Abstract
Malignant melanoma is one of the most common and aggressive tumors in the oral cavity of dog. The tumor has a poor prognosis, and methods for diagnosis and prediction of prognosis after treatment are required. Here, we examined metabolite profiling using gas chromatography-mass spectrometry (GC-MS) for development of a discriminant model for evaluation of prognosis. Metabolite profiles were evaluated in healthy and melanoma plasma samples using orthogonal projection to latent structure using discriminant analysis (OPLS-DA). Cases that were predicted to be healthy using the OPLS discriminant model had no advanced lesions after radiation therapy. These results indicate that metabolite profiling may be useful in diagnosis and prediction of prognosis of canine malignant melanoma.
Keywords: canine oral malignant melanoma, GC-MS, metabolomics
Malignant melanoma is a common and aggressive tumor in the oral cavity of dog [5, 14, 17]. The tumor is associated with a poor prognosis, because of rapid invasion to surrounding normal tissue and a high likelihood of regional and distant metastases early in the course of the disease [4, 8]. Current treatment recommendations are local control with curative-intent surgery and/or radiation therapy and systemic adjunctive therapy [20]. The median survival time (MST) in canine oral malignant melanoma is significantly shorter for tumors at more advanced stages in the World Health Organization (WHO) clinical staging system [3, 10, 12]. Thus, early detection is desirable, but is also difficult, because there are few opportunities to check the inside of the mouth. In addition, although an oral lesion can be observed with the naked eye, it is more difficult to detect a metastatic focus. Systemic imaging methods, such as radiography, ultrasound or computer tomography (CT), may be required for detection of metastasis, in addition to an ocular examination or palpation, since melanoma may metastasize to the abdominal lymph nodes, liver, adrenal glands and lung [20]. Williams and Packer found that approximately 70% of dogs with oral malignant melanoma had local lymph node metastasis when lymphadenomegaly was present, and approximately 40% had local lymph node metastasis in the absence of lymphadenomegaly [19]. Development of a biomarker for less-invasive evaluation of the condition of dogs would be value, since more aggressive examinations using CT or biopsy under anesthesia are not desirable for older dogs or those with advanced disease.
The metabolomics approach involves global analysis of all metabolites in a sample [18]. Metabolomic investigations attempt to detect and profile changes in metabolites, which reflect changes in metabolic pathways and may provide information concerning a disease state or the biological stress of an organism [2]. In recent years, metabolomics has combined data-rich advanced analytical techniques, such as mass spectrometry, with multivariate statistical analysis.
Serum metabolomics analysis has also been shown to be useful to identify potential biomarkers of several cancers [11, 13, 15]. Gas chromatography-mass spectrometry (GC-MS) provides high separation efficiency for resolving complex biological mixtures. In our laboratory, we have shown that metabolite profiles determined by GC-MS may be useful for detection of potential biomarkers and diagnosis of canine lymphoma [16] and canine epilepsy [9]. In this study, we used GC-MS to examine metabolite profiles in plasma from dogs with oral malignant melanoma, with the goal of identifying biomarkers for diagnosis of the tumor and prediction of prognosis.
Plasma samples from 9 healthy dogs, which were judged to be normal by physical examination and blood tests, and 32 dogs with oral malignant melanoma were obtained from the Animal Medical Center of Gifu University. The characteristics of the dogs are shown in Table 1. The tumors were classified as stage I (8 dogs), II (6 dogs), III (10 dogs) or IV (8 dogs) using WHO staging guidelines. All melanoma cases had undergone radiotherapy delivered with Radioflex 300EMG (Rigaku Corporation, Tokyo, Japan) or PRIMUS Mid-Energy (Toshiba Medical Systems, Tokyo, Japan), which have X-ray energy outputs of 300 kV and 4 MV, respectively. The radiation beam provided orthovoltage X-rays (10 dogs) or megavoltage X-ray (22 dogs). Blood samples from melanoma dogs were collected at the first visit and after radiotherapy. The blood test after radiotherapy was performed at the time of the visit for monitoring the effects of therapy.
