Abstract
Leber congenital amaurosis (LCA) and retinitis pigmentosa (RP) are two genetically heterogeneous retinal degenerative disorders. Despite the identification of a number of genes involved in LCA and RP, the genetic etiology remains unknown in many patients. In this study, we aimed to identify novel disease-causing genes of LCA and RP. Retinal capture sequencing was initially performed to screen mutations in known disease-causing genes in different cohorts of LCA and RP patients. For patients with negative results, we performed whole exome sequencing and applied a series of variant filtering strategies. Sanger sequencing was done to validate candidate causative IFT140 variants. Exome sequencing data analysis led to the identification of IFT140 variants in multiple unrelated non-syndromic LCA and RP cases. All the variants are extremely rare and predicted to be damaging. All the variants passed Sanger validation and segregation tests provided that the family members’ DNA was available. The results expand the phenotype spectrum of IFT140 mutations to non-syndromic retinal degeneration, thus extending our understanding of intraflagellar transport and primary cilia biology in the retina. This work also improves the molecular diagnosis of retinal degenerative disease.
Introduction
Leber congenital amaurosis (LCA, MIM# 204000) and retinitis pigmentosa (RP, MIM# 268000) are two types of inherited retinal degenerative diseases. LCA is featured by congenital or infantile-onset vision loss, nystagmus and absent electroretinogram (ERG) signals. RP is a more common and variable form of retinal degeneration (RD) and its onset ranges from childhood to mid-adulthood (Hartong et al. 2006). Both LCA and RP are highly genetically heterogeneous. To date, at least 21 LCA-causing and 64 RP-causing genes have been identified (RetNet, the Retinal Information Network) (SP Daiger). Mutations in these genes account for about 70 % of LCA and 60 % of RP cases, respectively, suggesting that the molecular basis of a significant number of cases is yet to be discovered (Wang et al. 2013, 2014).
Retinal degenerative disorders can be syndromic, in which case patients develop symptoms in other systems in addition to their ocular abnormalities. This phenomenon is frequently seen in ciliopathies with retinal involvement since photoreceptors develop highly specialized cilia structure and ciliated cells are widespread in the human body (Hildebrandt et al. 2011). Mutations in ciliary genes were identified in a number of syndromes with RD including Senior–Løken syndrome (SLSN, MIM# 266900) (Otto et al. 2005), Joubert syndrome (JBTS, MIM# 213300) (Dixon-Salazar et al. 2004), Bardet–Biedl syndrome (BBS, MIM#209900) (Mykytyn et al. 2001).
It has also been reported that mutations in syndromic ciliopathy genes can lead to non-syndromic LCA or RP. For example, IQCB1 mutations were originally identified to cause SLSN (Otto et al. 2005), but certain IQCB1 mutant alleles were found to cause LCA without renal symptoms (Estrada-Cuzcano et al. 2011). Similarly, while mutations in CEP290, a cilia basal body gene, can cause a series of syndromic ciliopathies including JBTS and BBS (Baala et al. 2007; Sayer et al. 2006; Valente et al. 2006), it is also a major contributor to non-syndromic LCA cases (den Hollander et al. 2006). Given the fact that cilia are responsible for numerous biological processes in multiple tissues, the diverse genotype–phenotype correlations observed by these studies can be explained by a combination of multi-functional nature of these ciliary genes and differential damaging effect of their mutant alleles.
Intraflagellar transport (IFT) is a biological process by which various proteins are transported along the microtubule-based cilia (Rosenbaum and Witman 2002). Specifically, the IFT-A complex is responsible for the return of proteins from the ciliary tip (Absalon et al. 2008). Defects in IFT-A particles have already been associated with a spectrum of human ciliopathies. Mutations in one IFT-A complex component, WDR19, were reported to cause cranioectodermal dysplasia (CED, MIM# 614378) (Bredrup et al. 2011), nephronophthisis (NPHP, MIM# 614377) (Halbritter et al. 2013b) as well as non-syndromic retinitis pigmentosa (Coussa et al. 2013). Similarly, mutations in IFT140, another IFT-A complex gene, were known to cause two types of rare recessive ciliopathies: Mainzer–Saldino syndrome (MZSDS, MIM# 266920) and Jeune asphyxiating thoracic dystrophy (JATD, MIM# 208500) (Khan et al. 2014; Perrault et al. 2012; Schmidts et al. 2013). MZSDS is featured by cone-shaped epiphysis, chronic renal disease, abnormality of the proximal femur and RD (Beals and Weleber 2007; Giedion 1979). JATD patients show constricted thoracic cage, short-limbed short stature, polydactyly and often develop multi-organ disorders including retinal abnormalities (Bard et al. 1978; de Vries et al. 2010; Oberklaid et al. 1977). Since both WDR19 and IFT140 are linked to ciliopathies with retinal involvement, it is intriguing for us to know, whether IFT140 defects, like WDR19 mutations, can also cause non-syndromic RD.
In this study, through whole exome sequencing (WES), mutations in IFT140 have been identified in seven patients diagnosed with non-syndromic LCA or RP. These patients come from diverse ethnicities and account for about 1 % of non-syndromic RD cases. Our results highlight a novel genotype–phenotype correlation of a ciliary gene, which can improve the molecular diagnosis of retinal degenerative diseases and our understanding of intraflagellar transport in the retina.
Methods and materials
Clinical diagnosis of patients and DNA sample collection—Dr. Ruifang Sui
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital (PUMCH) and adhered to the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. All the probands were diagnosed at the Department of Ophthalmology, PUMCH (Beijing, China) by ophthalmic examinations including best corrected visual acuity (BCVA) testing, fundus examination, optical coherence topography (OCT, 3D OCT-2000 Spectral Domain; Topcon, Tokyo, Japan), visual field tests (Octopus, Interzeag, Schlieren, Switzerland), autofluorescence (AF, Spectralis HRA+OCT; Heidelberg, Germany) and electroretinogram (ERG, RetiPort ERG system, Roland Consult, Wiesbaden, Germany). LCA and RP were diagnosed according to medical and family history, typical fundus and OCT features, visual field defects and attenuated or abolished ERG responses. Blood samples were obtained from all patients and their family members if available. DNA was extracted using QIAamp DNA Blood Midi Kit as instructed by the manufacturer (QIAGEN, Hilden, Germany).
