Abstract
Objective
To compare clinical manifestations and activity of Behçet syndrome (BS) in the United States versus Turkey using validated outcome measures.
Methods
Consecutive patients with BS from the US National Institutes of Health (NIH), New York University, and the University of Istanbul were evaluated. Disease activity was measured using the Behçet’s Syndrome Activity Scale (BSAS) and the Behçet’s Disease Current Activity Form (BDCAF) with quality of life measured by the Behçet Disease Quality of Life (BDQOL) form. One-way ANOVA, t-tests, and multivariate regression analyses were performed.
Results
Mean age did not differ between sites; however, more women were seen in the United States versus in Turkey (p < 0.001), and disease duration was longer in the United States (p = 0.02). Organ manifestations were similar for oral and genital ulcers, skin disease, arthralgia, eye disease, and thrombosis. However, more gastrointestinal (p < 0.001) and neurologic disease (p = 0.003) was seen in the United States. BSAS and BDCAF scores were worse in the United States compared to Turkey (p = 0.013 and < 0.001, respectively). Worse mean BDQOL scores were observed at the NIH compared to Istanbul (not significant). Multivariable regression models showed worse scores in ethnically atypical patients for BSAS and BDCAF (p = 0.04 and p = 0.001), American patients for BDCAF (p = 0.01), older age for BDCAF (p = 0.005), and women for BDQOL (p = 0.01).
Conclusion
Demographic and clinical manifestations of BS differ between sites with higher disease activity in the United States compared to Turkey. Referral patterns, age, sex, ethnicity, and country of origin may be important in these differences. These observations raise the question of whether pathogenic mechanisms differ in Turkish and American patients.
Key Indexing Terms: BEHÇET SYNDROME, TURKEY, UNITED STATES, DISEASE MANIFESTATIONS
Behçet syndrome (BS) is a multisystem disorder with varied manifestations including recurrent oral and genital ulcers, the pathergy test, ocular inflammation, skin disease including papulopustular lesions and erythema nodosum, gastrointestinal (GI) ulceration, neurologic disease, fevers, and arthritis without autoantibody production. The diagnosis of BS is based solely on clinical symptoms1 with no currently available diagnostic tests to aid in diagnosis.
BS has an ethnic predominance for patients from backgrounds along the Silk Route, with rates of 80 to 370 per 100,0002 in Turkey compared to 5.2 per 100,0003 in the United States. The 2 largest cohorts of American patients with BS are at New York University (NYU) and at the US National Institutes of Health (NIH), where patients come from both typical and atypical ethnic backgrounds. Careful descriptions of American cohorts are limited; however, preliminary data from NYU suggest that clinical characteristics differ between American patients and those in Turkey4,5, with more female patients, more GI involvement, and less severe disease manifestations, such as blindness and vascular involvement, found in the Americans. This raises the possibility that disease in America may have important differences in presentation and activity when compared to disease in Turkey.
Despite available data regarding longterm organ damage, American patients report substantial morbidity related to their disease. In our study, disease manifestations and disease activity of American patients from the NIH and NYU were compared to a more thoroughly studied and ethnically typical cohort of Turkish patients from the University of Istanbul, with an evaluation of the relative contributions of age, sex, ethnicity, and country of origin to these findings.
MATERIALS AND METHODS
Patients
Patients fulfilling the International Study Group criteria1 for BS were enrolled in this cross-sectional observational study. Criteria include recurrent oral ulcers and 2 of the following: recurrent genital ulcers, typical eye lesions, typical skin lesions, and pathergy. One patient did not fulfill all criteria but had a diagnosis of BS based on the investigator’s judgment (YY). Patients were recruited from NYU and the University of Istanbul as part of routine clinical care. Patients from the NIH were recruited after enrollment in a Natural History of Autoinflammatory Diseases protocol (NCT00059748). This study recruits patients from across the United States for a multidisciplinary evaluation of their condition after management by their primary specialists. Research was in compliance with the Helsinki Declaration, and the protocol was approved by the institutional review board (IRB) at each participating site. Patients at the NIH and University of Istanbul signed an informed consent with prospective collection of data other than disease duration, which was collected by retrospective chart review. At NYU, a retrospective analysis of prospectively collected data was performed. Informed consent was not performed in consultation with the IRB because data were collected as part of routine clinical care. Age, sex, country of origin, typical ethnic background, and current medications were recorded for each patient.
