Abstract
Introduction
Functional and aesthetic outcome after breast-conserving surgery are vital endpoints for patients with primary breast cancer. A large variety of oncoplastic techniques exist; however, it remains unclear which techniques yield the highest rates of local control at first surgery, omission of reexcision or subsequent mastectomy, and merits the highest degree of patient satisfaction.
Methods
In this retrospective case cohort trial with a customized investigational questionnaire for assessment of patient satisfaction with the surgical result, we analyzed 1,035 patients with primary, unilateral breast cancer and oncoplastic surgery from 2004 to 2009.
Results
Analysis of patient reported outcome (PRO) revealed that 88 % of the cohort was satisfied with their aesthetic result using oncoplastic techniques following the concept presented. These results also were achieved in difficult tumor localizations, such as upper inner and lower inner quadrant. Conversion rate from breast-conserving therapy to secondary mastectomy was low at 7.2 % (n = 68/944 patients). The systematization of oncoplastic techniques presented—embedded in a multimodal concept of breast cancer therapy—facilitates tumor control with a few number of uncomplicated techniques adapted to tumor site and size with a median resection of 32 (range 11–793) g. Five-year recurrence rate in our cohort was 4.0 %.
Conclusions
Patient´s satisfaction was independent from age, body mass index, resection volume, tumor localization, and type of oncoplastic surgery (p > 0.05). We identified postoperative pain as an important negative impact factor on patient´s satisfaction with the aesthetic result (p = 0.0001).
Electronic supplementary material
The online version of this article (doi:10.1245/s10434-015-4396-4) contains supplementary material, which is available to authorized users.
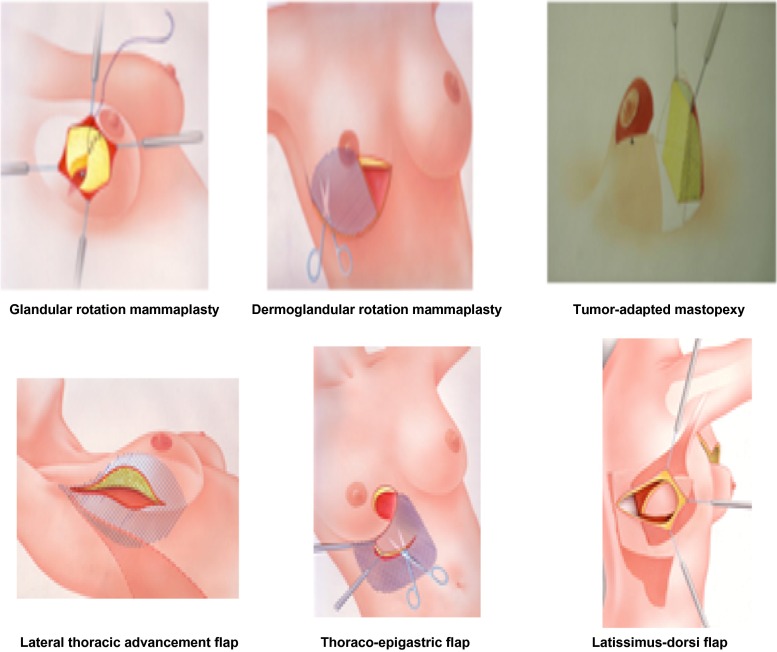
The oncologic outcome of breast-conserving surgery is equivalent to mastectomy, when free margins are achieved and adjuvant radiotherapy of the operated breast is applied.1–5 Oncoplastic breast conserving techniques combine two aspects: oncological safety with a resection of the tumor with free margins and optimal aesthetic aspects.6–8 Breast-conserving oncoplastic techniques divide into volume displacement and volume replacement techniques: the first are constituted by rotational mammaplasty techniques (glandular rotation mammaplasty, dermoglandular rotation mammaplasty and tumor-adapted mastopexy), the latter by latissimus-dorsi-flap and lateral thoracic advancement flap.9–11 We investigated the options and limitations of oncoplastic surgery as well as patient satisfaction in a large cohort of oncoplastic patients. As primary endpoints, we defined: the oncological safety of oncoplastic breast surgery (clear margins, low recurrence rate) and feasibility (reexcision rates, secondary mastectomy rates) as well as patient satisfaction [patient-reported outcome (PRO)].
Patients and Methods
We analysed data of 1,035 oncoplastic patients in a breast unit of maximum care from 2004 to 2009 retrieved from patient charts and used a customized questionnaire for evaluation of current patient satisfaction. An additional questionnaire as a validated instrument of perceived esthetic and functional status of the breast was used, i.e., “BCTOS” (Breast Cancer Treatment Outcome Scale), first described by Stanton et al.,12 and ratings in this scale were correlated to the ratings in our customized questionnaire.
