Abstract
Liver contrast X-ray computed tomography (CT) has been used for evaluation of hepatic vessels for liver transplantation, liver lobectomy, interventional radiology and diagnosis of hepatocellular carcinoma in humans. However, there remains scant available anatomical information on normal hepatic vessels in the veterinary field. In this study, visualization of hepatic vessels was evaluated in 32 normal beagle dogs by X-ray contrast CT using triple phase images. The following hepatic vessels were clearly visualized: arterial, portal and hepatic veins. With regards to the running patterns of the portal vein and hepatic vein, there were no significant differences between the dogs. However, the hepatic artery exhibited some differences in each dog. In particular, the hepatic artery of the quadrate lobe and the right lateral lobe had many running patterns. The results of the present study could be useful for veterinary diagnosis, surgery and interventional radiology.
Keywords: canine, computed tomography, hepatic artery, hepatic vascular system
Recently, diagnostic precision has been improving in veterinary medicine due to advanced equipment and techniques. One of the major diagnostic modalities is computed tomography (CT). It is noninvasive, fast and provides much information for the clinician, including information on the hepatic vessels [6, 10]. In recent years, three-dimensional (3D) CT has assisted preoperative surgical planning and has contributed to evaluation of the morphology of the liver, hepatic vessels and tumors in hepatic surgery [8].
There are many reports about the hepatic vascular system in humans, and its acceptance in clinical medicine is high [4, 5, 7]. However, in dogs, little information regarding hepatic vascular anatomy is available for hepatic surgery. Therefore, knowledge of the details of the hepatic vascular system is highly desirable for development of veterinary clinical medicine.
The present study aimed to define anatomical variations of the hepatic artery, portal vein and hepatic vein based on integrated 3D images in live, normal dogs.
MATERIALS AND METHODS
Animals: Thirty-two beagles, ranging from 2 to 12 years old, weighing 10.78 ± 1.50 kg (mean ± standard deviation (SD)), genetic relationship unknown and without clinical signs relating to liver disease, were entered into the study. Dogs meeting these criteria with liver parenchymal diseases have previously been evaluated with CT. The liver volume in the present study was 336.3 ± 46.7 ml (31.4 ± 4.4 ml/kg), and there were no anatomical abnormalities in liver parenchyma. The beagles were caged with free access to water, and food was withheld for 12 hr before the dogs were anesthetized.
Abdominal CT: All dogs has anesthesia induced by slow intravenous administration of propofol (7 mg/kg, intravenously) to effect. A cuffed endotracheal tube was placed in the trachea, and the dogs were connected to a partial rebreathing cycle system delivering isoflurane (1.0–2.0%) and oxygen. Anesthetic depth was monitored using clinical signs, and dogs were ventilated to maintain eucapnia.
Contrast-enhanced multi-detector computed tomography (MDCT) examinations were carried out using an eight-detector-row computed tomography system (ECLOS 8; Hitachi Medical Corp., Tokyo, Japan). Scans were obtained with a collimation of 8 × 1.25 mm, table pitch of 0.875, tube voltage of 120 kV and tube current of 350 mA. All dogs were placed in ventral recumbency. All CT studies were performed during apnea under anesthesia. All images were acquired using a soft tissue reconstruction algorithm.
The CT angiographic study was performed in four steps. In the first step, a survey helical CT scan of the abdomen was performed from most cranial aspect of the diaphragm to the cranial aspect of the pelvis, and then, imaging of the liver was performed from the most cranial aspect of the liver to the middle position of the light kidney. In the second step, dynamic CT was performed with MDCT. Dogs received 2 ml/kg of iopamidol with an iodine concentration of 370 mg/ml (Oiparomin 370; Fuji Pharmaceutical Co., Toyama, Japan) as intravenous contrast for vascular imaging. The contrast medium was injected with an automatic power injector (A-60, Nemoto Kyorindo Co., Ltd., Tokyo, Japan, at a speed of 1–2 ml/s), and arterial phase imaging was started 8–20 sec after the start of the injection of the contrast medium. The scan parameters were identical to the survey helical scan. The start time of the arterial phase CT scanning depended on patient’s heart rate, and CT scanning time was common to all phases, ranging from 10 to 15 sec. In the third step, portal phase imaging was started 45–60 sec after the start of the injection. In the fourth step, equilibrium phase imaging was started 150 sec after the start of the injection.
