Abstract
The ability of dopamine (DA) neurons to release other transmitters in addition to DA itself has been increasingly recognized, hence the concept of their multilingual nature. A subset of DA neurons, mainly found in the ventral tegmental area, express VGLUT2, allowing them to package and release glutamate onto striatal spiny projection neurons and cholinergic interneurons. Some dopaminergic axon terminals release GABA. Glutamate release by DA neurons has a developmental role, facilitating axonal growth and survival, and may determine in part the critical contribution of the ventral striatum to psychostimulant-induced behavior. Vesicular glutamate coentry may have synergistic effects on vesicular DA filling. The multilingual transmission of DA neurons across multiple striatal domains and the increasing insight into the role of glutamate cotransmission in the ventral striatum highlight the importance of analyzing DA neuron transmission at the synaptic level.
Keywords: dopamine, glutamate, GABA, cotransmission, vesicular
1 THE PARTICULAR NATURE OF DOPAMINE NEURONS
Dopamine (DA) neurons of the ventral midbrain, encompassing the substantia nigra (SN), and ventral tegmental area (VTA), number about 450,000 in humans and 10–25,000 in rodents (German et al., 1983; Oorschot, 1996). Despite their small number, they provide a massive innervation of the striatum and also innervate other cortical and subcortical structures (Gauthier et al., 1999; Prensa et al., 2009). Single DA neurons give rise to approximately half a meter of striatal axon in rat, which is so highly branched that a single axon can occupy about 3% of striatal volume (Matsuda et al., 2009). A single SN neuron may possess over 300,000 axon terminals (Arbuthnott and Wickens, 2007; see also Bolam and Pissadaki, 2012).
Ultrastructural studies show that in the striatum of the rat or the monkey, approximately 70% of the dopaminergic inputs forming synapses are established with dendritic shafts, about one-quarter with dendritic spines, and very few with neuronal cell bodies (Descarries et al., 1996; Freund et al., 1984; Smith et al., 1994). However, the fraction of dopaminergic axonal varicosities that contains synaptic membrane specializations is relatively low. Depending on the methods used, estimates range from 20% to 50% in the rat (Antonopoulos et al., 2002; Descarries et al., 1996; Groves et al., 1994; Pickel et al., 1981) and the incidence appears to be even lower in mice (Berube-Carriere et al., 2012). It has therefore been proposed that DA neurons have two types of axonal release sites: some that are synaptic and others, perhaps the majority, that are nonsynaptic or asynaptic (Beaudet and Descarries, 1978; Berube-Carriere et al., 2012; Bérubé-Carriere et al., 2006; Descarries et al., 1980, 2008). Three-dimensional reconstruction of electron micrographs indicates that synaptic vesicles in dopaminergic axon terminals are spread widely throughout the axon, including narrow regions (Gaugler et al., 2012). Release sites in DA neurons are clearly not yet as well characterized as those in the far more studied glutamatergic hippocampal and cholinergic neuromuscular synapses. In DA neurons, DA release may occur throughout the axon at heterogeneous sites that remain to be fully characterized at the molecular and functional levels. The use of fluorescent false neurotransmitters that act as DA analogs may allow visualization of these release sites, independent of their localization and configuration along axons (Rodriguez et al., 2013). In addition, since catecholamines such as DA can be oxidized, the study of DA exocytosis through amperometric techniques has provided important insights into some of the fundamental properties of DA release sites. For example, amperometric measurements of isolated dopaminergic vesicles provided estimates of approximately 30,000 molecules per vesicle (Omiatek et al., 2013), which is higher than the measured quantal size obtained by amperometry on vesicles fusing in intact axons (approximately 10,000 molecules), therefore supporting the hypothesis that exocytosis can occur by transient fusion (Pereira and Sulzer, 2012; Staal et al., 2004), a concept that remains controversial at other synapses. The rapid kinetics of amperometric measurements have also revealed the duration of the exocytotic events (<100 μs) and provided evidence for multiple transient fusion events (Staal et al., 2004), a phenomenon that could not be resolved with comparatively filtered postsynaptic recordings at conventional ionotropic synapses. In addition, much data suggest that quantal size is a parameter that can be modulated by a wide range of factors including transmitter synthesis, electrochemical gradients, and growth factors (Pereira and Sulzer, 2012; Staal et al., 2004; Sulzer and Pothos, 2000).
Another apparently unusual property of DA neurons is that they have long been known to release DA from their somatodendritic compartment (Geffen et al., 1976; Groves et al., 1975; Korf et al., 1976; Nieoullon et al., 1977), a mechanism that may lead to the activation of somatodendritic autoreceptors and regulate the firing of these neurons and their afferent input. Somatodendritic neurotransmitter release also occurs in other cell types including serotonin neurons (Hery and Ternaux, 1981) and has been well described in the context of neuropeptide release in some systems (Bergquist and Ludwig, 2008). As clusters of synaptic vesicles are essentially absent in dendrites, the most likely candidates for release are tubular structures that express VMAT2 (Nirenberg et al., 1996). The molecular machinery allowing somatodendritic DA release is still incompletely characterized, although a subset of SNARE proteins different from those present in axon terminals appears to be involved (Bergquist et al., 2002; Chen and Rice, 2001; Fortin et al., 2006; Mendez et al., 2011; Witkovsky et al., 2009). Differences between measured somatodendritic and terminal DA release could also involve differences in regional diffusion and reuptake in the VTA versus the striatum (Ford et al., 2010).
2 DISCOVERY OF COTRANSMITTERS IN DA NEURONS
DA neurons figure prominently in the elucidation of neurotransmission and cotransmission in the CNS. DA, which was discovered in the brain in 1957 (Montagu, 1957), was actually the first central neurotransmitter molecule to be identified due to its activation of motor responses in mice and rabbits (Carlsson, 1959; Carlsson and Waldeck, 1958). With the burgeoning of immunocytochemistry, DA neurons were more readily identified by the expression of the rate-limiting enzyme in DA production, tyrosine hydroxylase (TH). In addition to their complex axonal and dendritic arborization, allowing multiple modes of DA release, DA neurons have been known for many years to have the potential to release several other neurotransmitters, including neuropeptides, glutamate, and GABA. The physiological significance and pathological implications of these forms of cotransmission in DA neurons are still in the midst of being examined.
The widespread identification of neuropeptides in neurons otherwise using small-molecule, classical neurotransmitters established the idea of cotransmission in the CNS (Hökfelt et al., 1984). Following the discovery and characterization of the distribution of neuropeptides in the nervous system in the 1980s, it was soon reported that DA neurons of the hypothalamic arcuate nucleus contain neuropeptide Y (Everitt et al., 1986; Harfstrand, 1986), while those of the SN and VTA did not (Murakami et al., 1989). Neurotensin (NT), vasoactive intestinal polypeptide, and cholecystokinin were however reported to be present in subsets of hypothalamic and mesencephalic DA neurons, but with phylogenetic limitations in the case of NT (Hökfelt et al., 1980, 1984; Kalivas et al., 1985; Seroogy et al., 1988; Studler et al., 1988). Neuropeptides are loaded in vesicles at the Golgi apparatus and are thus not released by recycling small synaptic vesicles. Very little is presently known about the regulation of neuropeptide release from DA neurons as well as its physiological and pathological significance.
