SUMMARY
Defective Hippo/YAP signaling in the liver results in tissue overgrowth and development of hepatocellular carcinoma (HCC). Here, we uncover mechanisms of YAP-mediated hepatocyte reprogramming and HCC pathogenesis. YAP functions as a rheostat maintaining metabolic specialization, differentiation and quiescence within the hepatocyte compartment. Increased or decreased YAP activity reprograms subsets of hepatocytes to different fates associated with deregulation of the HNF4A, CTNNB1, and E2F transcriptional programs controlling hepatocyte quiescence and differentiation. Importantly, treatment with siRNA-lipid nanoparticles (siRNA-LNPs) targeting YAP restores hepatocyte differentiation and causes pronounced tumor regression in a genetically engineered mouse HCC model. Furthermore, YAP targets are enriched in an aggressive human HCC subtype characterized by a proliferative signature and absence of CTNNB1 mutations. Thus, our work reveals Hippo signaling as a key regulator of positional identity of hepatocytes, supports targeting YAP using siRNA-LNPs as a paradigm of differentiation-based therapy, and identifies an HCC subtype potentially responsive to this approach.
Keywords: Hippo pathway, hepatocellular carcinoma, differentiation therapy, siRNA lipid nanoparticles, YAP, liver differentiation, liver zonation
Introduction
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death worldwide, and the incidence is increasing in the United States (El-Serag, 2011; Forner et al., 2012). Curative resection or transplantation is only possible in ~30% of patients, and for advanced HCC, the multi-kinase inhibitor, sorafenib, remains the only approved agent with modest survival benefits (Cheng et al., 2009; Llovet et al., 2008). Genomic studies have classified HCC into subsets with distinct molecular and clinical features (Chiang et al., 2008; Guichard et al., 2012; Hoshida et al., 2009; Lachenmayer et al., 2012). Activating CTNNB1 mutations are the most common oncogenic drivers, present in ~30% of tumors, and define an HCC subtype characterized by a hepatocyte-like gene expression signature, well-differentiated histology, and improved survival. The other recurrent genetic alterations in HCC involve tumor suppressor inactivation (Fujimoto et al., 2012; Guichard et al., 2012) and have not pointed to readily targetable vulnerabilities. Thus, there is a need to more fully discern biologically distinct HCC subtypes and to identify therapeutic targets.
Hippo signaling is an important tumor suppressor mechanism in multiple tissues including the liver (Harvey et al., 2013; Mo et al., 2014). The core of the pathway involves a kinase cascade with the homologous MST1 and MST2 (STK3, STK4) proteins as upstream kinases that subsequently activate the LATS1/LATS2 kinases (Yu and Guan, 2013). Signaling inputs include G-protein coupled receptors, Rho GTPases, AMPK, and polarity complex proteins including NF2, AMOT, and α-CATENIN, which link Hippo activity to a variety of cues, including cell contact, mitotic failure, nutrients and growth factors (Ganem et al., 2014; Yu and Guan, 2013). Major downstream targets are the transcriptional co-activators, YAP and TAZ, which are phosphorylated by LATS1/LATS2, leading to their cytoplasmic retention and degradation. YAP and TAZ promote growth, survival and/or stem cell-like phenotypes in many cellular contexts (Mo et al., 2014), although the key mediators of these processes are not clearly established.
In the adult mouse liver, inactivation of Mst1/Mst2, Sav1 or Nf2, or ectopic expression of Yap all result in expansion of atypical ductal cells (ADCs, or “oval cells”), liver enlargement and progression to HCC or mixed HCC/cholangiocarcinoma; Mst1/Mst2 KO or Yap overexpression also cause hepatocyte overproliferation (Camargo et al., 2007; Dong et al., 2007; Lee et al., 2010; Lu et al., 2010; Yimlamai et al., 2014; Zender et al., 2006; Zhou et al., 2009). Lineage tracing in Yap transgenic and Nf2 KO livers indicate that the ADCs are derived from de-differentiation of hepatocytes (Yimlamai et al., 2014), consistent with emerging data demonstrating plasticity of these cells (Schaub et al., 2014; Yanger et al., 2014), and pointing to important functions of Hippo/YAP in controlling liver cell fate. Consistent with a role for activated YAP in human HCC, ~50% of these tumors exhibit nuclear YAP staining, and nuclear YAP levels correlate with decreased survival after resection (Tschaharganeh et al., 2013; Xu et al., 2009).
Here we sought to explore the role of YAP in HCC progression and maintenance in order to define its downstream oncogenic program, assess its potential as a therapeutic target, and establish biomarkers identifying the subsets of HCC where YAP targeting may be beneficial. By using genetic approaches and siRNA-lipid nanoparticle (siRNA-LNP) technology in genetically engineered mouse (GEM) models, we demonstrate that activation of endogenous YAP perturbs hepatocyte differentiation and maintains this state in advanced tumors. Correspondingly, YAP silencing in HCC restores hepatocyte differentiation and leads to tumor regression; this offers proof-of-concept that differentiation therapy has potential as a treatment strategy in an epithelial tumor. Moreover, the significant responses induced by siYAP-LNPs support ongoing clinical development of this therapeutic modality. Finally, our work shows that an aggressive subtype of human HCC — enriched for cell cycle gene expression and lacking CTNNB1 mutations — exhibits a YAP activation signature, pointing to biomarkers to guide the deployment of YAP-directed therapies in clinical trials.
Results
Role of YAP downstream of MST1/MST2 in the control of hepatocyte identity
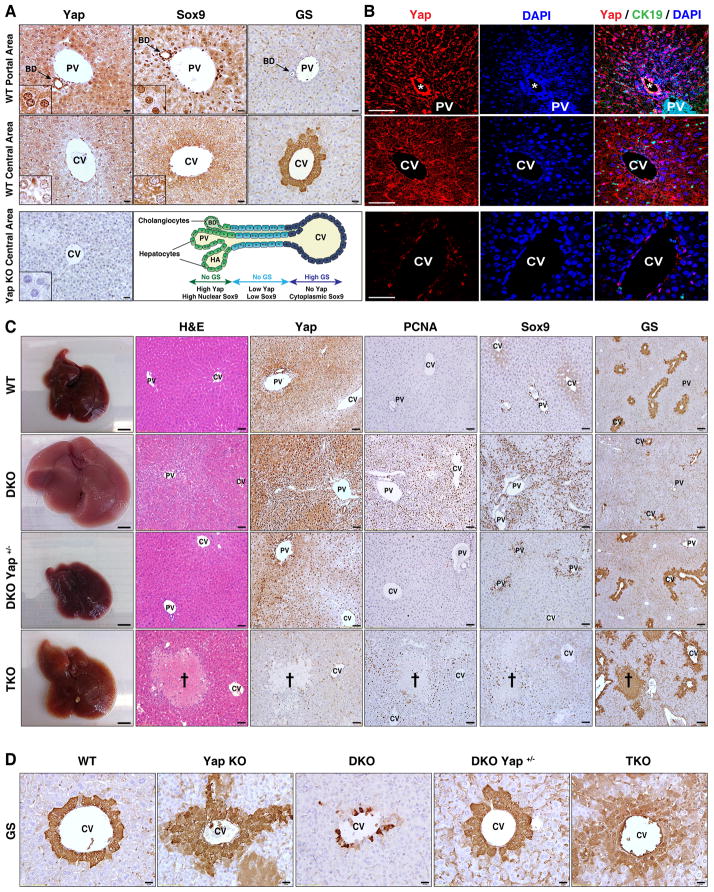
To begin to explore the relationship between Hippo pathway activity and liver cell differentiation, we examined Yap expression in normal mouse liver. The bile ducts showed strong nuclear YAP staining (Fig. 1A,B), consistent with prior observations (Yimlamai et al., 2014). We also observed a graded staining pattern in the hepatocyte compartment, with the periportal hepatocytes (adjacent to the bile duct) showing readily detectable Yap nuclear and cytoplasmic localization, and with pericentral hepatocytes (adjacent to the central vein) showing clear nuclear exclusion. SOX9 — like YAP, a marker of the bile ducts and liver cell plasticity (Antoniou et al., 2009; Shin et al., 2011; Yanger et al., 2013) — exhibited a similar expression pattern in hepatocytes along the porto-central axis (Fig. 1A, S1A). This staining gradient is suggestive of a role for YAP in the zonation of hepatocytes, which have specialized metabolic functions depending on their position in the liver lobule (Jungermann and Katz, 1989). The zonation program is thought to be governed by the interplay of the HNF4A and WNT/β-CATENIN pathways and is characterized by strict boundaries of expression of key metabolic genes in the pericentral region and periportal regions (e.g. glutamine synthase and glutaminase 2, respectively) (Benhamouche et al., 2006; Gougelet et al., 2014; Torre et al., 2011).
