Abstract
The sedative effects of intramuscular (IM) alfaxalone in 2-hydroxypropyl-beta-cyclodextrin (alfaxalone-HPCD) were evaluated in cats. The cats were treated with alfaxalone-HPCD in five occasions with a minimum 14-day interval between treatments: an IM injection of 1.0 mg/kg (IM1), 2.5 mg/kg (IM2.5), 5 mg/kg (IM5) or 10 mg/kg (IM10), or an intravenous injection of 5 mg/kg (IV5). The sedative effects were evaluated subjectively using a composite measurement scoring system (a maximum score of 16). Cardio-respiratory variables were measured non-invasively. The median sedation scores peaked at 10 min (score 9), 15 min (score 14), 10 min (score 16), 10 to 20 min (score 16) and 2 to 5 min (score 16) after the IM1, IM2.5, IM5, IM10 and IV5 treatments, respectively. The IM5 treatment produced longer lasting sedation, compared to the IV5 treatment. Durations of maintenance of lateral recumbency after the IM10 treatment (115 ± 22 min) were longer than those after the IM2.5 (40 ± 15 min), IM5 (76 ± 21 min) and IV5 treatments (50 ± 5 min). Cardio-respiratory variables remained within clinically acceptable ranges, except for each one cat that showed hypotension (<60 mmHg) after the IM10 and IV5 treatments. Tremors, ataxia and opisthotonus-like posture were observed during the early recovery period after the IM2.5, IM5, IM10 and IV5 treatments. In conclusion, IM alfaxalone-HPCD produced dose-dependent and clinically relevant sedative effect at 2.5 to 10 mg/kg in healthy cats. Hypotension may occur at higher IM doses of alfaxalone-HPCD.
Keywords: alfaxalone, feline, intramuscular administration, sedation
Chemical restraint of fractious, fearful or exited cats is an important clinical procedure to reduce the stress of handling and the risk of injury to both cats and handlers [26]. Judicious use of sedatives or other agents with anesthetic or analgesic properties can be useful for chemical restraint [17]. However, intravenous (IV) administration is usually difficult and/or impossible in these uncooperative animals. Chamber induction with inhalant anesthetics is useful to protect handlers from injury, but it has associated disadvantages, such as airway irritation and stress in patients, during the induction phase [24] and the waste gas pollution [3]. Thus, injectable agents that can be administered subcutaneously or intramuscularly (IM) are often used for chemical restraint in cats.
Because of their availability and efficacy, IM administration of ketamine, opioids, α2-adrenoceptor agonists or their combinations [6, 17, 27, 28, 31] has been widely used for chemical restraint in various veterinary species. However, ketamine has been designated as a legally controlled drug, and its usage has been severely restricted since January 2007 in Japan. In addition, α2-adrenoceptor agonists should be avoided in cats with significant disease and/or aging, because they induce dose-dependent peripheral vasoconstriction and cardiovascular depression through reduced cardiac output and blood perfusion [6]. Therefore, it is clinically important to develop new intramuscular sedatives that have minimal cardiovascular depression in cats.
Alfaxalone (3-alpha-hydroxy-5-alpha-pregnane-11,20-dione) is a synthetic neuroactive steroid molecule which modulates the gamma-aminobutyric acid A (GABAA) receptor-causing neuro-depression and muscular relaxation [1, 10, 18]. Lower concentrations of alfaxalone facilitate the open state of the GABAA receptor channel, similar to that produced by benzodiazepine [18]. On the other hand, alfaxalone at higher concentrations could directly activate the GABAA receptor channel as an agonist, similar to that produced by propofol or barbiturates [18]. Therefore, a sub-anesthetic IM dose of alfaxalone might produce sedative effect. Because of its water-insolubility, previous alfaxalone products (e.g. Saffan®) had been solubilized with 20% polyoxyethylated castor oil (Cremophor EL) and coformulated with a related neurosteroid, alfadolone. However, this product was voluntary withdrawn from the market, because of its side effects induced by histamine release associated with the solubilizing agent, such as hyperemia of the ear pinnae or forepaw in cats [8] and histamine induced anaphylactoid reaction in dogs [5].
Recently, alfaxalone was reformulated with another solubilizing agent, 2-hydroxypropyl-beta-cyclodextrin (HPCD), which does not cause histamine release [4]. This formulation is approved in some countries (e.g. Australia) as an injectable anesthetic agent for both IV and IM administrations in cats. An IV administration of alfaxalone-HPCD produces a smooth induction of anesthesia and rapid recovery with dose-dependent cardio-respiratory depression [23, 29, 33] similar to that of propofol [29] in cats. Based on the dosing recommendations from the product information sheet, an IM administration of alfaxalone-HPCD (10 mg/kg) is expected to induce deep sedation or light anesthesia. Grubb et al. [11] reported that the IM administration of alfaxalone-HPCD (5 mg/kg) to cats in combination with dexmedetomidine (0.01 mg/kg, IM) and hydromorphone (0.1 mg/kg, IM) produced light, general anesthesia with cardiovascular and respiratory stability. Grubb et al. [11] also mentioned that the IM administration of alfaxalone-HPCD seems to produce more undesirable events during recovery compared to the IV administration. However, as far as we know, the sedative and cardio-respiratory effects of sub-anesthetic IM dose of alfaxalone-HPCD alone have not been reported in cats. In addition, the influence of administration routes on the quality of recovery has not been compared between IV and IM administrations of alfaxalone-HPCD alone in cats. The aim of the present study was to evaluate sedative effects of sub-anesthetic IM doses (1 mg/kg, 2.5 mg/kg, 5 mg/kg and 10 mg/kg) and an IV anesthetic induction dose (5 mg/kg) of alfaxalone-HPCD in cats.
MATERIALS AND METHODS
Experimental animals: Six intact purpose-bred cats (3 males and 3 females), 1 to 3 years of age [1.8 ± 1.0 (mean ± standard deviation) years] and weighing from 3.0 to 5.4 kg [4.3 ± 0.8 (mean ± standard deviation) kg], were used in the present study. All cats were judged to be in good to excellent health based upon a physical examination. Food was withheld for at least 12 hr before drug administration, but the cats were allowed free access to water prior to each treatment. The cats were cared for according to the principles of the “Guide for the Care and Use of Laboratory animals” prepared by Rakuno Gakuen University. The Animal Care and Use Committee of Rakuno Gakuen University approved this study (approved No.: VH24B13).
