Abstract
Purpose
To evaluate prospectively the role of prostate-specific antigen (PSA) density in predicting Gleason score upgrading in prostate cancer patients eligible for active surveillance (T1/T2, biopsy Gleason score≤6, PSA≤10 ng/mL, and ≤2 positive biopsy cores).
Materials and Methods
Between January 2010 and November 2013, among patients who underwent greater than 10-core transrectal ultrasound-guided biopsy, 60 patients eligible for active surveillance underwent radical prostatectomy. By use of the modified Gleason criteria, the tumor grade of the surgical specimens was examined and compared with the biopsy results.
Results
Tumor upgrading occurred in 24 patients (40.0%). Extracapsular disease and positive surgical margins were found in 6 patients (10.0%) and 8 patients (17.30%), respectively. A statistically significant correlation between PSA density and postoperative upgrading was found (p=0.030); this was in contrast with the other studied parameters, which failed to reach significance, including PSA, prostate volume, number of biopsy cores, and number of positive cores. Tumor upgrading was also highly associated with extracapsular cancer extension (p=0.000). The estimated optimal cutoff value of PSA density was 0.13 ng/mL2, obtained by receiver operating characteristic analysis (area under the curve=0.66; p=0.020; 95% confidence interval, 0.53-0.78).
Conclusions
PSA density is a strong predictor of Gleason score upgrading after radical prostatectomy in patients eligible for active surveillance. Because tumor upgrading increases the potential for postoperative pathological adverse findings and prognosis, PSA density should be considered when treating and consulting patients eligible for active surveillance.
Keywords: Neoplasm grading, Prostate specific antigen, Prostatectomy
INTRODUCTION
In the era of prostate-specific antigen (PSA) screening, more and more patients are diagnosed with insignificant prostate cancer. Because many of these cancers will not become clinically symptomatic, active surveillance (AS) with delayed treatment has been introduced and has been reported to have similar therapeutic effects [1]. The criteria for defining patients who are suitable to enter AS protocols are mainly based on PSA, clinical stage, and tumor grade. However, many studies have shown that the tumor grade obtained by prostate biopsy does not always correlate with the final pathological grade of the surgical specimen resected in a radical prostatectomy [2,3]. In many cases, prostate cancer grade is primarily underestimated during the examination of biopsy cores. Gleason score (GS) upgrading of up to 57% of cases has been reported in some studies [4,5,6]. The incorrect assessment of tumor grade may lead to inappropriate estimation of cancer aggressiveness, resulting in under-treatment of these patients.
Thus, a critical factor for the success of AS is the use of appropriate entry criteria. Although a number of prognostic models have been developed to help to identify men who are appropriate candidates for AS, it remains controversial whether to adopt PSA density as an appropriate entry criterion. Accordingly, we conducted a prospective data analysis in patients with low-grade prostate cancer and evaluated the potential of several clinical and pathological variables to predict upgrading of the cancer.
MATERIALS AND METHODS
After we obtained approval from the Ethics Committee at Institutional Review Board of Daegu Catholic University Hospital, we conducted a prospective analysis of 60 patients with low-risk prostate cancer who were recruited from 4 university hospitals (GS≤6, PSA<10 ng/mL, T1/T2, ≤2 positive biopsy cores). All patients underwent a retropubic or laparoscopic radical prostatectomy between January 2010 and December 2013. All clinical, imaging, laboratory, and pathological data were collected and recorded prospectively and were analyzed retrospectively. We excluded patients who had received any preoperative therapy for prostate cancer (hormone therapy, radiation therapy) and patients who underwent less than 10-core transrectal ultrasound (TRUS)-guided biopsy. Biopsy cores were examined by different university-based pathologists and all radical prostatectomy samples were examined according to the standard protocol of the pathology department at each university hospital.