Figure 1. Hippo signaling is a rheostat controlling hepatocyte identity.
A, B. Immunohistochemistry (A) and immunofluorescence (B) of mouse liver in the region of the portal vein (top row) and central vein (middle row) demonstrating nuclear and cytoplasmic YAP staining in bile ducts (arrow) and periportal hepatocytes, and absence of YAP in the first layer of pericentral hepatocytes. SOX9 shows nuclear staining in bile ducts and weaker nuclear staining in periportal hepatocytes, whereas it is excluded from the nucleus in pericentral hepatocytes. GS is a pericentral marker. CK19 is a biliary marker. The schematic representation of the porto-central axis indicates the territories of expression for YAP, SOX9 and GS. Yap KO liver (bottom row) is a negative control for YAP staining. Insets show magnified periportal or pericentral hepatocytes in (A). Bile duct is indicated by an asterisk in (B). Scale bars: 20μm (A) and 50μm (B).
C. Gross, histologic and IHC analysis of livers of the indicated genotypes (Albumin-Cre models). Cross: necrotic region in TKO. Scale bars: 5mm (gross), 50μm (H&E, YAP, PCNA, SOX9) and 100 μm (GS).
D. GS staining in pericentral regions. Scale bar: 20μm.
PV: Portal vein, CV: Central vein, BD: Bile duct, HA: Hepatic artery.
See also Figure S1.
To study the impact of Hippo pathway modulation on liver cell identity and proliferation, we generated mice with liver-specific Mst1/Mst2 double-knockout (DKO), Yap deletion (Yap KO), and DKO combined with hemizygous or homozygous Yap deletion (designated DKO Yap+/− and TKO (triple-knockout) mice, respectively) via crosses of floxed strains to Albumin-Cre mice. Necropsy at 7 weeks revealed that DKO mice had massive liver overgrowth and high levels of hepatocyte proliferation, associated with upregulation of nuclear and total Yap levels throughout the hepatocyte compartment and marked expansion of SOX9+ and SOX9− ADCs in the periportal region (Fig. 1C, S1B,C), as previously reported in other Hippo pathway mutant mouse models (Benhamouche et al., 2010; Lee et al., 2010; Lu et al., 2010; Yimlamai et al., 2014). In addition, DKO animals exhibited a striking loss of hepatocyte zonation as reflected by decreased pericentral expression of glutamine synthase (GS) and ornithine aminotransferase (OAT) (Fig. 1C,D, S1D). Each abnormality was rescued in DKO Yap+/− and TKO mice, although the latter exhibited biliary defects, regional hepatocyte necrosis, cholestasis, fibrosis and swelling of the tissue as observed in Yap KO livers (Zhang et al., 2010). Notably, in Yap KO mice, we found that there was expansion of the GS-positive domain, whereby several layers of hepatocytes surrounding the central vein as well as scattered cells throughout the lobule showed aberrant induction of GS (Fig. 1D). These findings link aberrantly activated YAP to hepatocyte reprogramming downstream of MST1/MST2 and reveal an unexpected role of basal YAP function in maintenance of hepatocyte zonation.
Despite the ongoing expansion of ADCs, proliferating cells with hepatocyte morphology persist in the DKO liver and exhibit a higher frequency of PCNA staining than adjacent ADCs (Fig. S1C). Together the data imply that the response of hepatocytes to activation of endogenous YAP is distinct depending on their zonal location, creating a highly proliferative population of dedifferentiated, (GS-negative) pericentral hepatocytes as well as an expanded pool of ADCs emerging from periportal hepatocytes.
Acute deregulation of the Hippo pathway overrides hepatocyte zonation
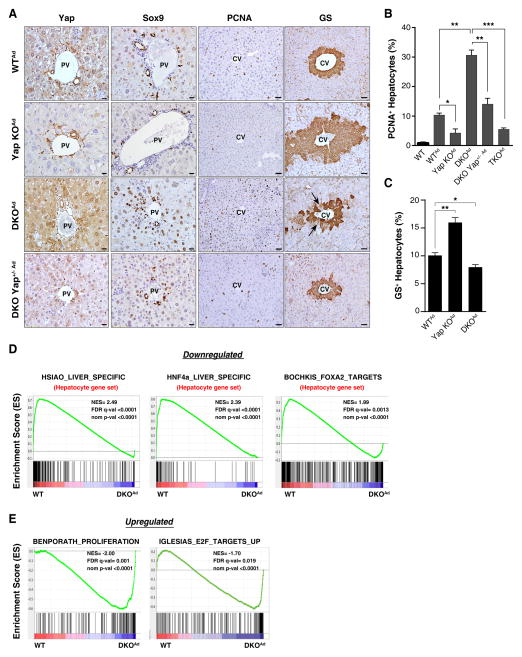
These phenotypes were recapitulated in the setting of acute deregulation of the Hippo pathway using intravenous injection of Cre adenovirus to conditionally delete Mst1/Mst2 and Yap in adult hepatocytes (designated as DKOAd, TKOAd and Yap KOAd models). Examination at 2 weeks post-injection revealed that DKOAd mice had increased hepatocyte proliferation, ADC expansion, and reduced pericentral GS expression compared to WTAd livers (Fig. 2A–C). These changes were rescued by Yap ablation (TKOAd and DKO Yap+/−Ad models) and moreover, Yap KOAd mice exhibited expansion of the GS-positive domain (10.1+/−0.5% of hepatocytes were GS+ in WTAd livers, versus 16.0+/−0.9% in Yap KOAd, and 8.0+/−0.5% in DKOAd) (Fig. 2C and see Methods). Thus, YAP serves as a rheostat for maintenance of quiescence and identity in adult hepatocytes. In particular, activation of YAP overrides the pericentral zonation program and also directs periportal hepatocytes to dedifferentiate to ADCs, whereas basal levels of Yap are required to prevent the aberrant induction of a pericentral phenotype in multiple layers of hepatocytes surrounding the central vein.
Figure 2. Acute effect of Hippo pathway modulation on hepatocyte identity.
A–C. Mice were administered Cre adenovirus intravenously and livers were analyzed 2 weeks later by IHC for YAP, SOX9, PCNA and GS. The graphs quantify PCNA (B) and GS (C) staining. N= 3 mice in the WT, WTAd, Yap KOAd, TKOAd groups; 4 mice in the DKO Yap+/− Ad group and 5 mice in the DKOAd group. Error bars indicate S.E.M. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Scale bars: 20μm (YAP, SOX9) and 50μm (PCNA, GS).
D, E. GSEA of mRNA expression data after acute deletion of Mst1/Mst2 in liver. DKO and WT mice were administered Adeno-Cre and liver mRNA was isolated after 8 days. D. GSEA revealed rapid loss of a broad program of hepatocyte differentiation genes and of targets of the master hepatocyte regulators, HNF4A and FOXA2. E) GSEA showing strong induction of a proliferation gene signature including established E2F targets. Liver-derived gene sets are indicated in red. NES: Normalized enrichment score; FDR: False discovery rate. Gene sets are from the Molecular Signatures Database except (HNF4a_LIVER_SPECIFIC) which is a curated list of liver-specific HNF4A targets (Saha et al., 2014).
