Abstract
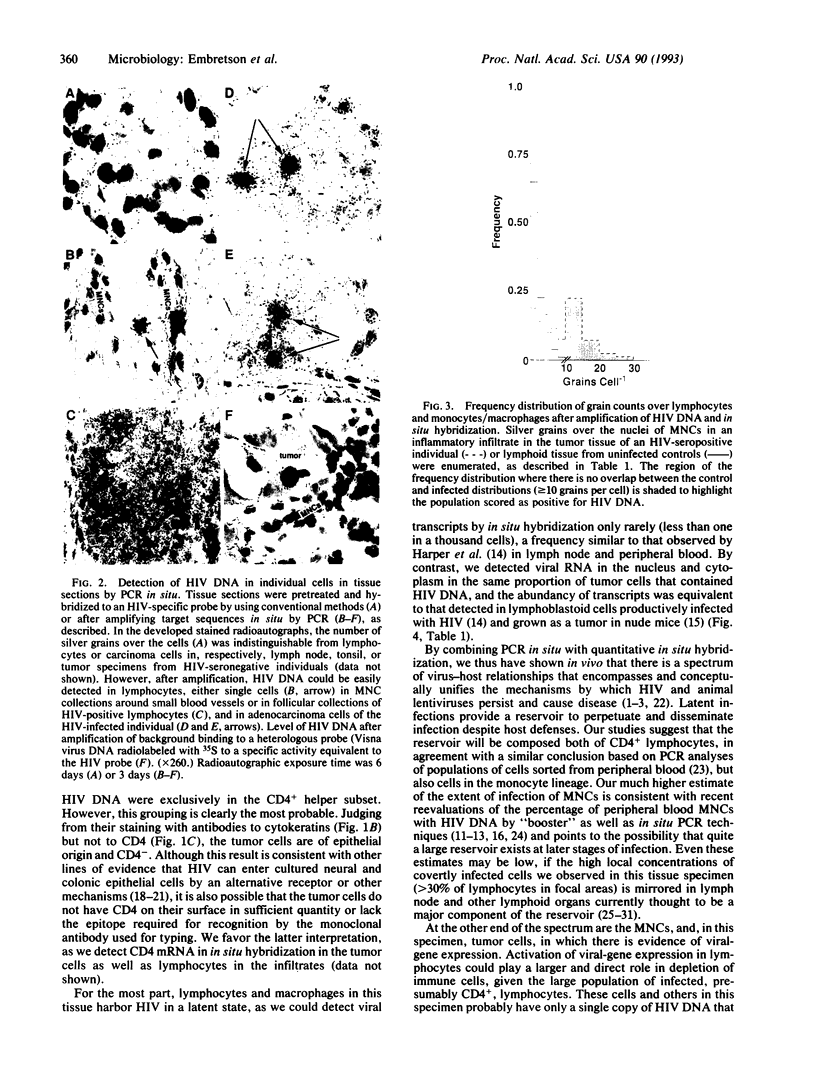
Latent and productive viral infections are at the extremes of the spectrum of virus-cell interactions that are thought to play a major role in the ability of such important human pathogens as human immunodeficiency virus (HIV) to elude host defenses and cause disease. The recent development of PCR-based methods to amplify target sequences in individual cells in routinely fixed tissues affords opportunities to directly examine the subtle and covert virus-cell relationships at the latent end of the spectrum that are inaccessible to analysis by conventional in situ hybridization techniques. We have now used PCR in situ with in situ hybridization to document latent and permissive HIV infection in routinely fixed and paraffin-embedded tissue. In one of the first specimens we examined, a tumor biopsy from an HIV-infected individual, we found many of the lymphocytes and lymphocytes infiltrating the tumor had HIV DNA that was detectable only by PCR in situ. The fraction of positive cells varied regionally, but there were foci where most of the cells contained HIV DNA. Most of these lymphocytes and macrophages are latently infected, as we could detect HIV RNA in fewer than one in a thousand of these cells. We also detected HIV RNA, surprisingly, in 6% of the tumor cells, where the number of copies of viral RNA per cell was equivalent to productively infected cell lines. The alternative states of HIV-gene expression and high local concentration of latently infected lymphocytes and monocytes revealed by these studies conceptually supports models of lentiviral pathogenesis that attribute persistence to the reservoir of latently infected cells and disease to the consequences of viral-gene expression in this population. The magnitude of infection of lymphocytes documented in this report is also consistent with the emerging view that HIV infection per se could contribute substantially to depletion of immune cells in AIDS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Hauptman S. P., Lischner H. W., Sachs M., Pomerantz R. J. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992 May 21;326(21):1385–1391. doi: 10.1056/NEJM199205213262103. [DOI] [PubMed] [Google Scholar]
- Baroni C. D., Pezzella F., Mirolo M., Ruco L. P., Rossi G. B. Immunohistochemical demonstration of p24 HTLV III major core protein in different cell types within lymph nodes from patients with lymphadenopathy syndrome (LAS). Histopathology. 1986 Jan;10(1):5–13. doi: 10.1111/j.1365-2559.1986.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Bhat S., Spitalnik S. L., Gonzalez-Scarano F., Silberberg D. H. Galactosyl ceramide or a derivative is an essential component of the neural receptor for human immunodeficiency virus type 1 envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7131–7134. doi: 10.1073/pnas.88.16.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Double-label techniques of in situ hybridization and immunocytochemistry. Curr Top Microbiol Immunol. 1989;143:9–20. doi: 10.1007/978-3-642-74425-9_2. [DOI] [PubMed] [Google Scholar]