Data cutoff was at February 2013. We explored the following characteristics, comorbidities, and surgery-related complications:
Patient characteristics (body-mass-index, age, menarche, menopause, family history of breast cancer)
Tumor characteristics (histology, TNM-classification, immunohistochemical subtype, tumor localization)
Surgical treatment characteristics (local therapy: operation—type of surgery, margins, reexcision rate, resection volume)
Physical sequelae/complications: (early: <14 days; late: ≥14 days)
Pain scale
Secondary mastectomy rates and its influencing factors
Patient satisfaction with the aesthetic outcome
Disease-free and overall survival
Intrinsic subtypes have been approximated by immunohistochemical characterization according to 12th St. Gallen International Consensus Conference.13 This study complies with the principles of the Declaration of Helsinki and was approved by the institutional review board.
Surgical Techniques
Surgical techniques were based first on tumour location, second on the condition whether the lesion was unicentric or multicentric, and whether resection volume would exceed >20 % of the breast. For all locations of the upper hemisphere of the breast and unicentric tumours, glandular rotation mammaplasty was the standard option for reshaping of the breast. With multicentricity or breast resection >20 % or tumours of the lower hemisphere of the breast, a reduction mammaplasty pattern was applied (inferior-pedicled technique described by Ribeiro in the modification of the author) to reconstitute the optimal breast form. This procedure avoids birds peak deformations for patients with gross resection of tissue in the lower quadrants of the breast. Where fat tissue was readily accessible for volume displacement without necessity of musculocutaneous flaps, this was incorporated in the concept of reshaping of the breast such as the thoracoepigastric flap for the lower quadrants (in cases of skin resection) and lateral thoracic advancement flap for the upper outer quadrant (in cases with need of additional volume replacement).9
Statistics
Because statistical tests—χ 2/Likelihood, Mantel–Haenszel test, and Wilcoxon’s test—for calculation were applied, p values must be seen as descriptive, not adjusted. Log-rank test and Wilcoxon rank-sum tests were used (Fig. 1). To calculate 5-year overall survival and disease-free survival, the date of death or local recurrence was defined as the endpoint, respectively, and the duration of follow-up was calculated as the date of breast-conserving surgery to this endpoint.
Fig. 1.
Surgical techniques
Results
Of 1,035 patients with oncoplastic operations, 944 patients met the inclusion criteria (REMARK diagram, see Supplement, Material 1); 70.7 % (624/882) of patients responded to the emission of questionnaires. Average age was 57.6 years (median 58, range 25–88 years). Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Cohort Prozent |
Responders | Nonresponders | ||||
|---|---|---|---|---|---|---|
| n = 944 | n = 624 | n = 320 | ||||
| Characteristics | No. | % | No. | % | No. | % |
| Age group at time of surgery (year) | ||||||
| 20–29 | 7 | 0.7 | 1 | 0.2 | 6 | 1.9 |
| 30–39 | 41 | 4.3 | 21 | 3.4 | 20 | 6.3 |
| 40–49 | 190 | 20.1 | 110 | 17.6 | 80 | 25.0 |
| 50–59 | 261 | 27.7 | 172 | 27.5 | 89 | 27.7 |
| 60–69 | 305 | 32.3 | 215 | 34.5 | 90 | 28.1 |
| 70–79 | 128 | 13.6 | 100 | 16.0 | 28 | 8.8 |
| 80–89 | 12 | 1.3 | 5 | 0.8 | 7 | 2.2 |
| Unknown | 0 | 0 | 0 | 0.0 | 0 | 0.0 |
| BMI | ||||||
| Underweight (BMI 15–19.9 kg/m2) | 56 | 5.9 | 27 | 4.3 | 29 | 9.0 |
| Normal weight (BMI 20–24.9 kg/m2) | 534 | 56.6 | 356 | 57.1 | 178 | 55.6 |
| Overweight (BMI 25–29.9 kg/m2) | 269 | 28.5 | 184 | 29.5 | 85 | 26.7 |
| Obesity (BMI > 30.0 kg/m2) | 72 | 7.6 | 51 | 8.2 | 21 | 6.6 |
| Unknown | 13 | 1.4 | 6 | 0.9 | 7 | 2.1 |
| Age of menarche (year) | ||||||
| <12 | 70 | 7.4 | 68 | 10.9 | ||
| 12–16 | 509 | 53.9 | 509 | 81.6 | ||
| 17–20 | 20 | 2.1 | 20 | 3.2 | ||
| >20 | 2 | 0.2 | 2 | 0.3 | ||
