Abstract
Aims
Ecological Momentary Assessment (EMA) captures real-time reports in subjects’ natural environments. This experiment manipulated EMA frequency to estimate effects on abstinence and peri-cessation subjective experiences.
Design
In this randomized trial, subjects had an equal chance of being assigned to low-frequency (once) or high-frequency (6 times) daily EMA for 4 weeks (1 week pre- and 3 weeks post-cessation). Participants completed 6 office visits over 5 weeks and 6- and 12-week follow-up telephone interviews.
Setting
Community participants were recruited from central New Jersey, USA.
Participants
110 adult daily smokers seeking to quit smoking were included in intent-to-treat analyses of tobacco abstinence; 94 were available for secondary analyses of peri-cessation subjective ratings.
Measurements
Primary outcomes were cessation (abstaining at least 24 hours within 2 weeks of attempting to quit) and prolonged abstinence (no relapse between weeks 2 and 12 post-quit). Secondary outcomes were mean levels and growth in ratings of cigarette craving, affect, and quitting motivation and self-efficacy.
Findings
EMA frequency was unrelated to cessation (Odds Ratio=1.37, 95% CI=0.60–3.10) or prolonged abstinence (Odds Ratio=1.04, 95% CI=0.45–2.39) in intent-to-treat analyses. High-frequency EMA was associated with lower craving (B=−.54, SE=.18, p=.004, anxiety (B=−.42, SE=.17, p=.015), anger (B=−.47, SE=.14, p=.001), hunger (B=−.39, SE=.17, p=0.25), and positive affect (B=−.43, SE=.20, p=.03).
Conclusions
In smokers trying to quit, more frequent ecological momentary assessment (EMA) self-monitoring results in lower craving, anxiety, anger, hunger, and positive affect. It is not clear whether this translates into higher rates of smoking abstinence.
Keywords: Ecological Momentary Assessment, Reactivity, Smoking Cessation, Self-Monitoring
Introduction
Ecological momentary assessment (EMA) [1] refers to collection of data in real-time in respondents’ natural environments. EMA is a useful tool in the study of smoking behavior and cessation [2]. Time-stamped EMA offers several benefits over traditional pen-and-paper measures, such as reducing recall biases and better establishing the temporal ordering of events [1–2]. In addition, EMA tracks the antecedents and consequences of smoking in smokers’ typical environments, which can help identify ways in which context and both distal and proximal factors combine to influence smoking [2–3]. Portable electronic devices such as personal digital assistants and cell phones make time-stamped EMA possible in many contexts.
Although EMA has many benefits, EMA may also induce assessment reactivity. Assessment reactivity refers to changes in participant experiences and behavior triggered by assessment. Research has shown that assessment of alcohol use, alcohol use consequences, mental health, and affect triggers reductions in drinking pre-treatment [4] and that assessment can serve as an intervention [5]. Early research suggested that minimal self-monitoring of behavior could reduce smoking [6–7]. Assessing behavior may be an intervention. Assessment may also induce immediate reactivity in subjective experiences that may alter behavior. Research suggests that binge eating episodes are reactive to self-monitoring of eating [8]. In one study, objective binges decreased while subjective binges increased during self-monitoring [8]. This highlights the potential for assessment to have different effects on experiences and behavior.
The few EMA studies of smokers that have explored assessment reactivity have produced mixed results. One study evaluated subjective reactivity in non-treatment-seeking smokers assigned EMA assessment versus a no-assessment control [9]. Smokers in the self-monitoring condition reported less worry but no change in perceived risk of smoking at the end of monitoring. The relation between monitoring and smoking behavior was not investigated. Another study compared smoking cessation outcomes and experiences at three levels of EMA assessment: no assessment, assessment pre-quit, or assessment post-quit [10]. Results suggested some immediate and some delayed reactivity in self-efficacy, negative affect, and select nicotine withdrawal symptoms, but did not support the hypothesis that EMA use or timing influenced early abstinence. An earlier study by Shiffman and colleagues assigned a subsample of EMA subjects to a reduced burden condition that completed only 30% of the full assessment condition [11–12]. The reports completed by the reduced burden group were also shorter versions of reports [13]. Although those in the reduced burden group initiated more temptation reports than subjects in the full assessment condition, there were no differences in smoking urge as a function of EMA burden [13]. Another quit smoking study compared subjects with either a 4- or 7-week pre-quit EMA assessment period [14]. If subjects were reactive to the onset of recording, we would expect to see differences 3 weeks pre-quit for those who just started recording, relative to those who had been recording for weeks. Results indicated that EMA duration was not related to withdrawal in the 3 weeks leading up to the quit day. This suggests that subjects may not be reactive to the onset of assessment, but this does not necessarily mean that subjects are not reactive to ongoing recording. For this reason, it is important to manipulate EMA directly rather than inferring a lack of reactivity based on a lack of change in scores during EMA [15].
Studies in other populations have failed to detect any subjective or behavioral EMA reactivity. For example, alcohol-abusing college students did not show changes in drinking frequency during EMA recording, relative to pre-EMA levels [16]. Another study [17] failed to demonstrate that requiring 0, 3, 6, or 12 EMA reports per day significantly affected pain patients’ recalled pain or the course of pain ratings over time. Another research group found no differences in retrospective reports of drinking or urges to drink among subjects in early recovery from alcohol asked to complete 0, 4, or 28 days of once-daily self-monitoring [18].
Although these negative results reduce concerns about reactivity during stable states (e.g., chronic pain, continued drinking), questions remain about EMA reactivity during change attempts. Many of the past studies of EMA reactivity are limited by small sample sizes [15, 16, 19] or unmatched control conditions [9–12]. As such, more systematic study of EMA reactivity during smoking cessation attempts is warranted. The current study randomly assigned adult smokers seeking to quit smoking to self-monitor via EMA either once per day or 6 times per day for 1 week pre-quit and 3 weeks post-quit. The primary aim of the study was to determine whether more frequent EMA self-monitoring would promote smoking cessation and maintenance of abstinence, as hypothesized based on earlier work showing that self-monitoring reduced smoking heaviness [6–7]. A secondary, exploratory aim was to estimate EMA frequency effects on craving, affect, quitting motivation and self-efficacy in the peri-cessation period in multilevel models. These secondary, subjective outcomes were selected because past EMA research has linked these variables to success in quitting smoking and shown them to mediate treatment effects on abstinence [14, 20]. A third aim was to estimate mediated (indirect) effects of EMA frequency on prolonged abstinence through changes in craving, affect, motivation, or self-efficacy.