Study design
Sequential patients were enrolled at the NIH (n = 35) and NYU (n = 77) between May 2009 and June 2011. Every other patient was enrolled at the University of Istanbul (n = 107) in December 2010 and between November 2011 and December 2011. Two disease activity questionnaires were administered at each site: (1) the Behçet’s Disease Current Activity Form9 (BDCAF) and (2) the Behçet’s Syndrome Activity Scale (BSAS)10. A quality of life questionnaire, the Behçet’s Disease Quality of Life Scale (BDQOL)8, was administered to patients at the NIH and University of Istanbul.
Ethnic background was recorded by patient report. “Typical” ethnic background was defined as at least 50% Turkish, Greek, Arabic, Middle Eastern, Israeli, Ashkenazi Jewish, Sephardic Jewish, Iranian, Northern African, Italian, Spanish, Portuguese, Mediterranean, Korean, Japanese, Chinese, or Far East ethnic background.
Assessment of Clinical Endpoints
Organ manifestations. A consensus was met between sites for the definitions for prior organ manifestations prior to patient enrollment. See Appendix 1 for definitions of organ involvement in BS.
Disease activity and quality of life outcomes. Each questionnaire was scored according to its respective scoring system. All questionnaires were developed in English and have been validated in Turkish-speaking populations6,7,8. The BDCAF assesses current activity based on current symptoms and is a composite of patient and physician assessments of disease scored on a scale of 0 to 12. Each increase of 1 corresponds to a new organ manifestation9. The “patient’s index score” version of the BDCAF was used for the final analysis. The BSAS is based solely on patient report and also assesses current symptoms and is scored on a scale of 0 to 100 with each increase of 10 corresponding to a new organ manifestation10. BDQOL measures quality of life on a scale of 0 to 308. For all questionnaires, higher scores indicate worse values.
Statistical analysis
The mean values, SD, and ranges for demographic data, medication usage, organ manifestations, and questionnaire data were descriptively summarized at each center. One-way ANOVA were performed to evaluate differences between sites followed by pairwise t-tests for significant ANOVA results (p < 0.05). Cohen d effect size score was calculated to estimate the pairwise effect size for all questionnaires. For questionnaire outcomes in which the ANOVA did not show significance (BDQOL), a linear regression analysis accounting for site was performed to calculate a 95% CI around the regression measure to assess for power limitations in detecting a true difference. All data were pooled for each questionnaire, and t-tests were performed to evaluate for differences in scores due to sex, country of origin, and ethnicity. A multivariate linear regression model was then performed. The following prespecified variables were included in the analysis: age, sex, country of origin, and ethnic background in an attempt to clarify which variables were the strongest predictors of outcome. For the BDQOL, an additional multivariate linear regression model was performed with the prespecified predictors in addition to BDCAF scores to account for disease activity as a contributor to any observed differences.
RESULTS
Clinical characteristics
Demographic data, medication usage, and prior organ manifestations are summarized in Table 1. The mean age across sites was similar. At the NIH, 2 of 35 patients were of typical ethnic background and at NYU, 28 of 77 patients were of typical ethnic background. More women were seen in the American sites than in Turkey (p < 0.001) and disease duration was longer at the American sites (p = 0.02). Of the 30 American patients with typical ethnic backgrounds, 4 of 30 (13.3%) were men, which is similar to the proportion of women in the overall American population (Table 1).
Table 1.
