to the work over the years in South Africa: Robyn Labrum, Heste Joubert, Claire Mitchell and Anthony Lane, and in the US: Colleen Callahan, Adam Rosenblatt, Susan Holmes, Elizabeth O’Hearn, Hyon S. Hwang, Juan C. Troncoso, Mary L. Franz, Lisa Gourley). Many clinicians have referred their patients for Huntington disease diagnostic testing. These patients formed the basis from which this study could proceed. Professor Kees van der Meyden is acknowledged for tracing some of the original patients. Thanks to Tasha Wainstein for assistance with formatting of the data and final document, and Professors Michele Ramsay and Jennifer Kromberg for their academic input.
References
- Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, Graham RK, Hayden MR. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat Genet. 1993;4:398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- Andrew SE, Goldberg YP, Kremer B, Squitieri F, Theilmann J, Zeisler J, Telenius H, Adam S, Almqvist E, Anvret M, Luckotte G, Stoessl AJ, Campanella G, Hayden MR. Huntington disease without CAG expansion: phenocopies or errors in assignment? Am J Hum Genet. 1994;54:852–863. [PMC free article] [PubMed] [Google Scholar]
- Baine FK, Kay C, Ketelaar ME, Collins JA, Semaka A, Doty CN, Krause A, Greenberg LJ, Hayden MR. Huntington disease in the South African population occurs on diverse and ethnically distinct genetic haplotypes. Eur J Hum Genet. 2013;21:1120–1127. doi: 10.1038/ejhg.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardien S, Abrahams F, Soodyall H, van der Merwe L, Greenberg J, Brink T, Carr J. A South African mixed ancestry family with Huntington disease-like 2: Clinical and genetic features. Mov Disord. 2007;22:2083–2089. doi: 10.1002/mds.21672. [DOI] [PubMed] [Google Scholar]
- Bauer P, Laccone F, Rolfs A, Wullner U, Bosch S, Peters H, Liebscher S, Scheible M, Epplen JT, Weber BHF, Holinski-Feder E, Weirich-Schwaiger H, Morris-Rosendahl DJ, Andrich J, Riess O. Trinucleotide repeat expansion in SCA17/TBP in white patients with Huntington’s disease-like phenotype. J Med Genet. 2004;41:230–232. doi: 10.1136/jmg.2003.015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilhos R, Souza A, Furtado G, Gheno T, Silva A, Vargas F, Lima M-A, Barsottini O, Pedroso J, Godeiro C, Salarini D, Pereira E, Lin K, Toralles M-B, Saute J, Rieder C, Quintas M, Sequeiros J, Alonso I, Saraiva-Pereira M, Jardim L. Huntington disease and Huntington disease-like in a case series from Brazil. Clin Genet. 2014;86:373–377. doi: 10.1111/cge.12283. [DOI] [PubMed] [Google Scholar]
- Chang CM, Yu YL, Fong KY, Wong MT, Chan YW, Ng TH, Leung CM, Chan V. Huntington’s Disease in Hong Kong Chinese: Epidemiology and Clinical Picture. Clin Exp Neurol. 1994;31:43–51. [PubMed] [Google Scholar]
- Duyao M, Ambrose C, Myers R, Novelletto A, Perischetti F, Frontali M, Folstein S, Ross C, Franz M, Abbott M, Gray J, Conneally P, Young A, Penney J, Hollingsworth Z, Shoulson I, Lazzarini A, Falek A, Koroshetz W, Sax D, Bird E, Vonsattel J, Bonilla E, Alver J, Bickham Conde J, Cha J-H, Dure L, Gomez F, Ramos M, Sanchez-Ramos J, Snodgrass S, De Young M, Wexler N, Moscowitz C, Penchaszadeh G, MacFarlane H, Anderson M, Jenkins B, Srinidhi J, Barnes G, Gusella J, MacDonald M. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat Genet. 1993;4:387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Garbino A, van Oort RJ, Dixit SS, Landstrom AP, Ackerman MJ, Wehrens XHT. Molecular evolution of the junctophilin gene family. Physiol Genomics. 2009;37:175–186. doi: 10.1152/physiolgenomics.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass J, Saffer DS. Huntington’s chorea in a black family. S Afr Med J. 1979;56:685–688. [PubMed] [Google Scholar]