While the modulatory actions of DA in the CNS were recognized quite early, physiological evidence later suggested that DA neurons also had direct excitatory actions (Wilson et al., 1982). The immunocytochemical visualization of glutamate in DA neurons provided a potential basis for these observations (Ottersen and Storm-Mathisen, 1984). The identification of the glutamate synthetic enzyme glutaminase in DA neurons (Kaneko et al., 1990) further suggested that DA neurons might use glutamate as an excitatory cotransmitter. However, later studies demonstrated that glutaminase is not an ideal and selective marker for glutamatergic neurons, as it is present at varying levels in many neurons and glial cells (Marquez et al., 2009). A new chapter in our understanding of the signaling and connectivity of DA neurons was opened with the report in 1998 of the capacity of rat DA neurons in culture to release glutamate at some of their synaptic contacts ( Joyce and Rayport, 2000; Sulzer et al., 1998) (Fig. 1), in a manner similar to serotonin neurons, which had been shown to do the same in 1994 ( Johnson, 1994). This capacity of DA neurons to mediate glutamate release was shown in vitro to be regulated by D2 autoreceptors and growth factors such as GDNF, suggesting that it can be regulated by some of the same signals that control DA release (Bourque and Trudeau, 2000; Congar et al., 2002; Joyce and Rayport, 2000; Sulzer et al., 1998).
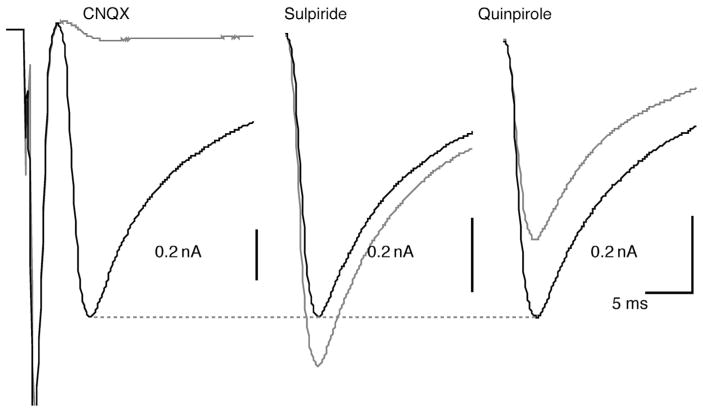
FIGURE 1.
Glutamate-mediated synaptic currents recorded in isolated dopamine neurons in vitro. In an isolated neuron, subsequently shown to be TH-positive, a large autaptic EPSC was recorded under voltage clamp. This was almost completely blocked by CNQX (EPSC was 4% of control; traces shown are averages of 10 stimulations; traces during drug application are shown in gray). The reversible D2 antagonist sulpiride enhanced the EPSC (117%; shown here and in subsequent traces without the initiating action current), whereas the D2 agonist quinpirole markedly attenuated the autaptic EPSC (76%). This suggests that concomitant DA release modulates the glutamate-mediated EPSC.
Over the last decade, the identification of the vesicular neurotransmitter transporters has permitted the demonstration that classical neurotransmitters may be cotransmitters (El Mestikawy et al., 2011; Hnasko and Edwards, 2012). Following the cloning of the vesicular glutamate transporters at the turn of the millennium (see El Mestikawy et al., 2011; Fremeau et al., 2004; Omote et al., 2011, for reviews), the ability of DA neurons to release glutamate was explained by demonstrating that they selectively express VGLUT2, one of the three vesicular glutamate transporters (Dal Bo et al., 2004). In situ hybridization studies mapped the distribution of VGLUT2-expressing DA neurons (Dal Bo et al., 2008; Kawano et al., 2006; Yamaguchi et al., 2007), as will be discussed in more detail later. Single-cell RT-PCR has also been used to demonstrate expression of VGLUT2 mRNA in TH-positive DA neurons (Birgner et al., 2010; Dal Bo et al., 2004; Fortin et al., 2012; Mendez et al., 2008). The proportion of DA neurons containing VGLUT2 mRNA appear higher in single-cell RT-PCR studies than in in situ hybridization studies probably because in situ hybridization does not visualize the low copy numbers of VGLUT2 mRNA found in many TH-positive DA neurons (Li et al., 2013). Interestingly, the expression of VGLUT2 by DA neurons appears to be tightly controlled, as it is upregulated in isolated neurons forming autapses in vitro (Dal Bo et al., 2004; Mendez et al., 2008) and downregulated by contact with GABAergic neurons, both in vitro and in vivo (Mendez et al., 2008). Pathological conditions such as partial 6-OHDA neurotoxic lesions also upregulate the gene in surviving DA neurons (Dal Bo et al., 2008).
Although the initial discoveries of glutamate release and VGLUT2 expression by DA neurons were made in vitro, multiple subsequent experiments validated these findings in vivo in intact mouse and rat brain. First, extracellular stimulation of dopaminergic cell bodies in the VTA was shown to induce glutamate-mediated synaptic responses in ventral striatal neurons (Chuhma et al., 2004) and in the prefrontal cortex (Lavin et al., 2005). Subsequently, conditional deletion (cKO) of the gene encoding VGLUT2 in DA neurons confirmed that such responses were largely mediated by glutamate release from DA neurons (Hnasko et al., 2010). With the advent of optogenetic techniques, the long-range projections of the major modulatory neurotransmitter systems became synaptically accessible. It was shown that channelrhodopsin-2 (ChR2)-mediated photoactivation of dopaminergic axons in striatal brain slices prepared from DA transporter (DAT)-Cre mice (Zhuang et al., 2005) injected with floxed ChR2 viral vectors also induced glutamate-mediated synaptic currents in GABAergic spiny projection neurons (SPNs) of the nucleus accumbens (NAcc) (Stuber et al., 2010; Tecuapetla et al., 2010), with comparatively little response detected in the dorsal striatum (Tritsch et al., 2012). The discovery that such responses are preferentially detected in the NAcc is compatible with the localization of most double-phenotype DA neurons to the VTA and not the SN (Fortin et al., 2012; Yamaguchi et al., 2007, 2013). The reported glutamate corelease in the dorsal striatum (Tritsch et al., 2012) may be dependent on the extent of ChR2 expression, striatal subregion (e.g., medial vs. lateral), or the younger ages of the mice studied, since VGLUT2 expression is developmentally downregulated. In these optogenetic experiments, the magnitude of the evoked currents in SPNs was comparatively small, indicating that few ionotropic postsynaptic glutamate receptors may be activated by glutamate release from DA axons (Koos et al., 2011). This suggests that there may be functional roles of glutamate in mature DA neurons other than to drive action potentials in SPNs.
Indeed, more recently, DA neurons have been shown to make more robust glutamatergic connections to cholinergic interneurons (ChIs), specifically in the medial shell of the NAcc (Chuhma et al., 2014). In the dorsal striatum, DA neurons make discrete dopaminergic connections to ChIs, without glutamatergic or GABAergic components (Chuhma et al., 2014). This work suggests that not only do single CNS neurons use multiple neurotransmitters, but also DA neurons can exert diametrically opposite actions on different target neurons, exhibiting regional and postsynaptic cellular target heterogeneity. Further experiments will be needed to define whether single DA neurons projecting to SPNs or ChIs display differential expression of VGLUT2.
Recent work showing GABA-mediated synaptic currents in striatal SPNs (Tritsch et al., 2012) and in lateral habenula neurons (Stamatakis et al., 2013) elicited by optogenetic activation of dopaminergic axons suggests the existence of yet another possible cotransmitter in DA neurons. Surprisingly, this release was noncanonical in the neurons projecting to the striatum, due to an apparent lack of both the GABA synthetic enzyme GAD and the vesicular GABA transporter vGAT (Tritsch et al., 2012). Such a capacity has also been demonstrated in retinal DA neurons (Hirasawa et al., 2012) and in olfactory bulb DA neurons (Liu et al., 2013) and likely occurs in hypothalamic DA neurons, which show extensive GABA immunoreactivity (Schimchowitsch et al., 1991). Remarkably, DA neurons projecting to ChIs in the dorsal striatum do not appear to release GABA (Chuhma et al., 2014), highlighting further the cell-type specificity of DA neuron cotransmission. Further work will be required to delineate the physiological significance of such GABA release, although a role for the dopaminergic GABA projections to the lateral habenula in reward has already been proposed.