Demographic information.
| Demographics | NIH, n = 35 | NYU, n = 77 | Istanbul, n = 107 | ANOVA p |
|---|---|---|---|---|
| Age, yrs, mean (SD) | 36.1 (± 13.4) | 36.8 (± 13.5) | 37.4 (± 11.9) | 0.85 |
| Men, n (%) | 7 (20) | 6 (7.8) | 42 (39.3) | < 0.001* |
| Disease duration, yrs, mean (SD) | 10.7 (± 11.5) | 8.5 (± 5.7) | 6.9 (± 6.2) | 0.02* |
| Medications, n (%) | ||||
| Colchicine | 8 (22.9) | 16 (20.8) | 51 (47.7) | < 0.001* |
| Prednisone | 17 (48.6) | 37 (48.1) | 11 (10.3) | < 0.001* |
| Disease-modifying antirheumatic drugs | 20 (57.1) | 43 (55.8) | 46 (43) | 0.15 |
| Prior organ manifestations, n (%) | ||||
| Oral ulcers | 35 (100) | 71 (93.4) | 107 (100) | 0.008* |
| Genital ulcers | 31 (88.6) | 59 (78.7) | 86 (80.4) | 0.45 |
| Arthralgia | 30 (85.7) | 59 (77.6) | 79 (73.8) | 0.35 |
| Skin disease | 32 (91.4) | 63 (84) | 99 (92.5) | 0.17 |
| Thrombosis | 8 (22.9) | 8 (10.7) | 13 (12.1) | 0.19 |
| Eye disease | 14 (40) | 30 (42.2) | 37 (34.6) | 0.57 |
| Gastrointestinal disease | 15 (42.9) | 27 (37) | 0 (0) | < 0.001* |
| Neurologic disease | 7 (20) | 13 (17.3) | 4 (3.7) | 0.003* |
Statistically significant. Study sites: NIH: US National Institutes of Health; NYU: New York University; Istanbul: University of Istanbul.
Colchicine use was more frequent in Turkey (p < 0.001) and prednisone use was more frequent in the United States (p < 0.001). The rate of other disease-modifying medications was similar.
Clinically, all patients at the NIH and University of Istanbul had experienced oral ulcers whereas this was not the case at NYU (p = 0.008). Neurologic disease was seen in 20% of patients at the NIH and 17.3% of patients at NYU compared to only 3.7% in Istanbul (p = 0.003). Moreover, GI ulceration was documented in 42.9% of patients at the NIH, 37% of patients at NYU, and no patients in Istanbul (p < 0.001). Disease duration did not explain the differences in neurologic and GI manifestations because no differences in disease duration were observed between American patients with and without these findings. Genital ulcers, skin disease, arthralgia, and eye disease did not differ between sites. Details on the severity of manifestations were not available.
For all significant clinical characteristics, pairwise comparisons confirmed significant differences between the NIH and Istanbul and between NYU and Istanbul, with the exception of oral ulcers, which were significantly different between NYU and the NIH and between NYU and Istanbul.
Questionnaire Results
Disease activity questionnaires
Differences were seen between sites for both the BDCAF (p < 0.001) and BSAS (p = 0.013, Figure 1). For the BDCAF, worse mean (SD) scores were observed at the NIH 5.9 (2.5) and NYU 5.7 (2.4) compared to the University of Istanbul 3.3 (2.1). This was similarly seen for the BSAS with mean (SD) scores of 42.5 (23.5) at the NIH, 36.5 (19.1) at NYU, and 30.4 (20) at the University of Istanbul.
Figure 1.
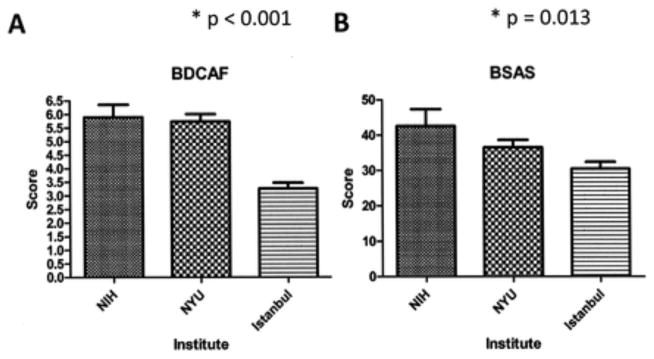
Disease activity measurements in Behçet syndrome. (A) Behçet’s Disease Current Activity Form (BDCAF) and (B) Behçet’s Syndrome Activity Scale (BSAS) values from the US National Institutes of Health (NIH), New York University (NYU), and University of Istanbul, with SD. Higher values indicate worse activity.