Method
Participants
Participants were recruited between 2007 and 2009 via mass media advertisements for a stop smoking study in central New Jersey. Only English-literate adults smoking at least 10 cigarettes per day who were motivated to quit and free from contraindications to nicotine lozenge use and serious mental illness were eligible (see online supporting material for details). Demographic characteristics of eligible subjects are shown in Table 1.
Table 1.
Characteristics of the enrolled sample (N=110) by EMA frequency condition
| Variable | Value |
Low Frequency (n=54) |
High Frequency (n=56) |
|---|---|---|---|
| Sex | Female | 27 (50.0%) | 25 (44.6%) |
| Ethnicity | Hispanic | 3 (5.6%) | 5 (9.3%) |
| Race | White | 43 (79.6%) | 44 (78.6%) |
| African-American | 4 (7.4%) | 5 (8.9%) | |
| Other | 7 (13.0%) | 7 (12.5%) | |
| Marital Status | Married or Cohabitating | 29 (53.7%) | 28 (50.0%) |
| Separated or Divorced | 6 (11.1%) | 16 (28.6%) | |
| Never Married | 16 (29.6%) | 11 (19.6%) | |
| Widowed | 3 (5.6%) | 1 (1.8%) | |
| Education | Less than high school degree | 3 (5.6%) | 1 (1.8%) |
| High school | 8 (14.8%) | 17 (30.4%) | |
| Some college | 27 (50.0%) | 27 (48.2%) | |
| College degree or greater | 16 (29.6%) | 11 (19.6%) | |
| Employment | Employed | 41 (75.9%) | 45 (80.4%) |
| Statusa | Unemployed or Disabled | 8 (14.8%) | 15 (26.8%) |
| Student | 10 (18.5%) | 5 (8.9%) | |
| Retired | 2 (3.7%) | 5 (8.9%) | |
| Household Incomeb | < $25,000 | 5 (9.3%) | 9 (16.1%) |
| $25,00-$49,999 | 15 (27.8%) | 14 (25.0%) | |
| $50,000-$74,999 | 13(24.1%) | 7 (12.5%) | |
| >$75,000 | 18 (33.3%) | 25 (44.6%) | |
| Time to First | Within 5 minutes | 19 (35.2%) | 22 (39.3%) |
| Cigarette | 6–30 minutes | 26 (48.1%) | 25 (44.6%) |
| After 30 minutes | 9 (16.7%) | 9 (16.1%) |
| t(108) | M (SD) | M (SD) | |
|---|---|---|---|
| Mean Age | 1.40 | 41.11 (14.02) | 44.80 (13.58) |
| Mean Cigarettes Smoked per Day | 0.56 | 20.70 (8.17) | 21.71 (10.48) |
| Mean Baseline FTND Score | 0.44 | 5.50 (1.77) | 5.66 (2.09) |
| Mean % of Daytime EMA Reports Completed | 0.36 | 54.67 (26.21) | 56.46 (26.38) |
| Mean Total Number of Reports Completedc | 12.81* | 26.31 (10.41) | 141.82 (65.48) |
| Baseline WSWS Craving | 0.30 | 2.70 (0.83) | 2.65 (0.84) |
| Baseline WSWS Sadness | 0.18 | 1.46 (0.78) | 1.44 (0.73) |
| Baseline WSWS Anxiety | 0.89 | 2.31 (0.74) | 2.18 (0.80) |
| Baseline WSWS Anger | −0.06 | 1.85 (1.01) | 1.86 (1.01) |
| Baseline PANAS Positive Affect | −0.30 | 35.22 (5.51) | 35.61 (7.75) |
| Baseline WSWS Hunger | −1.31 | 2.15 (0.81) | 2.34 (0.71) |
| Baseline Motivation | −1.02 | 8.61 (1.61) | 8.89 (1.29) |
| Baseline Confidence (n=109) | 0.91 | 5.47 (1.15) | 5.23 (1.55) |
p<.05
Sums to more than 100% because subjects could select more than one
Sums to less than 100% because of missing data
Includes unprompted and late reports, and reports completed outside target 28-day assessment period.
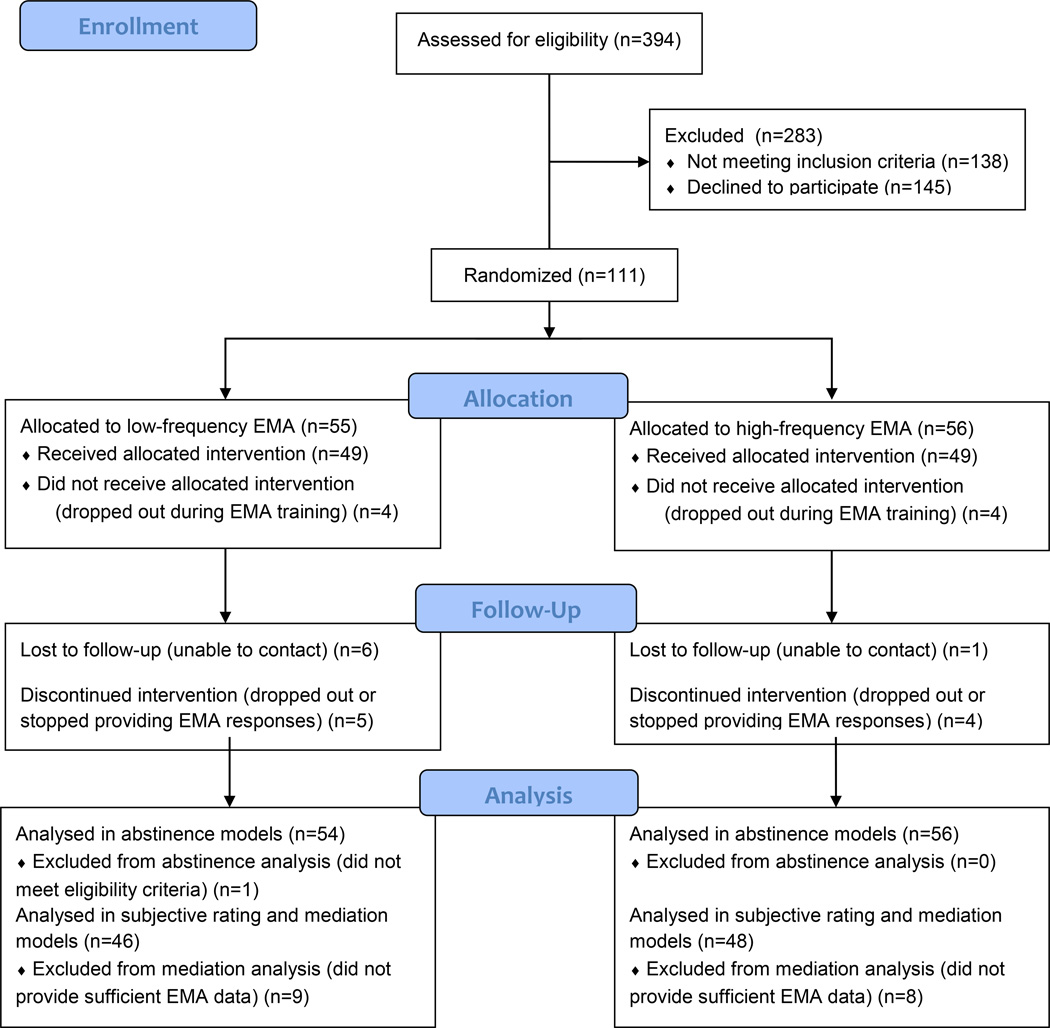
The target sample size was 100 based on a priori power analyses. This sample size would be sufficient (with power 0.7) to detect large (relative risk 1.75) EMA frequency effects on abstinence. Power would exceed 0.7 to detect medium to large effects on secondary outcomes [21–22] even with 90 participants. A total of 111 smokers were randomized. Of these, 1 was later found to be ineligible due to exclusive use of cigars, leaving 110 for primary analyses. Attrition and non-adherence reduced the sample available for secondary analyses to 94. The 94 retained did not differ from the 16 lost in age, gender, race, ethnicity, or smoking heaviness or dependence (all ps >.05), although 12 of the 16 lost were men.