Clinical diagnosis of patients and DNA sample collection—eyeGENE
For eyeGENE patients, we obtained informed consent from tested individuals or from parents or guardians for individuals under age 18. The eyeGENE is a genomic medicine initiative started by the National Eye Institute (NEI) in 2006. It aims to promote studies of inherited eye diseases and their genetic causes (Blain et al. 2013). The patients from eyeGENE were diagnosed in the United States and referred by eyeGENE-approved certified eye specialists. Clinical information and family history were provided by referring clinicians to the eyeGENE database, and further reviewed by the eyeGENE Working Group to confirm the patients’ diagnosis. For each patient enrolled in eyeGENE, a blood sample was collected and shipped to the eyeGENE coordinating center CLIA (Clinical Laboratory Improvement Amendments) laboratory on the NIH campus in Bethesda, MD. Genomic DNA was extracted from whole blood either manually or automatically using the Gentra Puregene (Qiagen). DNA concentration was measured by a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). A fraction of de-identified DNA was sent to Baylor College of Medicine for diagnostic research testing.
DNA library preparation and next generation sequencing (NGS)
Approximate 1 μg of genomic DNA sample was sheared into fragments of 200–500 bp in length. The sheared fragments were blunt-end repaired and a single adenine base was added to the 3′ ends using Klenow exonuclease. Illumina adapters were ligated to the repaired ends and DNA fragments were PCR amplified for eight cycles after ligation. In each capture reaction, 50 pre-capture DNA libraries were pooled together. The targeted DNA was captured by customized retinal disease gene panel for retinal capture sequencing to screen for variants in known disease-causing genes. The detailed information of the retinal capture panel was described previously (Wang et al. 2014). If no plausible causative variants were identified, the DNA was then captured by NimbleGenSeqCap EZ Hybridization and Wash kit (NimblegenSeqCap EZ Human Exome Library v.2.0) following the manufacturer's protocols for WES. Captured libraries were sequenced on Illumina HiSeq 2000 (Illumina, San Diego, CA) as 100 bp paired-end reads according to the manufacturer's protocol.
Bioinformatics analysis
Paired-end sequencing reads were obtained for each sample. Reads were mapped to human reference genome hg19 using Burrows-Wheeler Aligner (Li and Durbin 2009). Base quality recalibration, local realignment and variant calling were performed as previously described (Wang et al. 2014). We obtained the variant frequencies from a series of public and internal control databases (Wang et al. 2014) as well as the Exome Aggregation Consortium (ExAC) database. Since LCA and RP are rare Mendelian disorders, variants with a frequency higher than 1/200 (for a recessive model) or 1/1000 (for a dominant model) were filtered out. After frequency-based filtering, we filtered out synonymous variants, identified known retinal disease-causing variants and predicted the pathogenicity of variants using SIFT (Ng and Henikoff 2003), Polyphen2 (Adzhubei et al. 2010), LRT (Chun and Fay 2009), MutationTaster (Schwarz et al. 2010), MutationAssessor (Reva et al. 2011) as previously described (Wang et al. 2014; Xu et al. 2015).
Sanger sequencing
For each suspected causative mutation, a 500-bp flanking sequence at both sides was obtained from the UCSC genome browser (hg19 assembly). RepeatMasker (Smit et al. 1996–2010) was used to mask the repetitive sequences in human genome. Primer 3 (Untergasser et al. 2012) was used to design a pair of primers for generating a 400–600-bp PCR product to sequence the mutation site and at least 50-bp region surrounding it. After PCR amplification, the amplicons were sequenced on ABI 3730xl. Family members of patients were also Sanger sequenced when available for confirming allele segregation.
Results
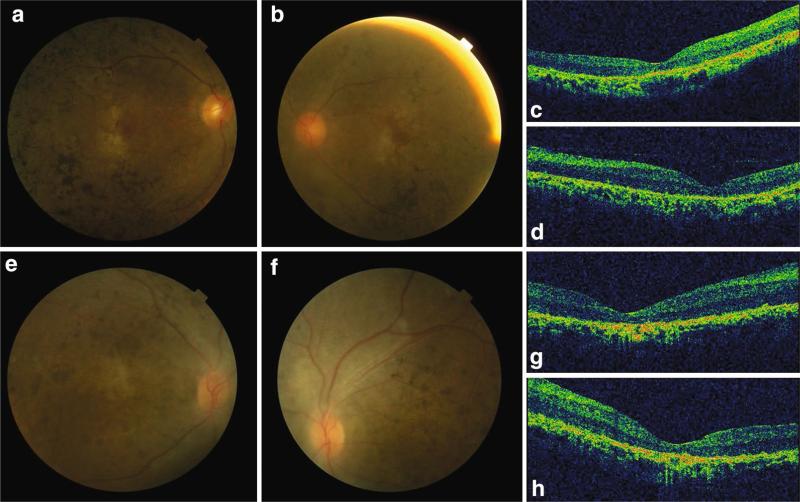
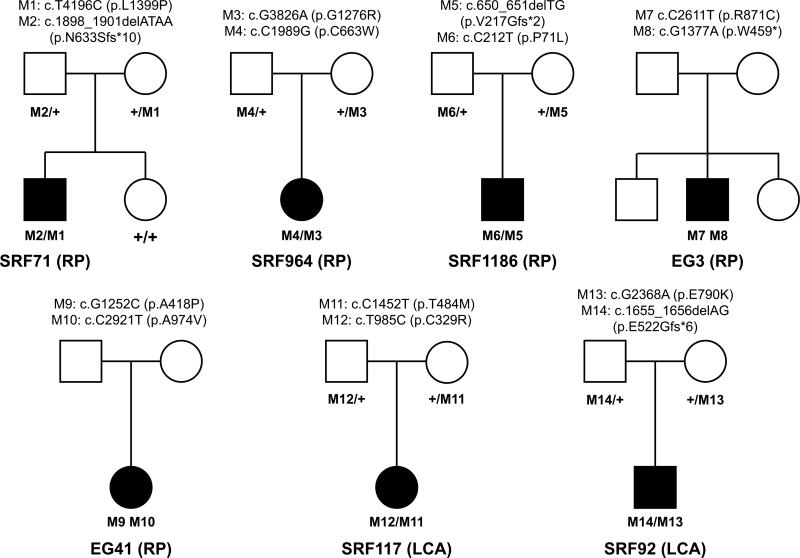