Cohen d for BDCAF scores at the NIH and NYU, respectively, compared to the University of Istanbul were 1.04 and 1. Both correspond to a large effect size. Cohen d for BSAS scores at the NIH and NYU, respectively, compared to the University of Istanbul were 0.51 and 0.31, indicative of a medium effect size.
For individual components of the BSAS, American patients had significantly worse (p < 0.05) scores for the following: number of genital ulcers, the bother of genital ulcers, number of acne lesions, the bother of acne lesions, amount of abdominal pain, and overall disease activity. The number of oral ulcers, bother of oral ulcers, amount of eye disease, and amount of clotting or swelling did not differ.
Patients from the NIH and Turkey were generally bothered a similar amount for the same number of oral ulcers, genital ulcers, and skin lesions. Two exceptions were that Turkish patients were bothered more by > 3 oral ulcers (p = 0.02) and American patients were bothered more by 1–3 skin lesions (p = 0.01; data not shown).
Quality of life questionnaire
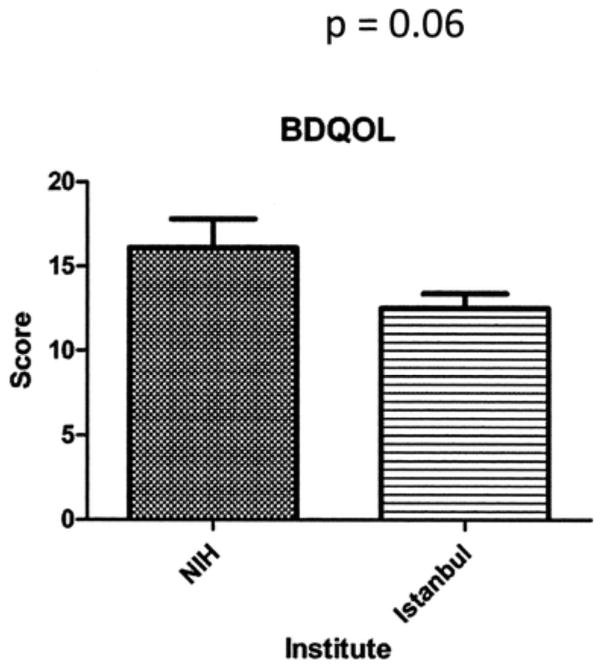
As patients at NYU did not complete the BDQOL, 26 American questionnaires from the NIH and 107 Turkish questionnaires were available for analysis. Worse mean values (SD) were observed at the NIH 16.1 (8.6) compared to the University of Istanbul 12.5 (9.1); however, this was not significant (Figure 2). Cohen d for BDQOL scores at the NIH compared to the University of Istanbul was 0.4, indicative of a medium effect size.
Figure 2.
Quality of life measurements in Behçet syndrome. Behçet’s Disease Quality of Life (BDQOL) measurements at the US National Institutes of Health (NIH) compared to University of Istanbul, with SD. Higher values indicate worse quality of life.
Questionnaire Multivariate Regression Model
BDCAF
Scores were worse in women patients from the United States, and patients without a typical ethnic background (all t-test p < 0.0001). In a multivariate linear regression model, age, country of origin, and atypical ethnicity were all independently associated with BDCAF scores (p = 0.005, 0.01, and 0.001, respectively, Table 2).
Table 2.
Multivariate linear regression model.
| B | 95% CI | p | |
|---|---|---|---|
| BDCAF | 5 | 4, 6 | |
| Age | 0.03 | 0.01, 0.06 | 0.005* |
| Male | −0.75 | −1.5, 0.02 | 0.06 |
| Typical ethnicity | −1.5 | −2.5, −0.6 | 0.001* |
| Living in Turkey | −1.2 | −2.1, −0.3 | 0.01* |
| Model R2 | 0.30 | ||
| BSAS | 26.1, 44.4 | ||
| Age | 0.15 | −0.07, 0.4 | 0.17 |
| Male | −2.9 | −9.9, 4 | |
| Living in Turkey | −1.9 | −10.2, 6.4 | 0.65 |
| Model R2 | 0.08 | ||
| BDQOL | 13.2 | 7.2, 19.2 | |
| Age | 0.1 | −0.02, .24 | 0.11 |
| Male | −8, −1 | 0.01* | |
| Typical ethnicity | −1.9 | −12.7, 9 | 0.73 |
| Living in Turkey | −1.3 | −11.5, 8.9 | 0.80 |
| Model R2 | 0.11 |
Statistically significant. BDCAF: Behçet’s Disease Current Activity Form; BSAS: Behçet’s Syndrome Activity Scale; BDQOL: Behçet’s Disease Quality of Life Scale; B: beta coefficient.