3 LOCALIZATION OF GLUTAMATERGIC AND MIXED PHENOTYPE DA NEURONS IN THE BRAIN
The DA neurons giving rise to the mesocorticolimbic system reside in the A10 region of the ventral midbrain, which comprises two major nuclei of the VTA, the parabrachial pigmented (PBP) nucleus and paranigral nucleus (PN), and three midline nuclei, the caudal linear nucleus (CLi), interfascicular nucleus (IF), and rostral linear nucleus (RLi) of the raphe (Swanson, 1982). Within the A10 region, DA neurons are interspersed with GABAergic and glutamatergic neurons, the latter expressing VGLUT2 (Kawano et al., 2006; Nair-Roberts et al., 2008; Yamaguchi et al., 2007). Some of these VGLUT2 neurons make local synapses (Dobi et al., 2010), countering the notion that all glutamatergic inputs to the A10 region are from extrinsic neurons. The relative prevalence of neurons expressing VGLUT2 mRNA in the PBP and PN nuclei of the VTA in the adult rat has been somewhat controversial, as some studies concluded that only very few VGLUT2 neurons were present in the PBP and PN in the adult rat (Kawano et al., 2006), and other studies reported a high prevalence of VGLUT2 neurons in these nuclei in the mature rat (Yamaguchi et al., 2007, 2011). As previously mentioned, such differences may involve the differential sensitivity of the in situ hybridization methods used for the cellular detection of the low level of VGLUT2 transcripts (Li et al., 2013).
The visualization of VGLUT2 transcripts in the ventral mesencephalon and brain stem made possible a closer evaluation of the specific localization of DA neurons and other catecholaminergic neurons capable of glutamate cotransmission. Indeed, the glutamatergic signaling capacity of catecholaminergic neurons has received strong support following the demonstration of the coexpression of VGLUT2 mRNA and TH mRNA or protein in neurons of the brain stem (Stornetta et al., 2002) and A10 region (Dal Bo et al., 2008; Kawano et al., 2006; Yamaguchi et al., 2007, 2011) (Fig. 2). Although some of the anatomical studies reported VGLUT2–TH coexpressing neurons to be abundant in the VTA of immature rats, but very rare in the adult rat (Bérubé-Carrière et al., 2009), single-cell RT-PCR studies support the hypothesis of continued expression of VGLUT2 in adult DA neurons in the mouse (Berube-Carriere et al., 2012; Mendez et al., 2008). More recent anatomical and molecular biological studies in the adult rat also provide evidence that VGLUT2–TH coexpressing neurons are present at all levels of CLi, RLi, and IF and are interspersed with neurons expressing VGLUT2 but lacking TH (glutamate-only neurons) in the medial portions of PBP and PN (Li et al., 2013; Yamaguchi et al., 2013). VGLUT2-only and VGLUT2–TH coexpressing neurons each innervate the prefrontal cortex and the NAcc, indicating that in addition to the well-recognized mesocorticolimbic DAergic and GABAergic pathways, there exists a parallel mesocorticolimbic glutamatergic pathway comprising both glutamate-only and glutamate–DA neurons (Gorelova et al., 2012; Hnasko et al., 2012; Li et al., 2013; Yamaguchi et al., 2011).
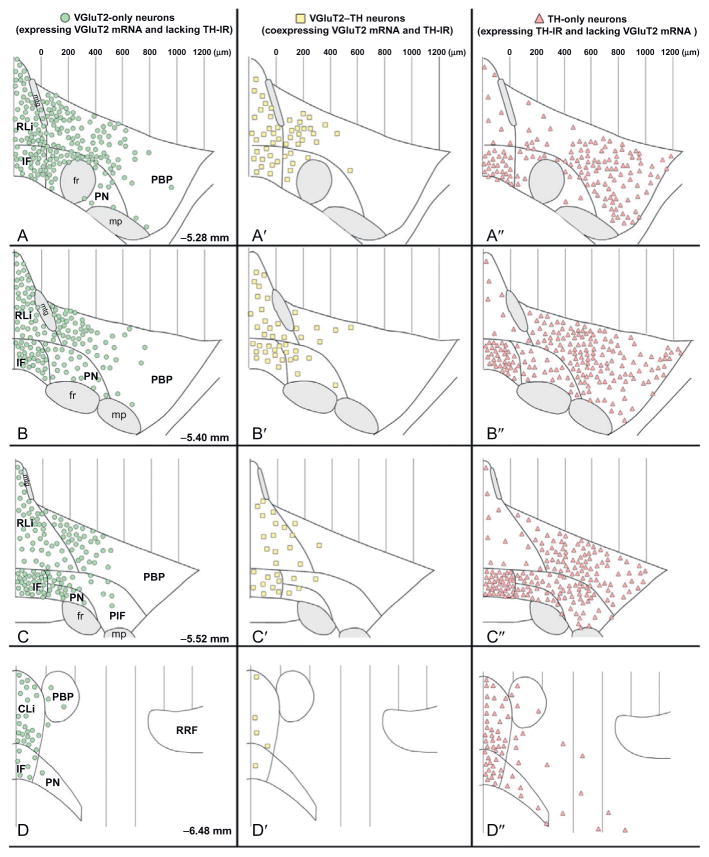
FIGURE 2.
Summary diagram of the cellular heterogeneity within the A10 region. Differential distribution of VGLUT2-only neurons, VGLUT2–TH neurons, and TH-only neurons within each subdivision of the A10 region. (A–D) Distribution of VGLUT2-only neurons (neurons expressing VGLUT2 mRNA but lacking TH immunoreactivity). These VGLUT2-only neurons are present throughout the rostrocaudal levels of each subdivision of the A10 region with a lateromedial increasing gradient of concentration. VGLUT2-only neurons are infrequent in the most lateral region of the PBP, a region with a high concentration of TH-only neurons (A″, B″, and C″). (A′–D′) Distribution of VGLUT2–TH neurons (neurons coexpressing VGLUT2 mRNA and TH immunoreactivity). VGLUT2–TH neurons are restricted to the most rostromedial aspects of the PBP and PN; however, they are present at all rostrocaudal and mediolateral levels of the RLi, CLi, and IF. (A″–D″) Distribution of TH-only neurons (neurons lacking expression of VGLUT2 mRNA but containing TH immunoreactivity). The TH-only neurons are present throughout the rostrocaudal levels of each subdivision of the A10 region, including the lateral aspects of the PBP. Each panel represents the average number of labeled neurons found in three sections, each section from a different rat. PIF, parainterfascicular nucleus; mtg, mammillotegmental tract; fr, fasciculus retroflexus; mp, mammillary peduncle; RRF, retrorubral field.
Taken from Yamaguchi et al. (2007).
The type of axon terminals established by VGLUT2–TH coexpressing neurons in their target areas is still relatively unclear. Although, in vitro, it has proved relatively easy to detect VGLUT2 protein in TH- or DAT-positive varicosities established by DA neurons (Berube-Carriere et al., 2012; Dal Bo et al., 2004; Mendez et al., 2008), in vivo, only sporadic VGLUT2–TH double-labeled varicosities have been detected in the target areas of these neurons, including the NAcc (Berube-Carriere et al., 2012; Fortin et al., 2012; Kawano et al., 2006). Moreover, ultrastructural evidence showed that VGLUT2–TH dual-labeled axon terminals are present in the NAcc of immature rats (Bérubé-Carrière et al., 2009), but absent either in the adult rat (Bérubé-Carrière et al., 2009; Moss et al., 2011) or at any age in mice (Berube-Carriere et al., 2012). Considering the relatively clear evidence for glutamate release in the NAcc in response to optogenetic stimulation of dopaminergic axons of adult mice (Chuhma et al., 2014; Stuber et al., 2010; Tecuapetla et al., 2010), the anatomical findings raise the intriguing hypothesis that double-phenotype DA neurons segregate their glutamatergic and dopaminergic terminals along the same axon in regions such as the NAcc, a possibility that has been considered by a number of groups (Berube-Carriere et al., 2012; Dal Bo et al., 2004; Descarries et al., 2008; El Mestikawy et al., 2011; Fortin et al., 2012; Hattori et al., 1991; Sulzer et al., 1998; S. Zhang and M. Morales, unpublished results) (Fig. 3).