In this study, we totally investigated seven unrelated non-syndromic RD patients, including five RP and two LCA cases. Among them, five of them are Han Chinese and the remaining two are of European ethnicity diagnosed in United States. The index case we investigated, SRF71, is a 43-year-old male RP patient of Han Chinese ethnicity. He was aware of night blindness during childhood and vision loss at the age of 36 (Table 1). His fundus images showed widespread retinal bone spicule pigmentation, and gold foil macular reflex. OCT images showed loss of inner segment (IS)/outer segment (OS) of photoreceptor cells (Fig. 1). His parents are normal and he has an unaffected sister. Preliminary screening by retinal capture sequencing found no causative mutations in known RP-causing genes. WES data show that he has biallelic variants in IFT140, including a missense (c.T4196C, p. L1399P) and a frameshift (c. 1898_1901delATAA, p. N633Sfs*10) variant. Neither variant was found more than once in ExAC controls (Table 1). The missense variant disrupts a highly conserved amino acid (AA) in TPR9 domain and is considered to be damaging by 5 out of 6 prediction algorithms (Fig. 3; Table S2). The frameshift variant does not lie in the last exon thus it probably leads to a nonsense-mediated decay of the IFT140 transcript. Sanger sequencing confirmed his unaffected sister is wild type at these two alleles and his parents possess one IFT140 variant each, supporting the pathogenicity of the IFT140 variants (Fig. 2). Given that IFT140 is associated with syndromic ciliopathies, we performed radiography of skeletons and laboratory tests for hepatic and renal functions. Interestingly, the patient does not show any other symptoms or abnormalities after detailed examination (Figure S4, Table S3). Therefore, these results raise the possibility that mutations in IFT140 might lead to non-syndromic retinal disease.
Table 1.
Clinical features and identified IFT140 variants of RD patients in this study
| ID/ethnicity | Dx | Sex | Age | Age VL | RN | SK | Other | IFT140 variants | O freq | EM freq |
|---|---|---|---|---|---|---|---|---|---|---|
| SRF71/Chi | RP | M | 43 | 36 | No | No | No | M: c.T4196C (p.L1399P) P: c.1898_1901delATAA (p.N633Sfs*10) |
1 in 73,058 Absent |
1 in 5762 Absent |
| SRF964/Chi | RP | F | 28 | 23 | No | No | No | M: c.G3826A (p.G1276R) P: c.C1989G (p.C663W) |
Absent Absent |
Absent Absent |
| SRF1186/Chi | RP | M | 25 | 25 | No | No | No | M: c.650_651delTG (p.V217Gfs*2) P: c.C212T (p.P71L) |
Absent 1 in 121,148 |
Absent Absent |
| EG3/Cau | RP | M | 59 | 21 | No | No | No | c.C2611T (p.R871C) c.G1377A (p.W459*) |
1 in 48,782 12 in 120,704 |
1 in 27,048 11 in 72,946 |
| EG41/Cau | RP | F | 55 | 33 | No | No | No | c.G1252C (p.A418P) c.C2921T (p.A974 V) |
1 in 114,034 2 in 120,106 |
1 in 68,816 Absent |
| SRF117/Chi | LCA | M | 9 | AB | No | No | No | M: c.C1451T (p.T484 M) P: c.T985C (p.C329R) |
5 in 120,980 Absent |
2 in 8640 Absent |
| SRF92/Chi | LCA | M | 11 | AB | No | No | Hypogonadism and mild fatty liver | M: c.G2368A (p.E790 K) P: c.1655_1656delAG (p.E522Gfs*6) |
2 in 121,412 Absent |
Absent Absent |
Non-Finnish European and Finnish European are the EM population groups for Caucasian patients; in variants column, M, indicates maternal allele; P indicates paternal allele. cDNA and protein changes are based on reference cDNA sequence NM_014714.3
Chi Han Chinese, Cau Caucasian, Dx primary diagnosis, Age VL age aware of vision loss, RN if the patient has renal abnormalities, SK if the patient has skeletal abnormalities, Other if the patient has other disorders. O Freq overall frequency, occurrence in ExAC database (all population groups), EM Freq ethnically matched frequency, occurrence in ExAC database within the ethnically matched (EM) population group. Specifically in ExAC database, East Asian is the EM population group for Han Chinese patients
Fig. 1.
Fundus photograph and OCT images of RP patient SRF71 and LCA patient SRF92. a, b Right and left fundus pictures of SRF71. Widespread retinal bone spicule pigmentation, gold foil macular reflex and attenuation of retinal vessels were observed in both eyes. c, d Right and left eye OCT images of SRF71. The retina was thin with loss of signal of IS/OS of photoreceptor cells. e, f Right and left fundus pictures of SRF92. Widespread pigment crumbs at posterior pole and mid-peripheral area. g, h Right and left eye OCT images of SRF92. IS/OS signal was lost and the retina was atrophic
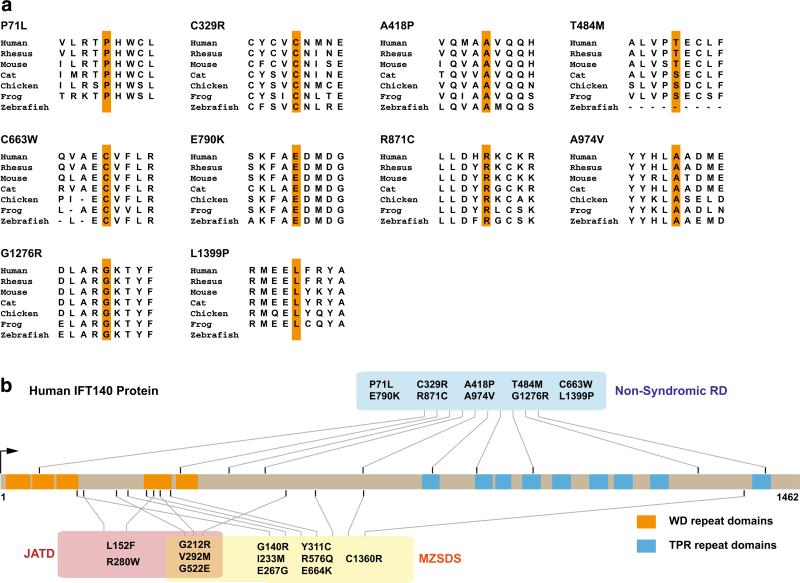
Fig. 3.