Table 1. Characteristics of samples.
| Sample (Number) | Age (Year) | Gender |
||
|---|---|---|---|---|
| Male (Cast) | Female (Spay) | |||
| Healthy | 9 | 6.0 ± 2.6 | 3 (3) | 6 (2) |
| Melanoma | 32 | 11.5 ± 2.4 | 22 (9) | 10 (4) |
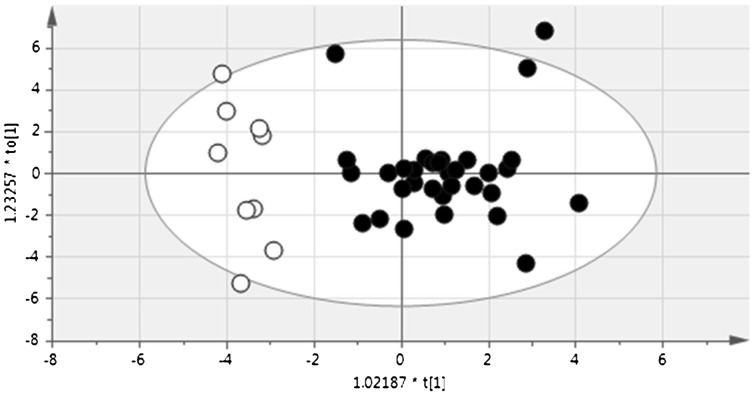
Plasma samples of 50 µl were used for GC-MS analysis, which was performed as described by Nishiumi et al. [13]. This GC-MS analysis permits detection of more than 130 metabolites simultaneously, and more than 60 metabolites were identified in canine plasma. Among these metabolites, 46 were present in all samples and were used in statistical analysis. The similarity of metabolic profiles was evaluated using orthogonal projection to latent structure with discriminant analysis (OPLS-DA) in SIMCA 13.0 (Umetrics, Umea, Sweden). Using this method, plasma metabolite profiles from healthy dogs were discriminated from those of melanoma cases at the first visit, which suggests that metabolite profiles in melanoma differ from those of healthy dogs (Fig. 1).
Fig. 1.
OPLS-DA plot for discrimination between healthy and melanoma dogs. Plasma metabolites profiles of melanoma dogs (black circles) were clearly discriminated from those of healthy dogs (white circles), and a discrimination model was built based on the data.
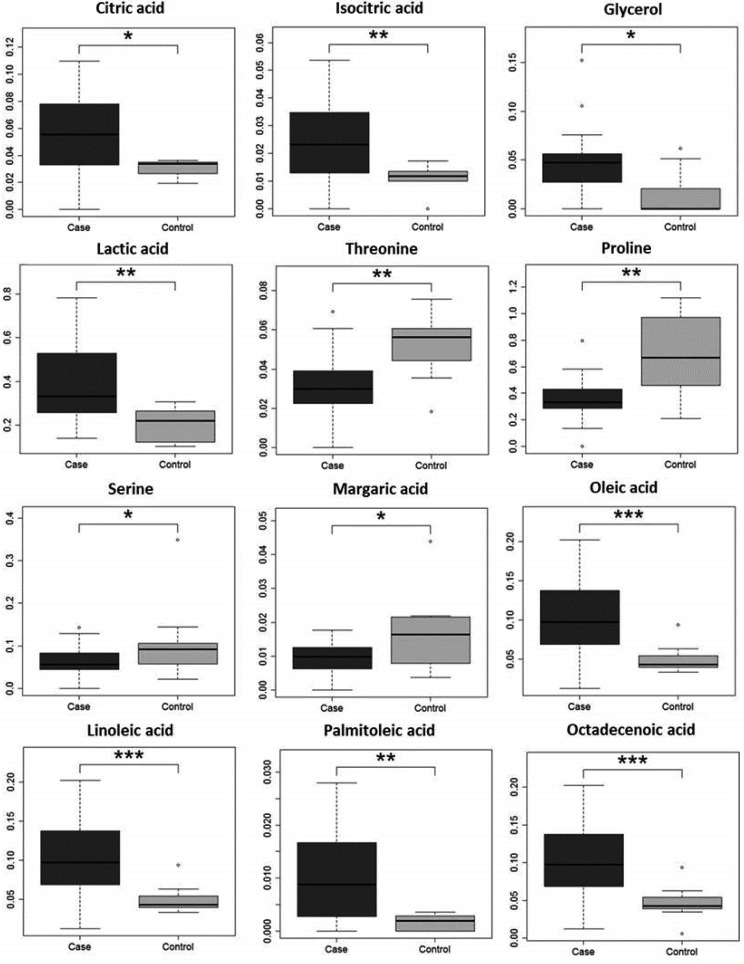
Twelve metabolites showed significant changes in melanoma plasma (Fig. 2). These included citric acid, an intermediate in energy metabolism; lactic acid, the end product of anaerobic energy metabolism; fatty acids including oleic acid, linoleic acid, palmitoleic acid and octadecenoic acid; and glycerol, a triglyceride component, all of which were elevated in melanoma cases. Elevation of fatty acids in human melanoma has been reported in volatile metabolomics with GC-MS [1]. These results show that energy metabolism is elevated in melanoma cases, which is a typical signature of cancer that is referred to as the Warburg effect [7]. These metabolites could be potential biomarkers for canine malignant melanoma.