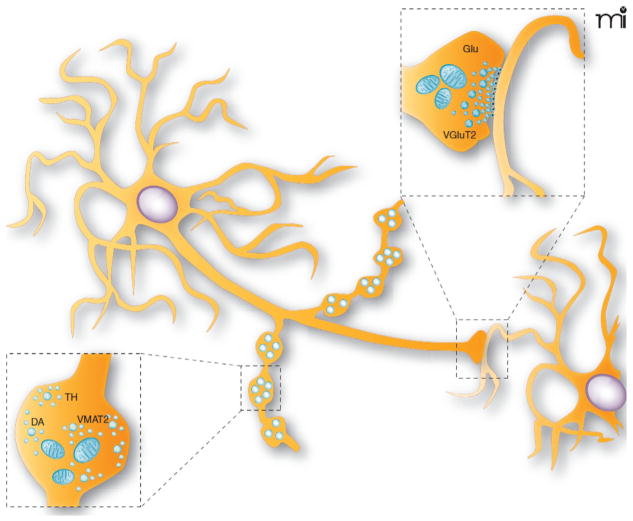
FIGURE 3.
Schematic representation of the synaptic and nonsynaptic axon terminals established by dopamine neurons. DA neurons are known to establish two morphologically distinguishable axon terminals: some that are nonsynaptic and others that are synaptic. The nonsynaptic terminals (varicose-like structures) display no obvious pre- and postsynaptic specializations (see lower illustration showing a magnified view of a single nonsynaptic terminal), contain tyrosine hydroxylase (TH), and could be specialized for the release of DA. The synaptic terminals display a more classical active zone, postsynaptic density, and synaptic cleft and could be the site of VGLUT2 expression and of glutamate (Glu) release (see upper illustration showing a magnified view of a synaptic axon terminal). Although not shown, some of the terminals can also contain both TH and VGLUT2.
Taken from Trudeau and Gutierrez (2007).
4 SYNAPTIC CONNECTIVITY AND PLASTICITY OF GLUTAMATE RELEASE BY DA NEURONS
With global expression of optogenetic probes such as ChR2 in a genetically defined population of neurons and recording systematically from identified target neurons, it becomes possible to define key metrics of synaptic connectivity, including the incidence of connections and their strength. In the initial application of this functional connectome approach, restricted expression of ChR2 in striatal SPNs allowed measurements of functional connections of SPNs to principal intrinsic neurons and their projections to the pallidum and ventral midbrain (Chuhma et al., 2011); this revealed distinctive connectivity, with quantitative measures, and projection-specific DA modulation.
DA neuron terminals are the molecular target of psychostimulant action, so it would be anticipated that addictive doses or chronic administration of stimulants should affect connectivity. With comprehensive viral transfection of DA neurons with ChR2, a functional connectome analysis can be applied to drug-induced plasticity (Fig. 4). Surprisingly, a recent study revealed that repeated cocaine exposure had minimal impact on DA neuron connections to SPNs (Ishikawa et al., 2013). In contrast, a single dose of amphetamine had profound effects on DA neuron connections to striatal ChIs. A few hours after a single low dose of amphetamine, which induced hyperlocomotion, functional connectome measures showed a profound reduction in DA neuron glutamatergic connections to ChIs in the medial shell of the NAcc (Chuhma et al., 2014). Consistent with the lack of repeated cocaine exposure on connections to SPNs, no impact was seen of amphetamine exposure on DA neuron connections to SPNs. Several hours after a single high dose of amphetamine, which induced stereotypy, dopaminergic connections to ChIs were significantly attenuated. This differential drug-induced plasticity in DA neuron connections shows further how DA neuron transmission can be heterogeneously affected on a dose- and region-dependent manner by drugs of abuse.
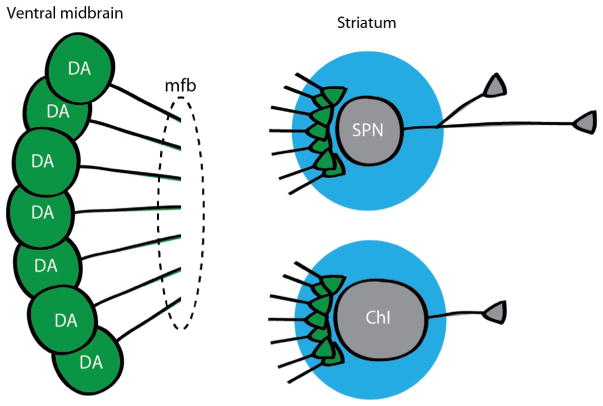
FIGURE 4.
Functional connectome analysis of DA neuron connections in striatum. Mice are generated with ChR2 expressed selectively in DA neurons. DA neuron cell bodies are shown in the ventral midbrain (left) with their axons projecting into the median forebrain bundle (mfb) toward the striatum. In the striatum (right), spiny projection neurons (SPNs) and cholinergic interneurons (ChIs) are recorded and DA neuron inputs activated by wide-field photostimulation (larger circle around cells and terminals). With repeated measurements, the incidence and strength of DA neuron inputs to SPNs and ChIs are quantitated to determine functional connectivity.
5 DEVELOPMENTAL ROLE OF GLUTAMATE RELEASE BY DA NEURONS
Although the developmental pattern of expression of the three VGLUTs has never been extensively examined, initial comparative evaluations of the expression of VGLUT1, VGLUT2, and VGLUT3 in the rat brain revealed that while at birth, expression of VGLUT1 or VGLUT3 was quite low, that of VGLUT2 was already quite high (Boulland et al., 2004; Schafer et al., 2002). Compatible with the finding of early expression of VGLUT2, in situ hybridization studies reported high expression of VGLUT2 in the embryonic ventral mesencephalon as early as E12 (Birgner et al., 2010) and E14 (Dal Bo et al., 2008). Single-cell RT-PCR data also support embryonic and perinatal expression of VGLUT2 in mesencephalic DA neurons (Birgner et al., 2010; Dal Bo et al., 2008; Mendez et al., 2008). Considering these findings and the recent demonstration that glutamate can positively regulate the growth and axonal branching of DA neurons (Schmitz et al., 2009, 2013), a plausible hypothesis is that one of the functions of glutamate release by mesencephalic DA neurons is to promote the growth and connectivity of these neurons during development.
Arguing in favor of this hypothesis, a recent paper reported that cKO of the gene encoding VGLUT2 in DA neurons caused a number of morphological and functional alterations that are compatible with reduced axonal development (Berube-Carriere et al., 2012). The cKO was carried out by using a DAT-Cre mouse (Ekstrand et al., 2007; Zhuang et al., 2005), which, based on the known developmental expression pattern of DAT, should drive Cre recombinase expression and gene deletion approximately by embryonic days 13 or 14 (Fujita et al., 1993; Le et al., 1992; Perrone-Capano et al., 1994). The cKO mice showed reduced numbers of TH-positive neurons in the VTA and SN and a reduced density of TH-positive axon terminals in the NAcc (Fortin et al., 2012). Intriguingly, in a separate study, no change in TH protein or enzymatic activity was detected in NAcc from similar mice (Hnasko et al., 2010). Examined in vitro, DA neurons from these mice showed reduced axonal growth (Fortin et al., 2012), compatible with the possibility that glutamate release from growth cones contributes to the growth potential of these neurons. The presence of glutamate NMDA receptors on the growth cones of cultured DA neurons has been demonstrated previously, making the autocrine action of glutamate plausible (Schmitz et al., 2009). Further work will be required to determine whether glutamate release from growth cones induces growth through autocrine activation of receptors on the growth cone itself or through activation of glutamate receptors on target cells, such as astrocytes, that could provide retrograde trophic factor support.