IFT140 missense variants identified in previous and present studies. a IFT140 protein sequence alignment between vertebrates at the loci of IFT140 missense variants identified in our studies. Nearly all variants show consistent high conservation in vertebrates. b Distribution of IFT140 missense variants identified in all the studies. Missense variants identified in JATD (red box) and MZSDS (yellow box) show a trend to be enriched in the N-terminus and WD repeat domains (orange blocks). We identified novel missense variants lying in TPR repeat domains (blue blocks) in non-syndromic RD patients (blue box). Variant position, protein and domain length were drawn in scale (color figure online)
Fig. 2.
Pedigrees of RD patients in this study. A total of seven unrelated non-syndromic RD patients with IFT140 biallelic variants were identified. Variant segregation was confirmed in five families (SRF71, SRF964, SRF1186, SRF92 and SRF117). Genotypes (cDNA and protein changes based on reference cDNA sequence NM_014714.3) are labeled for each family
To further test the prevalence of mutations in IFT140 in non-syndromic retinopathy, we screened IFT140 variants in a collection of 215 LCA and 243 RP patients who were negative for mutations in known retinal disease genes and had undergone WES. Strikingly, a total of additional six cases with IFT140 biallelic variants, including 4 RP patients and 2 LCA patients, have been identified (Table 1). Among the 4 RP patients, SRF964 and SRF1186 are also Han Chinese. SRF964 is a 28-year-old female RP patient who noticed vision loss at the age of 23. Her fundus images showed bone spicule and obvious salt–pepper changes in the retina with attenuated retinal vessels. OCT results featured disrupted IS/OS structure (Figure S1). WES identified that she possesses two IFT140 missense variants and Sanger sequencing confirmed they are in trans (Fig. 2). Both missense variants are absent in the ExAC database, indicating they are extremely rare (Table 1). The paternal variant (c.C1989G, p.C663W) affects a conserved AA site and is predicted to be damaging by all algorithms (Fig. 3; Table S2). It is also next to a disease-causing variant (c.1990G >A, p.E664K) previously reported in MZSDS patients (Perrault et al. 2012). The maternal variant (c.G3826A, p.G1276R) is predicted to be damaging by 5 out of 6 algorithms and the AA conservation is across vertebrates to zebrafish (Fig. 3; Table S2). SRF1186 is a 25-year-old male RP patient aware of recent vision loss. His fundus images featured RPE atrophy, gray pigments in the mid-peripheral retina and retinal fluorescent changes (Figure S2). WES data revealed he has two IFT140 variants (c.C212T, p.P71L and c.650_651delTG, p.V217Gfs*2). Sanger validation confirmed their segregation from both parents (Fig. 2). The P71L variant is found only once in over 60,000 controls in the ExAC database and the frameshift variant is absent (Table 1). The P71L variant affects a conserved residue in WD2 domain and is considered damaging by all algorithms (Fig. 3; Table S2). Both patients were revisited and no skeletal or renal disorders have been observed (Table 1, Table S3). The identification of these two cases further supports our argument that IFT140 mutations can cause non-syndromic RP.
In addition, two sporadic non-syndromic RP patients of Caucasian ethnicity from eyeGENE were identified to possess IFT140 biallelic variants. Patient EG3 is a 59-year-old male. He was aware of night blindness at the age of 7 and vision loss at 21. His retina shows intraretinal pigment spicules, vascular attenuation and degeneration. He was diagnosed with RP and does not report other syndromic phenotypes. The patient carries one missense (c.C2611T, p.R871C) and one stop-gain variant (c.G1377A, p.W459*) in IFT140. Both variants have a frequency lower than 1/10,000 in control individuals (Table 1). The R871C variant affects a conserved AA site and is considered damaging by all algorithms (Fig. 3; Table S2). The other patient, EG41, is a 55-year-old female diagnosed with non-syndromic RP. She noticed her night blindness and vision loss when she was 33 years old. Her ocular findings also featured intraretinal pigment spicules and vascular attenuation. NGS data indicated that she has two IFT140 missense variants (c.G1252C, p.A418P and c.C2921T, p.A974V). Both variants have a frequency lower than 1/60,000 in the ExAC database (Table 1). They both disrupt conserved residues and are predicted to be damaging (Fig. 3; Table S2). Unfortunately, we were unable to obtain the DNA samples of their family members to confirm these variants are in trans. Nevertheless, given the rareness and highly pathogenic nature of these variants, the concurrence of these IFT140 variants in non-syndromic RP patients significantly supports our results shown above.
In addition to five non-syndromic RP patients mentioned above, we also identified two non-syndromic Han Chinese LCA patients with IFT140 variants. SRF117 is a 9-year-old male patient with infantile-onset retinal problems and diagnosed with LCA. His fundus images featured RPE atrophy and a hyperfluorescence ring in the macular area and OCT results showed disruption of IS/OS and increased foveal retina thickness (Figure S3). The patient does not have other syndromic abnormalities (Table 1, Table S3). WES data show that he has biallelic missense variants in IFT140 gene (c.C1451T, p.T484M and T985C, p.C329R) and Sanger sequencing confirmed that the two variants are from one parent each (Fig. 2). Both variants have a frequency lower than 1/20,000 in ExAC controls and are considered to be damaging by nearly all algorithms (Tables 1 and S2). The other patient, SRF92, is an 11-year-old male diagnosed with LCA/early onset severe retinal dystrophy. His fundus image showed extensive retinal pigment crumbs including macula region and OCT results indicated IS/OS loss and retinal atrophy (Fig. 1). According to Sanger sequencing, this patient inherited a frameshift variant (c.1655_1656delAG, p.E522Gfs*6) from his father and a missense variant (c.G2368A, p.E790K) from his mother (Fig. 2). Both variants are extremely rare in control individuals (less than 1 in 60,000). Similar to previously described variants, the E790 K variant affects a conserved AA site and predicted to be damaging by five out of six algorithms. This patient also does not show typical MZSDS or JATD-like syndromic phenotypes but has hypogonadism and mild fatty liver disease (Table 1, Table S3). The discussion of this phenotype will be in the next section.