We analyzed age, body mass index (BMI), preoperative PSA value, PSA density, number of cores, and number of positive cores in biopsy material. We analyzed the association of these factors with upgrading of cancer in the radical prostatectomy specimen. Any increase in GS between core biopsy and radical prostatectomy specimen was considered an upgrade of cancer. Preoperative PSA was measured before the digital rectal exam, TRUS, or core biopsy. During TRUS, prostate volume was calculated according to information on the maximum transverse diameter (D1), the maximum antero-posterior diameter (D2), and the maximum longitudinal diameter (D3) and by using the formula based on the prostate ellipse dimension theory: D1×D2×D3×π/6. PSA density was calculated by dividing the preoperative PSA value and prostate volume. A histological report concerning tumor grade of the surgical specimen and pathological stage was obtained. The 2009 TNM classification for prostate cancer was used to classify the pathological stage. We estimated tumor grade by use of the contemporary criteria of the 2005 international society of urological pathology modified Gleason system [7].
Statistical analysis was performed by using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics are presented as the mean±standard deviation for continuous variables. The normality condition of the numerical variables was studied by means of the Kolmogorov-Smirnov test. PSA was the only variable with a normal distribution; consequently, Student t-test was used to compare means. To analyze other variables, the Mann-Whitney U test was used, and the chi-square test was used for categorical variables. Univariate and multivariate analysis was performed to identify significant predictive variables. The optimal cutoff values and sensitivity and specificity for quantitative variables that were found to be significant predictors were estimated by using receiver operating characteristic (ROC) curve analysis. Positive predictive value (true positive/[true positive+false positive]) and negative predictive value (true negative/[true negative+false negative]) were also estimated. All tests were described with p<0.05 as statistically significant.
RESULTS
In total, 60 patients met our inclusion criteria within the study period and were entered into the analysis. The median patient age was 63 years, the median preoperative PSA value was 6.26 ng/mL, and the median PSA density was 0.15 ng/mL2. As we previously described, every patient underwent TRUS biopsy with more than 10 cores; 58 patients (96.7%) underwent 12-core biopsy. On the basis of the pathological results from the radical prostatectomy, 2 patients (3.3%) were diagnosed with GS 5 prostate cancer, 40 patients (57.1%) had GS 6 cancer, 27 patients (38.6%) had GS 7 cancer, and 1 patient (1.4%) had GS 8. Clinical stage on the basis of the digital rectal examination and TRUS findings was categorized as follows: 61 patients (86.7%) with T1c disease and 9 patients (13.3%) with T2a prostate cancer, respectively. Following radical prostatectomy, a tumor upgrade was noticed in 28 patients (40.0%). Extracapsular extensions were noted in 6 patients (10.0%) and positive surgical margins in 8 patients (17.3%). No lymph node invasion was found. The demographic, clinical, and pathologic data regarding the presence of pathological tumor upgrading are described in Table 1.
Table 1. Demographics of low-risk prostate cancer (n=60).
| Variable | Value |
|---|---|
| Age (y) | 63 (47-75) |
| PSA level (ng/mL) | 6.4 (0.1-10.0) |
| PSA density | 0.15 (0.00-0.32) |
| Prostate volume (mL) | 36.5 (16.0-123.0) |
| No. of biopsy cores | 12 (10-12) |
| No. of positive biopsy cores | 1 (1-2) |
| Biopsy Institute | |
| Daegu Catholic University | 19 (31.7) |
| Kyungpook National University | 14 (23.3) |
| Keimyung University | 17 (28.3) |
| Yeungnam University | 10 (16.7) |
| Biopsy cores obtained | |
| 10 | 2 |
| 12 | 58 |
| Gleason score downgrade | 2 |
| Gleason score upgrade | 24 (40.0) |
| ≤6 to 7 | 23 (38.3) |
| ≤6 to ≥8 | 1 (1.7) |
Values are presented as median (range) or number (%).
PSA, prostate-specific antigen.
We statistically analyzed the significance of various variables with GS upgrading. A statistically significant correlation between preoperative PSA density and postoperative GS upgrading was noted (p=0.030). No statistical correlation with postoperative GS upgrading was found for age, BMI, preoperative PSA level, prostate volume, number of biopsy cores, number of positive biopsy cores, the institute where the biopsy was done, or the operation method. In the analysis of pathological outcomes after radical prostatectomy, tumor upgrading was highly associated with extracapsular cancer extension (p=0.000). In the multivariate analysis, PSA density was the only statistically significant variable to predict GS upgrading (p=0.030) (Table 2). The estimated optimal cutoff value of PSA density was 0.13 ng/mL2, as obtained by ROC analysis (area under the curve=0.66; p=0.020; 95% confidence interval, 0.53-0.78), as shown in Fig 1. The predictive parameters of PSA density are described in Table 3.