We conducted gene expression profiling at day 8 following Ad-Cre administration to determine the early transcriptional changes provoked by Mst1/Mst2 inactivation (WTAd versus DKOAd). Gene Set Enrichment Analysis (GSEA) revealed that acute Mst1/Mst2 DKO caused potent inactivation of the hepatocyte differentiation program, with downregulation of hundreds of hepatocyte-specific genes (Fig. 2D). Accordingly, the set of downregulated genes was highly enriched for established targets of the master regulators of hepatocyte identity, HNF4A and FOXA2. Conversely, there was upregulation of cell proliferation signatures enriched for E2F target genes (Fig. 2E). Experimentally determined binding sites for TEAD/TEF-1 were significantly enriched in the promoter elements of the differentially expressed genes (p<2.0 × 10−14) as was the consensus TEAD/TEF1 binding motif (Fig. S2A), consistent with TEAD family proteins being the key partners for YAP in the liver (Liu-Chittenden et al., 2012). We identified candidate direct YAP targets by integrating the expression analysis and chromatin immunoprecipitation data for TEAD binding in hepatic cells (HepG2 cells, ENCODE database)(Fig. S2B, Table S1). This gene set was highly enriched for predicted targets of HNF4A and FOXA1, which are master regulators of hepatocyte differentiation that cooperatively regulate many common targets (Fig. S2B)(Alder et al., 2014; Bochkis et al., 2012; Li et al., 2012). Notably, YAP has been shown to act as a switch for enhancer occupancy of HNF4A and FOXA2 in the liver, preventing expression of mature hepatocyte genes and promoting expression of liver stem cell (hepatoblast) genes (Alder et al., 2014). Moreover, deletion of Hnf4a in the adult liver results in hepatocyte dedifferentiation and the emergence of SOX9+ ADCs (Saha et al., 2014; Walesky et al., 2013). These observations suggest a potential mechanism involving interplay between activated YAP and an HNF4A/FOXA2/FOXA1 module for the control of hepatocyte identity and quiescence in DKO mice.
Yap status dictates HCC cell differentiation phenotypes downstream of Hippo inactivation
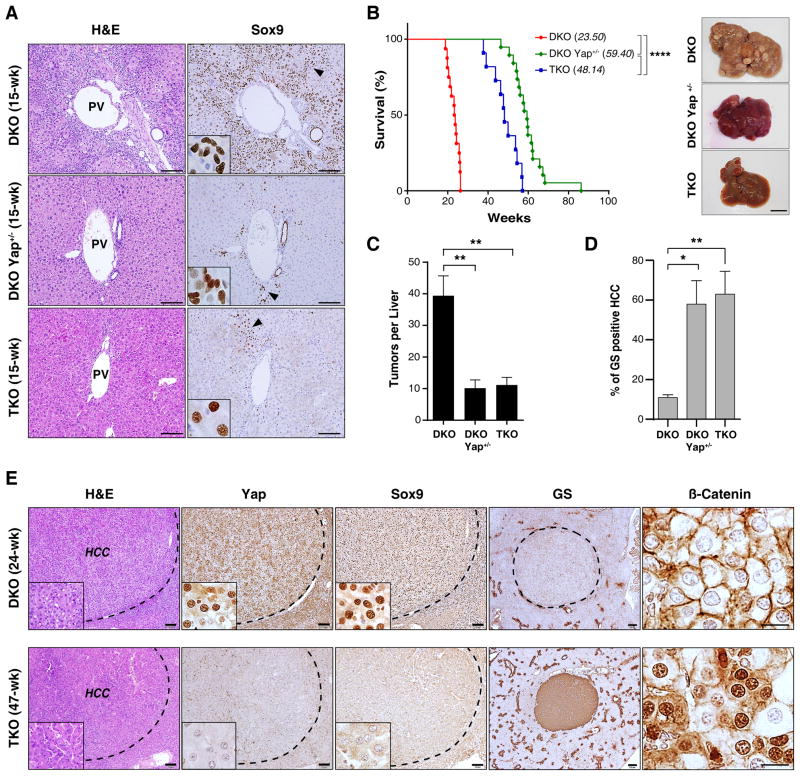
We next sought to assess the effects of YAP deficiency on the tumor phenotype caused by Mst1/Mst2 DKO (Lu et al., 2010; Zhou et al., 2009). To this end, mice were either necropsied at serial time points or monitored until signs of poor body condition necessitated euthanasia, which in all cases was due the presence of liver tumors. At 15 weeks, DKO mice had rampant ADC expansion, whereas DKO Yap+/− and TKO livers showed only focal appearance of hepatocyte-like cells staining strongly for SOX9, but limited or no cells with ADC morphology (Fig. 3A). In addition, TKO and DKO Yap+/− mice had a marked extension in survival as compared to DKO animals (48.1 and 59.4 weeks, versus 23.5 weeks, respectively), and had reduced tumor burden (TKO 11 tumors/mouse; DKO Yap+/− 10 tumors/mouse, DKO 39 tumors/mouse) (Fig. 3B,C). Immunohistochemistry confirmed that TKO tumors lacked Yap expression indicating that they did not emerge from rare cells that escaped Yap deletion (Fig. 3E, lower panel). Thus, Yap has a central role in tumorigenesis in the DKO model and even single copy Yap ablation greatly delays HCC progression. On the other hand, observations from TKO mice demonstrate that Mst1/Mst2 inactivation can still ultimately drive liver tumorigenesis through a YAP-independent, albeit less potent, program.
Figure 3. YAP suppresses the β-CATENIN pericentral program and drives invasive HCC downstream of Mst1/Mst2 DKO.
A. Analysis of the indicated genotypes by H&E and SOX9 staining at 15 weeks. The dedifferentiation of hepatocytes to ADCs caused by Mst1/Mst2 DKO is blocked by hemizygous or homozygous Yap deletion, although scattered SOX9-positive cells are observed by this time point. Insets: High magnification images of regions denoted by arrowheads in the main panels. Note differences in the morphology of SOX9-positive cells between genotypes. Scale bar: 100 μm
B. Left panel: Kaplan-Meier analysis showing time until animals reached endpoints for disease burden requiring euthanasia (see Methods). Median survival is indicated in brackets. N= 16 mice (DKO), 19 mice (DKO Yap+/−), 11 (TKO). ****p<0.0001. Right panels: Gross images of representative livers at sacrifice.
C. Mean tumor burden at sacrifice. N= 8 mice (DKO), 7 mice (DKO Yap+/−) and 7 mice (TKO). Error bars indicate S.E.M. **p<0.01.
D, E. Histologic and IHC analysis of tumors from DKO and TKO mice with end-stage disease. GS staining is quantified in (D). N= 4 mice per group. Error bars indicate S.E.M.*p<0.05 and **p<0.01. All tumors showed HCC histopathology, although distinct staining patterns were noted for liver markers. DKO tumors (E, top) stained positive for SOX9 and negative for GS and nuclear β-catenin, whereas the majority of TKO tumors (E, bottom) showed the opposite pattern. All TKO tumors lacked YAP staining. Scale bars: 20 μm (β-catenin), 100 μm (H&E, YAP, SOX9) and 200 μm (GS).
See also Figure S3.
Histopathologic analysis at end-stage revealed that the tumors in each genotype were invasive HCC (Fig. 3E). However, the groups expressed distinct sets of differentiation markers, with the DKO HCCs staining for SOX9 and lacking both GS and nuclear β-CATENIN, and the great majority of TKO and DKO Yap+/− tumors showing the opposite profile (Fig. 3D,E). The phenotypes caused by liver-specific inactivation of Nf2, which are partially due to YAP activation, overlap those in DKO mice (Benhamouche et al., 2010; Yin et al., 2013; Zhang et al., 2010). Examination of Nf2 KO HCCs revealed heterogeneous YAP staining in sharply demarcated regions. Those with higher nuclear and cytoplasmic YAP expressed SOX9 and lacked GS, whereas those with lower levels of YAP staining showed a reciprocal pattern (Fig. S3A), again correlating YAP levels and markers of HCC differentiation state. Together, these findings suggest a link between YAP-dependent hepatocyte reprogramming and HCC initiation, and connect YAP activity to the specific malignant phenotype of the ensuing tumors.