Fig. 2.
Metabolites showing significant changes in melanoma dog plasma. *P<0.05, **P<0.01, and ***P<0.001 by Steel-Dwass test.
Three amino acids, threonine, proline and serine, were decreased in melanoma plasma. In contrast, the levels of phenylalanine and glutamic acid were increased in serum of dogs with lymphoma [16]. Changes of amino acids in tumors may be related to invasiveness and viability [6], and further studies are required to determine the mechanisms underlying these changes and their possible use in cancer diagnosis.
Melanoma plasma samples obtained from each dog after radiotherapy were evaluated using the OPLS-DA model. One of the 32 dogs was predicted to be healthy at 6 months after radiotherapy, despite not being healthy just after radiotherapy. The dog was in stage I and was found to be in complete remission after radiotherapy, with no advanced lesions based on inspection and palpation. In a follow-up study, this dog lived for 316 days and died from an unrelated disease with no advanced melanoma lesion. In another melanoma case, the dog lived more than 300 days with no advanced lesion found in imaging or by inspection and palpation. The plasma sample collected just after radiotherapy was consistent with melanoma. We were unable to obtain subsequent blood samples. These findings suggest that the timing of blood collection might be an important factor in prediction of prognosis, and this requires further evaluation in a controlled clinical trial.
Our results show that the metabolic profile of canine malignant melanoma differs from that in healthy dogs. A further study of the specificity of plasma metabolites for melanoma is required to establish serum diagnostic markers for melanoma.
REFERENCES
- 1.Abaffy T., Möller M. G., Riemer D. D., Milikowski C., DeFazio R. A.2013. Comparative analysis of volatile metabolomics signals from melanoma and benign skin: a pilot study. Metabolomics 9: 998–1008. doi: 10.1007/s11306-013-0523-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bando K., Kawahara R., Kunimatsu T., Sakai J., Kimura J., Funabashi H., Seki T., Bamba T., Fukusaki E.2010. Influences of biofluid sample collection and handling procedures on GC-MS based metabolomic studies. J. Biosci. Bioeng. 110: 491–499. doi: 10.1016/j.jbiosc.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 3.Bergman P. J.2007. Canine oral melanoma. Clin. Tech. Small Anim. Pract. 22: 55–60. doi: 10.1053/j.ctsap.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 4.Bolon B., Calderwood Mays M. B., Hall B. J.1990. Characteristics of canine melanomas and comparison of histology and DNA ploidy to their biologic behavior. Vet. Pathol. 27: 96–102. doi: 10.1177/030098589002700204 [DOI] [PubMed] [Google Scholar]
- 5.Bradley R. L., MacEwen E. G., Loar A. S.1984. Mandibular resection for removal of oral tumors in 30 dogs and 6 cats. J. Am. Vet. Med. Assoc. 184: 460–463. [PubMed] [Google Scholar]
- 6.Fu Y. M., Yu Z. X., Li Y. Q., Ge X., Sanchez P. J., Fu X., Meadows G. G.2003. Specific amino acid dependency regulates invasiveness and viability of androgen-independent prostate cancer cells. Nutr. Cancer 45: 60–73. doi: 10.1207/S15327914NC4501_8 [DOI] [PubMed] [Google Scholar]
- 7.Gatenby R. A., Gillies R. J.2004. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4: 891–899. doi: 10.1038/nrc1478 [DOI] [PubMed] [Google Scholar]