Table 2. Univariate and multivariate analysis comparing Gleason score upgrading in low-risk prostate cancer.
| Variable | Group | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|
| No upgrade | Upgrade | p-value | p-value | |
| Age (y) | 62.34±5.93 | 65.08±5.62 | 0.080 | 0.080 |
| Body mass index (kg/m2) | 23.12±2.60 | 24.24±3.04 | 0.130 | 0.130 |
| Prostate volume (mL) | 41.13±18.04 | 36.17±14.54 | 0.260 | 0.250 |
| PSA (ng/mL) | 6.05±2.50 | 6.79±1.83 | 0.210 | 0.200 |
| PSA density | 0.16±0.09 | 0.21±0.10 | 0.030* | 0.030* |
| Total core | 11.83±1.01 | 11.92±0.40 | 0.670 | 0.660 |
| Cancer core | 1.40±0.55 | 1.44±0.51 | 0.780 | 0.770 |
| Pathology department | 0.999 | - | ||
| Daegu Catholic University | 11 (31.4) | 8 (32.0) | ||
| Kyungpook National University | 8 (22.9) | 6 (24.0) | ||
| Keimyung University | 10 (28.6) | 7 (28.0) | ||
| Yeungnam University | 6 (17.1) | 4 (16.0) | ||
| Operative method | 0.960 | 0.960 | ||
| RRP | 25 (71.4) | 18 (72.0) | ||
| LRP | 10 (28.6) | 7 (28.0) | ||
| Pathology | 0.000* | 0.000* | ||
| T2 | 35 (100) | 19 (76.0) | ||
| T3a | 0 (0) | 6 (24.0) | ||
| Resection margin | 0.100 | 0.040 | ||
| (-) | 33 (94.3) | 19 (76.0) | ||
| (+) | 2 (5.7) | 6 (24.0) | ||
Values are presented as mean±standard deviation or number (%).
PSA, prostate-specific antigen; RRP, radical retropubic prostatectomy; LRP, laparoscopic radical prostatectomy.
*p<0.05, statistically significant difference.
Fig. 1. Receiver operating characteristic curve analysis for prostatespecific antigen (PSA) density cutoff value of 0.13 ng/mL2.
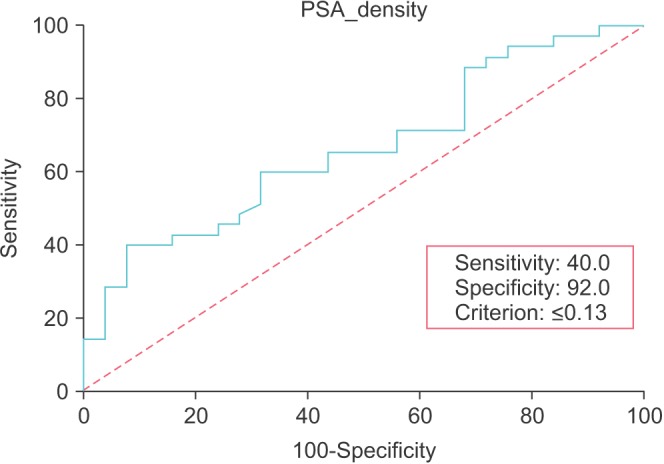
Table 3. Sensitivity, specificity, and positive and negative predictive value of prostate-specific antigen density values of 0.13 ng/mL2 for pathological Gleason score upgrade prediction.
| Value | Percentage |
|---|---|
| Sensitivity | 40.0 |
| Specificity | 92.0 |
| Positive predictive value | 86.7 |
| Negative predictive value | 51.1 |
DISCUSSION
It is well established that tumor grade is the most reliable and valuable parameter for estimating the prognosis of prostate cancer. By being a surrogate for tumor aggressiveness, the tumor grade allows us to stratify patients as either low, intermediate, or high risk. To date, the calculation of Gleason grade has been based on pathological evaluation of the cores obtained by prostate biopsy. For patients who enter an AS treatment program with close monitoring until disease progression and radical therapy, biopsy results, in combination with PSA levels and clinical stage, are the data that guide treatment decisions. Nevertheless, upgrading of the GS between the needle biopsy and the radical prostatectomy is not rare. Upgrading was found in 30% of patients in one recent meta-analysis [8], and higher rates have also been published [2,3,4,5,6]. This observation has important prognostic meaning because a significant percentage of these cases progress outside the prostate capsule [9].