- Fantini J., Yahi N., Baghdiguian S., Chermann J. C. Human colon epithelial cells productively infected with human immunodeficiency virus show impaired differentiation and altered secretion. J Virol. 1992 Jan;66(1):580–585. doi: 10.1128/jvi.66.1.580-585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J., Yahi N., Chermann J. C. Human immunodeficiency virus can infect the apical and basolateral surfaces of human colonic epithelial cells. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9297–9301. doi: 10.1073/pnas.88.20.9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fox C. H., Tenner-Rácz K., Rácz P., Firpo A., Pizzo P. A., Fauci A. S. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J Infect Dis. 1991 Dec;164(6):1051–1057. doi: 10.1093/infdis/164.6.1051. [DOI] [PubMed] [Google Scholar]
- Greene W. C. The molecular biology of human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Jan 31;324(5):308–317. doi: 10.1056/NEJM199101313240506. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Retzel E. F., Staskus K. A. Amplification and detection of lentiviral DNA inside cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4971–4975. doi: 10.1073/pnas.87.13.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia K., Spector S. A. Human immunodeficiency virus DNA is present in a high percentage of CD4+ lymphocytes of seropositive individuals. J Infect Dis. 1991 Sep;164(3):470–475. doi: 10.1093/infdis/164.3.470. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Mysteries of HIV: challenges for therapy and prevention. Nature. 1988 Jun 9;333(6173):519–522. doi: 10.1038/333519a0. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Gallery F., MacConnell P., Becker J., Bloch W. An improved technique for the in situ detection of DNA after polymerase chain reaction amplification. Am J Pathol. 1991 Dec;139(6):1239–1244. [PMC free article] [PubMed] [Google Scholar]
- Nuovo G. J., MacConnell P., Forde A., Delvenne P. Detection of human papillomavirus DNA in formalin-fixed tissues by in situ hybridization after amplification by polymerase chain reaction. Am J Pathol. 1991 Oct;139(4):847–854. [PMC free article] [PubMed] [Google Scholar]
- Nuovo G. J., Margiotta M., MacConnell P., Becker J. Rapid in situ detection of PCR-amplified HIV-1 DNA. Diagn Mol Pathol. 1992 Jun;1(2):98–102. [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P. A., Schnittman S. M., Kotler D. P., Fauci A. S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier H. K., van Wichen D., Sie-Go D. M., Goudsmit J., Borleffs J. C., Schuurman H. J. HIV-1 infection and virus production in follicular dendritic cells in lymph nodes. A case report, with analysis of isolated follicular dendritic cells. Am J Pathol. 1990 Aug;137(2):247–251. [PMC free article] [PubMed] [Google Scholar]
- Phillips D. M., Bourinbaiar A. S. Mechanism of HIV spread from lymphocytes to epithelia. Virology. 1992 Jan;186(1):261–273. doi: 10.1016/0042-6822(92)90080-9. [DOI] [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Kahl C., Feller A. C., Kern P., Dietrich M. Spectrum of morphologic changes of lymph nodes from patients with AIDS or AIDS-related complexes. Prog Allergy. 1986;37:81–181. doi: 10.1159/000318442. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Spiegel H., Herbst H., Niedobitek G., Foss H. D., Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol. 1992 Jan;140(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- Staskus K. A., Couch L., Bitterman P., Retzel E. F., Zupancic M., List J., Haase A. T. In situ amplification of visna virus DNA in tissue sections reveals a reservoir of latently infected cells. Microb Pathog. 1991 Jul;11(1):67–76. doi: 10.1016/0882-4010(91)90095-r. [DOI] [PubMed] [Google Scholar]