| Unknown | 343 | 36.3 | 25 | 4.0 | ||
| Age at menopause (year) | ||||||
| <30 | 3 | 0.3 | 3 | 0.5 | ||
| 30–39 | 32 | 3.4 | 32 | 5.2 | ||
| 40–49 | 239 | 25.3 | 239 | 38.3 | ||
| 50–59 | 206 | 21.8 | 206 | 33.0 | ||
| ≥60 | 7 | 0.7 | 7 | 1.1 | ||
| Unknown | 457 | 48.4 | 137 | 21.9 | ||
| Menopause status at time of surgery | ||||||
| Premenopausal | 61 | 6.5 | 61 | 9.8 | 0 | 0.0 |
| Perimenopausal | 11 | 1.2 | 11 | 1.8 | 0 | 0.0 |
| Postmenopausal | 576 | 61.8 | 469 | 75.2 | 107 | 33.4 |
| Unknown | 296 | 31.3 | 83 | 13.2 | 213 | 66,6 |
| Hormone replacement therapy | ||||||
| Administered, duration unknown | 152 | 16.1 | 109 | 17.5 | 43 | 13.5 |
| Administered up to 10 years | 123 | 13.0 | 120 | 19.2 | 3 | 0.9 |
| Administered 10 years or more | 270 | 28.6 | 269 | 43.1 | 1 | 0.3 |
| Not administered | 121 | 12.9 | 114 | 18.3 | 7 | 2.2 |
| Unknown | 278 | 29.4 | 12 | 1.9 | 266 | 83.1 |
| Family history of breast cancer | ||||||
| BRCA-positive | 18 | 1.9 | 18 | 2.9 | 0 | 0.0 |
| BRCA-negative | 235 | 24.5 | 194 | 31.1 | 41 | 12.8 |
| Negative | 398 | 42.2 | 397 | 63.6 | 1 | 0.3 |
| Unknown | 2934 | 31.4 | 15 | 2.4 | 278 | 86.9 |
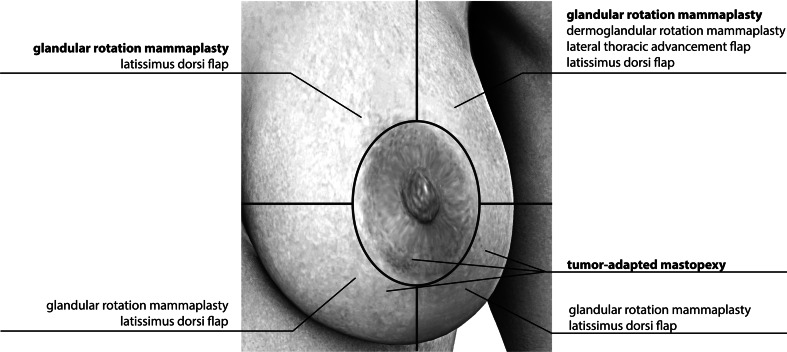
The selection of an oncoplastic technique presented follows a nomogram that we adapted to the tumor localization, tumor size, and the volume of the breast. The different choices of techniques are displayed in Fig. 2.
Fig. 2.
Selection of oncoplastic techniques
In the lower hemisphere of the breast, 55.4 % of tumors were operated by tumor-adapted mastopexy, whereas glandular rotation mammoplasty was less frequently used here (36.2 %). In the upper part of the breast, glandular rotation mammoplasty was the most frequently used technique (69.5 %), whereas tumor-adapted mastopexy was not commonly performed (11.3 %, p > 0.001). One-third (29.7 %) of multicentric or multifocal tumors were operated by tumor-adapted mastopexy. Dermoglandular rotation mammaplasty and lateral thoracic advancement flap are predominantly performed in cases of involvement of the upper outer quadrant of the breast. Glandular rotation mammaplasty and latissimus-dorsi-flap were not associated with specific tumor locations (Table 2). The tumor-adapted mastopexy was characterized by a significantly higher median resection volume (52 g) compared with the glandular rotation mammaplasty (29 g; p < 0.001). Tumor size was not a determining factor for the choice of a certain oncoplastic technique (p > 0.05).
Table 2.
Oncoplastic techniques by tumor location
| Tumor location | ||||||
|---|---|---|---|---|---|---|
| Oncoplastic techniques | Lowera | Multifocal/multicentric | NAC and horizontal transitionb | Upperc | Unknown | Total |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Glandular rotation mammaplasty | 47 (36.2) | 70 (63.1) | 68 (68.0) | 405 (69.5) | 12 (60.0) | 602 (63.8) |
| Dermoglandular rotation mammaplasty | 8 (6.2) | 2 (1.8) | 3 (3.0) | 49 (8.4) | 1 (5.0) | 63 (6.7) |
| Tumor-adapted mastopexy | 72 (55.4) | 33 (29.7) | 23 (23.0) | 66 (11.3) | 3 (15.0) | 197 (20.9) |
| Lateral thoracic advancement flap | 2 (1.5) | 1 (0.9) | 3 (3.0) | 36 (6.2) | 0 (0.0) | 42 (4.4) |
| Latissimus-dorsi flap | 0 | 1 (0.9) | 0 | 5 (0.9) | 1 (5.0) | 7 (0.7) |
| Others | 1 (0.8) | 2 (2.7) | 3 (3.0) | 19 (3.3) | 1 (5.0) | 27 (2.9) |
| Unknown | 0 | 1 (0.9) | 0 | 3 (0.5) | 2 (10.0) | 6 (0.6) |
| Total | 130 (13.8) | 111 (11.8) | 100 (10.6) | 583 (61.8) | 20 (2.1) | 944 (100) |
aLower inner quadrant, lower outer quadrant, 6 o’clock
b3 and 9 o’clock
cUpper outer quadrant, upper inner quadrant, 12 o’clock
Aesthetic Outcome
From 624 responders, 558 patients provided information about patient satisfaction with the aesthetic outcome (PRO). Results of PRO revealed a total of high degree of satisfaction with 78 % rating the aesthetic result as very good (55 %) or good (23 %). Combined with the rating “satisfactory” (10 %), a total of 88 % were satisfied with their surgical result. Five percent scored “fair,” 3 % “insufficient,” and 5 % “unsatisfactory.”