BSAS
BSAS scores were also worse in patients from the United States and patients without a typical ethnic background (t-test p = 0.008 and 0.001, respectively). The mean score in women was worse than in men, but this did not reach statistical significance (p = 0.062). A multivariate linear regression model accounting for age, sex, typical ethnicity, and country of origin showed that only atypical ethnic background was associated with worse BSAS scores (p = 0.042), suggesting that ethnicity was stronger than were environmental factors of country of origin in BSAS scores (Table 2).
BDQOL
No significant differences were seen in BDQOL scores between the NIH and the University of Istanbul or for an atypical ethnic background (t-test p = 0.06). The BDQOL regression CI based on site suggested insufficient power to detect a difference (-7.5, 0.26). Negative numbers indicate worse scores at the NIH. However, women had worse scores than men (t-test p = 0.0035) and this remained the case after accounting for age, country of origin, and atypical ethnic background in a multivariate linear regression model (p = 0.0118, Table 2). Women continued to show worse BDQOL scores after accounting for disease activity as measured by BDCAF scores in a regression model (data not shown).
Association with medication usage
Worse disease activity and quality of life were observed with the use of some medications. For the BDCAF, no difference was observed based on colchicine use; however, worse scores were seen with immunosuppressant use (mean 4.2 vs 5.0, p = 0.02) and prednisone use (mean 4.2 vs 5.3, p = 0.005). For the BSAS, no difference was observed based on colchicine or immunosuppressant use; however, worse scores were observed with prednisone use (37.7 vs 39.6, p = 0.01). Finally, for the BDQOL no difference was observed based on colchicine or immunosuppressant use; however, worse scores were also seen in association with the use of prednisone (12.4 vs 16.6, p = 0.03). In multivariate regression models accounting for age, sex, country of origin and atypical ethnic background the use of immunosuppressants or prednisone no longer showed a significant association with any of the outcome questionnaires.
DISCUSSION
While descriptive cohort studies in American and Turkish patients exist, to our knowledge this is the first study to date to directly compare clinical criteria and disease activity through validated outcome measures. Here we show that disease manifestations, treatment, and disease activity differ between patients in the United States and Turkey.
We find that 2 of the most severe manifestations of BS, GI disease and neurologic disease, occurred at higher rates in American patients than in Turkish patients. Both definitions were based on prespecified objective criteria — GI ulceration on endoscopy and dural sinus thrombosis or white matter changes on magnetic resonance imaging. The differences in disease rates are quite striking, with rates of GI disease seen between 37 and 42.9% of American patients compared to no patients in Turkey; and neurologic disease observed between 17.3 and 20% of American patients compared to 3.7% of Turkish patients. These American values are much higher than have been published in other cohorts, with the highest published rates of GI disease of 13% in Japan11 and for neurologic disease of 17% in Morocco12. An ascertainment bias in assessing clinical symptoms may be in effect because there is a lower threshold for endoscopy and radiographic studies at the American sites compared to Turkey. However, given the magnitude of differences, this is unlikely to completely explain the observed differences. Notably, the severity of eye disease, GI disease, and neurologic disease was not recorded. Other severe manifestations, specifically eye disease and vascular disease, did not differ between cohorts.
We also find that disease activity differs between sites with higher levels of activity in American patients. Quality of life may be worse in American patients; however, the power to detect a difference in this analysis is limited owing to sample size. In multivariate regression analyses, older age, female sex, an atypical ethnic background, and living in the United States were significant factors in some measures of higher disease activity and/or quality of life scores. Importantly, combined factors other than these prespecified variables of interest are more important in predicting outcomes because the largest R2 obtained in regression models was 0.30 for the BDCAF (Table 2).