Vascular analysis: 3D reconstruction of the hepatic vasculature was made using volume measurement software (Ziostation2; Amin, Tokyo, Japan). The resulting 3D images of the hepatic artery, the portal vein and the hepatic vein were carefully reviewed and compared with the axial source images to ensure that no important structures were inadvertently deleted from the 3D images. The 3D images of the hepatic artery, the portal vein and the hepatic vein were adjusted to the position to each other by focusing at the hepatic hilum. Axial, sagittal and coronal images were further integrated into a single image.
RESULTS
The present study visualized small vessels in the livers of 32 dogs using triple phase helical CT images. Contrast medium was used to distinguish the hepatic artery, portal vein and hepatic vein of the following liver segments: the papillary process of the caudate lobe, left lateral lobe, left medial lobe, quadrate lobe, right medial lobe, right lateral lobe and the caudate process of the caudate lobe. The paths of the hepatic artery, portal vein and hepatic vein were visualized in each lobe, and the results are discussed below (Figs. 1, 2, 3, 4).
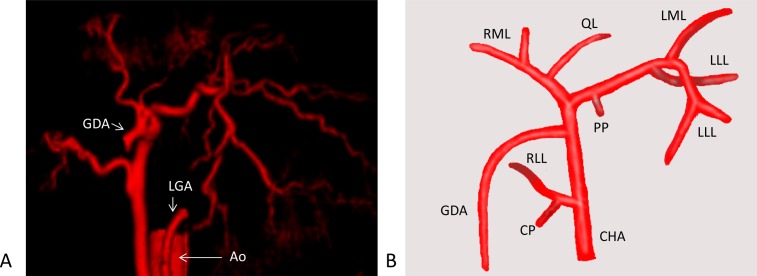
Fig. 1.
(A) Ventral-dorsal arterial phase of a normal dog. The abdominal artery, left gastric artery and gastroduodenal artery were removed from the images for clarity. (B) Schema of the hepatic artery. Shown is a main branch of the artery to each lobe. Ao, aorta; LGA, left gastric artery; GDA, gastroduodenal artery; CHA, common hepatic artery; PP, papillary process of the caudate lobe; LLL, left lateral lobe; LML, left medial lobe; QL, quadrate lobe; RML, right medial lobe; RLL, right lateral lobe; CP, caudate process of the caudate lobe.
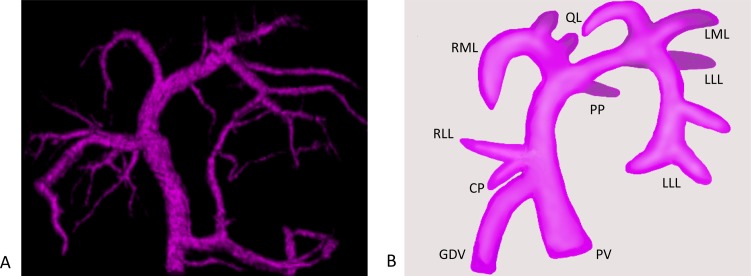
Fig. 2.
(A) Ventral-dorsal portal phase of normal dog. (B) Schema of portal vein. A main branch of the portal vein to each lobe is shown. PV, portal vein; GDV, gastroduodenal vein; PP, papillary process of the caudate lobe; LLL, left lateral lobe; LML, left medial lobe; QL, quadrate lobe; RML, right medial lobe; RLL, right lateral lobe; CP, caudate process of the caudate lobe.
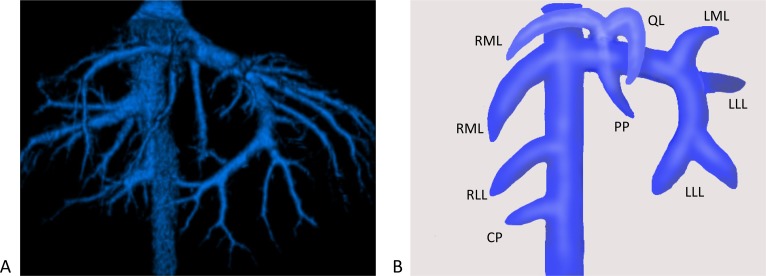
Fig. 3.
(A) Ventral-dorsal hepatic vein of a normal dog. (B) Schema of the hepatic vein, with a main branch of the hepatic vein to each lobe shown. PP, papillary process of the caudate lobe; LLL, left lateral lobe; LML, left medial lobe; QL, quadrate lobe; RML, right medial lobe; RLL, right lateral lobe; CP, caudate process of the caudate lobe.