Therapeutic efficacy of siRNA-lipid nanoparticles targeting YAP in advanced HCC
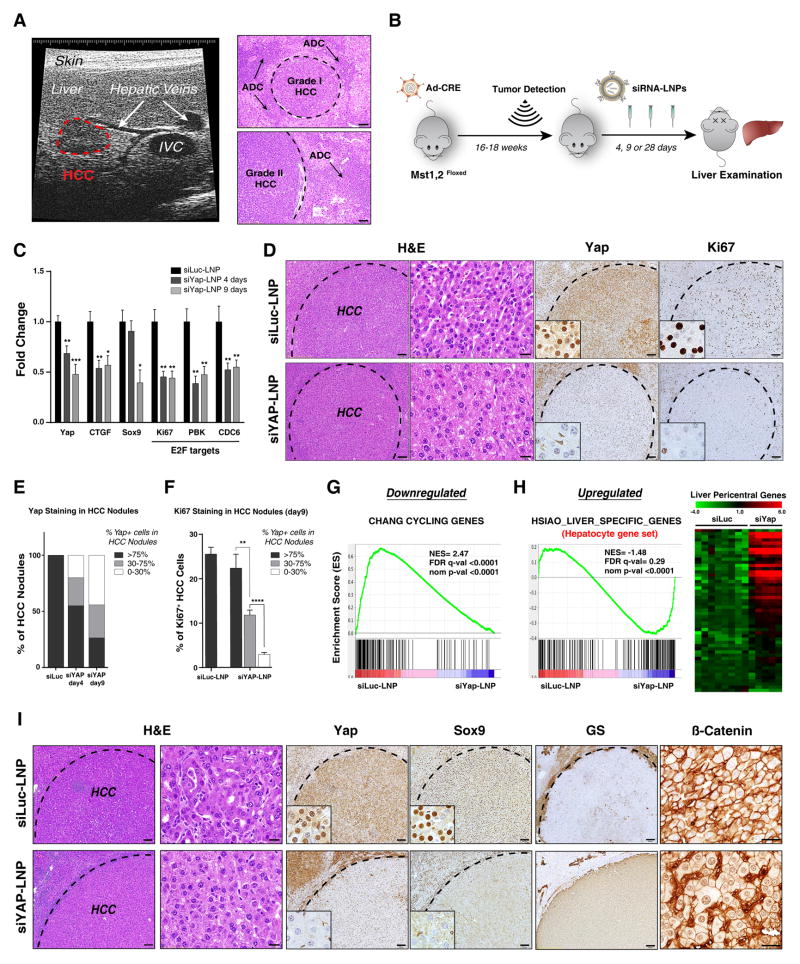
We next explored the functions of YAP in the clinically relevant setting of maintenance of established tumors. In this regard, the use of siRNA encapsulated into lipid nanoparticles (siLNPs) — which improves cellular uptake, stability and pharmacokinetics of the siRNA (Whitehead et al., 2009) — is an approach to YAP targeting with clinical potential. Primary HCC is a particularly plausible cancer context for siRNA-LNP use since the dual blood supply and fenestrated endothelium in the liver lead to high perfusion, which may facilitate siRNA-LNP delivery. To test this strategy, we developed protocols to monitor tumor development in the DKOAd model by ultrasound (US) imaging. Pilot studies with serial imaging followed by necropsy provided histologic verification that the lesions detected by US were invasive HCC (Fig. 4A). Subsequently, an additional cohort was imaged, and animals with comparable tumor burden were randomized to receive tail vein injections of siLNPs targeting YAP or Luciferase (Luc) control (Fig. 4B, also see Methods). Mice were euthanized after 4 and 9 days to assess the acute responses. qRT-PCR and IHC revealed progressive YAP knockdown by siYap-LNPs (Fig. 4C–E, S4A). At day 4, IHC showed that 45% of tumor nodules had partial or complete reduction in YAP protein (Fig. 4E, S4A) (defined as % of HCC cells within the nodule staining above background; see Fig. S4B and Methods), and by day 9 this figure increased to 73.5% of nodules (29.4% partial, 44.1% complete) (Fig. 4D,E). We also observed coordinate reduction in expression of Ctgf, an established YAP-TEAD target, as well as of a series of E2F targets (Fig. 4C).
Figure 4. siLNP-mediated YAP silencing induces proliferative arrest and a pericentral hepatocyte differentiation program in advanced HCC.
A. Ultrasound (US) detection of a liver tumor in DKOAd at 16 weeks after Adeno-Cre injection. The right panel shows corresponding histology of liver tumors. Interior vena cava (IVC). Scale bar: 100 μm.
B. Schematic of therapeutic study. Upon detection of tumors by US imaging, animals were randomized into groups receiving siYAP-LNPs or control siLuc-LNPs and euthanized at the indicated time points.
C. qRT-PCR analysis of tumors isolated after 4 or 9 days treatment. N= 32 tumors (siLuc), 18 tumors (siYAP 4 days) and 16 tumors (siYAP 9 days). Error bars indicate S.E.M. *p<0.05, **p<0.01, ***p<0.001.
D. Representative IHC staining for YAP and KI67 in HCCs following 9 days treatment. Note that at 9 days HCC cells are preferentially targeted by siYAP-LNPs, whereas all liver cells show knockdown at later time points (see Fig. 5C). Scale bars: 20 μm (H&E, right) and 100 μm (H&E left, YAP, KI67).
E. Quantification of YAP staining in HCC nodules at day 4 and 9. The chart shows the % of HCC nodules containing the indicated proportions of YAP-positive tumor cells (black, >75% of cells YAP-positive; grey, 30–75%; white, 0–30%). Note that YAP knockdown efficiency increases from day 4 to day 9.
F. Quantification of KI67 staining at day 9. The chart shows % KI67-positive HCC cells in different tumor nodules grouped according to degree of YAP knockdown. Note that the decrease in KI67 staining correlates with effectiveness of YAP knockdown. siLuc-LNP: N= 20 tumors, from 3 treated mice; siYAP-LNP: N= 34 tumors from 4 treated mice. Error bars indicate S.E.M. **p<0.01, ****p<0.0001.
G, H. GSEA of differentially expressed genes in response to siYAP-LNPs versus control siLuc-LNPs (day 9). G. Cell cycle genes are repressed. H. Left panel. Markers of hepatocyte differentiation are induced. Right panel. Heat map showing induction of markers specific to pericentral hepatocytes (Braeuning et al., 2006). NES: Normalized enrichment score. FDR: False discovery rate.
I. By day 9, siYAP-LNPs result in loss of SOX9 staining, nuclear translocation of β-CATENIN and induction of GS. Scale bar 20 μm (H&E right, β-catenin) and 100 μm (H&E left, YAP, SOX9, GS).
Importantly, siYAP-LNP treatment resulted in a prominent decrease in tumor cell proliferation that was proportional to the degree of knockdown at both day 4 and 9, as determined by KI67 and PCNA staining (Fig. 4D,F, S4A,B). At day 9, 25.5+/−1.6% of HCC cells in siLuc-LNP treated controls stained for proliferation markers, whereas the rates in the siYAP-LNP treated group were 2.9+/−0.4% and 11.8%+/−1.1% in nodules with complete or partial Yap knockdown, respectively (Fig. 4D,F). There was no evidence of apoptosis as determined by cleaved caspase-3 staining (Fig. S4A). These findings were corroborated using a second siLNP targeting a different YAP sequence (siYAP-LNP#2; Fig. S4C) and reveal a critical function of YAP in the proliferation of advanced HCC in this model.
To identify the transcriptional program underlying YAP-mediated tumor maintenance, we conducted RNA-sequencing (RNA-seq) on siLuc- and siYAP-treated tumors following 9 days treatment. GSEA revealed that YAP silencing caused potent inactivation of the E2F/cell proliferation pathway (Fig. 4G, S4D left panel). Reciprocally, there was a pronounced induction of a hepatocyte differentiation signature (Fig. 4H left panel), including activation of program of specific pericentral hepatocyte markers (Fig. 4H right panel), and enrichment of predicted HNF4A targets (Fig. S4D right panel). We integrated the set of genes altered by siYAP-LNP treatment in HCCs with ChIP data for TEAD occupancy in liver cancer cells in order to identify candidate direct YAP/TEAD target genes in these tumors (see Methods). To gain further insight into transcriptional circuits, we then searched this YAP/TEAD signature for enrichment of known binding sites for other transcription factors using the ENCODE database (Fig. S4E, Table S2). In line with our observations in the DKO liver, the YAP/TEAD signature was greatly enriched for HNF4 and FOXA1 binding sites, which were ranked first and second among all transcription factors analyzed, consistent with the interplay between these factors in dictating the balance between tumor maintenance and hepatocyte differentiation (Fig. S4E).