Aesthetic Outcome in Questionnaires and Breast Cancer Treatment Outcome Scale
We compared the ratings of aesthetic outcome in our customized questionnaires with the results of the Breast Cancer Treatment Outcome Scale (BCTOS).12 In the BCTOS ratings, patients evaluated the outcome of the treated vs. untreated breast in 80.3 and 83.5 %, respectively, as “no difference or almost no difference” regarding the size and form of the breast. We found a significant correlation for good functional and aesthetic outcome in the BCTOS (= no difference or almost no difference) with high ratings of satisfaction in our customized questionnaire (p < 0.001).
Factors that Influence the Perception of the Aesthetic Result
Factors that negatively influence the assessment of the aesthetic result were postoperative pain, wound infection, and issues related to scars. A higher intensity of pain on a visual analogue scale (VAS) ≥5 also was associated with less satisfaction with aesthetic outcome (p < 0.0001). Vice versa, those patients rating the aesthetic result as “very good” and “good” experienced low pain intensity in 86.3 % during the period of 14 days after surgery. This trend corresponded well with the perception of patients beyond the first 2 weeks after surgery.
Complication rate was low with 3.3 % wound infection (20/624), 9.5 % broadening of the scars (56/624), and 7.9 % occurrence of keloids (47/624). A total of 60.1 % of patients experienced perceptibility of the scar by palpation (346/624). The correlation of these complications with a lower rating of the aesthetic result was statistically significant [wound infection (p < 0.0001), broadening of the scars (p < 0.0001), perceptibility of the scars by palpation (p < 0.0001), and occurrence of keloids (p < 0.0001)].
Factors That Do Not Influence the Rating of the Aesthetic Result
The following factors did not exert any impact on the patient satisfaction (p > 0.05):
Resection volume
Type of oncoplastic technique
Age at time of surgery
Body mass index (BMI) at time of surgery
Tumor localization
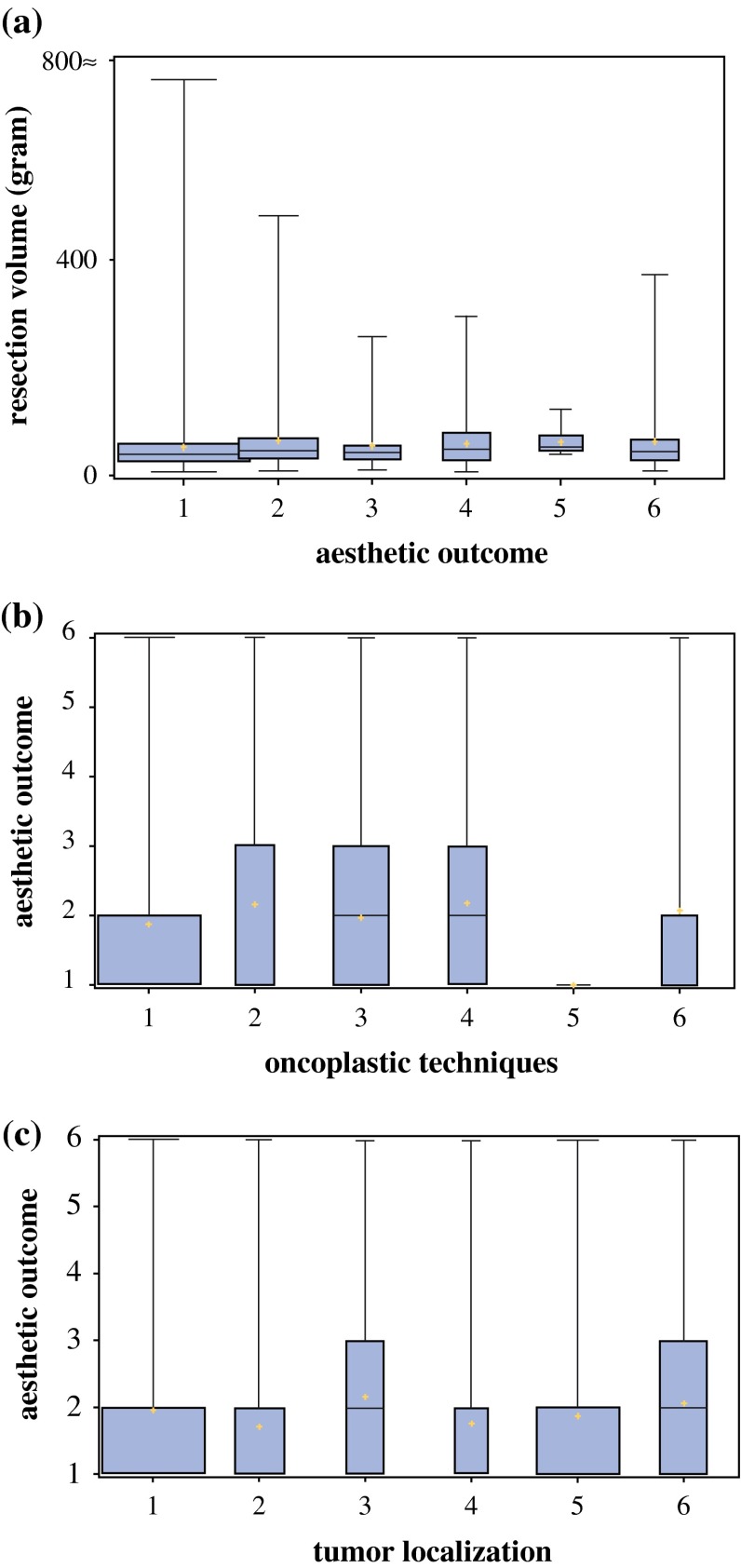
The following boxplots illustrate the independence of the aesthetic result from the factors: resection volume, type of surgery, and localization of the tumor (Fig. 3a–c).
Fig. 3.