The present data, which showed GS upgrading in 40.0% of samples, also revealed 24.0% extracapsular extension. Given that conservative treatment protocols are mainly applied in low-risk patients, these findings are very important. An underestimation of prostate cancer aggressiveness may lead to under-treatment and inappropriate monitoring of biologically aggressive tumors. Thus, it is clear that many patients classified as having clinically localized disease and being at low risk actually have highly malignant cancer with a risk of clinical progression.
Several reports have indicated that the likelihood of GS upgrading will decrease as the number of cores obtained by biopsy increases. King et al. [10] showed that in patients with a biopsy GS of 6, an extended biopsy strategy reduced GS upgrading from 66.7% to 36.8%. Capitanio et al.'s [11] study also showed that GS upgrading decreased from 47.9% to 23.5% by taking more than 18 cores in a cohort of patients with low-risk prostate cancer. In Coogan et al.'s [12] study, increasing the number of cores from 6 to10 significantly improved the accuracy of the biopsy GS.
The question remains as to what the optimal number of biopsy cores should be. The study by Chambo et al. [13] showed that at least 10-core biopsies may be needed in patients with low-risk prostate cancer. Adopting this concept, the present study recruited only patients who underwent biopsies of more than 10 cores. No statistically significant dif ferences were found in the present study when comparing 12-core biopsies with 10-core biopsies.
The results in the literature vary in relation to the number of positive cores. Whereas some groups have shown that an increase in positive cores correlates with an upgrading of GS [14,15,16], other studies could not find any association [17,18]. In the present study, no significant difference was found when comparing one positive core with two positive cores.
The association between preoperative PSA level and GS upgrading also varies. Whereas the studies of Mian et al. [18] and King et al. [10] did not show any association, those of Moussa et al. [19] showed that PSA level was a statistically significant predictor of GS upgrading. In the present study, we were not able to show an association between PSA level and GS upgrading.
Several reports have shown that an increase in prostate weight reduces the risk of GS upgrading [19,20,21]. An explanation for this finding might be that a small prostate size is a surrogate of low in vivo androgenicity, leading to reselection of aggressive cancers in an androgen-depleted hostile environment [21]. However, the results in the literature vary in relation to the weight of the prostate. In the present study, we also observed a lower prostate weight in the GS upgrading group. However, in the multivariate logistic regression analysis, prostate weight was not an independent predictor of GS upgrading.
Even though conf licting results regarding the interobserver reproducibility of Gleason scoring in prostate biopsies have been reported [22,23,24], we did not observe a significant difference in GS upgrading between university pathologists in a univariate analysis (Table 2). This result could partly be due to the proficiency of the pathologists and also to the number of biopsy cores being more than 10.
Few studies have evaluated the role of PSA density in upgrade prediction. Corcoran et al. [25] examined the predictive characteristics of PSA density in patients with low- and intermediate-risk disease on biopsy and subsequently treated with radical prostatectomy. They found that 58.3% of patients with low-grade disease after prostate biopsy were upgraded to higher scores and that PSA density was a significant predictor (p<0.001) of upgrading in patients with GS 6. Similar results were observed by Kojima et al. [26] and Magheli et al. [27], with p=0.019 and p=0.037, respectively. Our results add to the above data, revealing a beneficial role of PSA density in prediction of upgrading (p=0.030). A significant association of PSA density with the pathological outcomes following radical prostatectomy was observed. Gleason upgrading was correlated with increased rates of extracapsular disease (Table 2). We tried to produce a threshold level of PSA density, over which the possibility of Gleason upgrading increased significantly, triggering either a repeat biopsy or definitive therapy. By using the ROC analysis, a cutoff value of 0.13 ng/mL was produced. Even though the sensitivity and negative predictive value were low, an increased specificity and positive predictive value were found (Table 3). Given the complexity of prostate cancer, it seems to be more reasonable to use PSA density combined with other predictive factors.