- Goldman A, Ramsay M, Jenkins T. Ethnicity and myotonic dystrophy: a possible explanation for its absence in sub-Saharan Africa. Ann Hum Genet. 1996;60:57–65. doi: 10.1111/j.1469-1809.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Goldman A, Labrum R, Claustres M, Desgeorges M, Guittard C, Wallace A, Ramsay M. The molecular basis of cystic fibrosis in South Africa. Clin Genet. 2001;59:37–41. doi: 10.1034/j.1399-0004.2001.590106.x. [DOI] [PubMed] [Google Scholar]
- Greenberg LJ, Martell RW, Theilman J, Hayden MR, Joubert J. Genetic linkage between Huntington disease and the D4S10 locus in South African families: further evidence against non-allelic heterogeneity. Hum Genet. 1991;87:701–708. doi: 10.1007/BF00201729. [DOI] [PubMed] [Google Scholar]
- Harper PS. The epidemiology of Huntington’s disease. Hum Genet. 1992;89:365–376. doi: 10.1007/BF00194305. [DOI] [PubMed] [Google Scholar]
- Harper PS. Major Problems in Neurology. 2. W B Saunders; London: 1996. Huntington’s disease. [Google Scholar]
- Hayden MR, Hopkins HC, Macrae M, Beighton PH. The origin of Huntington’s chorea in the Afrikaner population of South Africa. S Afr Med J. 1980a;58:197–200. [PubMed] [Google Scholar]
- Hayden MR, MacGregor JM, Beighton PH. The prevalence of Huntington’s chorea in South Africa. S Afr Med J. 1980b;58:193–196. [PubMed] [Google Scholar]
- Hayden MR, MacGregor JM, Saffer DS, Beighton PH. The high frequency of juvenile Huntington’s chorea in South Africa. J Med Genet. 1982;19:94–97. doi: 10.1136/jmg.19.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, O’Hearn E, Rosenblatt A, Callahan C, Hwang HS, Ingersoll-Ashworth RG, Fleisher A, Stevanin G, Brice A, Potter NT, Ross CA, Margolis RL. A repeat expansion in the gene encoding junctophilin-3 is associated with Huntington Disease-like 2. Nat Genet. 2001;29:377–378. doi: 10.1038/ng760. [DOI] [PubMed] [Google Scholar]
- Joubert J, Botha MC. Huntington disease in South African blacks: a report of 8 cases. S Afr Med J. 1988;73:489–494. [PubMed] [Google Scholar]
- Kakizawa S, Moriguchi S, Ikeda A, Iino M, Takeshima H. Functional Crosstalk Between Cell-Surface and Intracellular Channels Mediated by Junctophilins Essential for Neuronal Functions. The Cerebellum. 2008;7:385–391. doi: 10.1007/s12311-008-0040-1. [DOI] [PubMed] [Google Scholar]
- Kambouris M, Bohlega S, Al-Tahan A, Meyer BF. Localization of the gene for a novel autosomal recessive neurodegenerative Huntington-like disorder to 4p15. 3. Am J Hum Genet. 2000;66:445–452. doi: 10.1086/302744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A, Temlett J, Van der Meyden K, Ross CA, Callahan C, Margolis RL. CAG/CTG repeat expansions at the HDL2 locus are a common cause of Huntington disease in black South Africans. Am J Hum Genet. 2002;71:528. [Google Scholar]
- Krause A, Greenberg J. Genetic testing for Huntington’s disease in South Africa. S Afr Med J. 2008;98:193–194. [PubMed] [Google Scholar]