Procedures
All study procedures were approved by an institutional review board. Participant flow is shown in Figure 1. Participants who passed initial telephone screening were enrolled at group orientation sessions at which written informed consent, CO testing, and baseline assessment occurred. Randomization to either the high- or low-frequency EMA condition, with 1:1 allocation blocked on gender, occurred between telephone screening and orientation to give investigators, who were not blind to condition, time to program EMA devices. Randomization was accomplished in blocks of 8 using a computer-generated list assigning a subject number and associated condition to each new enrollee. The PI generated the randomization log at the outset of the study and enrolled participants while research staff assigned subjects to conditions. At orientation, enrollees were trained to use an EMA device scheduled to administer alarms once (in the low-frequency condition) or 6 times (in the high-frequency condition) per day during their typical waking day. Subjects were not informed of the experimental manipulation; they were told that they could receive up to 6 alarms per day. Research staff conducting study visits were aware of study condition because they needed to program EMA data collection in accordance with each subject’s schedule and provide feedback about EMA adherence at each visit.
Figure 1.
CONSORT flow diagram.
Enrollees completed six 20–30-minute individual office visits (on days −10, −3, 0, 3, 10, and 21 relative to a target quit day set by investigators). At each visit, subjects were assessed for adverse events; completed CO testing and self-report measures of withdrawal, affect, and depressive symptoms; and received feedback about their EMA adherence and encouragement to complete all reports within 15 minutes of a prompt. All subjects received 15–20-minute individual Clinical Practice Guideline-based [23] smoking cessation counseling at 3 visits (days −3, 0, 3, see supplemental material for the counseling protocol). All subjects also received up to a 12-week supply of mint nicotine lozenges (2- or 4-mg depending on time to first cigarette upon waking, in accordance with drug packaging, GlaxoSmithKline, Research Triangle, NC) for use beginning on the quit day. Follow-up interviews assessing tobacco use were conducted by telephone 6 and 12 weeks post-quit, with CO (at an additional office visit) or collateral confirmation (if unable to come to the office for CO testing) of point-prevalence abstinence at 12 weeks. Subjects received $20 for each visit completed after orientation, for a maximum of $140.
Measures
At baseline, subjects completed a smoking history and demographic questionnaire, along with measures of cigarette dependence, nicotine withdrawal, negative and positive affect, and depressive symptoms. The Fagerström Test of Nicotine Dependence (FTND) [24] is a widely used, brief measure of physical dependence with modest internal consistency in this sample (Cronbach’s alpha=.51). The Wisconsin Smoking Withdrawal Scale (WSWS) [25] is a 7-scale (craving, anxiety, anger, sadness, difficulty concentrating, hunger, and sleep disturbance) measure of withdrawal severity with good internal consistency in this sample (alpha ranged from .71 to .91 for the subscales). The Positive and Negative Affect Schedule (PANAS) [26] was used to assess negative (alpha=.85) and positive affect (alpha=.83). The Center for Epidemiologic Studies-Depression (CES-D) [27] scale was used to screen for elevated depressive symptoms at enrollment (alpha=.66). At subsequent visits, subjects again completed the WSWS, PANAS, and CES-D, and a timeline follow-back interview assessing daily tobacco and treatment use retrospectively since the last visit.
Electronic diaries were programmed using Pendragon Forms 5.1 software (Pendragon Software Corporation, Libertyville, IL). Subjects in the high-frequency condition were prompted to complete 5 daytime and 1 bedtime reports daily. Subjects in the low-frequency condition were prompted to complete a daytime report on half the days they carried the EMA device and a bedtime report on the other days, with type of report randomly assigned by day within subjects. Daytime reports were prompted at random during the waking day. In the high-frequency condition, a prompt was scheduled at random in each of 5 equal intervals of the waking hours (e.g., a person who was awake 15 hours would have 1 prompt at random in every 3-hour window), with the constraint that no prompt occur within 30 minutes of another prompt. In the low-frequency condition, a daytime report was scheduled at random between wake up and 30 minutes before bedtime. Daytime reports assessed the 15 minutes before the prompt and took about 3 minutes to complete. We instructed participants to respond to prompts within 30 minutes. Bedtime reports occurred 5 minutes prior to the subject’s usual bedtime and assessed retrospective reports of the past 24 hours and were not analyzed for the current study.
The EMA items are listed in the supporting information online. Affect and withdrawal items were adapted for EMA from the WSWS and PANAS [14] and rated on 5-point scales ranging from 1=Disagree to 5=Agree for WSWS items and 0=Very slightly or not at all to 4=Extremely for PANAS items. Confirmatory factor analyses (see supporting information online) suggested that a model with separate facets for each negative emotion (sadness, anxiety, and anger) fit the data best. Motivation to quit and confidence in the ability to quit for good were assessed with single items rated on a 7-point scale ranging from 1=Not at all to 7=Extremely. The number of cigarettes smoked in the past two hours was assessed and recoded as binary (0=no smoking, 1=smoking). Additional items regarding context were administered at each report, but will not be discussed further.
Primary cessation outcomes included initial cessation (i.e., quitting for 24 hours within the first 2 weeks of the quit attempt) and 12-week prolonged abstinence. Prolonged abstinence indicates that no relapse (i.e., smoking for at least 7 days in a row) occurred between weeks 2 and 12 of a quit attempt. Initial cessation was coded as occurring if no smoking was reported on either EMA reports or retrospective timeline follow-back calendar reports for a given day within the first 14 days following a target quit day set by investigators.