Study design: The cats received 5 treatments with a minimum 14-day washout period (14 to 685 days) between treatments. In each treatment, the cats were administered one of the following 5 doses of alfaxalone-HPCD (ALFAXAN, Jurox Pty. Ltd., Rutherford, NSW, Australia): an IM dose of 1 mg/kg (IM1), 2.5 mg/kg (IM2.5), 5 mg/kg (IM5), 10 mg/kg (IM10) or an IV dose of 5 mg/kg (IV5). The total volumes of injection of alfaxalone-HPCD were 0.45 ± 0.08, 1.0 ± 0.2, 2.1 ± 0.4, 4.5 ± 0.8 and 1.9 ± 0.4 ml for the IM1, IM2.5, IM5, IM10 and IV5 treatments, respectively. The IM doses were injected into the dorsal lumbar muscle of the cats by using a 23-gauge, 1-inch needle (TOP injection needle, TOP Co., Ltd., Tokyo, Japan). The IV dose was administered for over approximately 60 sec through a 22-gauge catheter (Supercath, Medikit Co., Ltd., Tokyo, Japan) previously placed into the cephalic vein of each cat. The cats were allowed to breathe room air spontaneously throughout the experiment. Sedative effects and cardio-respiratory function were evaluated in the cats before (baseline) and at 2, 5, 10, 15, 20, 30, 45, 60, 90, 120, 150 and 180 min after starting the drug administration.
Evaluation of sedative effects: The sedative effects of alfaxalone-HPCD were subjectively evaluated using a composite measure scoring system modified from a sedative scale previously used in dogs [32]. The observer (J. T.) was not blinded, but responsible for the evaluation using this scoring system throughout the present study. The scoring system consisted of 5 categories: spontaneous posture, placement on side, response to noise, jaw relaxation and general attitude. These categories were rated in scores 0 to 2 for jaw relaxation, 0 to 3 for placement of side and general attitude, or 0 to 4 for spontaneous posture and response to noise based on responsiveness expressed by the cats (Table 1). The sedation score was calculated as a sum of scores in the 5 categories (a maximum of 16). In addition, times to onset of lateral recumbency, the first appearance of spontaneous movement, head lift and unaided standing after starting the drug administration, and the durations of lack of spontaneous movement and maintenance of lateral recumbency were recorded.
Table 1. Composite measure scoring system for evaluating sedative effects in cats.
| Score | ||
|---|---|---|
| Spontaneous posture | Standing | 0 |
| Tired and standing | 1 | |
| Lying but can rise | 2 | |
| Lying with difficulty rising | 3 | |
| Unable to rise | 4 | |
| Placement on side | Resists strongly | 0 |
| Modest resistance | 1 | |
| Slight resistance | 2 | |
| No resistance | 3 | |
| Response to noise | Jump | 0 |
| Hears and moves | 1 | |
| Hears and twitches ear | 2 | |
| Barely perceives | 3 | |
| No response | 4 | |
| Jaw relaxation | Poor | 0 |
| Slight | 1 | |
| Good | 2 | |
| General attitude | Excitable | 0 |
| Awake and normal | 1 | |
| Tranquil | 2 | |
| Stuporous | 3 | |
| Sedation score* | 0–16 | |
This scoring system consisted of 5 categories (spontaneous posture, placement on side, response to noise, jaw relaxation and general attitude). These categories were rated in scores 0 to 2, 0 to 3 or 0 to 4 based on responsiveness expressed by the cats. *The sedation score was calculated as a sum of scores for the 5 categories: spontaneous posture, placement on side, response to noise, jaw relaxation and general attitude.
Measurements of cardio-respiratory valuables: Lead II electrocardiography (ECG), heart rate (HR; beats/min), rectal temperature (RT;°C), mean arterial blood pressure (MABP; mmHg) and percutaneous oxygen saturation of hemoglobin (SpO2;%) were recorded before and after the drug administration until reliable data were collected. ECG, HR and SpO2 were recorded by a patient monitoring system (DS-7210, Fukuda Denshi Co., Ltd., Tokyo, Japan). HR was also counted by thoracic auscultation. Respiratory rate (RR; breaths/min) was counted by observing thoracic movements. RT was measured with a digital thermometer (Thermo flex for animal, Astec Co., Ltd., Tsukuba, Japan). MABP was indirectly measured by an oscillometric method (Pet MAP, Ramsey Medical, Inc., Hudson, OH, U.S.A.) using a blood pressure cuff approximately 40% circumference of the measuring site in width placed around the clipped tail base of each cat. The arterial blood pressure was measured three times at each assessment, and the average of these measurements was defined as arterial blood pressure.
Statistical analysis: The sedation score was reported as median ± quartile deviation and analyzed by the Kruskal-Wallis test to assess the changes with time for each treatment. Differences in the sedation score among the treatments were compared by the Kruskal-Wallis test with the Steel-Dwass test for post hoc comparisons. Times related to the sedative effects and cardio-respiratory variables were reported as mean ± standard deviation. Times related to the sedative effects were compared by one-way (treatment) factorial ANOVA with the Bonferroni test for post hoc comparisons among treatments. The cardio-respiratory variables were analyzed using one-way (time) factorial ANOVA with the Dunnet test for post hoc comparisons for each treatment. Observations and/or perceived adverse effects related to drug administration were compared between treatments by use of chi square test. The level of significant was set at P<0.05.
RESULTS
The times required for the IM injection were 5 ± 5, 11 ± 9, 8 ± 5 and 35 ± 18 sec in the IM1, IM2.5, IM5 and IM10 treatments, respectively. In the IM10 treatment, the time required for the IM injection was longer compared to the IM1, IM2.5 and IM5 treatments (P<0.001, P=0.006 and P=0.002, respectively). Vocalization during the IM administration was observed in 3 cats (50%), 2 cats (33%), 4 cats (67%) and 4 cats (67%) receiving the IM1, IM2.5, IM5 and IM10 treatments, respectively. On the other hand, no cat receiving the IV5 treatment showed discomfort (vocalization, attempting to bite at injection site or aggressively excited behavior) during the administration. There was no significant difference in the incidence of vocalization during the IM administration between the treatments (P=0.605). No cats showed discomfort (excessive grooming at injection site or avoiding touching the injection site), and swelling, redness and/or other inflammatory changes around the IM injection site after the experiment on each occasion.