Our study had several limitations. First, the sample size was relatively small and might have decreased the strength of the results. Another limitation is that the PSA density calculation was based on the estimation of prostate volume during TRUS made by 4 different operators. Even though a standard calculation protocol was followed, the interobserver differences in the volume measurement may have negatively affected the validity of the results. Last, even though the data collection was made prospectively, the analysis was done retrospectively. However, the strength of our study was that the evaluation of PSA density in low-risk prostate cancer was adjusted solely for patients who underwent biopsies with more than 10 cores. Equal distribution of the number of cores obtained by prostate biopsies might have reduced a statistical bias and increased the value of the results for the estimation of tumor grade.
CONCLUSIONS
With the commonly used criteria for AS, GS upgrading in a radical prostatectomy specimen is a challenging problem. PSA density was an independent predictor of GS upgrading and may be included as a criterion for patients eligible for AS. Further prospective study with larger sample sizes may be needed.
References
- 1.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg DM, Sauvageot J, Piantadosi S, Epstein JI. Correlation of prostate needle biopsy and radical prostatectomy Gleason grade in academic and community settings. Am J Surg Pathol. 1997;21:566–576. doi: 10.1097/00000478-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Cookson MS, Fleshner NE, Soloway SM, Fair WR. Correlation between Gleason score of needle biopsy and radical prostatectomy specimen: accuracy and clinical implications. J Urol. 1997;157:559–562. [PubMed] [Google Scholar]
- 4.Kvale R, Moller B, Wahlqvist R, Fossa SD, Berner A, Busch C, et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU Int. 2009;103:1647–1654. doi: 10.1111/j.1464-410X.2008.08255.x. [DOI] [PubMed] [Google Scholar]
- 5.Thickman D, Speers WC, Philpott PJ, Shapiro H. Effect of the number of core biopsies of the prostate on predicting Gleason score of prostate cancer. J Urol. 1996;156:110–113. [PubMed] [Google Scholar]
- 6.Corcoran NM, Hong MK, Casey RG, Hurtado-Coll A, Peters J, Harewood L, et al. Upgrade in Gleason score between prostate biopsies and pathology following radical prostatectomy significantly impacts upon the risk of biochemical recurrence. BJU Int. 2011;108(8 Pt 2):E202–E210. doi: 10.1111/j.1464-410X.2011.10119.x. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Hanley RS, Kurteva T, Ruthazer R, Silverman ML, Sorcini A, et al. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: the Lahey Clinic Medical Center experience and an international meta-analysis. Eur Urol. 2008;54:371–381. doi: 10.1016/j.eururo.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes ET, Sundaram CP, Long R, Soltani M, Ercole CJ. Biopsy Gleason score: how does it correlate with the final pathological diagnosis in prostate cancer? Br J Urol. 1997;79:615–617. doi: 10.1046/j.1464-410x.1997.00126.x. [DOI] [PubMed] [Google Scholar]
- 10.King CR, McNeal JE, Gill H, Presti JC., Jr Extended prostate biopsy scheme improves reliability of Gleason grading: implications for radiotherapy patients. Int J Radiat Oncol Biol Phys. 2004;59:386–391. doi: 10.1016/j.ijrobp.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Capitanio U, Karakiewicz PI, Valiquette L, Perrotte P, Jeldres C, Briganti A, et al. Biopsy core number represents one of foremost predictors of clinically significant gleason sum upgrading in patients with low-risk prostate cancer. Urology. 2009;73:1087–1091. doi: 10.1016/j.urology.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 12.Coogan CL, Latchamsetty KC, Greenfield J, Corman JM, Lynch B, Porter CR. Increasing the number of biopsy cores improves the concordance of biopsy Gleason score to prostatectomy Gleason score. BJU Int. 2005;96:324–327. doi: 10.1111/j.1464-410X.2005.05624.x. [DOI] [PubMed] [Google Scholar]