- 8.Harvey H. J., MacEwen E. G., Braun D., Patnaik A. K., Withrow S. J., Jongeward S.1981. Prognostic criteria for dogs with oral melanoma. J. Am. Vet. Med. Assoc. 178: 580–582. [PubMed] [Google Scholar]
- 9.Hasegawa T., Sumita M., Horitani Y., Tamai R., Tanaka K., Komori M., Takenaka S.2014. Gas chromatography-mass spectrometry-based metabolic profiling of cerebrospinal fluid from epileptic dogs. J. Vet. Med. Sci. 76: 517–522. doi: 10.1292/jvms.13-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry C. J., Buss M. S., Potter K. A., Wardrop K. J.1999. Mitoxantrone and cyclophosphamide combination chemotherapy for the treatment of various canine malignancies. J. Am. Anim. Hosp. Assoc. 35: 236–239. doi: 10.5326/15473317-35-3-236 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T., Nishiumi S., Ikeda A., Yoshie T., Sakai A., Matsubara A., Izumi Y., Tsumura H., Tsuda M., Nishisaki H., Hayashi N., Kawano S., Fujiwara Y., Minami H., Takenawa T., Azuma T., Yoshida M.2013. A novel serum metabolomics-based diagnostic approach to pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 22: 571–579. doi: 10.1158/1055-9965.EPI-12-1033 [DOI] [PubMed] [Google Scholar]
- 12.Konefal J. B., Emami B., Pilepich M. V.1987. Malignant melanoma: analysis of dose fractionation in radiation therapy. Radiology 164: 607–610. doi: 10.1148/radiology.164.3.3112864 [DOI] [PubMed] [Google Scholar]
- 13.Nishiumi S., Kobayashi T., Ikeda A., Yoshie T., Kibi M., Izumi Y., Okuno T., Hayashi N., Kawano S., Takenawa T., Azuma T., Yoshida M.2012. A novel serum metabolomics-based diagnostic approach for colorectal cancer. PLoS ONE 7: e40459. doi: 10.1371/journal.pone.0040459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith S. H., Goldschmidt M. H., McManus P. M.2002. A comparative review of melanocytic neoplasms. Vet. Pathol. 39: 651–678. doi: 10.1354/vp.39-6-651 [DOI] [PubMed] [Google Scholar]
- 15.Song H., Peng J. S., Dong-Sheng Y., Yang Z. L., Liu H. L., Zeng Y. K., Shi X. P., Lu B. Y.2012. Serum metabolic profiling of human gastric cancer based on gas chromatography/mass spectrometry. Braz. J. Med. Biol. Res. 45: 78–85. doi: 10.1590/S0100-879X2011007500158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamai R., Furuya M., Hatoya S., Akiyoshi H., Yamamoto R., Komori Y., Yokoi S., Tani K., Hirano Y., Komori M., Takenaka S.2014. Profiling of serum metabolites in canine lymphoma using gas chromatography mass spectrometry. J. Vet. Med. Sci. 76: 1513–1518. doi: 10.1292/jvms.14-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todoroff R. J., Brodey R. S.1979. Oral and pharyngeal neoplasia in the dog: a retrospective survey of 361 cases. J. Am. Vet. Med. Assoc. 175: 567–571. [PubMed] [Google Scholar]
- 18.Weckwerth W., Morgenthal K.2005. Metabolomics: from pattern recognition to biological interpretation. Drug Discov. Today 10: 1551–1558. doi: 10.1016/S1359-6446(05)03609-3 [DOI] [PubMed] [Google Scholar]
- 19.Williams L. E., Packer R. A.2003. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987–2001). J. Am. Vet. Med. Assoc. 222: 1234–1236. doi: 10.2460/javma.2003.222.1234 [DOI] [PubMed] [Google Scholar]
- 20.Withrow S. J., Vail D. M., Page R. L.2012. Small animal clinical oncology, 5th ed., Saunders, Philadelphia. [Google Scholar]