Comparison of the day 4 and day 9 time points indicated that the differentiation markers were gradually induced. At day 4, multiple HCC nodules with patchy staining for GS and nuclear β-CATENIN were observed in siYAP-LNP treated mice, with positively stained regions correlating with areas of more complete YAP knockdown (Fig. S4F). By day 9, uniform staining for both markers was frequently observed, coordinated with loss of SOX9 (Fig. 4I). Thus, YAP inhibition in aggressive HCC causes rapid downregulation of the E2F cell cycle pathway and progressive induction of a hepatocyte differentiation program associated with upregulation of HNF4/FOXA2/FOXA1 targets and increased activity of the pericentral/β-CATENIN program.
siYAP-LNPs induce a hepatocyte differentiation program in advanced HCC
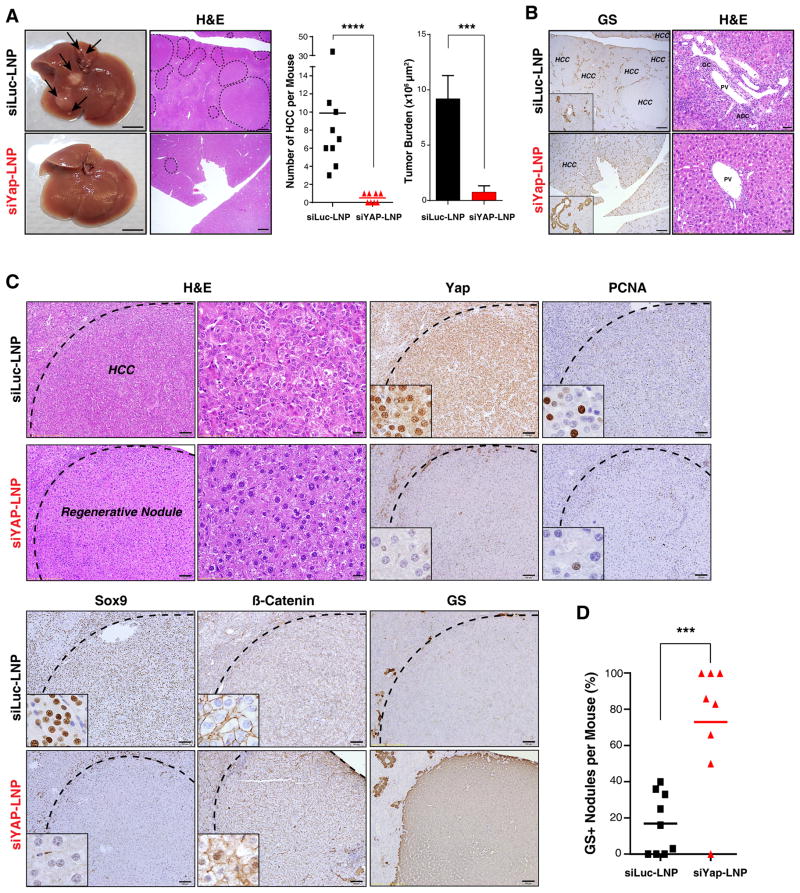
Tumor-bearing mice were treated with siRNA-LNPs for 4 weeks and then euthanized to determine the durability of the response and uncover any gradual consequences for tumor phenotypes. Consistent with the profound anti-proliferative effects seen upon acute YAP knockdown, the siYAP-LNP-treated animals showed a dramatic reduction in tumor burden as compared to controls, with marked decreases in number of HCCs (9.9 vs 0.5) and in their total cross-sectional area (9.2 ×106 μm2 vs 0.75 ×106 μm2) (Fig. 5A). Adjacent regions of liver tissue showed restoration of zonal GS expression and complete elimination of ADCs (Fig. 5B). Remarkably, siYAP-LNP treatment resulted in the replacement of virtually all of invasive HCCs by areas of cells resembling regenerative hepatocyte nodules; in comparison to the HCCs in control animals, these cells had lower nuclear-to-cytoplasmic ratio, lacked mitotic figures, and had a reduction in cell plate thickness (Fig. 5C). Correspondingly, these nodules had low proliferation rates and showed induction of GS and nuclear β-catenin (Fig. 5C,D). Thus, sustained YAP inhibition provoked a broad hepatocyte differentiation program in established tumors.
Figure 5. siLNP-mediated YAP silencing is an effective differentiation therapy in advanced HCC.
Following detection of tumors by US imaging, DKOAd mice were treated with siRNA-LNPs for 28 days and then euthanized. A. Representative gross liver images and H&E stained sections. Arrows: visible liver tumors. Dotted lines: histologically confirmed HCC lesions. The charts show the number of histologically-validated HCC lesions/mouse (left) and tumor area occupied per whole liver cross-section (right; see Methods). N= 9 mice (siLuc-LNP) and 8 mice (siYAP-LNP). Scale bar 5 mm (gross) and 1 mm (H&E). Error bars indicate S.E.M. ***p<0.001, ****p<0.0001. B. The liver parenchyma shows restoration of zonal GS expression and disappearance of ADCs upon siYAP-LNP treatment. HCC lesions are indicated. Scale bar: 1 μm (GS) and 50 μm (H&E) C. siYAP-LNP treatment results in replacement of invasive HCCs by benign nodules with histopathologic features consistent with differentiation to regenerative hepatocytes, associated with loss of SOX9, infrequent PCNA staining, focal induction of nuclear β-CATENIN, and strong upregulation of GS. Scale bars: 100 μm and 20 μm (H&E right panel). D. Chart quantifying the proportion of nodules staining positive for GS. Error bars indicate S.E.M. ***p<0.001.
See also Figure S5.
The rare remaining HCC foci in siYAP-LNP treated animals exhibited persistent YAP expression suggesting that resistance arose either from augmented YAP expression or altered uptake or activity of the siRNA-LNPs (Fig. S5A). Importantly, the treatment regimen was well tolerated, causing no weight loss or significant changes in liver function values, and slight inflammation in periportal areas that was fully resolved within 2–4 weeks following discontinuation of treatment (Fig. S5B–D). These data indicate a central function of YAP in HCC tumor maintenance in the DKO model and support the application of siLNPs targeting YAP to treat advanced HCC. In addition, these studies provide proof-of-concept for differentiation therapy as an oncologic approach in the context of an aggressive epithelial tumor.
YAP-mediated control of proliferative and hepatic differentiation programs in primary tumor cultures
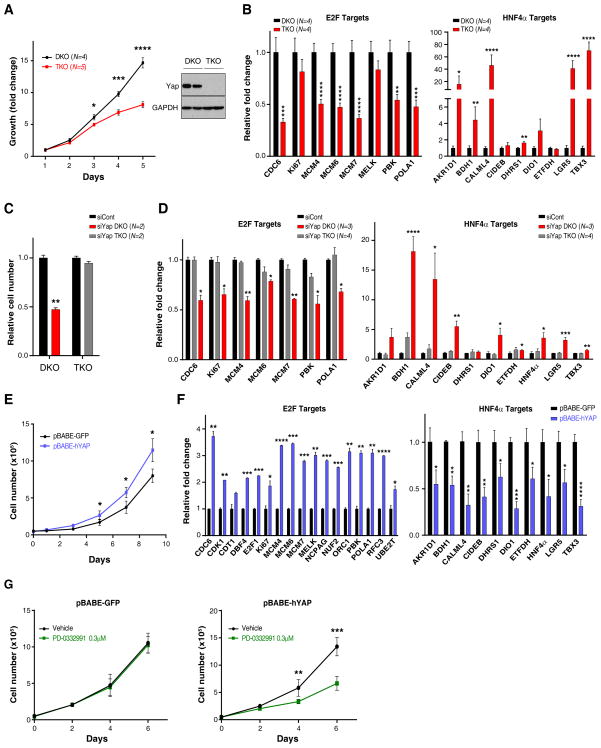
We used a series of early passage HCC tumor cultures derived from independent DKO and TKO mice to examine in vitro the relationship between Yap activity and proliferation and differentiation pathways. Consistent with our in vivo data, DKO tumor cultures grew more rapidly than TKO cultures, and were characterized by higher expression of multiple E2F-regulated cell cycle genes and lower expression of a set of HNF4A targets involved in specialized hepatocyte functions (Fig. 6A,B). Likewise, YAP knockdown in DKO cells caused proliferative arrest without evidence of cell death, whereas TKO cells were unaffected, demonstrating the specificity of the RNAi (Fig. 6C, S6A). The YAP-depleted DKO cells exhibited reduced expression of E2F target genes and pronounced activation of the HNF4A gene signature (Fig. 6D). Reciprocally, forced expression of YAP promoted the proliferation of TKO cultures (referred to as TKO-Yap cells) whereas it either slowed or did not affect the proliferation DKO cells that already harbor high YAP levels (Fig. 6E and data not shown). The TKO-Yap cells showed a corresponding induction of E2F target and suppression of the HNF4A targets (Fig. 6F). Finally, we found that TKO-Yap cells were sensitized to the CDK4 inhibitor, PD-0332991, at concentrations that did not affect TKO-Control cells (Fig. 6G, S6B). Thus, the role of YAP in sustaining proliferation and blocking differentiation is recapitulated in cultured HCC cells and points to direct functional roles for YAP in activating E2F and suppressing HN4A activity in these tumors.