- Kremer B, Goldberg P, Andrew SE, Theilmann J, Telenius H, Zeisler J, Squitieri F, Lin B, Bassett A, Almqvist E, Bird TD, Hayden MR. A worldwide study of the Huntington’s disease mutation. The sensitivity and specificity of measuring CAG repeats. N Engl J Med. 1994;330:1401–1406. doi: 10.1056/NEJM199405193302001. [DOI] [PubMed] [Google Scholar]
- Magazi DS, Krause A, Bonev V, Moagi M, Iqbal Z, Dludla M, Van der Meyden CH. Huntington’s disease: Genetic heterogeneity in black African patients. S Afr Med J. 2008;98:200–203. [PubMed] [Google Scholar]
- Margolis RL. Huntington Disease-Like 2. GeneReviews. 2012 htp://www.ncbi.nlm.nih.gov/books/NBK1529/
- Margolis RL, O’Hearn E, Rosenblatt A, Willour V, Holmes SE, Franz ML, Callahan C, Hwang HS, Troncoso JC, Ross CA. A disorder similar to Huntington’s disease is associated with a novel CAG repeat expansion. Ann Neurol. 2001;50:373–380. doi: 10.1002/ana.1312. [DOI] [PubMed] [Google Scholar]
- Margolis RL, Holmes SE, Rosenblatt A, Gourley L, O’Hearn E, Ross CA, Seltzer WK, Walker RH, Ashizawa T, Rasmussen A, Hayden M, Almqvist EW, Harris J, Fahn S, MacDonald ME, Mysore J, Shimohata T, Tsuji S, Potter N, Nakaso K, Adachi Y, Nakashima K, Bird T, Krause A, Greenstein P. Huntington’s disease-like 2 (HDL2) in North America and Japan. Ann Neurol. 2004;56:670–674. doi: 10.1002/ana.20248. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Xiang F, Monaghan J, Han D, Zhang Z, Edström L, Anvret M, Prusiner SB. Huntington disease phenocopy is a familial prion disease. Am J Hum Genet. 2001;69:1385–1388. doi: 10.1086/324414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NV, Essop F, Demouth I, De Ravel T, Jansen S, Tischkowitz M, Lewis CM, Wainwright L, Poole J, Joenje H, Digweed M, Krause A, Mathew CG. A common Fanconi anemia mutation in black populations of sub-Saharan Africa. Blood. 2005;105:3542–3544. doi: 10.1182/blood-2004-10-3968. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Watanabe Y, Kusumi M, Nanba E, Maeoka Y, Nakagawa M, Igo M, Irie H, Ishino H, Fujimoto A, Goto J, Takahashi K. Epidemiological and genetic studies of Huntington’s disease in the San-in area of Japan. Neuroepidemiology. 1996;15:126–131. doi: 10.1159/000109899. [DOI] [PubMed] [Google Scholar]
- Paradisi I, Ikonomu V, Arias S. Huntington disease-like 2 (HDL2) in Venezuela: Frequency and ethnic origin. J Hum Genet. 2013;58:3–6. doi: 10.1038/jhg.2012.111. [DOI] [PubMed] [Google Scholar]
- Prohaska R, Sibon OCM, Rudnicki DD, Danek A, Hayflick SJ, Verhaag EM, Vonk JJ, Margolis RL, Walker RH. Brain, blood, and iron: Perspectives on the roles of erythrocytes and iron in neurodegeneration. Neurobiol Dis. 2012;46:607–624. doi: 10.1016/j.nbd.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues GGR, Walker RH, Brice A, Cazeneuve C, Russaouen O, Teive HAG, Munhoz RP, Becker N, Raskin S, Werneck LC, Junior WM, Tumas V. Huntington’s disease-like 2 in Brazil-Report of 4 patients. Mov Disord. 2008;23:2244–2247. doi: 10.1002/mds.22223. [DOI] [PubMed] [Google Scholar]
- Rosenblatt A, Ranen NG, Rubinsztein DC, Stein OC, Margolis RL, Wagster MV, Becher MW, Rosser AE, Leggo J, Hodges JR, ffrench-Constant CK, Sherr M, Franz ML, Abbott MH, Ross CA. Patients with features similar to Huntington’s disease without CAG expansion in huntingtin. Neurology. 1998;51:215–220. doi: 10.1212/wnl.51.1.215. [DOI] [PubMed] [Google Scholar]