Data Analysis
To assess our primary aim, EMA frequency effects on initial cessation and 12-week prolonged abstinence were estimated using logistic regression analyses in SPSS 20.0 (IBM, Armonk, NY). Analyses were intent to treat and used all 110 eligible enrollees, with missing cases treated as smoking.
To address our secondary aim, multilevel models of real-time, daytime reports (level 1) nested within subjects (level 2) were fit with HLM 6.04 (Scientific Software International, Inc., Skokie, IL) [28] using full maximum likelihood estimation to examine EMA frequency effects on subjective ratings of craving, sadness, anxiety, anger, positive affect, motivation to quit, and quitting confidence. Both uncorrected and Bonferroni-corrected significance (using p<.006 for the 8 subjective ratings tested) are presented below. To balance the number of data points in the two EMA frequency conditions (so the models would have similar precision and standard errors in the two conditions), a subset of reports was randomly selected from high-frequency subjects to match the number of reports from a gender-matched low-frequency subject. Because there were 2 more male participants in the high-frequency group than the low-frequency condition, we randomly selected the average number of daytime reports by men in the low-frequency condition (pre-quit=2, post-quit=7) from the unmatched high-frequency participants. A total of 871 daytime reports from 94 subjects were analyzed. The remaining 16 subjects did not provide sufficient EMA data to be included in analyses of subjective ratings. We conducted analyses twice, once with all prompted reports and once with only reports completed within 30 minutes of a prompt, to see if including late reports (which may differ from on-time reports due to recall or other biases) changed the pattern of results.
Piecewise models were estimated so the mean levels and slopes could be estimated separately in the pre- and post-target-quit-day periods. Time was centered around the midpoint of each epoch (day 3.5 of the 7-day pre-quit period and day 10.5 of the 21-day post-quit period). The pre-quit intercept captures the mean level of the dependent variable (e.g., craving) pre-quit whereas the post-quit intercept captures the mean change from pre- to post-quit average levels of each subjective rating. Separate pre- and post-quit linear and quadratic slopes were included if their inclusion improved model fit. Intercepts and slopes were set to random if doing so improved model fit and coefficients varied significantly across subjects.
First unconditional models were fit, and then EMA frequency was added as a level-2 covariate predicting level-1 intercept and slope coefficients. This was retained when significant. Then additional covariates (age, gender, minority status, college education, FTND score, and baseline levels of the dependent variable) and a binary time-varying smoking covariate (any smoking in the past 2 hours) were added. Non-significant covariates were pruned from the model.
To address our tertiary aim, we first examined the extent to which subjective rating growth parameters from aim 2 were related to 12-week prolonged abstinence using logistic regression analyses where the predictors were individual subject-level empirical Bayes’ estimates of rating intercepts or slopes. For those subjective rating parameters that were both significantly influenced by EMA frequency and predictive of prolonged abstinence, we computed bootstrap confidence intervals for the mediated effect [5] to determine if these mediated effects were significant (i.e., confidence intervals did not contain 0).
Results
Manipulation Check
Participants in the high- and low-frequency EMA conditions were similar in baseline characteristics but differed significantly in terms of the total number of EMA reports completed, as intended (Table 1). Non-prorated adherence measured by the average percentage of scheduled daytime reports completed was similar, if somewhat low, in the two conditions. Reports were more timely (average time to answer the prompt =10.2 minutes, SD=30.2, Median=1) in the high-frequency condition than in the low-frequency condition (42.7 minutes, SD=103.0, Median=3), suggesting that some in the low-frequency condition may not have carried the EMA device with them as instructed. Most reports in both conditions were within 30 minutes of a prompt (78.2% in the low-frequency condition, 90.8% in the high-frequency condition) and the pattern of results did not change when only reports completed within 30 minutes of a prompt were analyzed, except where noted for quitting confidence.
Self-Monitoring Frequency Effects on Abstinence
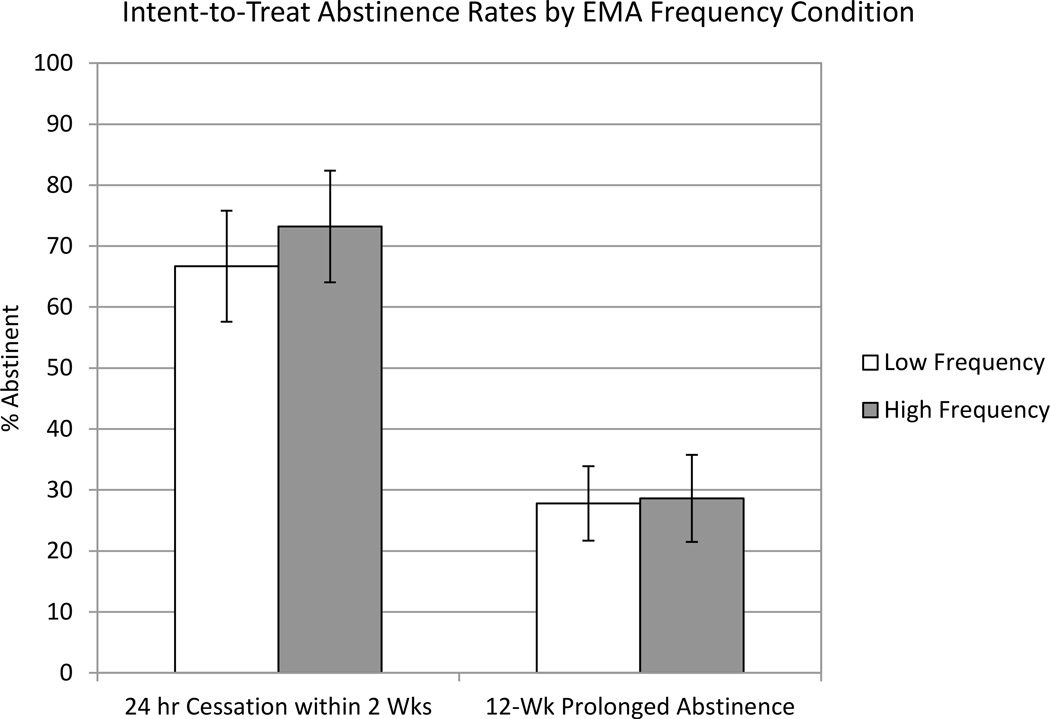
Intent-to-treat abstinence rates are shown as a function of EMA condition in Figure 2. Initial cessation rates did significantly differ between conditions (B=.31, SE=.42, Odds Ratio=1.37, 95% CI=0.60–3.10, p=.45, Number needed to treat [NNT]=16). Intent-to-treat 12-week prolonged abstinence rates also did not differ significantly between conditions (B=.04, SE=.42, Odds Ratio=1.04, 95% CI=0.45–2.39, p=.93, NNT=126).