a Aesthetic outcome (1 = very good; 2 = good; 3 = satisfactory; 4 = fair; 5 = insufficient; 6 = unsatisfactory) and the influence of resection volume (g). b Aesthetic outcome (1 = very good; 2 = good; 3 = satisfactory; 4 = fair; 5 = insufficient; 6 = unsatisfactory) and the influence of the choice of the primary surgical technique (1 = glandular rotation mammoplasty; 2 = dermoglandular rotation mammoplasty; 3 = tumor-adapted mastopexies; 4 = lateral thoracic advancement flap; 5 = latissimus-dorsi-flap; 6 = others). c Aesthetic outcome (1 = very good; 2 = good; 3 = satisfactory; 4 = fair; 5 = insufficient; 6 = unsatisfactory) and the influence of the tumor localization (1 = upper outer; 2 = upper inner; 3 = lower outer; 4 = lower inner; 5 = transition; 6 = multicentric and multifocal)
Patients across all ages and all BMI groups reported a high degree of satisfaction with the aesthetic result. BMI did not have any impact on the aesthetic result (p > 0.05). The majority of patients denoted that oncoplastic surgery did not have an impact on partnership (88 %) or body image (74 %).
Reexcision Rate and Necessity of Secondary Mastectomy to Achieve Local Control
In 11.4 % (108/944) of patients, margins were unclear after first oncoplastic surgery, of which 89.8 % (97/108) underwent reexcision. This resulted in a margin clearance of 96.9 % for all patients opting for reexcision. However 10.2 % (11/108) of patients did not undergo reexcision. Finally, a proportion of 1.5 % (14/944) of the whole oncoplastic cohort remained with unclear margins.
Factors that influenced the clearness of margins were multicentricity or multifocality of tumors (p < 0.001). Neither T stage nor resection volume had an impact on primarily achieved margin status (p > 0.05).
In a total of 7.2 % (68/944) of the cohort, a secondary mastectomy had to be performed. We identified ductal carcinoma in situ (DCIS) (p = 0.001) with and without invasive subtype as an independent risk factor for a subsequent mastectomy, and likewise this was the case for lobular histology (p = 0.001). A total of 13.6 % (n = 15/110) of lobular histology underwent mastectomy, whereas 5.8 % (n = 33/572) of invasive ductal histological subtype underwent this procedure.
Mastectomy as a subsequent procedure did not correlate with the choice of oncoplastic technique used for primary surgery (p > 0.05).
In 77.4 % (n = 731/944) of patients, the first oncoplastic operation was the definitive and final procedure. In 22.6 % (213/944) of cases, patients underwent two more surgical procedures. Reasons other than clearance of margins for a subsequent operation were bleeding in 5.0 % (47/944), contralateral alignment in 1.9 % (18/944), and dehiscence of scars in 0.4 % (4/944).