The VGLUT2 cKO also led to reduced action potential-evoked DA release in the NAcc (Fortin et al., 2012; Hnasko et al., 2010). This decreased release could result from the reduced number of DA neurons and reduced axonal growth. Intriguingly, although the number of DA neurons was reduced both in the VTA and in the SN, DA release was only reduced in the NAcc and not in the dorsal striatum (Fortin et al., 2012). One possibility that remains to be evaluated is that the reduced number of DA neurons in the SN was due to loss of the small contingent of SN neurons that project to the NAcc (Lynd-Balta and Haber, 1994a,b; Prensa and Parent, 2001; Prensa et al., 2009). In a separate cKO mouse, potassium-evoked DA release was found to be reduced in both the NAcc and the dorsal striatum (Alsiö et al., 2011). An alternate explanation of the reduced DA in these cKO mice is that the loss of vesicular synergy (El Mestikawy et al., 2011; Gras et al., 2008) between VGLUT2 and VMAT2 leads to reduced packaging of DA inside vesicles, as recently suggested (Hnasko et al., 2010, 2012). As previously mentioned, evidence for the partial segregation of VGLUT2 in a distinct set of axon terminals in DA neurons (Berube-Carriere et al., 2012; Dal Bo et al., 2004; Fortin et al., 2012; Sulzer et al., 1998) may argue against contribution of vesicular synergy in the adult, at least at some presynaptic sites. The reduced number of TH-positive DA neurons and terminals in VGLUT2 cKO mice could be due to the degeneration of DA neurons lacking the trophic signaling provided by glutamate. Further work will be required to test this and to consider alternate possibilities such as that the reduced number of neurons results from downregulation of the dopaminergic phenotype of these neurons.
6 POSSIBLE CONTRIBUTION OF VGLUT2 IN DA NEURONS TO VESICULAR SYNERGY
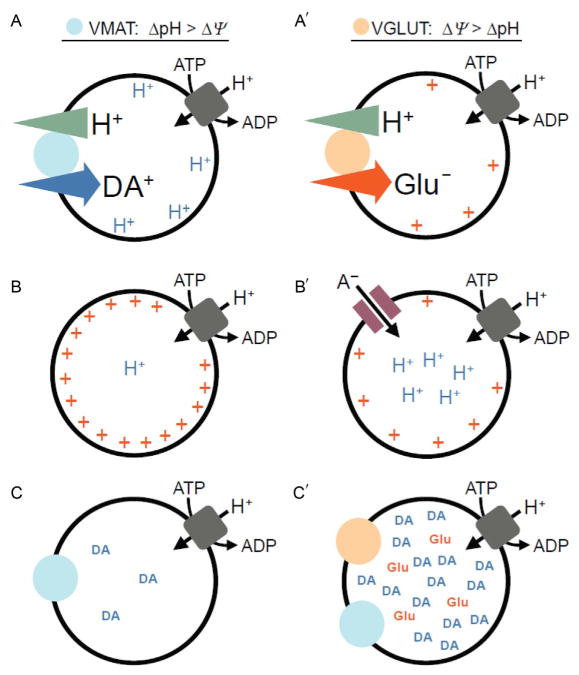
Vesicular neurotransmitter loading is dependent on the transport of protons (H+) into the vesicle lumen mediated by the vacuolar H+-ATPase (vATPase), thereby establishing a H+ electrochemical gradient (ΔμH+) composed of a chemical gradient (ΔpH) and membrane potential (ΔΦ). However, the vesicular accumulation of different transmitters by distinct transport mechanisms is differentially dependent on ΔpH versus ΔΦ (Edwards, 2007). DA and other substrates of the VMAT2 rely heavily on ΔpH, while glutamate transport through the VGLUT is dependent most on ΔΦ (Fig. 5A). Because the stoichiometry of the VMAT2 requires the exchange of two lumenal H+ for one charged cytosolic DA+, DA accumulation results in depletion of ΔpH relative to ΔΦ; efficient DA filling thus requires mechanisms to dissipate ΔΦ ( Johnson et al., 1981; Knoth et al., 1981). One such mechanism could involve glutamate; because glutamate is an anion, its entry through the VGLUT dissipates ΔΦ leading to secondary activation of the vATPase and increased ΔpH (Fig. 5B) (Maycox et al., 1988; Tabb et al., 1992).
FIGURE 5.
Vesicular coloading of glutamate with dopamine leads to synergistic effects on vesicle filling. Vesicular neurotransmitter loading is dependent on both the ΔpH and ΔΦ established by the vATPase. However, due to both the stoichiometry of the transporters and the opposite charges of the substrates, (A) dopamine loading through VMAT is dependent mostly on ΔpH, while (A′) glutamate uptake through VGLUT depends principally on ΔΦ. Although the efficient filling of vesicles with dopamine relies on ΔpH—in the absence of a counterion (B)—the transport of H+ by the vATPase sets up a large ΔΦ that impedes vesicle acidification. The generation of a large ΔpH therefore requires dissipation of ΔΦ, for example, by the (B′) entry of a permeant anion. Chloride anions are abundant at the nerve terminal and serve this function. However, glutamate anions are also present, and their vesicular entry through VGLUT can facilitate acidification (i.e., increase ΔpH) of dopamine-containing synaptic vesicles. (C) This model predicts increased dopamine quantal content for a subset of (C′) VGLUT2- and VMAT2-cocontaining vesicles. Abbreviations: ΔpH, vesicular proton gradient; ΔΦ, vesicular membrane potential; H+, protons; A−, anion; DA, dopamine; Glu, glutamate.
Because vesicular monoamine loading is principally dependent on ΔpH and because glutamate loading increases ΔpH, the potential for glutamate coentry to increase DA quantal content has been suggested (Fig. 5C). This mechanism requires localization of VGLUT2 and VMAT2 in the same synaptic vesicles. Consistent with this possibility, vesicular coimmunoisolation of VMAT2 with VGLUT2 has been demonstrated in rat NAcc (Hnasko et al., 2010). It was further shown that glutamate selectively increases uptake of serotonin into synaptic vesicles isolated from the NAcc of 3-week-old rats, providing biophysical evidence that vesicular glutamate coentry contributes to an increase in vesicular DA filling in a native vesicle preparation. Similar experiments have not yet been performed in synaptic vesicles prepared from mice or from adult rats. Along with a developmental impairment in the mesolimbic DA pathway, discussed in the preceding text, vesicular synergy provides an alternate explanation for the reduction in NAcc DA tissue content, evoked DA release, and psychostimulant-induced locomotion in cKO mice lacking VGLUT2 selectively in DA neurons (discussed in the succeeding text) (Alsiö et al., 2011; Birgner et al., 2010; Fortin et al., 2012; Hnasko et al., 2010). Importantly, a similar role for vesicular glutamate coentry promoting the loading of other cationic transmitters has been observed, suggesting this mechanism may be general (Amilhon et al., 2010; El Mestikawy et al., 2011; Gras et al., 2008; Hnasko and Edwards, 2012).
Although the colocalization of VGLUT2 and TH by dual immuno-EM in DA terminals of young (P15) rats (Dal Bo et al., 2008; Descarries et al., 2008) is consistent with the possibility that VGLUT2 and VMAT2 colocalize in vesicles in a subset of dopaminergic axon terminals in young rats, the lack of TH and VGLUT2 colocalization in young mice and in adult rats or mice (Berube-Carriere et al., 2012; Bérubé-Carrière et al., 2009; Moss et al., 2011) argue that vesicular synergy may be a developmentally or species-restricted phenomenon. Finally, it is possible that L-dopa synthesized by TH diffuses to neighboring axon terminals lacking TH or DAT where it is released after conversion to DA. Further experiments will be required to explore the possibility that vesicular colocalization and vesicular synergy may be restricted to subpopulations of DA neuron terminals, on the developmental stage, and on species.