Figure 2.
Abstinence outcomes as a function of EMA frequency condition among the 110 subjects randomized to condition. Error bars reflect one standard error above and below the observed abstinence rate.
Self-Monitoring Frequency Effects on Subjective Ratings
Cravings to smoke
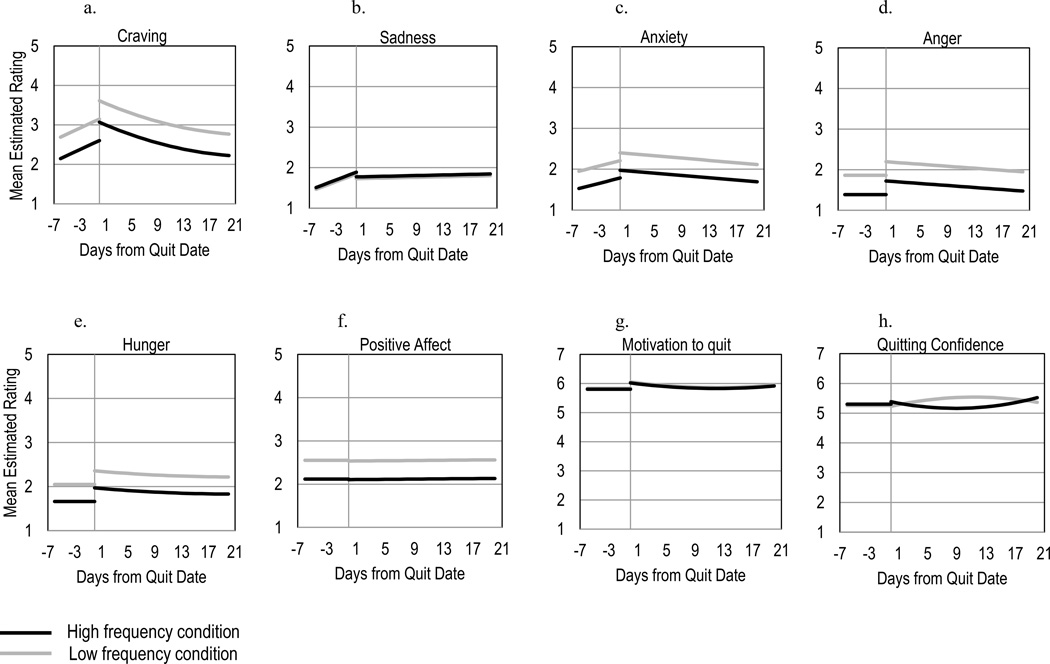
Average craving ratings were significantly (p=.004) lower pre-quit for smokers in the high-frequency condition than in the low-frequency condition, controlling for baseline craving (see Table 2 and Figure 3a). Momentary craving increased pre-quit [p=.008, non-significant (n.s.) at .006 Bonferroni-corrected alpha] and decreased significantly post-quit on average (p<.001), regardless of condition. Time-varying smoking effects are reported in supporting information online.
Table 2.
HLM analysis of EMA condition effects on momentary subjective experiences
| Fixed Effect | Coefficient | Standard Error | T-ratio | Approx. df | p-value | |
|---|---|---|---|---|---|---|
| Craving to smoke | ||||||
| Pre-quit average | 2.918 | 0.150 | 19.462 | 91 | <.001* | |
| High EMA frequency condition | −0.544 | 0.183 | −2.970 | 91 | 0.004* | |
| Baseline craving | 0.311 | 0.113 | 2.740 | 91 | 0.008 | |
| Change in pre-post quit average | 0.124 | 0.133 | 0.931 | 93 | 0.355 | |
| Pre-quit slope | 0.076 | 0.028 | 2.696 | 864 | 0.008 | |
| Post-quit slope | −0.042 | 0.007 | −5.740 | 93 | <.001* | |
| Quadratic post-quit slope | 0.001 | 0.001 | 1.053 | 93 | 0.296 | |
| Sadness | ||||||
| Pre-quit average | 1.656 | 0.069 | 23.892 | 91 | <.001* | |
| Baseline sadness | 0.240 | 0.079 | 3.024 | 91 | 0.004* | |
| Age | −0.012 | 0.004 | −2.944 | 91 | 0.005* | |
| Change in pre-post quit average | 0.110 | 0.060 | 1.840 | 93 | 0.069 | |
| Pre-quit slope | 0.063 | 0.019 | 3.248 | 93 | 0.002* | |
| Post-quit slope | 0.003 | 0.003 | 0.985 | 93 | 0.328 | |
| Anxietya | ||||||
| Pre-quit average | 2.080 | 0.127 | 16.296 | 89 | <.001* | |
| High EMA frequency condition | −0.424 | 0.170 | −2.497 | 89 | 0.015 | |
| Baseline anxiety | 0.391 | 0.114 | 3.426 | 89 | 0.001* | |
| Age | −0.016 | 0.006 | −2.590 | 89 | 0.012 | |
| Nicotine dependence | 0.111 | 0.045 | 2.469 | 89 | 0.016 | |
| Change in pre-post quit average | 0.178 | 0.086 | 2.049 | 93 | 0.043 | |
| Pre-quit slope | 0.043 | 0.024 | 1.796 | 863 | 0.072 | |
| Post-quit slope | −0.014 | 0.007 | −2.010 | 93 | 0.047 | |
| Anger | ||||||
| Pre-quit average | 1.865 | 0.102 | 18.157 | 91 | <.001* | |
| High EMA frequency condition | −0.474 | 0.139 | −3.409 | 91 | 0.001* | |
| Baseline anger | 0.257 | 0.068 | 3.756 | 91 | <.001* | |
| Change in pre-post quit average | 0.210 | 0.070 | 2.984 | 93 | 0.004* | |
| Post-quit slope | −0.012 | 0.006 | −2.156 | 93 | 0.033 | |
| Hunger | ||||||
| Pre-quit average | 2.047 | 0.126 | 16.253 | 91 | <.001* | |
| High EMA frequency condition | −0.388 | 0.170 | −2.281 | 91 | 0.025 | |
| Baseline hunger | 0.190 | 0.109 | 1.746 | 91 | 0.084 | |
| Change in pre-post quit average | 0.208 | 0.081 | 2.566 | 93 | 0.012 | |
| Post-quit slope | −0.006 | 0.004 | −1.445 | 865 | 0.149 | |
| Quadratic post-quit slope | 0.000 | 0.001 | 0.260 | 93 | 0.796 | |
| Positive Affect | ||||||
| Pre-quit intercept | 2.549 | 0.146 | 17.457 | 91 | <.001* | |
| High EMA frequency condition | −0.430 | 0.196 | −2.197 | 91 | 0.030 | |
| Baseline positive affect | 0.038 | 0.015 | 2.527 | 91 | 0.014 | |
| Change in pre-post quit average | −0.001 | 0.074 | −0.009 | 93 | 0.993 | |
| Post-quit slope | 0.001 | 0.005 | 0.264 | 93 | 0.792 | |
| Motivation to Quitb | ||||||
| Pre-quit average | 5.837 | 0.110 | 52.804 | 90 | <.001* | |
| Baseline motivation | 0.393 | 0.057 | 6.894 | 90 | <.001* | |
| Completed college | −0.401 | 0.202 | −1.989 | 90 | 0.049 | |
| Change in pre-post quit average | 0.030 | 0.098 | 0.314 | 92 | 0.754 | |
| Post-quit slope | −0.005 | 0.005 | −1.008 | 92 | 0.317 | |
| Quadratic post-quit slope | 0.001 | 0.001 | 1.295 | 92 | 0.199 | |
| Quitting Confidence | ||||||
| Pre-quit average | 5.258 | 0.117 | 44.783 | 91 | <.001* | |
| Baseline confidence | 0.438 | 0.077 | 5.695 | 91 | <.001* | |
| Change in pre-post quit average | 0.276 | 0.143 | 1.930 | 91 | 0.056 | |
| High EMA frequency conditionc | −0.410 | 0.191 | −2.148 | 91 | 0.034 | |
| Post-quit slope | 0.006 | 0.006 | 1.093 | 92 | 0.278 | |
| Quadratic post-quit slope | −0.002 | 0.001 | −1.654 | 91 | 0.101 | |
| High EMA frequency condition | 0.005 | 0.002 | 2.649 | 91 | 0.010 | |
Note: Bolded values p<.05
p < 0.006, Bonferroni-corrected alpha for examination of 8 candidate mediators
n=89, missing baseline anxiety for two people
n=90, missing baseline motivation for one person
Reduced to non-significance if only timely reports were included (662 reports from 90 subjects)
Figure 3.