Discussion
Here, we identified a total of seven (five RP and two LCA) unrelated patients with IFT140 causative variants. The identified variants are consistently rare and damaging, strongly supporting their pathogenicity. None of the patients show typical MZSDS or JATD-like abnormalities other than ocular phenotypes, indicating they are non-syndromic RD cases. This is the first report showing IFT140 mutations can cause non-syndromic retinal degenerative disease.
Primary cilia are microtubule-based organelles widely distributed in the human body (Badano et al. 2006). The cilia structure in the photoreceptor, named connecting cilium, is the only path linking the IS and OS of photoreceptor cells (Young 1976). The connecting cilium is responsible for protein transport and turnover between the IS and OS, relying on IFT (Rosenbaum and Witman 2002). Disruption of IFT components in vertebrate photoreceptors causes abnormal protein localization and subsequent photoreceptor degeneration (Keady et al. 2011; Krock and Perkins 2008; Marszalek et al. 2000). These data demonstrate that the IFT process is critical for proper photoreceptor function and explain the vulnerability of photoreceptor cells to disruption of ciliary genes. Consistent with this notion, in a recent study, acute deletion of ift140 in cone photoreceptors of adult mice causes abnormal accumulation of opsins in the nuclear layer and the IS (Crouse et al. 2014), suggesting disrupted protein transport as a potential mechanism of retinal degeneration caused by IFT140 deficiency. In addition to its function in adult photoreceptor maintenance, IFT140 is also likely involved in early photoreceptor development based on its critical role during ciliogenesis (Miller et al. 2013). This could explain the congenital retinal problems shown in IFT140-related syndromes (Perrault et al. 2012).
Mutations in IFT140 were previously identified to cause two forms of ciliopathies: MZSDS and JATD, featuring systemic disorders including skeletal abnormalities, renal disease, and often retinal degeneration (Perrault et al. 2012; Schmidts et al. 2013). In our study, none of the LCA and RP patients have skeletal or renal phenotypes as manifested in MZSDS and JATD, thus significantly expanding the phenotype spectrum of IFT140-related disorders. Several studies have also reported the wide phenotype spectrum caused by mutations in ciliary genes (Bujakowska et al. 2014; Coene et al. 2009; den Hollander et al. 2006; Riazuddin et al. 2010) and multiple underlying mechanisms have been proposed, including different variant positions (Coene et al. 2009), retina-specific splicing (Riazuddin et al. 2010) and hypomorphic alleles (Bujakowska et al. 2014; den Hollander et al. 2006). When looking at IFT140 missense variants identified in patients, the variants identified previously in MZSDS and JATD (Perrault et al. 2012; Schmidts et al. 2013) seem to be enriched in the N-terminus, with a number of them in WD repeat domains (Fig. 3). Interestingly, our study first identified several missense variants in C-terminal TPR repeats, raising the possibility that mutation position may play a role in determining the patient phenotype (Fig. 3). Since both WD (Xu and Min 2011) and TPR (D'Andrea and Regan 2003) repeats are important for protein–protein interactions, future studies on their specific roles for IFT140 function are warranted for revealing the mechanism of the wide phenotype spectrum we have observed. In addition, we did not observe a clear difference between IFT140 variants in RP cases and those in LCA cases, probably due to a limited number of patients. The severity of pathogenic allele combinations may explain the various retinal phenotypes we observed in the patients. We also cannot exclude the possibility that additional alleles in other genes may contribute to the variable expressivity of RD.
Mutations in other IFT-A complex components including WDR19, IFT122, WDR35, IFT43 and TTC21B, can also lead to a spectrum of ciliopathies (Arts et al. 2011; Bredrup et al. 2011; Coussa et al. 2013; Davis et al. 2011; Mill et al. 2011; Walczak-Sztulpa et al. 2010). However, it seems that IFT140 is more extensively involved in retinal pathology compared with other components. During our mutation screening in non-syndromic LCA and RP cohorts, we in total identified about 10 patients with probably causative IFT140 variants and much fewer cases with WDR19 variants (data not shown). There were no candidate variants identified in other IFT-A components. Interestingly, mutations in IFT172, which encodes an IFT-B complex component, can lead to JATD with retinal involvement (Halbritter et al. 2013a). A recent report identified multiple non-syndromic RP cases caused by IFT172 mutations (Bujakowska et al. 2014). The results in our study show a striking similarity between IFT140 and IFT172 in terms of human phenotype spectrum, suggesting a functional linkage. More intriguingly, IFT172 has been shown to be the only IFT-B component which can interact with IFT140 (Follit et al. 2009). Given the fact that the IFT-A complex is responsible for the return of IFT-B components from the ciliary tip (Absalon et al. 2008), future studies focusing on IFT140–IFT172 interaction will help us understand its role in the IFT-A and B complex crosstalk and further, the ciliary protein dynamics for proper cilia function.
Another finding, which should be noted, is that one of our identified patients, SRF92, has hypogonadism and mild fatty liver, which phenotypically overlap with BBS. Strikingly, in the recent IFT172 study (Bujakowska et al. 2014), researchers also identified a BBS-like phenotype caused by IFT172 mutations. The sporadic findings of these non-canonical phenotypes in both studies might be explained by the specific damaging effect of certain mutant alleles. It might also further corroborate the notion that mutation load in other ciliary genes can be modifiers of ciliopathy phenotype spectrum (Badano et al. 2003; Katsanis 2004).