Local Recurrence Rates
Thirty-eight women (4.0 %) experienced a local recurrence at a median follow-up time of 5.2 years. We detected no significant difference between the oncoplastic techniques. Five-year disease-free survival was 90.9 %, and 5-year overall survival was 94.5 %.
Discussion
Optimal local tumor control and the prevention of recurrence or metastatic spread by surgery, radiotherapy, and systemic therapy are the primary goal of breast cancer treatment.14 The systematization of oncoplastic operations presented in our study facilitates a high degree of local oncological control for any tumor localization and almost any tumor size. Nomograms for oncoplastic surgery were published by Veronesi et al., who presented reconstructive variations after quadrantectomy with higher aesthetic outcomes.15 The local recurrence rate in our study of 4.0 % is low in the context of international literature, where recurrence rates up to 9 % are reported in similar cohorts.16,17 In a recent meta-analysis of Losken et al., local recurrence occurred in oncoplastic patients only at a rate of 4 % compared with patients operated with breast-conserving therapy (7 %).1 The rate of subsequent operations performed in our cohort corresponds with international data, in which reexcision rates from 10 to 18 % are described.18–20 The conversion rate from oncoplastic procedure to mastectomy is low at 7.2 % in our cohort. This emphasizes the fact that extensive autologous or heterologous reconstructions may be spared when appropriate oncoplastic techniques are primarily applied.21 DCIS, multicentricity, and multifocality are known factors for a higher rate of local recurrence and consecutively mastectomy.14 Multicentric DCIS has been described as an indication for a subsequent mastectomy.22
Clear margins go along with a reduced risk of local recurrence whatever the distance of margins has been.23 In international literature, rates of unclear margins from 10.6 to 38 % are described.24–28 Our results were comparably low with a rate of 11.4 % of unclear margins after primary surgery.
Only a few patients of our cohort refused reexcision of unclear margins (n = 11). We did not detect any recurrences in these patients during the period of 5-year follow-up.
There was no significant correlation between a certain oncoplastic technique and the rate of unclear margins. In 2013, Down et al. demonstrated an advantage of oncological safety (lower rate of unclear margins) through oncoplastic techniques in a cohort of 158 patients. Oncoplastic techniques have been applied whenever the estimated volume of resection was higher than 10 % of breast volume in the inner quadrants and 20 % in the outer quadrants.29 Similar recommendations were given by Veronesi et al.30
Not only oncological safety but also aesthetic aspects are centrally incorporated in the oncoplastic concept as Cardoso and Heneghan et al. stated.31,32 Controversial data are reported as to patient satisfaction with the aesthetic result with a range of 40–89.5 %.1,33 We recorded a high degree of patient satisfaction with the aesthetic result at the upper range of internationally published data with 88 % of patients being satisfied with the aesthetic result.
Over a wide range of 11–793 (median 32) g, breast conservation appears feasible following this nomogram. Breast-tumor ratio and relative excision volume goes along with generally worse cosmetic outcome if conventional breast-conserving therapies are applied.34–37 Yang et al. demonstrated a high degree of satisfaction with aesthetic results independent of the extent of excision volume by using oncoplastic surgical techniques.7 Waljee et al. reported as treatment-related factors predictive for asymmetry: reexcision, postoperative seroma, and radiotherapy.38 These factors were comparatively low in our cohort. Tumor localizations in the upper inner and lower outer quadrant impose a high challenge to the surgeon´s skills with the risk of asymmetry.39 Even in difficult tumors locations, we did not find a deterioration of patient satisfaction; likewise, it was published in a smaller case series by Fitoussi et al.40 Most recently, smaller studies with oncoplastic techniques reported 72 patients that underlined the necessity of contralateral alignment during the same operation, which was performed in 53 of 72 patients (73.6 %) published by Rose et al.20 We report a low rate of 1.9 % (18/944 patients) with contralateral alignment operation. BMI and age did not have a negative impact on the rating of the aesthetic outcome in our cohort contrary to other study data.31,41
Conclusions
The systematization of oncoplastic techniques in the concept presented in this study (Fig. 3) embedded in a multimodal concept of breast cancer therapy1–3 facilitates tumor control with the use of uncomplicated techniques adapted to tumor site and size with a median resection of 32 (range 11–793) g in this cohort. It demonstrated a high level of satisfaction of patient-reported outcome using this concept. These favourable results were independent from age, BMI, resection volume, tumor localization, and type of oncoplastic surgery. We identified postoperative pain as an important factor to deteriorate patient satisfaction. This underlines the need for a well-structured pain management schedule postoperatively to eliminate this negative factor that influences patient assessment of the surgical result.
Electronic supplementary material
Below is the link to the electronic supplementary material.