Estimates of mean subjective craving, sadness, anxiety, anger, hunger, positive affect, motivation to quit, and quitting confidence pre- and post-quit as a function of EMA frequency condition derived from multilevel models.
Sadness
EMA frequency did not have a significant effect on sadness (see Figure 3b). Sadness increased significantly (p=.002) pre-quit regardless of condition.
Anxiety
Average anxiety ratings pre-quit were lower (p=.015, n.s.) in high-frequency participants than in low-frequency participants (see Figure 3c). The pre-quit slope was not significant, and average anxiety decreased (p=.047, n.s.) post-quit regardless of condition. Younger age (p=.012, n.s.), greater nicotine dependence (p=.016, n.s.), and greater baseline anxiety (p<.001) were associated with higher average pre-quit anxiety.
Anger
Average anger ratings pre-quit were lower (p=.015, n.s.) for high-frequency participants than low-frequency participants at p<.05 (see Figure 3d). Anger marginally increased from pre- to post-quit (p=.043, n.s.) and subsequently decreased (p=.047, n.s.), regardless of condition. None of the tested covariates improved model fit.
Hunger
High-frequency participants had lower (p=.025, n.s.) pre-quit average hunger compared to low-frequency participants (see Figure 3e). Hunger ratings were stable pre-quit and increased from pre- to post-quit (p=.012, n.s.) in both conditions. Average linear and quadratic growth in hunger post-quit were not significant, but inclusion of these terms improved model fit and exhibited significant variability across subjects.
Positive Affect
Pre-quit positive affect was lower (p=.030, n.s.) on average for high-frequency participants than low-frequency participants (see Figure 3f). Growth in positive affect was not significant and not related to EMA frequency.
Motivation to Quit
Motivation to quit was stable over time and not influenced by EMA frequency (see Figure 3g). Smokers with higher baseline motivation (p<.001) and those with less than a college education (p=.049, n.s.) had higher motivation pre-quit.
Quitting Confidence
Confidence decreased from pre- to post-quit for high-frequency subjects, while confidence increased for low-frequency subjects, on average (p=.034, n.s.; see Figure 3h). High- and low-frequency subjects displayed opposite patterns of curvilinear growth in confidence post-quit (p=.01, n.s.). Restricting analyses to on-time reports (completed within 30 minutes of a prompt) reduced the EMA frequency effect on the change in confidence from pre- to post-quit to non-significance (B=−0.26, SE=0.19, t(88)=−1.34, p=.184), but the condition effect on curvilinear growth remained significant.
Relations between Subjective Ratings and Abstinence
In logistic regression models, 12-week prolonged abstinence was regressed on empirical Bayes’ estimates of random coefficients from HLM models of subjective ratings. In all models that contained daily change slope variables (pre- or post-quit), the standard errors were very large, suggesting that the individual slopes could not be estimated reliably. These slope estimates were therefore dropped from regression models.
Pre-quit intercepts (mean levels) of several subjective ratings were predictive of prolonged abstinence. Estimated means in sadness, anxiety, anger, and confidence in the week preceding the quit attempt were all significantly related to abstinence at p<.05, and sadness and anxiety means were significantly associated with abstinence at p<.006.. At alpha .05, higher pre-quit confidence predicted greater odds of abstinence whereas higher levels of negative emotions predicted lower odds of abstinence. Pre-quit craving was significantly predictive of later abstinence in some models, but this effect was not robust when entered as a sole predictor or with level-two covariates. In contrast, the increase in mean craving from pre- to post-quit was negatively predictive of prolonged abstinence in all models.
Mediated Effects
As there were effects of EMA frequency on anger (p<.001) and anxiety (p=.015, n.s.), and these pre-quit intercepts predicted prolonged abstinence at p<.05 (only anxiety predicted abstinence at p<.006), we estimated mediated effects. The mediated effect is the product of the path between EMA frequency and anger or anxiety (a paths) and the path between anger or anxiety and prolonged abstinence (b paths). Standard errors and confidence intervals around the mediated effect (ab) were computed using the RMediation program [28]. Both mediated effects were significantly different from zero at p<.05 (anxiety: ab=.493, SE=.273, 95% CI=.060, 1.116; anger: ab=.477, SE=.274, 95% CI=.027–1.091). If we reduce alpha to .006, the mediated effects are no longer significant (anxiety; 99.4% CI=−.064, 1.455; anger: 99.4% CI=−0.144, 1.420).