- Rudnicki DD, Holmes SE, Lin MW, Thornton CA, Ross CA, Margolis RL. Huntington’s disease-like 2 is associated with CUG repeat-containing RNA foci. Ann Neurol. 2007;61:272–282. doi: 10.1002/ana.21081. [DOI] [PubMed] [Google Scholar]
- Rudnicki DD, Pletnikova O, Vonsattel JG, Ross CA, Margolis RL. A comparison of Huntington disease and Huntington Disease-Like 2 Neuropathology. J Neuropathol Expir Neurol. 2008;67:366–374. doi: 10.1097/NEN.0b013e31816b4aee. [DOI] [PubMed] [Google Scholar]
- Samuels BL, Gelfand M. Huntington’s chorea in a Black Rhodesian family. S Afr Med J. 1978;54:648–651. [PubMed] [Google Scholar]
- Scrimgeour EM. Huntington’s disease in Tanzania. J Med Genet. 1981;18:200–203. doi: 10.1136/jmg.18.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimgeour EM. The Huntington’s chorea register of Tanzania. East Afr Med J. 1982;59:280–282. [PubMed] [Google Scholar]
- Scrimgeour EM, Pfumojena JW. Huntington Disease in black Zimbabwean families living near the Mozambique border. Am J Med Genet. 1992;44:762–766. doi: 10.1002/ajmg.1320440610. [DOI] [PubMed] [Google Scholar]
- Scrimgeour EM, Simpson SA. Huntington disease in black African populations. Hum Genet. 1992;90:186–187. doi: 10.1007/BF00210775. [DOI] [PubMed] [Google Scholar]
- Scrimgeour EM, Samman Y, Brock DJH. Huntington’s disease in a Sudanese family from Khartoum. Hum Genet. 1995;96:624–625. doi: 10.1007/BF00197424. [DOI] [PubMed] [Google Scholar]
- Seixas AI, Holmes SE, Takeshima H, Pavlovich A, Sachs N, Pruitt JL, Silveira I, Ross CA, Margolis RL, Rudnicki DD. Loss of junctophilin-3 contributes to huntington disease-like 2 pathogenesis. Ann Neurol. 2012;71:245–257. doi: 10.1002/ana.22598. [DOI] [PubMed] [Google Scholar]
- Semaka A, Creighton S, Warby S, Hayden M. Predictive testing for Huntington disease: interpretation and significance of intermediate alleles. Clin Genet. 2006;70:283–294. doi: 10.1111/j.1399-0004.2006.00668.x. [DOI] [PubMed] [Google Scholar]
- Silber E, Kromberg J, Temlett JA, Krause A, Saffer D. Huntington’s disease confirmed by genetic testing in five African families. Mov Disord. 1998;13:726–730. doi: 10.1002/mds.870130420. [DOI] [PubMed] [Google Scholar]
- Sizer EB, Haw T, Wessels T-M, Kromberg JGR, Krause A. The Utilization and Outcome of Diagnostic, Predictive, and Prenatal Genetic Testing for Huntington Disease in Johannesburg, South Africa. Genet Test Mol Biomark. 2012;16:58–62. doi: 10.1089/gtmb.2011.0007. [DOI] [PubMed] [Google Scholar]
- Snell RG, MacMillan JC, Cheadle JP, Fenton I, Lazarou LP, Davies P, MacDonald ME, Gusella JF, Harper PS, Shaw DJ. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington’s Disease. Nat Genet. 1993;4:393–397. doi: 10.1038/ng0893-393. [DOI] [PubMed] [Google Scholar]
- Squitieri F, Andrew SE, Goldberg YP, Kremer B, Spence N, Zeisler J, Nichol K, Theilmann J, Greenberg J, Goto J, Kanazawa I, Wesa J, Peltonen L, Almqvist E, Anvret M, Telenius H, Lin B, Napolitano G, Morgan K, Hayden MR. DNA haplotype analysis of Huntington disease reveals clues to the origins and mechanisms of CAG expansion and reasons for geographic variations of prevalence. Hum Mol Genet. 1994;3:2103–2114. doi: 10.1093/hmg/3.12.2103. [DOI] [PubMed] [Google Scholar]