Figure 6. YAP positively regulates an E2F proliferation program and suppresses the Hnf4α hepatocyte differentiation pathway.
A. Left panel: Low passage HCC cultures derived from DKO mice display a proliferative advantage compared to TKO lines. N = number of independent cultures tested in each group. Right panel: Western blot analysis confirming that YAP expression is restricted to the DKO lines. B. qRT-PCR analysis shows that DKO lines are characterized by higher basal expression of E2F targets (left panel) and lower expression of HNF4α targets (right panel). Error bars indicate S.E.M. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
C, D. DKO and TKO cell lines were transfected with siRNA against YAP or control siRNA. C. Quantification of cell growth data showing that siRNA-mediated YAP knockdown causes proliferative arrest of DKO lines but does not affect TKO cells. D. qRT-PCR analysis showing that YAP knockdown in DKO cells results in a significant decrease in the expression of multiple E2F-regulated cell cycle genes (left panel) and pronounced activation of HNF4A targets (right panel). Error bars indicate S.E.M. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
E–G. TKO cells were transduced with retroviruses expressing human YAP (hYAP) or GFP control. E. YAP expression significantly increases the proliferation of TKO cells (left panel). F. Forced expression of YAP induces E2F targets (left panel) and inhibits HNF4A targets (right panel). Error bars indicate S.E.M. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. G. Treatment with a CDK4/6 inhibitor (PD-0332991, 0.3 μM) impairs proliferation of TKO cells expressing YAP (right panel), without affecting GFP-expressing TKO control cells (left panel). Error bars indicate S.E.M. **p<0.01, ***p<0.001.
See also Figure S6.
YAP activation is associated with the proliferative, CTNNB1 wild type subclass of HCC in humans
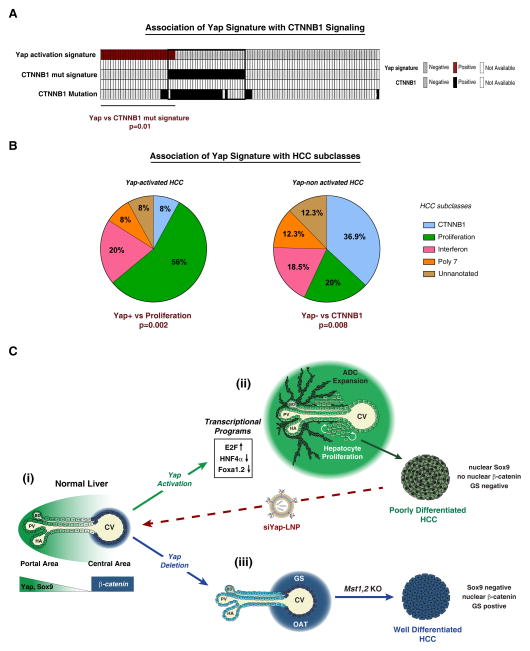
Mutations in CTNNB1 define a distinct subtype of HCC with less aggressive clinical features (Chiang et al., 2008). Since we observed an inverse correlation between YAP and β-CATENIN activity in the DKO HCC model, our data predict that YAP activation and CTNNB1 mutations may be associated with different HCC subtypes. In this regard, it is notable that nuclear β-CATENIN staining—which is closely correlated with CTNNB1 mutations—and nuclear YAP staining show a strong negative association in human HCC specimens (Tao et al., 2014). We sought to extend these data by using our YAP activity signature to identify HCC subtypes potentially driven by YAP. This signature of presumptive direct YAP targets was derived by integrating the set of genes altered by siYAP-LNP treatment in HCCs with ChIP data for TEAD occupancy in liver cancer cells; see Fig. S4E and Methods). To this end, enrichment for the YAP activation signature was calculated for each of the 111 HCC patients included in our human cohort. Correspondingly, nearest template prediction (NTP) revealed that the YAP gene expression signature had a strong negative correlation with CTNNB1 mutation signature in this set of human HCCs (Lachenmayer et al., 2012) (Fig. 7A). These findings were corroborated using three additional independent HCC classification systems (Fig. S7A–C). Moreover, patients positive for the YAP expression signature showed strong enrichment in the “Proliferation”, poor prognosis gene expression subtype of HCC (Fig. 7B, S7A) (Chiang et al., 2008). In addition, YAP levels showed close correlation with an E2F target gene/proliferation signature across both HCC cell lines and human specimens (Fig. S7D, E) Together, these data suggest that YAP specifically contributes to the growth of an aggressive CTNNB1 WT subtype of human HCC and point to the potential therapeutic value in targeting YAP in these currently intractable tumors.
Figure 7. The YAP activity signature is enriched in human HCC subtypes defined by lack of CTNNB1 mutation and a proliferation gene profile.
A. Analysis of 111 human HCC specimens reveals inverse correlation between the CTNNB1-mutation signature and a YAP activation signature. B. Using a second HCC classification system (Chiang et al., 2008), the YAP activation signature (YAP+) is positively correlated with the aggressive Proliferation subclass, whereas absence of the signature (YAP-) significantly correlates with the CTNNB1 subclass. Fisher’s exact test was used for statistical analysis. C. Model illustrating the impact of Hippo/YAP modulation on liver biology. (i) In normal liver, YAP shows a decreasing gradient of nuclear and cytoplasmic expression along the porto-central axis. The final layer of hepatocytes adjacent to the central vein completely lacks YAP expression, whereas active β-catenin and GS are restricted to these cells. (ii) Activation of YAP in hepatocytes results in induction of proliferation-related E2F target genes, downregulation of the targets of the HNF4A and FOXA1,2, and loss of pericentral β-CATENIN targets (e.g. GS, OAT), as well as hepatocyte proliferation throughout the lobule and dedifferentiation of hepatocytes to ADCs in the portal area. The resulting HCCs are poorly differentiated, express SOX9 and lack nuclear β-CATENIN and its target, GS. Treatment with siYAP-LNPs leads to tumor regression and hepatocyte differentiation. (iii) Yap deletion leads to an expansion of β-CATENIN targets in the central area. Additionally, Yap ablation completely blocks hepatocyte proliferation and oval cell expansion resulting from Mst1/Mst2 loss, and delays HCC development. The HCCs that develop display distinct differentiation markers and histopathology as compared to Yap-driven HCCs.
ADC: Atypical Ductal Cells, BD: Bile duct, CV: Central vein, GS: glutamine synthetase, HA: Hepatic artery, OAT: ornithine aminotransferase, PV: Portal vein. See also Figure S7.
DISCUSSION
We have shown that siRNA-LNP-mediated inhibition of YAP is an effective therapeutic in a GEM model of advanced HCC. In response to YAP inactivation, tumor cells undergo rapid proliferative arrest and subsequently acquire features of hepatocyte differentiation. These findings demonstrate the potential of YAP as a drug target in HCC and suggest a paradigm for differentiation therapy for the treatment of this aggressive epithelial malignancy.