- 13.Chambo RC, Tsuji FH, de Oliveira Lima F, Yamamoto HA, Nobrega de. What is the ideal core number for ultrasound-guided prostate biopsy? Korean J Urol. 2014;55:725–731. doi: 10.4111/kju.2014.55.11.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong SK, Han BK, Lee ST, Kim SS, Min KE, Jeong SJ, et al. Prediction of Gleason score upgrading in low-risk prostate cancers diagnosed via multi (> or = 12)-core prostate biopsy. World J Urol. 2009;27:271–276. doi: 10.1007/s00345-008-0343-3. [DOI] [PubMed] [Google Scholar]
- 15.Gofrit ON, Zorn KC, Taxy JB, Lin S, Zagaja GP, Steinberg GD, et al. Predicting the risk of patients with biopsy Gleason score 6 to harbor a higher grade cancer. J Urol. 2007;178:1925–1928. doi: 10.1016/j.juro.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 16.Dong F, Jones JS, Stephenson AJ, Magi-Galluzzi C, Reuther AM, Klein EA. Prostate cancer volume at biopsy predicts clinically significant upgrading. J Urol. 2008;179:896–900. doi: 10.1016/j.juro.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 17.Garnett JE, Oyasu R, Grayhack JT. The accuracy of diagnostic biopsy specimens in predicting tumor grades by Gleason's classification of radical prostatectomy specimens. J Urol. 1984;131:690–693. doi: 10.1016/s0022-5347(17)50583-2. [DOI] [PubMed] [Google Scholar]
- 18.Mian BM, Lehr DJ, Moore CK, Fisher HA, Kaufman RP, Jr, Ross JS, et al. Role of prostate biopsy schemes in accurate prediction of Gleason scores. Urology. 2006;67:379–383. doi: 10.1016/j.urology.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Moussa AS, Li J, Soriano M, Klein EA, Dong F, Jones JS. Prostate biopsy clinical and pathological variables that predict significant grading changes in patients with intermediate and high grade prostate cancer. BJU Int. 2009;103:43–48. doi: 10.1111/j.1464-410X.2008.08059.x. [DOI] [PubMed] [Google Scholar]
- 20.Kassouf W, Nakanishi H, Ochiai A, Babaian KN, Troncoso P, Babaian RJ. Effect of prostate volume on tumor grade in patients undergoing radical prostatectomy in the era of extended prostatic biopsies. J Urol. 2007;178:111–114. doi: 10.1016/j.juro.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23:7546–7554. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 22.Paulson DF. Impact of radical prostatectomy in the management of clinically localized disease. J Urol. 1994;152(5 Pt 2):1826–1830. doi: 10.1016/s0022-5347(17)32395-9. [DOI] [PubMed] [Google Scholar]
- 23.Allsbrook WC, Jr, Mangold KA, Johnson MH, Lane RB, Lane CG, Epstein JI. Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum Pathol. 2001;32:81–88. doi: 10.1053/hupa.2001.21135. [DOI] [PubMed] [Google Scholar]
- 24.Allsbrook WC, Jr, Mangold KA, Johnson MH, Lane RB, Lane CG, Amin MB, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: urologic pathologists. Hum Pathol. 2001;32:74–80. doi: 10.1053/hupa.2001.21134. [DOI] [PubMed] [Google Scholar]
- 25.Corcoran NM, Casey RG, Hong MK, Pedersen J, Connolly S, Peters J, et al. The ability of prostate-specific antigen (PSA) density to predict an upgrade in Gleason score between initial prostate biopsy and prostatectomy diminishes with increasing tumour grade due to reduced PSA secretion per unit tumour volume. BJU Int. 2012;110:36–42. doi: 10.1111/j.1464-410X.2011.10681.x. [DOI] [PubMed] [Google Scholar]
- 26.Kojima M, Troncoso P, Babaian RJ. Use of prostate-specific antigen and tumor volume in predicting needle biopsy grading error. Urology. 1995;45:807–812. doi: 10.1016/s0090-4295(99)80088-0. [DOI] [PubMed] [Google Scholar]
- 27.Magheli A, Hinz S, Hege C, Stephan C, Jung K, Miller K, et al. Prostate specific antigen density to predict prostate cancer upgrading in a contemporary radical prostatectomy series: a single center experience. J Urol. 2010;183:126–131. doi: 10.1016/j.juro.2009.08.139. [DOI] [PubMed] [Google Scholar]