References
- 1.Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg. 2013. [DOI] [PubMed]
- 2.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 4.Clough KB, Ihrai T, Oden S, Kaufman G, Massey E, Nos C. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg. 2012;99(10):1389–1395. doi: 10.1002/bjs.8877. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Saccozzi R, Del Vecchio M, Banfi A, Clemente C, De Lena M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305(1):6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 6.Yang JD, Bae SG, Chung HY, Cho BC, Park HY, Jung JH. The usefulness of oncoplastic volume displacement techniques in the superiorly located breast cancers for Korean patients with small to moderate-sized breasts. Ann Plast Surg. 2011;67(5):474–480. doi: 10.1097/SAP.0b013e318201fdf4. [DOI] [PubMed] [Google Scholar]
- 7.Yang JD, Lee JW, Cho YK, Kim WW, Hwang SO, Jung JH, et al. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderate-sized breasts (part 2): volume replacement. J Breast Cancer. 2012;15(1):7–14. doi: 10.4048/jbc.2012.15.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabel MS. Surgical considerations in early-stage breast cancer: lessons learned and future directions. Semin Radiat Oncol. 2011;21(1):10–19. doi: 10.1016/j.semradonc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Kramer S, Darsow M, Kummel S, Kimmig R, Rezai M. Breast-conserving treatment of breast cancer–oncological and reconstructive aspects. Gynakol Geburtshilfliche Rundsch. 2008;48(2):56–62. doi: 10.1159/000118932. [DOI] [PubMed] [Google Scholar]
- 10.Rezai M, Nestle-Krämling C. Oncoplastic surgical techniques in breast-conserving therapy for carcinoma of the breast. Der Gynäkologe. 1999;32(2):83–90. [Google Scholar]
- 11.Masetti R, Di Leone A, Franceschini G, Magno S, Terribile D, Fabbri MC, et al. Oncoplastic techniques in the conservative surgical treatment of breast cancer: an overview. Breast J. 2006;12(5 Suppl 2):S174–S180. doi: 10.1111/j.1075-122X.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- 12.Stanton AL, Krishnan L, Collinc CA. Form or function? Part 1. Subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 91;2273–81. [PubMed]
- 13.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nijenhuis MV, Rutgers EJT. Who should not undergo breast conservation? Breast. 2013;22:S110–S114. doi: 10.1016/j.breast.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Veronesi U, Zurrida S. Breast conservation: current results and future perspectives at the European Institute of Oncology. Int J Cancer. 2007;120(7):1381–1386. doi: 10.1002/ijc.22529. [DOI] [PubMed] [Google Scholar]
- 16.Fatouros M, Roukos DH, Arampatzis I, Sotiriadis A, Paraskevaidis E, Kappas AM. Factors increasing local recurrence in breast-conserving surgery. Expert Rev Anticancer Ther. 2005;5(4):737–745. doi: 10.1586/14737140.5.4.737. [DOI] [PubMed] [Google Scholar]
- 17.Moran MS, Haffty BG. Local-regional breast cancer recurrence: prognostic groups based on patterns of failure. Breast J. 2002;8(2):81–87. doi: 10.1046/j.1524-4741.2002.08202.x. [DOI] [PubMed] [Google Scholar]
- 18.Chagpar AB, Martin RC, 2nd, Hagendoorn LJ, Chao C, McMasters KM. Lumpectomy margins are affected by tumor size and histologic subtype but not by biopsy technique. Am J Surg. 2004;188(4):399–402. doi: 10.1016/j.amjsurg.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Jung W, Kang E, Kim SM, Kim D, Hwang Y, Sun Y, et al. Factors associated with re-excision after breast-conserving surgery for early-stage breast cancer. J Breast Cancer. 2012;15(4):412–419. doi: 10.4048/jbc.2012.15.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose M, Manjer J, Ringberg A, Svensson H. Surgical strategy, methods of reconstruction, surgical margins and postoperative complications in oncoplastic breast surgery. Eur J Plast Surg. 2014;37:205–214. doi: 10.1007/s00238-013-0922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezai M, Darsow M, Kummel S, Kramer S. Autologous and alloplastic breast reconstruction–overview of techniques, indications and results. Gynakol Geburtshilfliche Rundsch. 2008;48(2):68–75. doi: 10.1159/000118934. [DOI] [PubMed] [Google Scholar]
- 22.Patani N, Mokbel K. Oncological and aesthetic considerations of skin-sparing mastectomy. Breast Cancer Res Treat. 2008;111(3):391–403. doi: 10.1007/s10549-007-9801-7. [DOI] [PubMed] [Google Scholar]