Fig. 4.
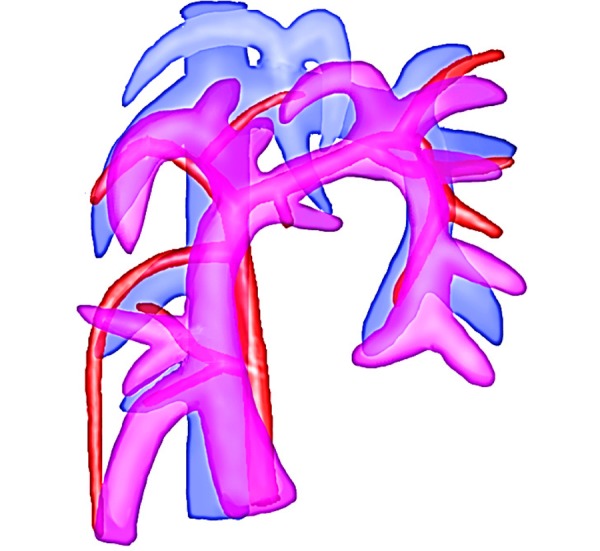
Schema of the ventral-dorsal hepatic artery, portal vein and hepatic vein.
Papillary process (PP) of the caudate lobe: Hepatic artery. In four of the 32 dogs (13%), one main artery was confirmed, and the artery arose proximally from the left hepatic artery.
Portal vein. In all dogs, one main portal vein was confirmed. It arose from the portal vein and entered PP at the level of the bifurcation of the right medial portal vein, running parallel and dorsal to the papillary lobar hepatic vein.
Hepatic vein: All dogs had one hepatic vein that originated from the bifurcation of the right medial lobar and quadrate lobar portal vein. The papillary lobar hepatic vein was dorsal to the portal vein of the PP.
Left lateral lobe (LLL): Hepatic artery. In all dogs, the hepatic artery was confirmed. Most dogs had two main arteries supplying the LLL, and each of the arteries branched to the cranial and caudal directions.
Portal vein. All dogs had two main portal vein branches supplying the cranial and caudal LLL. Each portal vein entered the LLL at the bifurcation of the quadrate and left medial lobar portal vein.
Hepatic vein. All dogs had two main hepatic veins that branched from the left hepatic vein to the cranial and caudal LLL as the portal vein. The hepatic vein was ventral and parallel to the portal vein of the LLL in all dogs.
Left medial lobe (LML): Hepatic artery. In all dogs, the hepatic artery was confirmed. Twenty nine dogs (91%) had an artery arising from the left hepatic artery. The other dogs (9%) had an artery arising from the right medial lobar artery.
Portal vein. All dogs had one main branch supplying the LML. The main branch originated from the left portal vein and was parallel to the right medial lobar hepatic vein.
Hepatic vein. All dogs had one main branch originating from the left hepatic vein. The hepatic vein of the LML was a single vessel, while the portal vein of the LML gave off a branch to the quadrate lobe.
Quadrate lobe (QL): Hepatic artery. In 25 of the 32 dogs (78%) the hepatic artery of the QL was confirmed. Fifteen dogs (47%) had a hepatic artery arising from the right medial lobar hepatic artery. Ten dogs (31%) had a hepatic artery arising from the left hepatic artery. The hepatic artery was immediately adjacent to the right of the portal vein of the QL.
Portal vein. All dogs had one main branch supplying the QL. The main branch had a common bifurcation of the left medial lobar portal vein and originated from the left portal vein.
Hepatic vein. In all dogs the QL and right medial lobe had a common branch. The common branch originated from left hepatic vein in close proximity to vena cava. In the bed of the gallbladder, the quadrate lobar hepatic vein arose from the common branch to right division.
Right medial lobe (RML): Hepatic artery. In all dogs, the hepatic artery was confirmed and arose from the proper hepatic artery after the gastroduodenal artery.
Portal vein. All dogs’ RML were supplied by one portal vein which was located to the right of the gallbladder and originated from the left portal vein at the bifurcation of the papillary division.
Hepatic vein. In all dogs, two hepatic veins were confirmed. One hepatic vein directly branched from the vena cava and was found to the right of the portal vein. Another hepatic vein originated from the common hepatic vein with the QL.