The sedative effects: Times associated with the sedative effects of each treatment are shown in Table 2. The cats ceased to move after being recumbent, except for one cat each after the IM1 and IM2.5 treatments. The IV5 treatment produced lateral recumbency in all cats during injection. All cats after the IM2.5, IM5 and IM10 treatments showed lateral recumbency within 7 min after starting the injection. On the other hand, the IM1 treatment produced lateral recumbency in only 3 cats (50%), and consequently, it was impossible to attempt statistical comparison using this treatment group. Therefore, the times related to the sedative effects were only statistically compared amongst the IM2.5, IM5, IM10 and IV5 treatments. The times to head lift and unaided standing and duration of maintenance of lateral recumbency were significantly longer in the IM5 (P=0.004, P=0.014 and P=0.009, respectively) and IM10 treatments (P<0.001, P<0.001 and P<0.001, respectively), compared to the IM2.5 treatment. The times to first appearance of spontaneous movement and durations of lack of spontaneous movement were markedly longer in the IM10 treatment, compared to the IM2.5 (P<0.001 and P<0.001) and IM5 treatments (P<0.001 and P=0.005). The times to head lift and unaided standing were significantly longer in the IM5 than those in the IV5 treatment (P=0.033 and P=0.011). All observations were completed at 45 min after the IM1 treatment, at 90 min after the IM2.5 and IV5 treatments and at 120 min after the IM5 treatment, because the cats fully recovered and could walk normally.
Table 2. Times related to sedative effects after starting intramuscular (IM) or intravenous (IV) administration of alfaxalone-HPCD in cats.
| Alfaxalone administration |
|||||
|---|---|---|---|---|---|
| 1 mg/kg IM | 2.5 mg/kg IM | 5 mg/kg IM | 10 mg/kg IM | 5 mg/kg IV | |
| Time to onset of lateral recumbency (sec) | 451 ± 129* | 213 ± 143d) | 152 ± 85 | 128 ± 22 | 27 ± 14a) |
| Time to the first appearance of spontaneous movement (min) | 15 ± 10* | 27 ± 7c) | 42 ± 12c) | 74 ± 12a, b) | 29 ± 12c) |
| Time to head lift (min) | 22 ± 10* | 40 ± 15b, c) | 75 ± 18a, d) | 100 ± 18a, d) | 48 ± 6b, c) |
| Time to unaided standing (min) | 30 ± 10* | 60 ± 10b, c) | 95 ± 24a, d) | 121 ± 22a, d) | 59 ± 6b, c) |
| Duration of lack of spontaneous movement (min) | 10 ± 11* | 21 ± 8c) | 35 ± 16c) | 72 ± 12a, b) | 29 ± 12c) |
| Duration of maintenance of lateral recumbency (min) | 16 ± 12* | 40 ± 15b, c) | 76 ± 21a, c) | 115 ± 22a, b, d) | 50 ± 5c) |
Data are expressed as mean ± standard deviation. a) Significant difference from 2.5 mg/kg IM (P<0.05). b) Significant difference from 5 mg/kg IM (P<0.05). c) Significant difference from 10 mg/kg IM (P<0.05). d) Significant difference from 5 mg/kg IV (P<0.05). *Statistical analysis was performed among three treatments (IM2.5, IM5 and IM10), because of small samples in the IM1 treatment.
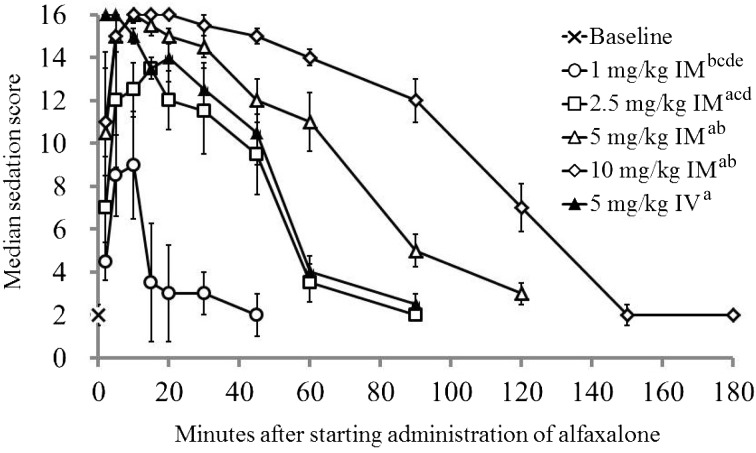
The sedation scores for each treatment are presented in Fig. 1. Each treatment produced a higher sedation score compared to each baseline value (P<0.001). A maximum sedation score of 16 was observed in 2 cats (33%) after the IM2.5 treatment and all cats (100%) each after the IM5, IM10 and IV5 treatments. The median sedation scores peaked at 10 min after the IM1 treatment, 15 min after the IM2.5 treatment, 10 min after the IM5 treatment, 10 to 20 min after the IM10 treatment and 2 to 5 min after the IV5 treatment. The sedation scores in cats administered the IM5 and IM10 treatments were significantly higher compared to the IM1 (P<0.001 and P<0.001) and IM2.5 treatments (P=0.029 and P<0.001). There was no significant difference in the sedation score between the IM5 and IV5 treatments (P=0.984).
Fig. 1.
Median (± quartile deviation) sedation score in 6 cats before and after starting intramuscular (IM) or intravenous (IV) administration of alfaxalone-HPCD. Based on responsiveness expressed by the cats, the categories of a composite measure scoring system were rated in scores 0 to 2 for jaw relaxation, 0 to 3 for placement of side and general attitude, or 0 to 4 for spontaneous posture and response to noise (see Table 1). The sedation score was calculated as a sum of scores in these 5 categories. a: Significant difference from 1 mg/kg IM (P<0.05). b: Significant difference from 2.5 mg/kg IM (P<0.05). c: Significant difference from 5 mg/kg IM (P<0.05). d: Significant difference from 10 mg/kg IM (P<0.05). e: Significant difference from 5 mg/kg IV (P<0.05).