Whereas YAP activation can induce anti-apoptotic genes and enhance cell survival in multiple contexts including livers treated with FAS ligand (Camargo et al., 2007; Zhou et al., 2009), we found no evidence of cell death upon siYAP-LNP administration in our HCC model. Rather, there was inhibition of E2F target genes and pronounced reduction in cell proliferation followed by a gradual activation of a hepatocyte differentiation program, culminating in the morphologic differentiation of the HCCs into nodules resembling regenerative hepatocytes. Thus, YAP inhibition in advanced HCC results in a remarkable reversion of transformed cells toward a growth arrested and histopathologically benign phenotype.
Anti-cancer drug development has focused largely on the identification of agents that incite death of the tumor cells. An alternative approach is to attempt to instruct cancer cells to reactivate differentiation programs thereby abrogating self-renewal and replicative lifespan. To date, this approach has been used to great success in acute promyelocytic leukemia harboring the PML/RARA fusion gene whereby all-trans retinoic acid and arsenic trioxide induce terminal differentiation of the leukemic cells into mature granulocytes and block their self-renewal (de The and Chen, 2010). Whether adult epithelial malignancies retain a latent capacity to differentiate and whether distinct nodes in cell regulation can be identified as targets to restore this differentiation potential are key questions in the broader application of such strategies. Our work provides a proof-of-concept for this approach in an autochthonous model of aggressive HCC and identifies YAP as such a targetable node. Differentiated hepatocytes survive for an extended period of time, therefore it remains to be seen whether a durable response can be attained via YAP targeting or whether sustained inhibition or combination therapies are required. Notably, pharmacologic inhibition of YAP has recently been shown to induce differentiation and tumor regression in embryonal rhabdomyosarcoma xenografts suggesting that this approach may extend to multiple cancer types (Tremblay et al., 2014).
Our transcriptomic analyses linked YAP activity in the normal liver and HCC to the negative regulation of targets of HNF4A and FOXA2/FOXA1. It is notable that the differentially expressed genes harboring TEAD binding sites — and thus potential direct YAP targets — exhibited a striking enrichment for HNF4A and FOXA1 binding sites (Fig. S4E). Although further studies will be required to resolve the associated transcriptional circuitry, these results suggest that the reported functions of YAP in switching the occupancy of HNF4A/FOXA1 from enhancers of mature hepatocyte genes to those of hepatic progenitor genes (Schaub et al., 2014) may also be operative in dictating sustained tumorigenic growth versus hepatocyte differentiation in advanced HCC. Accordingly, deletion of Hnf4a in the adult mouse liver leads to phenotypes overlapping those observed upon YAP activation, including loss of hepatocyte identity, proliferation of dedifferentiated hepatocytes and expansion of ADCs (Bonzo et al., 2012; Saha et al., 2014; Walesky et al., 2013). We also found that expression of E2F target genes is positively associated with YAP function in vivo (Fig. S4D, S7D, E), consistent with prior work demonstrating cooperative interactions between YAP and E2F in the liver (Ehmer et al., 2014). Notably, we observed YAP-mediated modulation of HNF4A targets and E2F targets in vitro using primary HCC cultures, suggesting that the regulatory interactions may be direct (Fig. 6). YAP activity was also associated with SOX9 expression and inhibition of β-CATENIN nuclear translocation, although these pathways were not as prominently altered in vitro (data not shown).
Our studies provide a number of insights into the role of Hippo/YAP in control of cell fate in the adult liver. In the normal liver, we find that nuclear YAP expressed at highest levels in the bile ducts and shows a decreasing gradient of expression along the porto-central axis, with the final row of pericentral hepatocytes completely lacking YAP expression (Fig. 1A,B). Activation of endogenous YAP following acute Mst1/Mst2 deletion not only results in conversion of hepatocytes in the periportal area to ADCs but also overrides the zonation program in pericentral hepatocytes as reflected by losses of expression of GS and OAT (Fig. 2A,C, also see Fig. 1C,D, S1D for Alb-Cre model). Moreover, acute deletion of YAP caused aberrant expansion of the GS-positive domain to multiple layers of pericentral hepatocytes (Fig. 2A,C). Therefore, we demonstrate an unexpected role for basal YAP function in maintaining liver zonation and show that the differentiation state of pericentral hepatocytes is sensitive to precise tuning of YAP activity (Fig. 7C). Liver zonation is an intriguing mechanism for regional compartmentalization of metabolic functions (Jungermann and Katz, 1989; Torre et al., 2011) and our data establish YAP as a major determinant of zonal identity with a role opposing that of β-CATENIN, which activates a pericentral program. It will be of interest to determine the basis for graded YAP activity within the parenchyma and to specifically relate YAP function to the control of the diverse metabolic processes carried out by this organ.
While Yap deletion delayed tumorigenesis in DKO mice, it did not completely prevent formation of HCC. Rather, DKO Yap+/− and TKO animals all eventually developed invasive HCC. These data are consistent with MST1/MST2 acting to suppress HCC development through targets in addition to YAP. It is possible that TAZ — which is expressed in the HCCs and derivative cell lines (data not shown) — partially compensates for YAP inactivation in this context. Since YAP deficiency eliminated ADCs in TKO mice and resulted in a distinct tumor phenotype—well-differentiated histology, β-CATENIN and GS positive, and SOX9 negative (Fig. 3D, 7C), it appears that this secondary pathway does not fully recapitulate the broad reprogramming of hepatocyte differentiation induced by YAP.
The link between YAP activity and differentiation status in HCC appears to be relevant in the corresponding human tumors. Using the transcriptional alterations provoked by YaAP inactivation in our HCC model as a read-out for YAP activity, we find that the YAP signature is negatively correlated with the CTNNB1 mutant subclass of HCC and positively correlated with the “proliferation” subclass (Fig. 7A,B, S7A–C). These data extend findings from IHC analysis showing inverse correlation between nuclear YAP and nuclear β-CATENIN (Tao et al., 2014), and point to a molecular subtype of HCC that may benefit from anti-YAP therapies.
Targeting YAP as a cancer therapy is particularly compelling due to the clear dosage dependence of its oncogenic activity. Approaches to inhibit YAP using small molecules and peptides have been described (Jiao et al., 2014; Liu-Chittenden et al., 2012). Our data suggest that there may be a clinical path forward using siYAP-LNPs to treat primary HCC. Importantly as a genetically-targeted therapy, siYAP-LNPs would be expected to have more specificity and lower toxicity than most small molecule inhibitors, and indeed, we observed mild and reversible side effects in our studies (Fig. S5B–D). Finally, siRNA-LNPs can readily be adapted to inhibit multiple targets, enabling development of combinatorial strategies.
EXPERIMENTAL PROCEDURES
Mouse models
Mice were housed in pathogen-free animal facilities at Massachusetts General Hospital (MGH). All experiments were conducted under protocol 2005N000148 approved by the Subcommittee on Research Animal Care at MGH. The following mouse strains were used: Mst1−, Mst2Flox, YapFlox and Albumin-Cre (Postic and Magnuson, 2000; Schlegelmilch et al., 2011; Zhou et al., 2009). The Albumin-Cre transgene efficiently deletes floxed sequences in all liver lineages by 4–6 weeks of age. Mice were maintained on a mixed genetic background and each genotype was generated from intercrosses from the same colony. For Adeno-Cre studies, 5×109 pfu of CMV-Cre adenovirus resuspended in a final volume of 200 μl PBS was injected in the tail vein of 6-week old mice. All mice included in the survival analysis were euthanized when criteria for disease burden were reached (including abdominal distension that impeded movement, loss of >15% of weight body weight, labored breathing and/or abnormal posture).
siLNP formulations
LNPs were formed by mixing equal volumes of lipids dissolved in alcohol (the lipid solution contains a cationic lipid, a helper lipid (cholesterol), a neutral lipid (DSPC) and a PEG lipid), with siRNA (dissolved in sodium citrate: sodium chloride buffer, pH 4.0) by an impinging jet process. Following an incubation period, the solution was concentrated and diafiltered by ultrafiltration process using membranes with molecular weight cutoff from 100 KD. The product was sterile filtered and stored at 4°C. The total siRNA was determined by anion exchange chromatography. The targeted sequences for Yap are 5′-TTAAGAAGTATCTTTGACC-3′ (siYap-LNP #1) and 5′-TTAAGAAAGGGATCGGAAC-3′ (siYap-LNP #2). A formulation targeting luciferase (siLuc-LNP) was used as a control. Formulations were intravenously injected (via tail vein) at 3 mg/kg per injection every other day (for short term treatment, i.e. < 10 days treatment) or for three consecutive days with a resting period of 2 days between every round of treatment for long-term analysis.