Right lateral lobe (RLL): Hepatic artery. In 30 of the 32 dogs (94%), the right lateral lobar hepatic artery was confirmed. Twenty six dogs (81%) had one artery arising from a common hepatic artery before the gastroduodenal artery. Four of those 26 dogs had another artery arising from the right medial hepatic artery, and one of those 26 dogs had another artery arising from gastroduodenal artery. Three dogs (9%) had an artery arising from the right medial hepatic artery. One dog (3%) had an artery arising from the gastroduodenal artery.
Portal vein. In all dogs, one portal vein was confirmed. Some dogs had one to three main branches supplying the RLL. The main branch originated from a main portal vein after the gastroduodenal vein entered a main portal vein and shared the foundation with the caudate lobe portal vein.
Hepatic vein. In all dogs, the hepatic vein was confirmed. The hepatic veins directly branched from caudal vena cava. All branches were dorsal to the lobar portal vein and located deep within the hepatic parenchyma. In some dogs, an additional smaller vessel of the RLL entered the caudate lobe.
Caudate process of the caudate lobe (CP): Hepatic artery. In 21 of the 32 dogs (66%), the hepatic artery was confirmed. The hepatic artery branched from the right lateral lobar hepatic artery and was caudal to the lobar portal vein.
Portal vein: In all dogs, the portal vein was common with the bifurcation of the right lateral lobar portal vein. The portal vein of CP branched from the right lateral lobe portal vein to the caudal direction.
Hepatic vein. In all dogs, the hepatic vein was confirmed and directly branched from the vena cava. Most dogs had one main caudate lobar hepatic vein with one to three accessory branches. All vessels were caudal and slightly dorsal to the portal vein of the CP.
DISCUSSION
In the veterinary field, early detection and diagnosis has become possible due to advanced equipment including CT scanners. However, the available literature on normal blood supply of the hepatic lobes in the dog is scarce and to some extent is also conflicting [1,2,3, 9]. Therefore, the present study tried to define the running patterns of the hepatic vascular system in healthy beagles. Hepatic arteries, portal veins and hepatic veins in live dogs were successfully visualized by using 3D-CT, and the hepatic vessels were identified in detail. In the present study, some running patterns of the hepatic arteries and the vascular anatomy of the portal vein and hepatic vein were found to be consistent between normal beagle dogs.
According to Ursic et al. [9], the branching pattern of the portal vein was found to be similar in all examined dogs. This is in agreement with the present results which found that there were no significant differences between running patterns. For the hepatic artery, they showed that the right lateral, right medial and left branches were actually major arteries which supply the liver. However, their origin, course and ramification patterns differed among individual livers. This was especially true for the right medial branches. In their study [9], there was no description about the running pattern of the hepatic vein. In contrast to their report [9], in the present study, the right medial hepatic artery was not significantly different between the 32 beagles. There were two patterns of origin of the quadrate lobar artery: arising from the left hepatic artery and right medial hepatic artery. The right lateral hepatic artery arose from a common hepatic artery, the right medial hepatic artery or the gastroduodenal artery. In addition, the hepatic artery usually adjoined the portal vein. Occasionally, a part of the hepatic artery twisted around the portal vein. These findings may prove to be useful for interventional radiologists. The origin, course and number of the hepatic artery in the present study were different from Ursic’s report [9]. According to Hall et al. [3], there was a broadly consistent pattern in the hepatic and portal supply to the individual liver lobes. The branching pattern of the major portal vein and hepatic vein in their study [3] agrees to a certain extent with the present study; however, in their study, there was no description about the hepatic artery.
These two previous reports [3, 9] using corrosion casting technique lacked visualization of the hepatic vein or hepatic artery. However, triple phase CT can visualize the entire hepatic vascular system at one time. Unfortunately, the present report failed to identify some vascular branches, such as the arteries of PP, CP and QL, in several dogs. Although previous reports [3, 9] showed no minimum diameter of hepatic vessels observed in corrosion casting, the diameter of the vessels that could not be identified in the present study might be smaller than 1 mm. Recently, CT with thinner slices and multi detector CT have become available, and these modalities may achieve better spatial resolution to visualize small vessels. The present study used CT scanning to investigate hepatic vascular anatomy in live dogs, whereas previous reports [1,2,3, 9] used the corrosion casting technique to confirm hepatic vascular anatomy. Corrosion casting can visualize tiny vessels better than CT. However, the diameter and position of hepatic vessels may differ in live dogs. In clinical practice, it is important to obtain biological information while the individual dog is alive. Therefore, the present report tried to define hepatic vessels using CT in normal, living dogs. As a result, it was possible to visualize small vessels better using CT imaging than with the corrosion casting technique [2, 3, 9]. Interestingly, it was found that the hepatic artery differed between each individual dog (Table 1), whereas the portal vein and hepatic veins were consistent. Therefore, the anatomy of the hepatic artery of each individual dog should be confirmed in situations involving interventional radiology.