During the recovery period, ataxia was observed in all cats (100%) administered the IM2.5, IM5, IM10 and IV5 treatments. Transient muscular tremors were observed in 2 cats (33%), 4 cats (67%), 6 cats (100%), 6 cats (100%) and 5 cats (83%) administered the IM1, IM2.5, IM5, IM10 and IV5 treatments, respectively. Opisthotonus-like posture was also observed in one cat (17%), 4 cats (67%), 3 cats (50%) and 3 cats (50%) administered the IM2.5, IM5, IM10 and IV5 treatments, respectively (Fig. 2). In addition, paddling of the forelimbs was observed in 4 cats (67%) and one cat (17%) administered the IM10 and IV5 treatments, respectively, and running was observed in one cat (17%) each administered the IM2.5, IM5 and IV5 treatments. In addition, one cat (17%) vomited during the recovery period after the IM10 treatment.
Fig. 2.
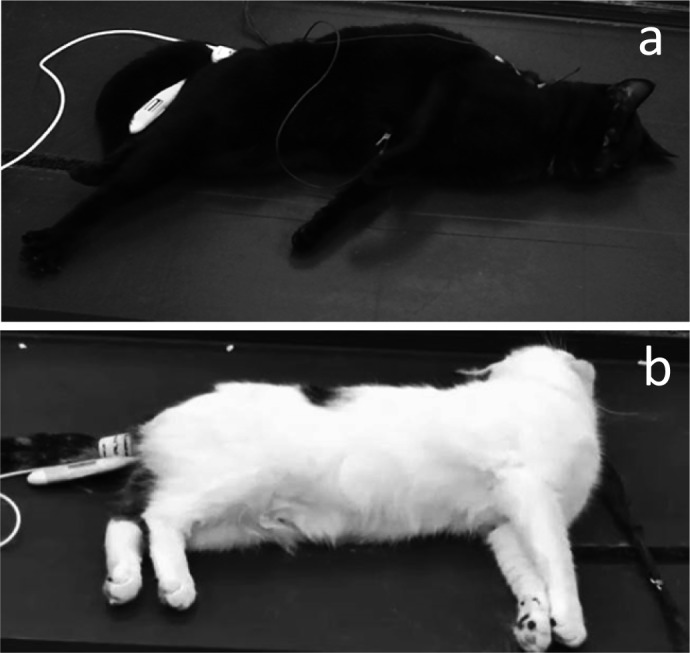
Opisthotonus-like posture observed in cats during the recovery period. During the recovery period, opisthotonus-like posture was observed in one cat (17%) received 2.5 mg/kg of intramuscular (IM) alfaxalone, 4 cats (67%) received 5 mg/kg of IM alfaxalone, 3 cats (50%) received 10 mg/kg of IM alfaxalone and 3 cats (50%) received 5 mg/kg of intravenous (IV) alfaxalone. A cat showed opisthotonus-like posture from 60 to 90 min after the IM5 treatment (a). Another cat showed opisthotonus-like posture from 90 to 120 min after the IM10 treatment (b).
Changes in cardio-respiratory valuables: The cardio-respiratory variables are summarized in Table 3. The RT significantly decreased from baseline at 20 min, at 30 min, at 45 min and at 15 min after the M2.5, IM5, IM10 and IV5 treatment, respectively. A mild hypothermia was detected in 4 cats (34.9 to 35.9°C) from 90 to 150 min after the IM10 treatment. A transient increase in HR from 2 to 5 min after the IM10 treatment was observed. A transient increase in HR during the recovery period was also detected in the IM5, IM10 and IV5 treatments. The MABPs decreased from baseline in all treatment groups. In particular, clinically relevant hypotension (MABP <60 mmHg) was observed in each one cat from 10 to 30 min after the IM10 treatment (50 to 57 mmHg) and from 2 to 20 min after the IV5 treatment (55 to 58 mmHg). Spontaneous breathing was maintained in all cats, but the RR significantly decreased from baseline after the IM2.5, IM5, IM10 and IV5 treatments. The IV5 treatment produced a transient hypoxemia in 2 cats (SpO2 87% and 88%) early after injection.
Table 3. Changes in cardio-respiratory variables before and after starting intramuscular (IM) or intravenous (IV) administration of alfaxalone-HPCD.
| Minutes after starting administration of alfaxalone |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 | 5 | 10 | 15 | 20 | 30 | 45 | 60 | 90 | 120 | 150 | 180 | ||
| RT (°C) | IM1 | 38.1 ± 0.4 | 38.4 ± 0.4 | 38.1 ± 0.2 | 38.2 ± 0.3 | 38.0 ± 0.3 | 38.0 ± 0.3 | 37.8 ± 0.4 | 37.7 ± 0.6 | N.D. | N.D. | N.D. | N.D. | N.D. |
| IM2.5 | 38.4 ± 0.3 | 38.5 ± 0.3 | 38.5 ± 0.2 | 38.3 ± 0.2 | 38.0 ± 0.2 | 37.9 ± 0.2* | 37.5 ± 0.1* | 37.4 ± 0.2* | 37.7 ± 0.4* | 37.8 ± 0.3* | N.D. | N.D. | N.D. | |
| IM5 | 38.3 ± 0.2 | 38.8 ± 0.4* | 38.7 ± 0.4 | 38.5 ± 0.3 | 38.2 ± 0.3 | 38.1 ± 0.3 | 37.7 ± 0.2* | 37.2 ± 0.2* | 37.0 ± 0.2* | 37.3 ± 0.5* | 38.2 ± 0.4 | N.D. | N.D. | |
| IM10 | 38.3 ± 0.4 | 38.5 ± 0.4 | 38.4 ± 0.5 | 38.3 ± 0.5 | 38.1 ± 0.5* | 37.9 ± 0.5 | 37.6 ± 0.4 | 37.1 ± 0.4* | 36.6 ± 0.4* | 35.7 ± 0.7* | 36.3 ± 1.0* | 36.9 ± 1.0* | 36.9 | |
| IV5 | 38.5 ± 0.5 | 38.3 ± 0.5 | 38.1 ± 0.4 | 37.8 ± 0.4 | 37.7 ± 0.4* | 37.4 ± 0.4* | 37.0 ± 0.4* | 36.8 ± 0.4* | 37.2 ± 0.8* | 37.7 ± 0.4* | N.D. | N.D. | N.D. | |
| HR (beats/min) | IM1 | 171 ± 29 | 180 ± 23 | 188 ± 46 | 178 ± 31 | 177 ± 30 | 176 ± 29 | 179 ± 47 | 177 ± 38 | N.D. | N.D. | N.D. | N.D. | N.D. |
| IM2.5 | 172 ± 21 | 178 ± 29 | 179 ± 42 | 161 ± 31 | 157 ± 30 | 155 ± 35 | 167 ± 43 | 207 ± 44 | 211 ± 36 | 218 ± 34 | N.D. | N.D. | N.D. | |
| IM5 | 169 ± 11 | 191 ± 25 | 188 ± 24 | 185 ± 15 | 173 ± 19 | 172 ± 14 | 154 ± 29 | 171 ± 29 | 210 ± 34* | 203 ± 38 | 179 ± 25 | N.D. | N.D. | |
| IM10 | 161 ± 9 | 200 ± 17* | 194 ± 9* | 175 ± 5 | 168 ± 6 | 161 ± 7 | 152 ± 5 | 142 ± 8 | 142 ± 16 | 192 ± 36 | 203 ± 49* | 188 ± 32 | 192 | |
| IV5 | 160 ± 13 | 180 ± 12 | 167 ± 11 | 148 ± 13 | 137 ± 11 | 143 ± 24 | 152 ± 43 | 189 ± 32 | 216 ± 33* | 185 ± 12 | N.D. | N.D. | N.D. | |