- Stevanin G, Camuzat A, Holmes SE, Julien C, Sahloul R, Dode C, Hahn-Barma V, Ross CA, Margolis RL, Durr A, Brice A. CAG/CTG repeat expansions at the Huntington’s disease–like 2 locus are rare in Huntington’s disease patients. Neurology. 2002;58:965–967. doi: 10.1212/wnl.58.6.965. [DOI] [PubMed] [Google Scholar]
- Stevanin G, Fujigasaki H, Lebre AS, Camuzat A, Jeannequin C, Dode C, Takahashi J, San C, Bellance R, Brice A, Durr A. Huntington’s disease-like phenotype due to trinucleotide repeat expansions in the TBP and JPH3 genes. Brain. 2003;126:1599–1603. doi: 10.1093/brain/awg155. [DOI] [PubMed] [Google Scholar]
- Stevens G, Yawitch T, Rodda J, Verhaart S, Krause A. Different molecular basis for spinal muscular atrophy in South African black patients. Am J Med Genet. 1999;86:420–426. [PubMed] [Google Scholar]
- Toyoshima Y, Yamada M, Onodera O, Shimohata M, Inenaga C, Fujita N, Morita M, Tsuji S, Takahashi H. SCA17 homozygote showing Huntington’s disease-like phenotype. Ann Neurol. 2004;55:281–286. doi: 10.1002/ana.10824. [DOI] [PubMed] [Google Scholar]
- Vuillaume I, Meynieu P, Schraen-Maschke S, Destee A, Sablonniere B. Absence of unidentified CAG repeat expansion in patients with Huntington’s Disease-Like phenotype. J Neurol Neurosurg Psychiatry. 2000;68:672–675. doi: 10.1136/jnnp.68.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RH, Jankovic J, O’Hearn E, Margolis RL. Phenotypic features of Huntington’s disease-like 2. Mov Disord. 2003;18:1527–1530. doi: 10.1002/mds.10587. [DOI] [PubMed] [Google Scholar]
- Warby SC, Montpetit A, Hayden AR, Carroll JB, Butland SL, Visscher H, Collins JA, Semaka A, Hudson TJ, Hayden MR. CAG Expansion in the Huntington Disease Gene Is Associated with a Specific and Targetable Predisposing Haplogroup. Am J Hum Genet. 2009;84:351–366. doi: 10.1016/j.ajhg.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warby SC, Visscher H, Collins JA, Doty CN, Carter C, Butland SL, Hayden AR, Kanazawa I, Ross CJ, Hayden MR. HTT haplotypes contribute to differences in Huntington disease prevalence between Europe and East Asia. Eur J Hum Genet. 2011;19:561–566. doi: 10.1038/ejhg.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilburn B, Rudnicki DD, Zhao J, Weitz TM, Cheng Y, Gu X, Greiner E, Park CS, Wang N, Sopher BL, La Spada AR, Osmand A, Margolis RL, Sun YE, Yang XW. An Antisense CAG Repeat Transcript at JPH3 Locus Mediates Expanded Polyglutamine Protein Toxicity in Huntington’s Disease-like 2 Mice. Neuron. 2011;70:427–440. doi: 10.1016/j.neuron.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang F, Almqvist EW, Huq M, Lundin A, Hayden MR, Edström L, Anvret M, Zhang Z. A Huntington disease–like neurodegenerative disorder maps to chromosome 20p. Am J Hum Genet. 1998;63:1431–1438. doi: 10.1086/302093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Fimmel A, Fung D, Trent RJ. Polymorphisms in the CAG repeat–a source of error in Huntington disease DNA testing. Clin Genet. 2000;58:469–472. doi: 10.1034/j.1399-0004.2000.580607.x. [DOI] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MAC, Nan Z, Forster C, Low WC, Schoser B, Somia NV, Clark HB, Schmechel S, Bitterman PB, Gourdon G, Swanson MS, Moseley M, Ranum LPW. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.