Table 1. Origination of vessels of the hepatic artery for each lobe.
| Liver lobe | Number of cases | Originated vessels |
|---|---|---|
| PP | 4/32 (12.5%) | Left hepatic artery |
| 28/32 (87.5%) | Unconfirmed | |
| LLL | 32/32 (100%) | Left hepatic artery |
| LML | 29/32 (90.6%) | Left hepatic artery |
| 3/32 (9.4%) | RMLa | |
| QL | 15/32 (46.9%) | RMLa |
| 10/32 (31.2%) | Left hepatic artery | |
| 7/32 (21.9%) | Unconfirmed | |
| RML | 32/32 (100%) | A main hepatic artery |
| RLL | 26/32 (81.2%) | Only CHA (21), CHA + RMLa (4), CHA + GDA (1) |
| 3/32 (9.4%) | RMLa | |
| 1/32 (3.1%) | GDA | |
| 2/32 (6.3%) | Unconfirmed | |
| CP | 21/32 (65.6%) | RLLa |
| 11/32 (34.4%) | Unconfirmed |
In conclusion, consistencies in vascular anatomy were found in the livers of 32 beagles. This information could be useful for diagnosis of liver failure and in creating a standardized description of the approaches to liver lobectomy in the dog. In addition, the hepatic vascular anatomy described in the present report provides the surgeon with an anatomic guideline to consult when planning and performing liver lobe resection and interventional radiology in the dog.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 25234567.
REFERENCES
- 1.Berent A. C., Tobias K. M.2012. Hepatic vascular anomalies. Veterinary Surgery Small Animal 1: 1624–1658. [Google Scholar]
- 2.Covey J. L., Degner D. A., Jackson A. H., Hofeling A. D., Walshaw R.2009. Hilar liver resection in dogs. Vet. Surg. 38: 104–111. doi: 10.1111/j.1532-950X.2008.00467.x [DOI] [PubMed] [Google Scholar]
- 3.Hall J. L., Mannion P., Ladlow J. F.2015. Canine intrahepatic vasculature: is a functional anatomic model relevant to the dog? Vet. Surg. 44: 27–34. [DOI] [PubMed] [Google Scholar]
- 4.Hiatt J. R., Gabbay J., Busuttil R. W.1994. Surgical anatomy of the hepatic arteries in 1000 cases. Ann. Surg. 220: 50–52. doi: 10.1097/00000658-199407000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hlaing K. P., Othman F.2012. Complex pattern of a variant hepatic artery. Singapore Med. J. 53: e186–e188. [PubMed] [Google Scholar]
- 6.Jeong Y., Lim C., Oh S., Jung J., Chang J., Yoon J., Choi M.2008. Three-dimensional CT angiography of the canine hepatic vasculature. J. Vet. Sci. 9: 407–413. doi: 10.4142/jvs.2008.9.4.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katou K.2003. 3D Anatomy. pp. 80–139, Nihonijishinpousya, Tokyo. [Google Scholar]
- 8.Uchida K., Taniguchi M., Shimamura T., Suzuki T., Yamashita K., Ota M., Kamiyama T., Matsushita M., Furukawa H., Todo S.2010. Three-dimensional computed tomography scan analysis of hepatic vasculatures in the donor liver for living donor liver transplantation. Liver Transpl. 16: 1062–1068. doi: 10.1002/lt.22109 [DOI] [PubMed] [Google Scholar]
- 9.Ursic M., Ravnik D., Hribernik M., Pecar J., Butinar J., Fazarinc G.2007. Gross anatomy of the portal vein and hepatic artery ramifications in dogs: corrosion cast study. Anat. Histol. Embryol. 36: 83–87. doi: 10.1111/j.1439-0264.2006.00719.x [DOI] [PubMed] [Google Scholar]
- 10.Zwingenberger A. L., Schwarz T.2004. Dual-phase CT angiography of the normal canine portal and hepatic vasculature. Vet. Radiol. Ultrasound 45: 117–124. doi: 10.1111/j.1740-8261.2004.04019.x [DOI] [PubMed] [Google Scholar]