| RR (breaths/min) | IM1 | 48 ± 21 | 80 ± 24 | 44 ± 14 | 33 ± 13 | 40 ± 27 | 38 ± 24 | 47 ± 19 | 78 ± 59 | N.D. | N.D. | N.D. | N.D. | N.D. |
| IM2.5 | 64 ± 31 | 65 ± 30 | 33 ± 7* | 28 ± 7* | 28 ± 11* | 27 ± 15* | 22 ± 4* | 27 ± 8* | 31 ± 9* | 46 ± 16 | N.D. | N.D. | N.D. | |
| IM5 | 57 ± 14 | 46 ± 7 | 35 ± 11* | 26 ± 8* | 24 ± 5* | 21 ± 5* | 19 ± 4* | 19 ± 5* | 25 ± 7* | 33 ± 8* | 38 ± 13* | N.D. | N.D. | |
| IM10 | 55 ± 14 | 56 ± 27 | 27 ± 14* | 21 ± 9* | 18 ± 6* | 17 ± 5* | 16 ± 4* | 14 ± 5* | 19 ± 14* | 30 ± 25* | 29 ± 9* | 30 ± 8* | 32 | |
| IV5 | 46 ± 11 | 17 ± 2* | 21 ± 2* | 19 ± 3* | 19 ± 3* | 17 ± 3* | 21 ± 9* | 22 ± 8* | 33 ± 12* | 40 ± 9 | N.D. | N.D. | N.D. | |
Data are expessed as mean ± standard deviation. RT: rectal temperature, HR: heart rate, RR: respiratory rate. IM1: IM administration of 1 mg/kg alfaxalone, IM2.5: IM administration of 2.5 mg/kg alfaxalone, IM5: IM administration of 5 mg/kg alfaxalone, IM10: IM administration of 10 mg/kg alfaxalone, IV5: IV administration of 5 mg/kg alfaxalone. N.D.: not done. The observation was completed at 45 min after the IM1 treatment, at 90 min after the IM2.5 and IV5 treatments, and at 120 min after the IM5 treatments, because the cats recovered from the sedation and could walk up and down normally. *Significant difference from baseline value (P<0.05).
DISCUSSION
In the present study, the IM administration of alfaxalone-HPCD produced a dose-dependent sedation and immobilization with mild cardio-respiratory depression in healthy cats. The IM alfaxalone-HPCD at 10 mg/kg produced a similar degree, but longer lasting sedation and immobilization, compared to the IM alfaxalone-HPCD at 5 mg/kg. The IM alfaxalone-HPCD at 5 mg/kg produced a similar degree of sedation and immobilization to the IV alfaxalone-HPCD at 5 mg/kg. The duration of sedation, however, was longer lasting after the IM alfaxalone-HPCD at 5 mg/kg, compared to that after the IV alfaxalone-HPCD at 5 mg/kg. During recovery, tremors, ataxia, opisthotonus-like posture and transient paddling were observed in cats administered IM or IV alfaxalone-HPCD. One cat vomited during recovery from the IM alfaxalone-HPCD at 10 mg/kg.
Based on the dosing recommendations from the product information sheet, an IM administration of alfaxalone-HPCD (10 mg/kg) is expected to induce deep sedation or light anesthesia. Thus, we chose four increasing IM doses of alfaxalone-HPCD up to 10 mg/kg in the present study. Some cats vocalized during the IM administration of alfaxalone-HPCD, but there was no significant difference in the incidence of vocalization between the IM treatments. In the IV5 treatment, no cat showed discomfort during the administration. Alfaxalone-HPCD has a neutral pH and does not cause pain and tissue irritation after IV administration [21] or perivascular injection [14]. In addition, Grubb et al. [11] mentioned that the IM alfaxalone-HPCD at 10 mg/kg seemed to cause moderate to profound discomfort in cats because of its excessive injection volume (1 ml/kg). Therefore, we propose that the discomfort observed in the study was a volume associated effect and not a property of the alfaxalone-HPCD formulation itself. Actually, there was no swelling, redness or changes in the skin observed around the site of injection during and after the experiment. The European Federation of Pharmaceutical Industries Associations and the European Centre for the Validation of Alternative Methods provide a guideline for the administration IM volumes [7]. The reported IM volume considered as good practice is 0.25 ml/kg and the maximal dose volume is 0.5 ml/kg in the guideline [7], although there is no description for dose volume in cats. According to this guideline [7], the IM alfaxalone-HPCD dose considered as good practice is 2.5 mg/kg, and the maximal dose is 5 mg/kg based on the concentration of alfaxalone in the approved product (10 mg/ml). We considered that the IM10 treatment was not a practical volume because the IM10 treatment (1 ml/kg of the approved alfaxalone-HPCD product) produced discomfort during injection and required longer duration of IM administration. The development of a more concentrated alfaxalone-HPCD product is desirable in order to reduce the discomfort associated with large IM injection volumes and promote practical convenience.