In summary, our findings provide new evidence that non-syndromic LCA and RP can be caused by mutations in syndromic ciliopathy-causing genes. This finding will lead us to dissect more detailed functions of IFT complex components as well as other ciliary proteins in the photoreceptors. In addition, based on our results, we estimate that IFT140 mutations possibly account for 1 % non-syndromic LCA and RP cases, which should not be ignored based on the highly heterogeneous genetic architecture of RD. Since there are still ~30 and ~40 % of unsolved LCA and RP cases, respectively, further screening of variants in ciliary genes can help to discover the mysterious molecular etiology of remaining unsolved cases.
Supplementary Material
Acknowledgments
We thank all the patients and their family members for participating in this study. We thank the eyeGENE Working Group (https://nei.nih.gov/eyegene/staff_eyegene). The eyeGENE study was supported by the Department of Health and Human Services/National Institutes of Health/National Eye Institute intramural program under eyeGENE—Protocol 06-EI-0236 and 10-EI-N164 which has been funded in part under Contract No. HHS-N-260-2007-00001-C. We thank the Exome Aggregation Consortium and the groups that provided exome variant data. We thank Mr. Zachry T. Soens, Mr. Jason S. Salvo and Mr. Evan M. Jones for reviewing and editing the manuscript. NGS was conducted at the Functional Genomic Core (FGC) facility at Baylor College of Medicine supported by NIH shared instrument grant 1S10RR026550 to R. C. This work was supported by grants from National Eye Institute (R01EY022356, R01EY018571), Retinal Research Foundation, Foundation Fighting Blindness (BR-GE-0613-0618-BCM) to R. C. This study was also supported by grants from Foundation Fighting Blindness (CD-CL-0214-0631-PUMCH), National Natural Science Foundation of China (81470669) and Beijing Natural Science Foundation (7152116) to R. S. M. X. is supported by Cullen Foundation endowment to the Molecular and Human Genetics Graduate Program, Baylor College of Medicine. F. W. is supported by a predoctoral fellowship funded by the Burroughs Wellcome Trust Fund: The Houston Laboratory and Population Sciences Training Program in Gene Environment Interaction. Z. G. is supported by NIH T32 funding 2T32EY007102-21A1.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00439-015-1586-x) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Ethical standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest The authors declare that they have no conflict of interest.
References
- Absalon S, Blisnick T, Kohl L, Toutirais G, Dore G, Julkowska D, Tavenet A, Bastin P. Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol Biol Cell. 2008;19:929–944. doi: 10.1091/mbc.E07-08-0749. doi:10.1091/mbc.E07-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. doi:10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts HH, Bongers EM, Mans DA, van Beersum SE, Oud MM, Bolat E, Spruijt L, Cornelissen EA, Schuurs-Hoeijmakers JH, de Leeuw N, Cormier-Daire V, Brunner HG, Knoers NV, Roepman R. C14ORF179 encoding IFT43 is mutated in Sensen-brenner syndrome. J Med Genet. 2011;48:390–395. doi: 10.1136/jmg.2011.088864. doi:10.1136/jmg.2011.088864. [DOI] [PubMed] [Google Scholar]
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audol-lent S, Ozilou C, Faivre L, Laurent N, Foliguet B, Munnich A, Lyonnet S, Salomon R, Encha-Razavi F, Gubler MC, Boddaert N, de Lonlay P, Johnson CA, Vekemans M, Antignac C, Attie-Bitach T. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet. 2007;80:186–194. doi: 10.1086/510499. doi:10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Kim JC, Hoskins BE, Lewis RA, Ansley SJ, Cutler DJ, Castellan C, Beales PL, Leroux MR, Katsanis N. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet–Biedl patients with two mutations at a second BBS locus. Hum Mol Genet. 2003;12:1651–1659. doi: 10.1093/hmg/ddg188. [DOI] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. doi:10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Bard LA, Bard PA, Owens GW, Hall BD. Retinal involvement in thoracic-pelvic-phalangeal dystrophy. Arch Ophthalmol. 1978;96:278–281. doi: 10.1001/archopht.1978.03910050146008. doi:10.1001/archopht.1978.03910050146008. [DOI] [PubMed] [Google Scholar]
- Beals RK, Weleber RG. Conorenal dysplasia: a syndrome of cone-shaped epiphysis, renal disease in childhood, retinitis pigmentosa and abnormality of the proximal femur. Am J Med Genet Part A. 2007;143A:2444–2447. doi: 10.1002/ajmg.a.31948. doi:10.1002/ajmg.a.31948. [DOI] [PubMed] [Google Scholar]
- Blain D, Goetz KE, Ayyagari R, Tumminia SJ. eyeGENE(R): a vision community resource facilitating patient care and paving the path for research through molecular diagnostic testing. Clin Genet. 2013;84:190–197. doi: 10.1111/cge.12193. doi:10.1111/cge.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbo M, Filhol E, Bole-Feysot C, Nitschke P, Gilissen C, Haugen OH, Sanders JS, Stolte-Dijkstra I, Mans DA, Steenbergen EJ, Hamel BC, Matignon M, Pfundt R, Jeanpierre C, Boman H, Rodahl E, Veltman JA, Knappskog PM, Knoers NV, Roepman R, Arts HH. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet. 2011;89:634–643. doi: 10.1016/j.ajhg.2011.10.001. doi:10.1016/j.ajhg.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujakowska KM, Zhang Q, Siemiatkowska AM, Liu Q, Place E, Falk MJ, Consugar M, Lancelot ME, Antonio A, Lonjou C, Carpen-tier W, Mohand-Said S, den Hollander AI, Cremers FP, Leroy BP, Gai X, Sahel JA, van den Born LI, Collin RW, Zeitz C, Audo I, Pierce EA. Mutations in IFT172 cause isolated retinal degeneration and Bardet–Biedl syndrome. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu441. doi:10.1093/hmg/ddu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19:1553–1561. doi: 10.1101/gr.092619.109. doi:10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coene KL, Roepman R, Doherty D, Afroze B, Kroes HY, Letteboer SJ, Ngu LH, Budny B, van Wijk E, Gorden NT, Azhimi M, Thauvin-Robinet C, Veltman JA, Boink M, Kleefstra T, Cremers FP, van Bokhoven H, de Brouwer AP. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am J Hum Genet. 2009;85:465–481. doi: 10.1016/j.ajhg.2009.09.002. doi:10.1016/j.ajhg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussa RG, Otto EA, Gee HY, Arthurs P, Ren H, Lopez I, Keser V, Fu Q, Faingold R, Khan A, Schwartzentruber J, Majewski J, Hilde-brandt F, Koenekoop RK. WDR19: an ancient, retrograde, intraflagellar ciliary protein is mutated in autosomal recessive retinitis pigmentosa and in Senior–Loken syndrome. Clin Genet. 2013;84:150–159. doi: 10.1111/cge.12196. doi:10.1111/cge.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse JA, Lopes VS, Sanagustin JT, Keady BT, Williams DS, Pazour GJ. Distinct functions for IFT140 and IFT20 in opsin transport. Cytoskeleton (Hoboken) 2014;71:302–310. doi: 10.1002/cm.21173. doi:10.1002/cm.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger SPBR, Greenberg J, Christoffels A, Hide W. [30th Jan 2015];RetNet. http://www.sph.uth.tmc.edu/RetNet/.