7 DOES GLUTAMATE CORELEASE MEDIATE A REWARD-RELEVANT SIGNAL?
Several experimental approaches have demonstrated that phasic increases in mesencephalic DA neuron firing occur in response to unexpected rewards or to reward predictive cues; such observations have led to the influential hypothesis that phasic DA responses encode a reward-prediction error (Schultz, 2013; Schultz et al., 1997). The cellular and molecular mechanisms by which released DA shape learning remain less clear but involve neuromodulatory effects on excitatory inputs to the striatum and other regions. Although DA-mediated synaptic effects can sometimes be relatively fast (a few tens of ms) (Beckstead et al., 2004; Chuhma et al., 2014; Gantz et al., 2013; Schmitz et al., 2002), postsynaptic actions mediated by metabotropic DA receptors are nonetheless generally considered slower in onset and duration compared to the very rapid effects of ionotropic neurotransmitter receptors. The utility of DA to mediate a temporally precise postsynaptic reward-prediction error signal has therefore been questioned (Lapish et al., 2007; Seamans and Yang, 2004), and testing the potential for glutamate corelease as mediating a reward-relevant fast excitatory synaptic signal has driven much of the recent work on the possible functional roles of the glutamate cophenotype of DA neurons.
To define the functional consequences of glutamate release by DA neurons, several laboratories have employed a conditional Cre–Lox strategy to disrupt the gene encoding VGLUT2 (reviewed in Wallén-Mackenzie et al., 2010) and hence vesicular glutamate packaging and release, specifically in DA neurons. In each case, cKO mice carrying floxed Slc17a6 (i.e., VGLUT2) alleles were crossed with mice expressing Cre recombinase under the control of DAT (Slc6a3) regulatory sequences (Alsiö et al., 2011; Birgner et al., 2010; Fortin et al., 2012; Hnasko et al., 2010). Although the precise mutant mouse lines and background strains have varied across laboratories, several key results have been independently corroborated and indicate that VGLUT2 expression in DA neurons shapes behavioral responses to psychostimulants and other reinforcers.
Acute behavioral responses to the psychostimulants amphetamine and cocaine, which elicit hyperlocomotion, were blunted in cKO mice (Birgner et al., 2010; Fortin et al., 2012; Hnasko et al., 2010). Notably, when tested with higher doses of amphetamine, behavioral activation was still blunted, but the distance traveled was actually higher in cKO than control mice, suggesting that cKO mice are less sensitive to the stereotypy inducing effects of higher-dose amphetamine (Birgner et al., 2010). In a cocaine (and morphine)-conditioned place preference paradigm, a commonly used test for measuring the reinforcing properties of drugs of abuse, cKO mice did not display any deviations from controls (Hnasko et al., 2010; Å. Wallén-Mackenzie, unpublished). However, on operant self-administration tasks, cKO mice consumed higher levels of both sugar and cocaine and displayed a significant elevation in cue-induced drug seeking, a behavioral model of relapse (Alsiö et al., 2011). The cKO mice show no significant differences in cognition measured in the radial arm maze, anxiety measured in the elevated-plus test, or depression measured in the forced swimming test (Birgner et al., 2010; Fortin et al., 2012). However, in the rotarod test of motor coordination, one study found a decrease in performance (Fortin et al., 2012), while others did not (Birgner et al., 2010; Hnasko et al., 2010).
cKO mice thus show diminished behavioral responses to acute and repeated psychostimulant administration but display heightened reward seeking for both sugar and cocaine. These behavioral changes appear to be consistent with the reductions in DA stores and release seen in the cKO (Alsiö et al., 2011; Fortin et al., 2012; Hnasko et al., 2010) and may indicate that in the absence of VGLUT2, rewards produce a weaker DA response and are consequently more sought-after. An alternate possibility is that the changes in drug intake result indirectly from developmental alteration of the mesotelencephalic DA system due to embryonic deletion of the gene encoding VGLUT2, as suggested by biochemical assays revealing multiple adaptations including altered DA receptor levels (Alsiö et al., 2011). However, it remains to be definitively established whether these behavioral perturbations are due to the effects of VGLUT2 on DA packaging, direct alterations in glutamate signaling, developmental changes, or some combination thereof.
In conclusion, the work discussed in this chapter suggests that DA neurons establish a highly heterogeneous set of axonal release sites, allowing the release of DA, glutamate, GABA, or combinations thereof onto target neurons at different sites in the brain, adding further dimensions to the multilingual nature of DA neuron transmission. Further work will be required to extend our understanding of the fine localization and function of individual release sites established by individual DA neurons. While glutamate release by DA neurons contributes to the acute response of animals to natural rewards and psychostimulant drugs, additional work will be required to establish the specific mechanisms involved.
Acknowledgments
Work in the Trudeau laboratory was supported by grants from the Canadian Institutes of Health Research (MOP-106556), by the Parkinson Society Canada, by the Brain Canada Foundation and Krembil Foundation, and by an infrastructure grant from the Fonds de recherche du Québec (Santé). Work in the Wallén-Mackenzie laboratory was supported by the Swedish Medical Research Council (SMRC 2007–5742 and 2011–4747), Uppsala University, the Swedish Brain Foundation, Parkinsonfonden, and the foundations of Bertil Ha °llsten, Major Gösta Lind, Åhlén, and Åke Wiberg. Work in the Hnasko laboratory was supported by NIH grant K01DA026504. Work in the Morales group was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA, NIH). Work in the Rayport laboratory was supported by grants from NIDA (DA017978), NIMH (MH086404), and NARSAD. Work in the Sulzer laboratory was supported by NIDA 10154 and 07418; NIAAA 19801, a Udall Center of Excellence Award; and the Parkinson’s Disease and JPB Foundations. Thanks to Yvonne Schmitz, Daniela Pereira, and Guillaume Fortin for their comments on the manuscript.