The objective of the present study was to investigate the sedative effects of IM alfaxalone-HPCD in cats without any other medicants. Therefore, we adopted the experimental design that had the least amount of nociceptive stimulation (i.e. manipulations) during the assessment of sedation effect and the cardio-respiratory variables. For similar reasons, we did not perform tracheal intubation during the experiment. We adopted the composite measure scoring system for the evaluation of sedative or anesthetic effect. The scoring system was modified from the existing scoring system used for evaluating sedative and analgesic effects of medetomidine in dogs [32] that consisted of 6 categories (spontaneous posture, placement on side, response to noise, jaw relaxation, general attitude and nociceptive response to interdigital pad pinch). These categories seemed to be suitable for the evaluation of the extent of sedation in cats, however, it was also anticipated that nociceptive stimulation would have some influence on the evaluation of sedative effect produced by alfaxalone-HPCD because of its poor analgesic property [23]. Therefore, we modified the existing scoring system [32] by removing the category of nociceptive response to interdigital pad pinch.
In the present study, the IM administration of alfaxalone-HPCD at 2.5, 5 and 10 mg/kg produced a clinically relevant sedative effect. The IM alfaxalone-HPCD at 1 mg/kg did not provide reliable sedation for handling in healthy cats. The sedative scores after the IM5 and IM10 treatments were higher than that after the IM2.5 treatment. In addition, the times to first appearance of spontaneous movement and durations of lack of spontaneous movement were markedly longer in the IM10 treatment, compared to the IM2.5 and IM5 treatments. Muir et al. [23] reported that IV alfaxalone-HPCD produced a dose-dependent unresponsiveness in cats. The observations in the present study show that IM alfaxalone-HPCD produced a dose-dependent sedative effect in cats. The IM alfaxalone-HPCD at higher dose (5 and 10 mg/kg) produced a marked and prolonged sedation and immobilization in cats. An IM dose of alfaxalone-HPCD at 2.5 mg/kg may provide enough sedation for securing vascular access in healthy cats.
The IV administration of alfaxalone-HPCD at 5 mg/kg is a recommended dose for anesthetic induction in cats [23, 30]. Whittem et al. [30] reported that all the 8 cats receiving 5 mg/kg of IV alfaxalone-HPCD became anesthetized shortly after the start of injection and the times to first head lift, and the times to unaided standing were 45 and 69 min, respectively. These findings are consistent with our results in the cats administered the IV5 treatment. In the present study, the IM5 and IM10 treatments produced slower onset, but achieved a maximum sedation score (Score 16) in all cats equivalent to the IV5 treatment. It is possible that IM alfaxalone-HPCD at 5 or 10 mg/kg may achieve anesthetic induction as well as reliable sedation in cats. Further investigation including an examination of the degree of difficulty in tracheal intubation will be necessary to determine the proper IM anesthetic induction doses of alfaxalone-HPCD in cats.
It has been reported that an IV administration of alfaxalone-HPCD resulted in uneventful recovery in dogs [2, 22] and cats [23, 29]. On the other hand, it has also been reported that the IV alfaxalone-HPCD produced a poor quality of recovery in dogs [16] and cats [20, 33]. Mathis et al. [20] reported that twitching, paddling, face rubbing, opisthotonus and tremors during recovery were observed in cats receiving IV alfaxalone-HPCD or propofol. It has also been reported that an IV alfaxalone-HPCD induction was associated with more episodes of paddling and trembling during recovery, compared to the propofol induction [20]. These results may be related to the differences in drug clearance and the proportion of occupied GABAA receptor between alfaxalone and propofol [9]. Abnormal behavior including ataxia, increased motor activity, hyperreflexia, sensitivity to touch and violent recovery may also occur during the recovery from ketamine anesthesia, and these reactions are probably attributable to depression of the inferior colliculus and medial geniculate nucleus leading to misperception of auditory and visual stimuli [19]. In the present study, almost the cats receiving the IM treatment at 2.5 to 10 mg/kg or the IV treatment at 5 mg/kg of alfaxalone-HPCD on its own exhibited tremors, ataxia and opisthotonus-like posture during the earlier period of recovery, but no cat was excited and behaved aggressively. On the other hand, less frequency of ataxia and tremors during recovery was observed after the IM treatment of alfaxalone-HPCD at 1 mg/kg. In addition, the IM alfaxalone-HPCD at the highest dose (10 mg/kg) was associated with paddling and vomiting. Vomiting has not been reported before as an alfaxalone-HPCD side effect. Further studies are needed to clarify whether the perceived adverse effects during recovery are regarded as a direct side effect of alfaxalone-HPCD or not.
The incidences of these episodes during recovery in the present study were higher than those in previous studies [20, 33]. Mathis et al. [20] reported that twitching, paddling, face rubbing, opisthotonus and tremors were observed during recovery from anesthesia in 14.3, 21.3, 4.3, 12.8 and 21.3% of 47 client owned cats receiving IV alfaxalone-HPCD for induction of anesthesia, respectively [20]. In that study, all the cats were premedicated with acepromazine (0.05 mg/kg IM) and buprenorphine (0.01 mg/kg IM) before the IV alfaxalone-HPCD induction [20]. Zaki et al. [33] showed that premedication (acepromazine 0.03 mg/kg and butorphanol 0.3 mg/kg administered subcutaneously) improved the quality of recovery after the IV alfaxalone-HPCD induction. Similarly, it was reported that the quality of recovery from anesthesia with alfaxalone-HPCD was superior in cats premedicated with acepromazine or medetomidine compared to those in cats without premedication [13]. These findings indicate that premedication with sedative and analgesic drugs would improve the quality of recovery from alfaxalone-HPCD in cats. In contrast, Grubb et al. [11] reported that premedication with dexmedetomidine (0.01 mg/kg IM) or dexmedetomidine plus hydromorphone (0.1 mg/kg IM) did not improve the quality of recovery from the IM alfaxalone-HPCD (5 mg/kg) and mentioned that the sedative effect of dexmedetomidine might have dissipated before the recovery period. Further studies will be necessary to determine the influence of premedication on the quality of recovery from the IM alfaxalone-HPCD in cats.