It could be hypothesized that observed differences may be due to different and likely increased availability of immunosuppressants or other medications used to treat BS in the United States. We did not observe a difference in immunosuppressant use between sites; however, Turkish patients were more likely to be treated with colchicine and American patients more likely to be treated with prednisone. Because immunosuppressant use did not differ between sites, and differences in scores were not observed with colchicine, observed differences in cohorts could not be due to altered usage of these medications. For the BDCAF, BSAS, and BDQOL, prednisone use was associated with worse scores. The higher mean scores observed with the use of prednisone and immunosuppressants in the case of the BDCAF suggests that these medications are being used in patients with worse activity rather than resulting in a sufficient therapeutic effect to lower scores. Based on this and assuming these medications are effective, the worse scores in American patients are not due to a less aggressive therapeutic strategy.
These results are surprising in view of published studies on the longterm morbidity and mortality in patients with BS. Also at the University of Istanbul, a 20-year retrospective study on the morbidity and mortality of patients with BS showed that 10.9% of patients died and that the vast majority of patients who died were young men with a mean age of 324. Moreover, men were more likely to develop uveitis, vascular disease, and neurologic disease. This is in contrast to the higher level of disease activity, GI disease, and neurologic disease observed in the American patients in our study; patients that are predominantly women. It is possible that the organ manifestations seen in American patients may be less severe than these manifestations in Turkish patients. For example, less than 1% of patients in both American sites are blind (visual acuity < 20/200) compared to much higher rates in Turkey4. Current disease activity as measured by BDCAF and BSAS may also not directly correlate with longterm morbidity and mortality. Moreover, because of the unweighted structure of these scales, a patient with active oral ulcers and papulopustular lesions may score higher than a patient with active eye involvement. A prospective followup will be helpful in understanding the longterm consequences of GI and neurologic disease in these American patients.
Given these conflicting studies, it is increasingly important to understand the pathogenesis of this pleiomorphic disease to better interpret the differences observed in cohorts. As the prevalence of disease is much lower in the American population and there are no absolute criteria or diagnostic tests, false-positive diagnoses will occur more frequently in American patients, which may account for some of the observed cohort differences. Disease in American patients must be more heterogeneous than that seen in Turkey, an area of higher disease prevalence. As the diagnosis of BS is made on purely clinical criteria, the establishment of better diagnostic methods based on well-defined classical disease would greatly aid in understanding our patients.
Our study is limited by the lack of an objective overall disease activity gold standard measure. All measures of disease activity and quality of life are based on patient report as there is no validated disease index available in BS. This is an area requiring further work. Another limitation is the lack of a reliable tool to assess severity. Although we were able to compare the frequency of each type of involvement in the Turkish and American cohorts in our study, we were not able to compare the severity of each involvement. As currently available disease activity measurements do not address severity, they are of marginal value in clinical care.
The differences observed need to be interpreted with caution because of several biases. One is referral bias. Because of the rarity of the disease in the United States, the patients seen at NYU and the NIH often have complicated cases that have been referred from across the country. The University of Istanbul is also a referral center but not to the same extent as the NIH and NYU. There is also a diagnostic bias in effect because American physicians are not as familiar with the diagnosis, and there is a concern that only the most complicated or sickest patients may be diagnosed. In addition, the heterogeneity observed in American patients may be responsible for differing manifestations due to diagnostic uncertainty rather than pathophysiologic mechanisms. Cultural differences may also have influenced our observations. A previous study comparing pain and fatigue perception among patients with RA from Turkey and the United States has shown that less pain is reported by Turkish patients with RA despite similar Multidimensional Health Assessment Questionnaire Functional Score and 28-joint Disease Activity Score13. Finally, clinical expertise may differ between the United States and Turkey because many American physicians have never seen a patient with BS whereas it is quite common in Turkey. All of these factors may influence organ manifestations and disease activity measures.