Discussion
This experimental manipulation of EMA self-monitoring frequency did not support the primary hypothesis that more frequent monitoring would promote abstinence, but identified significant reactivity in subjective experiences, particularly in reducing craving and anger. Results also suggested reactivity in reduced anxiety, hunger, positive affect, and post-quit confidence, but these effects were smaller and did not survive Bonferroni correction for multiple comparisons. Some subjective experiences were significantly and negatively related to 12-week prolonged abstinence, particularly sadness and anxiety. Other ratings (anger, quit-induced craving, confidence) had smaller associations with abstinence that did not survive corrections for multiple comparisons. Some subjective experiences (anger, anxiety) were both affected by EMA frequency and predictive of prolongs abstinence, and appeared to mediate monitoring effects on abstinence at conventional alpha levels, but not at a conservative alpha level.
We failed to detect direct effects of EMA use on abstinence, as in previous research [10], and the effects appear quite small (NNT 16–126, quite large compared to first-line or even minimal interventions [30–31]). Despite the lack of direct effects of EMA frequency on abstinence, we detected mediated effect whereby EMA frequency influenced prolonged abstinence by reducing anxiety and anger (at p<.05, although these were not significant at p<.006). Although it is important to protect family-wise error, this alpha correction may be too conservative at this early, exploratory stage of inquiry into subjective reactivity and its role in cessation. All the results that met conventional, but not Bonferroni-corrected significance were robust to inclusion of covariates and examination of only on-time reports, with the exception of pre-quit craving and confidence. Although this robustness does not rule out effects occurring due to chance, it does suggest that these effects are worthy of future investigation, particularly since there was partial support (significant a or b paths, even at p<.006) for the importance of all three negative emotions assessed (anger, anxiety, and sadness).
Some effects of EMA frequency on subjective experiences seemed favorable (i.e., lower craving), but some seemed unfavorable (i.e., lower positive affect and a possible small drop in confidence post quit). This may explain why we did not see a direct effect of EMA condition on later abstinence. Perhaps there is an additive mix of favorable and unfavorable EMA effects that yield a null net effect on abstinence. Despite this, some of these changes predicted later abstinence, and anxiety and anger mediated EMA frequency effects on abstinence,
The current results and those of Rowan et al. [10] suggest that EMA reactivity effects are not consistent over time (pre vs. post-quit) or across variables. In the current study, EMA reactivity effects for all but confidence were present as soon as monitoring began. The lack of EMA frequency effects thereafter suggest that reactivity happens upon EMA commencement and do not reverse or decay during monitoring. It is possible that attrition over the course of 3 weeks post-quit reduced our ability to detect later frequency effects, however.
The current results have implications for clinical and EMA research. These results add to the growing evidence that self-monitoring systematically changes subjective experiences [9–10]. Frequent self-monitoring may have therapeutic consequences. Future research may benefit from examining mechanisms by which more frequent monitoring may reduce withdrawal, or rare recording may exacerbate withdrawal. For example, greater awareness of withdrawal through self-monitoring may increase coping. Alternatively, rare, unexpected prompts to monitor may induce stronger reactivity than frequent monitoring. The differences observed as a function of EMA frequency suggest that assessments of key constructs may be distorted (suppressed by frequent responding, exaggerated by rare responding, or both). More work is needed to identify the optimal assessment frequency and duration.
Results of this study must be interpreted with the following limitations in mind. First, our results speak only to the effects of frequency of monitoring smoking-related constructs and do not speak to possible reactivity to carrying a monitoring device or engaging in any self-focused monitoring (a control condition in which people completed monitoring of other, neutral targets would be needed to determine the degree to which the reactivity observed here is specific to tobacco-related constructs). In addition, several of the effects of EMA monitoring, including the mediated effects through anger and anxiety reductions, were not be significant when we applied a Bonferroni correction for the number of candidate mediators examined. The family-wise alpha for the uncorrected tests may well exceed 0.05. In addition, unadjusted (i.e., non-pro-rated) adherence rates were lower than the 80% rate recommended [32] and missingness may not be random (e.g., people may be least likely to respond when distressed or unmotivated). Adherence rates were not significantly different in the two conditions, however. Providing incentives for responding or making adherence a criterion for retention might have improved adherence. In contrast, timeliness of reports was unequal across conditions and suggested that some low-frequency subjects were not carrying their EMA device consistently. Late reports may differ systematically from on-time reports and bias results, although we found the same pattern of significant treatment effects in all instances (except for change in confidence pre- to post-quit) when analyzing only on-time reports. In addition, frequent prompting may have induced unique biases (e.g., toward consistency) that once-daily prompting did not. If any such biases contributed to results, they seem to have done so in the first days of recording, as EMA condition appeared to have immediate and lasting effects. Finally, the sample comprised treatment-seeking, heavy daily smokers. Results may differ in other populations of smokers, particularly non-daily smokers or those not engaged in quitting.
Conclusions
This study adds to the literature suggesting that the frequency of momentary self-monitoring may alter experiences. Frequent self-monitoring appears to suppress affect and this may support smoking cessation efforts. Collection of intensive longitudinal data from subjects engaged in behavior change may be an intervention that alters experiences in some ways that promote change. There has been rapid growth in the availability and adoption of mobile assessment tools that may influence the negative affect that predicts difficulty in changing behavior.
Supplementary Material
Table 3.
Logistic regression models predicting 12-week prolonged abstinence from empirical Bayes estimates of pre- and post-quit intercepts in subjective ratings.
| Predictor | B (SE) | OR | 95% CI | |
|---|---|---|---|---|
| Craving to smoke (n=94) | ||||
| Pre-quit intercept | −.583 (.271) | .558 | .328–.949a | |
| Change in pre-post quit average | −.643 (.317) | .526 | .282–.978 | |
| Sadness (n=94) | ||||
| Pre-quit intercept | −1.590 (.576) | .204 | .066–.630* | |
| Change in pre-post quit average | −.444 (.796) | .641 | .135–3.051 | |
| Anxiety (n=94) | ||||
| Pre-quit intercept | −1.161 (.414) | .313 | .139–.705* | |
| Change in pre-post quit average | −.522 (.541) | .593 | .205–1.714 | |
| Anger (n=94) | ||||
| Pre-quit intercept | −1.007 (.477) | .365 | .143–.931 | |
| Change in pre-post quit average | −.646 (.719) | .524 | .128–2.145 | |
| Hunger (n=94) | ||||
| Pre-quit intercept | −.587 (.383) | .556 | .262–1.178 | |
| Change in pre-post quit average | .943 (.612) | 2.567 | .774–8.516 | |
| Positive Affect (n=94) | ||||
| Pre-quit intercept | .107 (.229) | 1.113 | .711–1.743 | |
| Change in pre-post quit average | .918 (.674) | 2.503 | .668–9.375 | |
| Motivation to quit (n=93) | ||||
| Pre-quit intercept | .335 (.268) | 1.398 | .826–2.365 | |
| Change in pre-post quit average | .467 (.357) | 1.596 | .792–3.214 | |
| Quitting Confidence (n=93) | ||||
| Pre-quit intercept | .805 (.297) | 2.237 | 1.249–4.008 | |
| Change in pre-post quit average | .724 (.396) | 2.063 | .950–4.480 | |
Note Bolded values retained significance when covariates were included
significant at Bonferroni-corrected p <.006 for 8 candidate mediators
Non-significant at p<0.05 when entered as only predictor (without empirical Bayes’ estimate of post-quit intercept) or with baseline covariates.