Muir et al. [23] reported that IV administration of alfaxalone-HPCD produced dose-dependent cardio-respiratory depression and an IV dose of 5 mg/kg produced hypoxia associated with hypoventilation mainly caused by decreases in respiratory rate and a transient apnea in cats. These findings are consistent with our results in the cats receiving the IV5 treatment. In the present study, spontaneous breathing was maintained, but the RR decreased in all the cats receiving the IM2.5, IM5, IM10 and IV5 treatments. The cardio-respiratory changes were within normal ranges in all the cats receiving the IM1, IM2.5 and IM5 treatments. The IM alfaxalone-HPCD at the highest dose (10 mg/kg) produced clinically relevant hypotension in one cat. These findings suggest that the IM administration of alfaxalone-HPCD produces a mild dose-dependent cardio-respiratory depression which is not clinically relevant up to doses of 5 mg/kg. However, there was a limitation in the present study, because we adopted indirect methods to measure cardio-respiratory valuables in order to achieve less nociceptive stimulation. In cats, the oscillometric method may result in an underestimation of the arterial blood pressure compared with the direct arterial blood pressure measurement [12, 25]. Tremors during the recovery phase may affect the accuracy of the arterial blood pressure measured by the oscillometric method. In addition, inadequate light transmission and animal movement are the greatest limitations of SpO2 measurement [15]. We applied the SpO2 sensor to the tongue during the lack of spontaneous movement and changed periodically. Further sophisticated cardiorespiratory measurement including arterial blood gas analysis and measurement of cardiac output or tidal volume will be required to confirm the cardiopulmonary depression produced by the IM alfaxalone-HPCD in cats.
In conclusion, IM alfaxalone-HPCD at doses of 2.5, 5 and 10 mg/kg produced dose-dependent sedation and immobilization in cats. The 10 mg/kg dose rate produced a similar degree of sedation, but longer lasting sedation compared to the IM alfaxalone-HPCD at 5 mg/kg. IM alfaxalone-HPCD caused a mild dose-dependent cardio-respiratory depression at doses up to 5 mg/kg. At 10 mg/kg, IM alfaxalone-HPCD caused clinical hypotension in one cat and was associated with vomiting in another cat. Some cats vocalized during the IM administration of alfaxalone-HPCD, however, it was thought that the vocalization was related to the act of administering an IM injection and not a pain response. During the early recovery period, undesirable effects including tremors, ataxia and opisthotonus-like posture were associated with IM alfaxalone-HPCD at 2.5 to 10 mg/kg. Nevertheless, we do not recommend the IM alfaxalone-HPCD at 10 mg/kg, because of the excessive IM volume (1 ml/kg). Premedication with sedatives and/or analgesics should be a focus of future investigations in order to reduce the injection volume of IM alfaxalone-HPCD and improve the quality of recovery.
REFERENCES
- 1.Albertson T. E., Walby W. F., Joy R. M.1992. Modification of GABA-mediated inhibition by various injectable anesthetics. Anesthesiology 77: 488–499. doi: 10.1097/00000542-199209000-00014 [DOI] [PubMed] [Google Scholar]
- 2.Ambros B., Duke-Novakovski T., Pasloske K. S.2008. Comparison of the anesthetic efficacy and cardiopulmonary effects of continuous rate infusions of alfaxalone-2-hydroxypropyl-beta-cyclodextrin and propofol in dogs. Am. J. Vet. Res. 69: 1391–1398. doi: 10.2460/ajvr.69.11.1391 [DOI] [PubMed] [Google Scholar]
- 3.Bednarski R. M.2007. Dogs and cats. pp. 705–716. In: Lumb and Jones’ Veterinary Anesthesia and Analgesia, 4th ed. (Tranquilli, W. J., Thurmon, J. C. and Grimm, K. A. eds.), Blackwell Publishing, Ames. [Google Scholar]
- 4.Branson K. R.2007. Injectable and alternative anesthetic techniques. pp. 273–300. In: Lumb and Jones’ Veterinary Anesthesia and Analgesia, 4th ed. (Tranquilli, W. J., Thurmon, J. C. and Grimm, K. A. eds.), Blackwell Publishing, Ames. [Google Scholar]
- 5.Child K. J., Currie J. P., Dis B., Dodds M. G., Pearce D. R., Twissell D. J.1971. The pharmacological properties in animals of CT1341—a new steroid anaesthetic agent. Br. J. Anaesth. 43: 2–13. doi: 10.1093/bja/43.1.2-a [DOI] [PubMed] [Google Scholar]
- 6.Cullen L. K.1996. Medetomidine sedation in dogs and cats: a review of its pharmacology, antagonism and dose. Br. Vet. J. 152: 519–535. doi: 10.1016/S0007-1935(96)80005-4 [DOI] [PubMed] [Google Scholar]
- 7.Diehl K. H., Hull R., Morton D., Pfister R., Rabemampianina Y., Smith D., Vidal J. M., van de Vorstenbosch C., European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods2001. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 21: 15–23. doi: 10.1002/jat.727 [DOI] [PubMed] [Google Scholar]
- 8.Dodman N. H.1980. Complications of saffan anaesthesia in cats. Vet. Rec. 107: 481–483. doi: 10.1136/vr.107.21.481 [DOI] [PubMed] [Google Scholar]
- 9.Ferchichi S., Troncy E., Guillot M., Rialland P., Truchetti G., del Castillo J. R.2013. Excitement in dogs recovering from alfaxalone anaesthesia: is the absent drug blamed in error? Vet. Anaesth. Analg. 40: 655–656. doi: 10.1111/vaa.12085 [DOI] [PubMed] [Google Scholar]
- 10.Goodchild C. S., Guo Z., Nadeson R.2000. Antinociceptive properties of neurosteroids I. Spinally-mediated antinociceptive effects of water-soluble aminosteroids. Pain 88: 23–29. doi: 10.1016/S0304-3959(00)00301-8 [DOI] [PubMed] [Google Scholar]