Gene-set enrichment analysis
GSEA (http://www.broadinstitute.org/gsea/index.jsp) of the expression data was used to assess enrichment of gene signatures. Depending upon the data set, there were several different methods used to rank genes: In the siRNA-LNP studies, a pairwise GSEA was performed by creating ranked lists of genes using the log2 ratio of siYAP-LNPs to siLuc-LNPs and p-values were obtained by permuting the gene set. For the identification of direct target genes (Fig. S2B and S4E), TEAD ChIP peaks for HEPG2 cells were downloaded from ENCODE (http://genome.ucsc.edu/ENCODE/) and TEAD-target lists were generated by associating genes to TEAD peaks using PeakAnalyzer (Salmon-Divon et al., 2010) with the “closest-TSS” routine. We defined YAP signatures by comparing the differentially regulated genes in D8 or D9 and TEAD-targets. For transcription factor enrichment analysis, the ENCODE ChIP-seq significance tool was utilized (Auerbach et al., 2013). In brief, YAP-TEAD lists for D8 or D9 were uploaded in the web interface (http://encodeqt.simple-encode.org) and transcription factor binding sites were detected in the region +5000 bp to −2500 bp relative to the TSS of each gene. Significance of enrichment of a transcription factor was calculated by the hypergeometric test and corrected for multiple testing using the Benjamini-Hochberg FDR.
Supplementary Material
Acknowledgments
We thank Bardeesy lab members as well as A. Kimmelman and W. Kim for helpful comments and F. Camargo for generously providing the Yapfl mice. The authors are thankful to the Novartis siRNA formulation group for siLNPs preparation, K. Ross for bioinformatics assistance, and D. Zhou for initiation of the mouse cohort. This work was supported by grants from the NIH/NCI (R01CA136567 and P50CA127003 to N.B. and J.A.), NIH/NIDDK (R01DK099558 to Y.H.), and the TargetCancer Foundation (to N.B.). N.B. holds the Gallagher Chair in Gastrointestinal Cancer Research at Massachusetts General Hospital. N.B. and J.M.L. are members of the Waxman Institute without Walls. J.F and S.B are recipients of Long-term postdoctoral fellowships from EMBO and the International Human Frontiers Science Program, respectively, and both were supported by ECOR Fund for Medical Discovery Postdoctoral Fellowships. C.P. is recipient of a CIHR postdoctoral fellowship. J.M.L. and D.S. are supported by the Asociación Española Contra el Cáncer. A.L., B.M., D.M., and L.R. are employees of Novartis, and J.M.L. is a consultant with Novartis. This work is dedicated to the bright memory of Rolande Fitamant (1959–2014).
Footnotes
ACCESSION NUMBERS
The NCBI GEO accession number for the data reported in this paper is pending
AUTHOR CONTRIBUTIONS
J.F., S.B., L.R. and N.B. designed experiments. J.F. performed experiments with the assistance of F.K., S.B., H.S.T., N.C., C.A.P., J.M.N., R.M.P., M.L., B.M. and G.C.
J.F., F.K., S.B., V.D., A.X.Z., A.L., Y.H., J.A., D.S., A.I.M., J.M.L., D.M., L.R. and N.B. analyzed the data. F.K. and S.B. contributed equally. J.F. and N.B. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alder O, Cullum R, Lee S, Kan AC, Wei W, Yi Y, Garside VC, Bilenky M, Griffith M, Morrissy AS, et al. Hippo Signaling Influences HNF4A and FOXA2 Enhancer Switching during Hepatocyte Differentiation. Cell reports. 2014;9:261–271. doi: 10.1016/j.celrep.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach RK, Chen B, Butte AJ. Relating genes to function: identifying enriched transcription factors using the ENCODE ChIP-Seq significance tool. Bioinformatics. 2013;29:1922–1924. doi: 10.1093/bioinformatics/btt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, McClatchey AI. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, Kuo CJ, Kahn A, Perret C, Colnot S. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Bochkis IM, Schug J, Ye DZ, Kurinna S, Stratton SA, Barton MC, Kaestner KH. Genome-wide location analysis reveals distinct transcriptional circuitry by paralogous regulators Foxa1 and Foxa2. PLoS Genet. 2012;8:e1002770. doi: 10.1371/journal.pgen.1002770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzo JA, Ferry CH, Matsubara T, Kim JH, Gonzalez FJ. Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4alpha in adult mice. J Biol Chem. 2012;287:7345–7356. doi: 10.1074/jbc.M111.334599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeuning A, Ittrich C, Kohle C, Hailfinger S, Bonin M, Buchmann A, Schwarz M. Differential gene expression in periportal and perivenous mouse hepatocytes. The FEBS journal. 2006;273:5051–5061. doi: 10.1111/j.1742-4658.2006.05503.x. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Current biology : CB. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Sole M, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nature reviews Cancer. 2010;10:775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmer U, Zmoos AF, Auerbach RK, Vaka D, Butte AJ, Kay MA, Sage J. Organ size control is dominant over Rb family inactivation to restrict proliferation in vivo. Cell reports. 2014;8:371–381. doi: 10.1016/j.celrep.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44:760–764. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Cornils H, Chiu SY, O’Rourke KP, Arnaud J, Yimlamai D, Thery M, Camargo FD, Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougelet A, Torre C, Veber P, Sartor C, Bachelot L, Denechaud PD, Godard C, Moldes M, Burnol AF, Dubuquoy C, et al. T-cell factor 4 and beta-catenin chromatin occupancies pattern zonal liver metabolism in mice. Hepatology. 2014;59:2344–2357. doi: 10.1002/hep.26924. [DOI] [PubMed] [Google Scholar]
- Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nature reviews Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Jungermann K, Katz N. Functional specialization of different hepatocyte populations. Physiological reviews. 1989;69:708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, Villanueva A, Minguez B, Newell P, Tsai HW, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4997–5007. doi: 10.1158/1078-0432.CCR-11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, Lim DS. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, Johnson RL. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H, et al. Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon-Divon M, Dvinge H, Tammoja K, Bertone P. PeakAnalyzer: genome-wide annotation of chromatin binding and modification loci. BMC bioinformatics. 2010;11:415. doi: 10.1186/1471-2105-11-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub JR, Malato Y, Gormond C, Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell reports. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, Smirnova O, Dorrell C, Erker L, Chu AS, et al. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev. 2011;25:1185–1192. doi: 10.1101/gad.2027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Calvisi DF, Ranganathan S, Cigliano A, Zhou L, Singh S, Jiang L, Fan B, Terracciano L, Armeanu-Ebinger S, et al. Activation of beta-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014;147:690–701. doi: 10.1053/j.gastro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre C, Perret C, Colnot S. Transcription dynamics in a physiological process: beta-catenin signaling directs liver metabolic zonation. The international journal of biochemistry & cell biology. 2011;43:271–278. doi: 10.1016/j.biocel.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Tremblay AM, Missiaglia E, Galli GG, Hettmer S, Urcia R, Carrara M, Judson RN, Thway K, Nadal G, Selfe JL, et al. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell. 2014;26:273–287. doi: 10.1016/j.ccr.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542. e1512. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walesky C, Gunewardena S, Terwilliger EF, Edwards G, Borude P, Apte U. Hepatocyte-specific deletion of hepatocyte nuclear factor-4alpha in adult mice results in increased hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G26–37. doi: 10.1152/ajpgi.00064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nature reviews Drug discovery. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, Pikarsky E, Stanger BZ. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell stem cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.