References
- Alsiö J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, Trudeau LE, Kullander K, Levesque D, Wallén-Mackenzie Å. Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J Neurosci. 2011;31:12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos J, Dori I, Dinopoulos A, Chiotelli M, Parnavelas JG. Postnatal development of the dopaminergic system of the striatum in the rat. Neuroscience. 2002;110:245–256. doi: 10.1016/s0306-4522(01)00575-9. [DOI] [PubMed] [Google Scholar]
- Arbuthnott GW, Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30:62–69. doi: 10.1016/j.tins.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Beaudet A, Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3:851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Ludwig M. Dendritic transmitter release: a comparison of two model systems. J Neuroendocrinol. 2008;20:677–686. doi: 10.1111/j.1365-2826.2008.01714.x. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Niazi HS, Nissbrandt H. Evidence for different exocytosis pathways in dendritic and terminal dopamine release in vivo. Brain Res. 2002;950:245–253. doi: 10.1016/s0006-8993(02)03047-0. [DOI] [PubMed] [Google Scholar]
- Bérubé-Carriere N, Riad M, Dal Bo G, Trudeau L-E, Descarries L. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2006. Colocalisation of Dopamine and Glutamate in Axon Terminals of VTA Neurons Innervating the Nucleus Accumbens. Online:722.711. [Google Scholar]
- Bérubé-Carrière N, Riad M, Dal Bo G, Lévesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Berube-Carriere N, Guay G, Fortin GM, Kullander K, Olson L, Wallén-Mackenzie Å, Trudeau LE, Descarries L. Ultrastructural characterization of the mesostriatal dopamine innervation in mice, including two mouse lines of conditional VGLUT2 knockout in dopamine neurons. Eur J Neurosci. 2012;35:527–538. doi: 10.1111/j.1460-9568.2012.07992.x. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallen-Mackenzie Å. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci USA. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Pissadaki EK. Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord. 2012;27:1478–1483. doi: 10.1002/mds.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RTJ, Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959;11:490–493. [PubMed] [Google Scholar]
- Carlsson A, Waldeck B. A fluorimetric method for the determination of dopamine (3-hydroxytyramine) Acta Physiol Scand. 1958;44:293–298. doi: 10.1111/j.1748-1716.1958.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81:901–912. doi: 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congar P, Bergevin A, Trudeau LE. D2 receptors inhibit the secretory process downstream from calcium influx in dopaminergic neurons: implication of k(+) channels. J Neurophysiol. 2002;87:1046–1056. doi: 10.1152/jn.00459.2001. [DOI] [PubMed] [Google Scholar]
- Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88:1398–1405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- Dal Bo G, Bérubé-Carrière N, Mendez JA, Leo D, Riad M, Descarries L, Lévesque D, Trudeau LE. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 2008;156:59–70. doi: 10.1016/j.neuroscience.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Descarries L, Bosler O, Berthelet F, Des Rosiers MH. Dopaminergic nerve endings visualised by high-resolution autoradiography in adult rat neostriatum. Nature. 1980;284:620–622. doi: 10.1038/284620a0. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Bosler O, Doucet G. Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: a quantitative autoradiographic and immunocytochemical analysis. J Comp Neurol. 1996;375:167–186. doi: 10.1002/(SICI)1096-9861(19961111)375:2<167::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Descarries L, Bérubé-Carrière N, Riad M, Bo GD, Mendez JA, Trudeau LE. Glutamate in dopamine neurons: synaptic versus diffuse transmission. Brain Res Rev. 2008;58:290–302. doi: 10.1016/j.brainresrev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci USA. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mestikawy S, Wallén-Mackenzie Å, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Meister B, Hokfelt T, Melander T, Terenius L, Rokaeus A, Theodorsson-Norheim E, Dockray G, Edwardson J, Cuello C, et al. The hypothalamic arcuate nucleus-median eminence complex: immunohistochemistry of transmitters, peptides and DARPP-32 with special reference to coexistence in dopamine neurons. Brain Res. 1986;396:97–155. doi: 10.1016/s0006-8993(86)80192-5. [DOI] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PE, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin GD, Desrosiers CC, Yamaguchi N, Trudeau LE. Basal somatodendritic dopamine release requires snare proteins. J Neurochem. 2006;96:1740–1749. doi: 10.1111/j.1471-4159.2006.03699.x. [DOI] [PubMed] [Google Scholar]
- Fortin GM, Bourque MJ, Mendez JA, Leo D, Nordenankar K, Birgner C, Arvidsson E, Rymar VV, Berube-Carriere N, Claveau AM, Descarries L, Sadikot AF, Wallén-Mackenzie Å, Trudeau LE. Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J Neurosci. 2012;32:17477–17491. doi: 10.1523/JNEUROSCI.1939-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Fujita M, Shimada S, Nishimura T, Uhl GR, Tohyama M. Ontogeny of dopamine transporter mRNA expression in the rat brain. Brain Res Mol Brain Res. 1993;19:222–226. doi: 10.1016/0169-328x(93)90031-j. [DOI] [PubMed] [Google Scholar]
- Gantz SC, Bunzow JR, Williams JT. Spontaneous inhibitory synaptic currents mediated by a G protein-coupled receptor. Neuron. 2013;78:807–812. doi: 10.1016/j.neuron.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler MN, Genc O, Bobela W, Mohanna S, Ardah MT, El-Agnaf OM, Cantoni M, Bensadoun JC, Schneggenburger R, Knott GW, Aebischer P, Schneider BL. Nigrostriatal overabundance of alpha-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 2012;123:653–669. doi: 10.1007/s00401-012-0963-y. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Parent M, Lévesque M, Parent A. The axonal arborization of single nigrostriatal neurons in rats. Brain Res. 1999;834:228–232. doi: 10.1016/s0006-8993(99)01573-5. [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- German DC, Schlusselberg DS, Woodward DJ. Three-dimensional computer reconstruction of midbrain dopaminergic neuronal populations: from mouse to man. J Neural Transm. 1983;57:243–254. doi: 10.1007/BF01248996. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cereb Cortex. 2012;22:327–336. doi: 10.1093/cercor/bhr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- Groves PM, Wilson CJ, Young SJ, Rebec GV. Self-inhibition by dopaminergic neurons. Science. 1975;190:522–528. doi: 10.1126/science.242074. [DOI] [PubMed] [Google Scholar]
- Groves PM, Linder JC, Young SJ. 5-hydroxydopamine-labeled dopaminergic axons: three-dimensional reconstructions of axons, synapses and postsynaptic targets in rat neostriatum. Neuroscience. 1994;58:593–604. doi: 10.1016/0306-4522(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Harfstrand A. Intraventricular administration of neuropeptide Y (NPY) induces hypotension, bradycardia and bradypnoea in the awake unrestrained male rat. Counteraction by NPY-induced feeding behaviour. Acta Physiol Scand. 1986;128:121–123. doi: 10.1111/j.1748-1716.1986.tb07957.x. [DOI] [PubMed] [Google Scholar]
- Hattori T, Takada M, Moriizumi T, Van der Kooy D. Single dopaminergic nigrostriatal neurons form two chemically distinct synaptic types: possible transmitter segregation within neurons. J Comp Neurol. 1991;309:391–401. doi: 10.1002/cne.903090308. [DOI] [PubMed] [Google Scholar]
- Hery F, Ternaux JP. Regulation of release processes in central serotoninergic neurons. J Physiol. 1981;77:287–301. [PubMed] [Google Scholar]
- Hirasawa H, Betensky RA, Raviola E. Corelease of dopamine and GABA by a retinal dopaminergic neuron. J Neurosci. 2012;32:13281–13291. doi: 10.1523/JNEUROSCI.2213-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Lundberg JM, Schultzberg M, Johansson O, Skirboll L, Anggard A, Fredholm B, Hamberger B, Pernow B, Rehfeld J, Goldstein M. Cellular localization of peptides in neural structures. Proc R Soc Lond B Biol Sci. 1980;210:63–77. doi: 10.1098/rspb.1980.0119. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J Comp Neurol. 1984;222:543–559. doi: 10.1002/cne.902220407. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Johansson O, Goldstein M. Chemical anatomy of the brain. Science (New York, NY) 1984;225:1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Otaka M, Neumann PA, Wang Z, Cook JM, Schlüter OM, Dong Y, Huang YH. Exposure to cocaine regulates inhibitory synaptic transmission from the ventral tegmental area to the nucleus accumbens. J Physiol Lond. 2013;591:4827–4841. doi: 10.1113/jphysiol.2013.262915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD. Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron. 1994;12:433–442. doi: 10.1016/0896-6273(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Carty SE, Scarpa A. Proton: substrate stoichiometries during active transport of biogenic amines in chromaffin ghosts. J Biol Chem. 1981;256:5773–5780. [PubMed] [Google Scholar]