- 11.Grubb T. L., Greene S. A., Perez T. E.2013. Cardiovascular and respiratory effects, and quality of anesthesia produced by alfaxalone administered intramuscularly to cats sedated with dexmedetomidine and hydromorphone. J. Feline Med. Surg. 15: 858–865. doi: 10.1177/1098612X13478265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberman C. E., Morgan J. D., Kang C. W., Brown S. A.2004. Evaluation of doppler ultrasonic and oscillometric methods of indirect blood pressure measurement in cats. Intern .J. Appl. Res. Vet. Med. 2: 279–289. [Google Scholar]
- 13.Heit M., Schnell M., Whiltem T., Pasloske K.2004. Cardiovascular and respiratory safety of ALFAXAN®-CD RTU in cats premedicated with acepromazine, medetomidine, midazolam or butorphanol. J. Vet. Intern. Med. 18: 419–420. [Google Scholar]
- 14.Heit M., Schnell M., Whiltem T., Pasloske K.2004. Safety and efficacy of ALFAXAN®-CD RTU administrated once to cats subcutaneously at 10 mg/kg. J. Vet. Intern. Med. 18: 432. [Google Scholar]
- 15.Jacobson J. D., Miller M. W., Matthews N. S., Hartsfield S. M., Knauer K. W.1992. Evaluation of accuracy of pulse oximetry in dogs. Am. J. Vet. Res. 53: 537–540. [PubMed] [Google Scholar]
- 16.Jiménez C. P., Mathis A., Mora S. S., Brodbelt D., Alibhai H.2012. Evaluation of the quality of the recovery after administration of propofol or alfaxalone for induction of anaesthesia in dogs anaesthetized for magnetic resonance imaging. Vet. Anaesth. Analg. 39: 151–159. doi: 10.1111/j.1467-2995.2011.00678.x [DOI] [PubMed] [Google Scholar]
- 17.Karas A. Z.1999. Sedation and chemical restraint in the dog and cat. Clin. Tech. Small Anim. Pract. 14: 15–26. doi: 10.1016/S1096-2867(99)80023-1 [DOI] [PubMed] [Google Scholar]
- 18.Lambert J. J., Belelli D., Peden D. R., Vardy A. W., Peters J. A.2003. Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 71: 67–80. doi: 10.1016/j.pneurobio.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 19.Lin H. C.2007. Dissociative anesthetics. pp. 301–353. In: Lumb and Jones’ Veterinary Anesthesia and Analgesia, 4th ed. (Tranquilli, W. J., Thurmon, J. C. and Grimm, K. A. eds.), Blackwell Publishing, Ames. [Google Scholar]
- 20.Mathis A., Pinelas R., Brodbelt D. C., Alibhai H. I.2012. Comparison of quality of recovery from anaesthesia in cats induced with propofol or alfaxalone. Vet. Anaesth. Analg. 39: 282–290. doi: 10.1111/j.1467-2995.2011.00707.x [DOI] [PubMed] [Google Scholar]
- 21.Michou J. N., Leece E. A., Brearley J. C.2012. Comparison of pain on injection during induction of anaesthesia with alfaxalone and two formulations of propofol in dogs. Vet. Anaesth. Analg. 39: 275–281. doi: 10.1111/j.1467-2995.2012.00709.x [DOI] [PubMed] [Google Scholar]
- 22.Muir W., Lerche P., Wiese A., Nelson L., Pasloske K., Whittem T.2008. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Vet. Anaesth. Analg. 35: 451–462. doi: 10.1111/j.1467-2995.2008.00406.x [DOI] [PubMed] [Google Scholar]
- 23.Muir W., Lerche P., Wiese A., Nelson L., Pasloske K., Whittem T.2009. The cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in cats. Vet. Anaesth. Analg. 36: 42–54. doi: 10.1111/j.1467-2995.2008.00428.x [DOI] [PubMed] [Google Scholar]
- 24.Mutoh T., Kanamaru A., Suzuki H., Tsubone H., Nishimura R., Sasaki N.2001. Respiratory reflexes in spontaneously breathing anesthetized dogs in response to nasal administration of sevoflurane, isoflurane, or halothane. Am. J. Vet. Res. 62: 311–319. doi: 10.2460/ajvr.2001.62.311 [DOI] [PubMed] [Google Scholar]
- 25.Pedersen K. M., Butler M. A., Ersbøll A. K., Pedersen H. D.2002. Evaluation of an oscillometric blood pressure monitor for use in anesthetized cats. J. Am. Vet. Med. Assoc. 221: 646–650. doi: 10.2460/javma.2002.221.646 [DOI] [PubMed] [Google Scholar]
- 26.Rodan I., Sundahl E., Carney H., Gagnon A. C., Heath S., Landsberg G., Seksel K., Yin S., American Animal Hospital Association2011. AAFP and ISFM feline-friendly handling guidelines. J. Feline Med. Surg. 13: 364–375. doi: 10.1016/j.jfms.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selmi A. L., Mendes G. M., Lins B. T., Figueiredo J. P., Barbudo-Selmi G. R.2003. Evaluation of the sedative and cardiorespiratory effects of dexmedetomidine, dexmedetomidine-butorphanol, and dexmedetomidine-ketamine in cats. J. Am. Vet. Med. Assoc. 222: 37–41. doi: 10.2460/javma.2003.222.37 [DOI] [PubMed] [Google Scholar]
- 28.Smith A. A., Posner L. P., Goldstein R. E., Ludders J. W., Erb H. N., Simpson K. W., Gleed R. D.2004. Evaluation of the effects of premedication on gastroduodenoscopy in cats. J. Am. Vet. Med. Assoc. 225: 540–544. doi: 10.2460/javma.2004.225.540 [DOI] [PubMed] [Google Scholar]
- 29.Taboada F. M., Murison P. J.2010. Induction of anaesthesia with alfaxalone or propofol before isoflurane maintenance in cats. Vet. Rec. 167: 85–89. doi: 10.1136/vr.b4872 [DOI] [PubMed] [Google Scholar]
- 30.Whittem T., Pasloske K. S., Heit M. C., Ranasinghe M. G.2008. The pharmacokinetics and pharmacodynamics of alfaxalone in cats after single and multiple intravenous administration of Alfaxan at clinical and supraclinical doses. J. Vet. Pharmacol. Ther. 31: 571–579. doi: 10.1111/j.1365-2885.2008.00998.x [DOI] [PubMed] [Google Scholar]
- 31.Wiese A. J., Muir W. W.2007. Anaesthetic and cardiopulmonary effects of intramuscular morphine, medetomidine and ketamine administered to telemetered cats. J. Feline Med. Surg. 9: 150–156. doi: 10.1016/j.jfms.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young L. E., Brearley J. C., Richards D. L. S., Bartram D. H., Jones R. S.1990. Medetmidine as a premedicant in dogs and its reversal by atipamezol. J. Small Anim. Pract. 31: 554–559. doi: 10.1111/j.1748-5827.1990.tb00685.x [DOI] [Google Scholar]
- 33.Zaki S., Ticehurst K., Miyaki Y.2009. Clinical evaluation of Alfaxan-CD® as an intravenous anaesthetic in young cats. Aust. Vet. J. 87: 82–87. doi: 10.1111/j.1751-0813.2009.00390.x [DOI] [PubMed] [Google Scholar]