Nonetheless, we observe that American patients report differences in clinical manifestations and higher disease activity than Turkish patients. We find that country of origin, sex, and ethnicity may contribute to these differences. Despite the lack of data regarding longterm morbidity, American patients report significant disease activity that may be underrecognized in the medical community. The mechanisms underlying these observed differences remain to be elucidated and are under active investigation.
Supplementary Material
Definitions of Organ Involvement in Behçet syndrome.
Acknowledgments
Supported by the Intramural Research Program of the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) at the National Institutes of Health (NIH), New York University, and the University of Istanbul. Yusuf Yazici is a consultant for Abbvie, BMS, Celgene, Genentech, Pfizer, UCB, Horizon, and Medac; and holds research grants from BMS, Genentech, and Celgene. Raphaela Goldbach-Mansky has received grant support from Regeneron, Novartis, and BIovitrum Inc.
References
- 1.Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 2.Yurdakul S, Hamuryudan V, Yazici H. Behcet syndrome. Curr Opin Rheumatol. 2004;16:38–42. doi: 10.1097/00002281-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behcet’s disease in the US: a population-based study. Arthritis Rheum. 2009;61:600–4. doi: 10.1002/art.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, et al. and morbidity of Behcet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine. 2003;82:60–76. doi: 10.1097/00005792-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Yazici Y, Filopoulos MT, Schimmel E, McCraken A. Clinical characteristics, treatment and ethnic/racial differences in the manifestations of 518 Behcet’s syndrome patients in the United States [abstract] Arthritis Rheum. 2010;62 (Suppl):S535. [Google Scholar]
- 6.Hamuryudan V, Fresko I, Direskeneli H, Tenant MJ, Yurdakul S, Akoglu T, et al. Evaluation of the Turkish translation of a disease activity form for Behcet’s syndrome. Rheumatology. 1999;38:734–6. doi: 10.1093/rheumatology/38.8.734. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz S, Simsek I, Cinar M, Erdem H, Kose O, Yazici Y, et al. Patient-driven assessment of disease activity in Behcet’s syndrome: cross-cultural adaptation, reliability and validity of the Turkish version of the Behcet’s Syndrome Activity Score. Clin Exp Rheumatol. 2013;31 (Suppl 77):77–83. [PubMed] [Google Scholar]
- 8.Gilworth G, Chamberlain MA, Bhakta B, Haskard D, Silman A, Tennant A. Development of the BD-QoL: a quality of life measure specific to Behcet’s disease. J Rheumatol. 2004;31:931–7. [PubMed] [Google Scholar]
- 9.Bhakta BB, Brennan P, James TE, Chamberlain MA, Noble BA, Silman AJ. Behçet’s disease: evaluation of a new instrument to measure clinical activity. Rheumatology. 1999;38:728–33. doi: 10.1093/rheumatology/38.8.728. [DOI] [PubMed] [Google Scholar]
- 10.Forbess C, Swearingen C, Yazici Y. Behçet’s Syndrome Activity Score (BSAS): a new disease activity assessment tool, composed of patient-derived measures only, is strongly correlated with the Behçet’s Disease Current Activity Form (BDCAF) [abstract] Arthritis Rheum. 2008;58 (Suppl):S854. [Google Scholar]
- 11.Ideguchi H, Suda A, Takeno M, Ueda A, Ohno S, Ishigatsubo Y. Behcet disease: evolution of clinical manifestations. Medicine. 2011;90:125–32. doi: 10.1097/MD.0b013e318211bf28. [DOI] [PubMed] [Google Scholar]
- 12.Benamour S, Naji T, Echchilali K, Hamimouch S, Moudatir M, Alaoui FZ, et al. Study of 1034 cases of Behcet’s disease. Clin Exp Rheumatol. 2006;24 (Suppl 42):S13. [Google Scholar]
- 13.Celik S, Fresko I, Sut N, Batumlu NM, Yazici H, Yazici Y. Differences in pain and fatigue perception among a group of rheumatoid arthritis patients in the United States and in Turkey who have similar disease activity and functional status. Clin Exp Rheumatol. 2010;28:884–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definitions of Organ Involvement in Behçet syndrome.