- Joyce MP, Rayport S. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience. 2000;99:445–456. doi: 10.1016/s0306-4522(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Deutch AY, Maggio JE, Mantyh PW, Roth RH. Substance K and substance P in the ventral tegmental area. Neurosci Lett. 1985;57:241–246. doi: 10.1016/0304-3940(85)90498-7. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Immunohistochemical demonstration of glutaminase in catecholaminergic and serotoninergic neurons of rat brain. Brain Res. 1990;507:151–154. doi: 10.1016/0006-8993(90)90535-j. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Knoth J, Zallakian M, Njus D. Stoichiometry of H+-linked dopamine transport in chromaffin granule ghosts. Biochemistry. 1981;20:6625–6629. doi: 10.1021/bi00526a016. [DOI] [PubMed] [Google Scholar]
- Koos T, Tecuapetla F, Tepper JM. Glutamatergic signaling by midbrain dopaminergic neurons: recent insights from optogenetic, molecular and behavioral studies. Curr Opin Neurobiol. 2011;21:393–401. doi: 10.1016/j.conb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf J, Zieleman M, Westerink BH. Dopamine release in substantia nigra? Nature. 1976;260:257–258. doi: 10.1038/260257a0. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007;191:609–625. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci. 2005;25:5013–5023. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le WD, Bostwick JR, Appel SH. Use of [3H]-GBR12935 to measure dopaminergic nerve terminal growth into the developing rat striatum. Brain Res Dev Brain Res. 1992;67:375–377. doi: 10.1016/0165-3806(92)90238-r. [DOI] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct. 2013;218:1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Liu S, Plachez C, Shao Z, Puche A, Shipley MT. Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J Neurosci. 2013;33:2916–2926. doi: 10.1523/JNEUROSCI.3607-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994a;59:609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994b;59:625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Marquez J, Tosina M, de la Rosa V, Segura JA, Alonso FJ, Mates JM, Campos-Sandoval JA. New insights into brain glutaminases: beyond their role on glutamatergic transmission. Neurochem Int. 2009;55:64–70. doi: 10.1016/j.neuint.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycox PR, Deckwerth T, Hell JW, Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988;263:15423–15428. [PubMed] [Google Scholar]
- Mendez JA, Bourque MJ, Dal Bo G, Bourdeau ML, Danik M, Williams S, Lacaille JC, Trudeau LE. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci. 2008;28:6309–6318. doi: 10.1523/JNEUROSCI.1331-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JA, Bourque MJ, Fasano C, Kortleven C, Trudeau LE. Somatodendritic dopamine release requires synaptotagmin 4 and 7 and the participation of voltage-gated calcium channels. J Biol Chem. 2011;286:23928–23937. doi: 10.1074/jbc.M111.218032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagu KA. Catechol compounds in rat tissues and in brains of different animals. Nature. 1957;180:244–245. doi: 10.1038/180244a0. [DOI] [PubMed] [Google Scholar]
- Moss J, Ungless MA, Bolam JP. Dopaminergic axons in different divisions of the adult rat striatal complex do not express vesicular glutamate transporters. Eur J Neurosci. 2011;33:1205–1211. doi: 10.1111/j.1460-9568.2011.07594.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Okamura H, Pelletier G, Ibata Y. Differential colocalization of neuropeptide Y- and methionine-enkephalin-Arg6-Gly7-Leu8-like immunoreactivity in catecholaminergic neurons in the rat brain stem. J Comp Neurol. 1989;281:532–544. doi: 10.1002/cne.902810404. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A, Cheramy A, Glowinski J. Release of dopamine in vivo from cat substantia nigra. Nature. 1977;266:375–377. doi: 10.1038/266375a0. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;15:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omiatek DM, Bressler AJ, Cans AS, Andrews AM, Heien ML, Ewing AG. The real catecholamine content of secretory vesicles in the CNS revealed by electrochemical cytometry. Sci Rep. 2013;3:1447. doi: 10.1038/srep01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote H, Miyaji T, Juge N, Moriyama Y. Vesicular neurotransmitter transporter: bioenergetics and regulation of glutamate transport. Biochemistry. 2011;50:5558–5565. doi: 10.1021/bi200567k. [DOI] [PubMed] [Google Scholar]
- Oorschot DE. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol. 1996;366:580–599. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984;229:374–392. doi: 10.1002/cne.902290308. [DOI] [PubMed] [Google Scholar]
- Pereira DB, Sulzer D. Mechanisms of dopamine quantal size regulation. Front Biosci. 2012;17:2740–2767. doi: 10.2741/4083. [DOI] [PubMed] [Google Scholar]
- Perrone-Capano C, Tino A, di Porzio U. Target cells modulate dopamine transporter gene expression during brain development. Neuroreport. 1994;5:1145–1148. doi: 10.1097/00001756-199405000-00031. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Beckley SC, Joh TH, Reis DJ. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225:373–385. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- Prensa L, Parent A. The nigrostriatal pathway in the rat: a single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci. 2001;21:7247–7260. doi: 10.1523/JNEUROSCI.21-18-07247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensa L, Gimenez-Amaya JM, Parent A, Bernacer J, Cebrian C. The nigrostriatal pathway: axonal collateralization and compartmental specificity. J Neural Transm Suppl. 2009:49–58. doi: 10.1007/978-3-211-92660-4_4. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Pereira DB, Borgkvist A, Wong MY, Barnard C, Sonders MS, Zhang H, Sames D, Sulzer D. Fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. Proc Natl Acad Sci USA. 2013;110:870–875. doi: 10.1073/pnas.1213569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Schimchowitsch S, Vuillez P, Tappaz ML, Klein MJ, Stoeckel ME. Systematic presence of GABA-immunoreactivity in the tuberoinfundibular and tuberohypophyseal dopaminergic axonal systems: an ultrastructural immunogold study on several mammals. Exp Brain Res. 1991;83:575–586. doi: 10.1007/BF00229836. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22:8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Luccarelli J, Kim M, Wang M, Sulzer D. Glutamate controls growth rate and branching of dopaminergic axons. J Neurosci. 2009;29:11973–11981. doi: 10.1523/JNEUROSCI.2927-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Castagna C, Mrejeru A, Lizardi-Ortiz JE, Klein Z, Lindsley CW, Sulzer D. Glycine transporter-1 inhibition promotes striatal axon sprouting via NMDA receptors in dopamine neurons. J Neurosci. 2013;33:16778–16789. doi: 10.1523/JNEUROSCI.3041-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Ceccatelli S, Schalling M, Hokfelt T, Frey P, Walsh J, Dockray G, Brown J, Buchan A, Goldstein M. A subpopulation of dopaminergic neurons in rat ventral mesencephalon contains both neurotensin and cholecystokinin. Brain Res. 1988;455:88–98. doi: 10.1016/0006-8993(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- Staal RG, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol. 2002;444:207–220. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studler JM, Kitabgi P, Tramu G, Herve D, Glowinski J, Tassin JP. Extensive co-localization of neurotensin with dopamine in rat mesocortico-frontal dopaminergic neurons. Neuropeptides. 1988;11:95–100. doi: 10.1016/0143-4179(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Pothos EN. Regulation of quantal size by presynaptic mechanisms. Rev Neurosci. 2000;11:159–212. doi: 10.1515/revneuro.2000.11.2-3.159. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tabb JS, Kish PE, Van Dyke R, Ueda T. Glutamate transport into synaptic vesicles. Roles of membrane potential, pH gradient, and intravesicular pH. J Biol Chem. 1992;267:15412–15418. [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Gutierrez R. On cotransmission and neurotransmitter phenotype plasticity. Mol Interv. 2007;7(3):138–146. doi: 10.1124/mi.7.3.5. [DOI] [PubMed] [Google Scholar]
- Wallén-Mackenzie Å, Wootz H, Englund H. Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: what have we learnt about functional glutamatergic neurotransmission? Ups J Med Sci. 2010;115:11–20. doi: 10.3109/03009730903572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Origins of postsynaptic potentials evoked in identified rat neostriatal neurons by stimulation in substantia nigra. Exp Brain Res. 1982;45:157–167. doi: 10.1007/BF00235775. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Patel JC, Lee CR, Rice ME. Immunocytochemical identification of proteins involved in dopamine release from the somatodendritic compartment of nigral dopaminergic neurons. Neuroscience. 2009;164:488–496. doi: 10.1016/j.neuroscience.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Morales M. Glutamate neurons in the substantia nigra compacta and retrorubral field. Eur J Neurosci. 2013;38:3602–3610. doi: 10.1111/ejn.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]