- D'Andrea LD, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci. 2003;28:655–662. doi: 10.1016/j.tibs.2003.10.007. doi:10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, Muzny DM, Young AC, Wheeler DA, Cruz P, Morgan M, Lewis LR, Cherukuri P, Maskeri B, Hansen NF, Mullikin JC, Blakesley RW, Bouffard GG, Program NCS, Gyapay G, Rieger S, Tonshoff B, Kern I, Soliman NA, Neuhaus TJ, Swoboda KJ, Kayserili H, Gallagher TE, Lewis RA, Bergmann C, Otto EA, Saunier S, Scambler PJ, Beales PL, Gleeson JG, Maher ER, Attie-Bitach T, Dollfus H, Johnson CA, Green ED, Gibbs RA, Hildebrandt F, Pierce EA, Katsanis N. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet. 2011;43:189–196. doi: 10.1038/ng.756. doi:10.1038/ng.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J, Yntema JL, van Die CE, Crama N, Cornelissen EA, Hamel BC. Jeune syndrome: description of 13 cases and a proposal for follow-up protocol. Eur J Pediatr. 2010;169:77–88. doi: 10.1007/s00431-009-0991-3. doi:10.1007/s00431-009-0991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KE, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, Hoyng CB, van den Born LI, Rohrschneider K, Cremers FP. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. doi:10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, Gleeson JG. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75:979–987. doi: 10.1086/425985. doi:10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Koenekoop RK, Coppieters F, Kohl S, Lopez I, Collin RW, De Baere EB, Roeleveld D, Marek J, Bernd A, Rohr-schneider K, van den Born LI, Meire F, Maumenee IH, Jacobson SG, Hoyng CB, Zrenner E, Cremers FP, den Hollander AI. IQCB1 mutations in patients with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2011;52:834–839. doi: 10.1167/iovs.10-5221. doi:10.1167/iovs.10-5221. [DOI] [PubMed] [Google Scholar]
- Follit JA, Xu F, Keady BT, Pazour GJ. Characterization of mouse IFT complex B. Cell Motil Cytoskeleton. 2009;66:457–468. doi: 10.1002/cm.20346. doi:10.1002/cm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedion A. Phalangeal cone shaped epiphysis of the hands (PhCSEH) and chronic renal disease—the conorenal syndromes. Pediatr Radiol. 1979;8:32–38. doi: 10.1007/BF00973675. doi:10.1007/BF00973675. [DOI] [PubMed] [Google Scholar]
- Halbritter J, Bizet AA, Schmidts M, Porath JD, Braun DA, Gee HY, McInerney-Leo AM, Krug P, Filhol E, Davis EE, Airik R, Czar-necki PG, Lehman AM, Trnka P, Nitschke P, Bole-Feysot C, Schueler M, Knebelmann B, Burtey S, Szabo AJ, Tory K, Leo PJ, Gardiner B, McKenzie FA, Zankl A, Brown MA, Hartley JL, Maher ER, Li C, Leroux, Scambler PJ, Zhan SH, Jones SJ, Kayserili H, Tuysuz B, Moorani KN, Constantinescu A, Krantz ID, Kaplan BS, Shah JV, Consortium UK, Hurd TW, Doherty D, Katsanis N, Duncan EL, Otto EA, Beales PL, Mitchison HM, Saunier S, Hildebrandt F. Defects in the IFT-B component IFT172 cause Jeune and Mainzer–Saldino syndromes in humans. Am J Hum Genet. 2013a;93:915–925. doi: 10.1016/j.ajhg.2013.09.012. doi:10.1016/j.ajhg.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbritter J, Porath JD, Diaz KA, Braun DA, Kohl S, Chaki M, Allen SJ, Soliman NA, Hildebrandt F, Otto EA. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet. 2013b;132:865–884. doi: 10.1007/s00439-013-1297-0. doi:10.1007/s00439-013-1297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. doi:10.1016/s0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. doi:10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis N. The oligogenic properties of Bardet–Biedl syndrome. Hum Mol Genet 13 Spec No. 2004;1:R65–R71. doi: 10.1093/hmg/ddh092. doi:10.1093/hmg/ddh092. [DOI] [PubMed] [Google Scholar]
- Keady BT, Le YZ, Pazour GJ. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol Biol Cell. 2011;22:921–930. doi: 10.1091/mbc.E10-09-0792. doi:10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AO, Bolz HJ, Bergmann C. Early-onset severe retinal dystrophy as the initial presentation of IFT140-related skeletal ciliopathy. J aapos. 2014;18:203–205. doi: 10.1016/j.jaapos.2013.11.016. doi:10.1016/j.jaapos.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. doi:10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. doi:10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MA, Keighren M, Bahlo M, Bromhead CJ, Budd P, Aftimos S, Delatycki MB, Savarirayan R, Jackson IJ, Amor DJ. Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet. 2011;88:508–515. doi: 10.1016/j.ajhg.2011.03.015. doi:10.1016/j.ajhg.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Ah-Cann CJ, Welfare MF, Tan TY, Pope K, Caruana G, Freckmann ML, Savarirayan R, Bertram JF, Dobbie MS, Bateman JF, Farlie PG. Cauli: a mouse strain with an Ift140 mutation that results in a skeletal ciliopathy modelling Jeune syndrome. PLoS Genet. 2013;9:e1003746. doi: 10.1371/journal.pgen.1003746. doi:10.1371/journal.pgen.1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, Shastri M, Beck G, Wright AF, Iannaccone A, Elbedour K, Riise R, Baldi A, Raas-Rothschild A, Gorman SW, Duhl DM, Jacobson SG, Casavant T, Stone EM, Sheffield VC. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet. 2001;28:188–191. doi: 10.1038/88925. doi:10.1038/88925. [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberklaid F, Danks DM, Mayne V, Campbell P. Asphyxiating thoracic dysplasia. Clinical, radiological, and pathological information on 10 patients. Arch Dis Child. 1977;52:758–765. doi: 10.1136/adc.52.10.758. doi:10.1136/adc.52.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O'Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, De Paepe A, Reinhardt R, Klages S, Tsuda M, Kawakami I, Kusakabe T, Omran H, Imm A, Tippens M, Raymond PA, Hill J, Beales P, He S, Kispert A, Margolis B, Williams DS, Swaroop A, Hildebrandt F. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior–Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–288. doi: 10.1038/ng1520. doi:10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- Perrault I, Saunier S, Hanein S, Filhol E, Bizet AA, Collins F, Salih MA, Gerber S, Delphin N, Bigot K, Orssaud C, Silva E, Baudouin V, Oud MM, Shannon N, Le Merrer M, Roche O, Pietrement C, Goumid J, Baumann C, Bole-Feysot C, Nitschke P, Zahrate M, Beales P, Arts HH, Munnich A, Kaplan J, Antignac C, Cormier-Daire V, Rozet JM. Mainzer–Saldino syndrome is a ciliopathy caused by IFT140 mutations. Am J Hum Genet. 2012;90:864–870. doi: 10.1016/j.ajhg.2012.03.006. doi:10.1016/j.ajhg.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucl Acids Res. 2011;39:e118. doi: 10.1093/nar/gkr407. doi:10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin SA, Iqbal M, Wang Y, Masuda T, Chen Y, Bowne S, Sullivan LS, Waseem NH, Bhattacharya S, Daiger SP, Zhang K, Khan SN, Riazuddin S, Hejtmancik JF, Sieving PA, Zack DJ, Katsanis N. A splice-site mutation in a retina-specific exon of BBS8 causes nonsyndromic retinitis pigmentosa. Am J Hum Genet. 2010;86:805–812. doi: 10.1016/j.ajhg.2010.04.001. doi:10.1016/j.ajhg.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. doi:10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O'Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. doi:10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- Schmidts M, Frank V, Eisenberger T, Al Turki S, Bizet AA, Antony D, Rix S, Decker C, Bachmann N, Bald M, Vinke T, Toenshoff B, Di Donato N, Neuhann T, Hartley JL, Maher ER, Bogdanovic R, Peco-Antic A, Mache C, Hurles ME, Joksic I, Guc-Scekic M, Dobricic J, Brankovic-Magic M, Bolz HJ, Pazour GJ, Beales PL, Scambler PJ, Saunier S, Mitchison HM, Bergmann C. Combined NGS approaches identify mutations in the intraflagellar transport gene IFT140 in skeletal ciliopathies with early progressive kidney Disease. Hum Mutat. 2013;34:714–724. doi: 10.1002/humu.22294. doi:10.1002/humu.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. doi:10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- Smit A, Hubley R, Green P. [30 Dec 2014];RepeatMasker Open-3.0. 1996–2010 http://www.repeatmasker.org.
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3—new capabilities and interfaces. Nucl Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. doi:10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, Fazzi E, Signorini S, Louie CM, Bellacchio E, Bertini E, Dallapiccola B, Gleeson JG. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Jou-bert syndrome. Nat Genet. 2006;38:623–625. doi: 10.1038/ng1805. doi:10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, Szczepanska M, Krawczynski M, Zachwieja J, Zwolinska D, Beales PL, Ropers HH, Latos-Bielenska A, Kuss AW. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet. 2010;86:949–956. doi: 10.1016/j.ajhg.2010.04.012. doi:10.1016/j.ajhg.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Sun V, Tuan HF, Keser V, Wang K, Ren H, Lopez I, Zaneveld JE, Siddiqui S, Bowles S, Khan A, Salvo J, Jacobson SG, Iannaccone A, Wang F, Birch D, Heckenlively JR, Fishman GA, Traboulsi EI, Li Y, Wheaton D, Koenekoop RK, Chen R. Comprehensive molecular diagnosis of 179 Leber congenital amaurosis and juvenile retinitis pigmentosa patients by targeted next generation sequencing. J Med Genet. 2013;50:674–688. doi: 10.1136/jmedgenet-2013-101558. doi:10.1136/jmedgenet-2013-101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang H, Tuan HF, Nguyen DH, Sun V, Keser V, Bowne SJ, Sullivan LS, Luo H, Zhao L, Wang X, Zaneveld JE, Salvo JS, Siddiqui S, Mao L, Wheaton DK, Birch DG, Branham KE, Heckenlively JR, Wen C, Flagg K, Ferreyra H, Pei J, Khan A, Ren H, Wang K, Lopez I, Qamar R, Zenteno JC, Ayala-Ramirez R, Buentello-Volante B, Fu Q, Simpson DA, Li Y, Sui R, Silvestri G, Daiger SP, Koenekoop RK, Zhang K, Chen R. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet. 2014;133:331–345. doi: 10.1007/s00439-013-1381-5. doi:10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Min J. Structure and function of WD40 domain proteins. Protein Cell. 2011;2:202–214. doi: 10.1007/s13238-011-1018-1. doi:10.1007/s13238-011-1018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Gelowani V, Eblimit A, Wang F, Young MP, Sawyer BL, Zhao L, Jenkins G, Creel DJ, Wang K, Ge Z, Wang H, Li Y, Hartnett ME, Chen R. ATF6 is mutated in early onset photoreceptor degeneration with macular involvement. Invest Ophthalmol Vis Sci. 2015;56:3889–3895. doi: 10.1167/iovs.15-16778. doi:10.1167/iovs.15-16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976;15:700–725. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.