- 23.Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al. Society of Surgical Oncology-American Society for Radiation Oncology Consensus Guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):553–564. doi: 10.1016/j.ijrobp.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz D, Rawlinson E, Narod SA, Sun P, Lickley HL, McCready DR, et al. The role of reexcision for positive margins in optimizing local disease control after breast-conserving surgery for cancer. Breast J. 2006;12(4):331–337. doi: 10.1111/j.1075-122X.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 25.Dzierzanowski M, Melville KA, Barnes PJ, MacIntosh RF, Caines JS, Porter GA. Ductal carcinoma in situ in core biopsies containing invasive breast cancer: correlation with extensive intraductal component and lumpectomy margins. J Surg Oncol. 2005;90(2):71–76. doi: 10.1002/jso.20242. [DOI] [PubMed] [Google Scholar]
- 26.Rath MG, Heil J, Domschke C, Topic Z, Schneider S, Sinn HP, et al. Predictors of resectability in breast-conserving therapy. Arch Gynecol Obstet. 2012. [DOI] [PubMed]
- 27.Semprini G, Cattin F, Vaienti L, Brizzolari M, Cedolini C, Parodi PC. Oncoplastic surgery and cancer relapses: cosmetic and oncological results in 489 patients. Breast. 2013. [DOI] [PubMed]
- 28.Gulcelik MA, Dogan L, Yuksel M, Camlibel M, Ozaslan C, Reis E. Comparison of outcomes of standard and oncoplastic breast-conserving surgery. J Breast Cancer. 2013;16(2):193–197. doi: 10.4048/jbc.2013.16.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Down SK, Jha PK, Burger A, Hussien MI. Oncological advantages of oncoplastic breast-conserving surgery in treatment of early breast cancer. Breast J. 2013;19(1):56–63. doi: 10.1111/tbj.12047. [DOI] [PubMed] [Google Scholar]
- 30.Veronesi U, Zurrida S. Preserving life and conserving the breast. LancetOncol. 2009;10(7):736-2045(09)70117-2. [DOI] [PubMed]
- 31.Cardoso MJ, Cardoso J, Santos AC, Vrieling C, Christie D, Liljegren G, et al. Factors determining esthetic outcome after breast cancer conservative treatment. Breast J. 2007;13(2):140–146. doi: 10.1111/j.1524-4741.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- 32.Heneghan HM, Prichard RS, Lyons R, Regan PJ, Kelly JL, Malone C, et al. Quality of life after immediate breast reconstruction and skin-sparing mastectomy: a comparison with patients undergoing breast conserving surgery. Eur J Surg Oncol. 2011;37(11):937–943. doi: 10.1016/j.ejso.2011.08.126. [DOI] [PubMed] [Google Scholar]
- 33.Kelly DA, Wood BC, Knoll GM, Chang SC, Crantford JC, Bharti GD, et al. Outcome analysis of 541 women undergoing breast conservation therapy. Ann Plast Surg. 2012;68(5):435–437. doi: 10.1097/SAP.0b013e31823b6ae3. [DOI] [PubMed] [Google Scholar]
- 34.Cochrane RA, Valasiadou P, Wilson AR, Al-Ghazal SK, Macmillan RD. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg. 2003;90(12):1505–1509. doi: 10.1002/bjs.4344. [DOI] [PubMed] [Google Scholar]
- 35.Heil J, Breitkreuz K, Golatta M, Czink E, Dahlkamp J, Rom J, et al. Do reexcisions impair aesthetic outcome in breast conservation surgery? Exploratory analysis of a prospective cohort study. Ann Surg Oncol. 2012;19(2):541–547. doi: 10.1245/s10434-011-1947-1. [DOI] [PubMed] [Google Scholar]
- 36.Al-Ghazal SK, Blamey RW, Stewart J, Morgan AA. The cosmetic outcome in early breast cancer treated with breast conservation. Eur J Surg Oncol. 1999;25(6):566–570. doi: 10.1053/ejso.1999.0707. [DOI] [PubMed] [Google Scholar]
- 37.Ozmen T, Polat AV, Polat AK, Bonaventura M, Johnson R, Soran A. Factors affecting cosmesis after breast conserving surgery without oncoplastic techniques in an experienced comprehensive breast center. Surgeon. 2014. [DOI] [PubMed]
- 38.Waljee JF, Hu ES, Ubel PA, Smith DM, Newman LA, Alderman AK. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol. 2008;26(20):3331–3337. doi: 10.1200/JCO.2007.13.1375. [DOI] [PubMed] [Google Scholar]
- 39.Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of breast asymmetry after breast-conserving operation for breast cancer. J Am Coll Surg. 2008;206(2):274–280. doi: 10.1016/j.jamcollsurg.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Fitoussi AD, Berry MG, Fama F, Falcou MC, Curnier A, Couturaud B, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases [outcomes article. Plast Reconstr Surg. 2010;125(2):454–462. doi: 10.1097/PRS.0b013e3181c82d3e. [DOI] [PubMed] [Google Scholar]
- 41.Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38(5):375–381. doi: 10.1016/j.ejso.2012.02.179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.