Acknowledgments
This work was supported by the Charles and Johanna Busch Memorial Fund from Rutgers, The State University of New Jersey and National Institute on Drug Abuse Grants and 1RC1DA028129-01 and 1R21DA026511-01A1 awarded to Dr. McCarthy. The authors wish to thank the staff of the Rutgers University Smoking Cessation Laboratory for their assistance with this project.
Footnotes
Declaration of Interests: GlaxoSmithKline provided nicotine lozenges used in this study at a discounted clinical research price. GlaxoSmithKline was not involved in the design, data collection, analysis, or reporting of this study.
References
- 1.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Ann Behav Med. 1994;16:199–206. [Google Scholar]
- 2.Shiffman S. Ecological Momentary Assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- 4.Epstein EE, Drapkin ML, Yusko DA, Cook SM, McCrady BS, Jensen NK. Is alcohol assessment therapeutic? Pretreatment change in drinking among alcohol-dependent women. J Stud Alcohol. 2005;66:36–378. doi: 10.15288/jsa.2005.66.369. [DOI] [PubMed] [Google Scholar]
- 5.Clifford PR, Maisto SA, Davis CM. Alcohol treatment research assessment exposure subject reactivity effects: Part I. Alcohol use and related consequences. J Stud Alcohol Drugs. 2007;68:519–528. doi: 10.15288/jsad.2007.68.519. [DOI] [PubMed] [Google Scholar]
- 6.Abrams DB, Wilson GT. Self-monitoring and reactivity in the modification of cigarette smoking. J Consult Clin Psychol. 1979;47:243–251. doi: 10.1037//0022-006x.47.2.243. [DOI] [PubMed] [Google Scholar]
- 7.McFall RM. Effects of self-monitoring on normal smoking behavior. J Consult Clin Psychol. 1970;35:135–142. doi: 10.1037/h0030087. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrandt T, Latner J. Effect of self-monitoring on binge eating: Treatment response or ‘binge drift’? Eur Eat Disord Rev. 2006;14:17–22. [Google Scholar]
- 9.Magnan RE, Koblitz AR, McCaul KD, Dillard AJ. Self-monitoring effects of ecological momentary assessment on smokers’ perceived risk and worry. Psychol Assess. 2013;25:416–423. doi: 10.1037/a0031232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowan PJ, Cofta-Woerpel L, Mazas CA, Irvin Vidrine J, Reitzel LR, Cinciripini PM, Wetter DW. Evaluating reactivity to Ecological Momentary Assessment during smoking cessation. Exp Clin Psychopharm. 2007;15:382–389. doi: 10.1037/1064-1297.15.4.382. [DOI] [PubMed] [Google Scholar]
- 11.Shiffman S, Paty JA, Gnys M, Kassel JD, Hickcox M. First lapses to smoking: Within-subjects analysis of real time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman S, Balabanis MH, Paty JA, Engberg J, Gwaltney CJ, Liu KS, Gnys M, Hickcox M, Paton SM. Dynamic effects of self-efficacy on smoking lapse and relapse. Health Psychol. 2000;19:315–323. doi: 10.1037//0278-6133.19.4.315. [DOI] [PubMed] [Google Scholar]
- 13.Shiffman S, Engberg J, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: Predicting smoking lapses from daily urge. J Abnormal Psychol. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: An electronic diary study. J Abnormal Psychol. 2006;115:454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- 15.Cruise CE, Broderick J, Porter L, Kaell A, Stone AA. Reactive effects of diary self-assessment in chronic pain patients. Pain. 1996;67:253–258. doi: 10.1016/0304-3959(96)03125-9. [DOI] [PubMed] [Google Scholar]
- 16.Hufford MR, Shields AL, Shiffman S, Paty J, Balabanis M. Reactivity to ecological momentary assessment: An example using undergraduate problem drinkers. Psychol Addict Behav. 2002;16:205–211. [PubMed] [Google Scholar]
- 17.Stone AA, Broderick JE, Schwartz JE, Shiffman S, Litcher-Kelly L, Calvanese P. Intensive momentary reporting of pain with an electronic diary: Reactivity, compliance, and patient satisfaction. Pain. 2003;104:343–351. doi: 10.1016/s0304-3959(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 18.Simpson TL, Kivlahan DR, Bush KR, McFall ME. Telephone self-monitoring among alcohol use disorder patients in early recovery: A randomized study of feasibility and measurement reactivity. Drug Alcohol Depend. 2005;79:241–250. doi: 10.1016/j.drugalcdep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Litt MD, Cooney NL, Morse P. Ecological Momentary Assessment (EMA) with treated alcoholics: Methodological problems and potential solutions. Health Psychol. 1998;17:48–52. doi: 10.1037//0278-6133.17.1.48. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion SR treatment for smoking cessation. Addiction. 2008;103:1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E. GPOWER: A priori-, post hoc-, and compromise power analyses for MS-DOS. Bonn, Germany: Bonn University; 1992. [Computer program] [Google Scholar]
- 22.Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. J Educ Behav Statistics. 1999;24:70–93. [Google Scholar]
- 23.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. Available at http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf (Accessed June 27, 2014). (Archived at http://www.webcitation.org/6RPLPpIIs 38 July 2014) [Google Scholar]
- 24.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 25.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharm. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 26.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28.Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] Skokie, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- 29.Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. (Available at http://www.amp.gatech.edu/RMediation). (Accessed April 18, 2014). (Archived at http://www.webcitation.org/6RPLXYgvp 28 July 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmaotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011;79:34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes JR. A quantitative estimate of the clinical significance of treatment tobacco dependence. Am J Prev Med. 2011;39:285–286. doi: 10.1016/j.amepre.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone AA, Shiffman S. Capturing momentary, self-report data: a proposal for reporting guideliens. Ann Behav Med. 2002;24:236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.