Abstract
A 66-year-old man underwent computed tomography-guided needle biopsy of a suspicious renal mass. Two months later he underwent partial nephrectomy. Histology revealed a 30-mm clear cell renal cell carcinoma, up to Fuhrman grade 3. An area of the capsule was interrupted, which corresponded to a hemorrhagic area on the cortical surface. Under microscopy, this area showed a tongue of tumor tissue protruding through the renal capsule. A tumor deposit was found in the perinephric fat. These features suggest that tumor seeding may have occurred during the needle biopsy.
Keywords: Biopsy, Carcinoma, Histology, Neoplasm seeding
INTRODUCTION
Incidental detection of small renal masses is increasing. This has led to an increase in biopsy of small renal masses, a proportion of which are then proven to be benign. Needle biopsy of small renal masses is controversial owing to the risk of seeding malignant cells along the needle tract. Needle tract seeding is a rare event; the incidence is estimated to be less than 1 in 10,000 cases of all biopsies [1]. Eight other cases of needle tract seeding in a renal mass biopsy have been described in the medical literature, two as recently as 2013 (Table 1). Complications in these patients include distant metastasis and death. We report our experience of a man with renal cell carcinoma (RCC) seeding along a biopsy tract and compare the circumstances and biopsy techniques with reported cases in the literature.
Table 1. Comparison of circumstances and outcomes with previous case reports.
| Source | Time to presentation | Tumor type | Location of seeding | Needle size (G/gauge) | Coaxial needle |
|---|---|---|---|---|---|
| Gibbons et al [6] | 20 Months | RCC | Subcutaneous | 18 | No |
| Auvert et al. [7] | 84 Months | Oncocytoma | Subcutaneous tissue | N/A | No |
| Wehle et al. [8] | 4 Years | Papillary RCC | Subcutaneous tissue | 20 | No |
| Kiser et al. [3] | 24 Days | Papillary RCC | Gerota's fascia | 14 and 20 | No |
| Shenoy et al. [2] | 12 Months | RCC | Subcutaneous tissue | 23 | No |
| Abe and Saitoh [4] | 30 Months | Angiomyoliposarcoma | N/A | 14 | No |
| Giorgadze et al. [9] | 4 Years | Papillary RCC | Retroperitoneal tissue | 20 and 22 | No |
| Mullins and Rodriguez [10] | Noted in histopathology | Papillary RCC | Perinephric fat | 20 and 22 | No |
| This case | 66 Days after biopsy (noted in histopathology) | Clear cell RCC | Perinephric fat | 16 and 22 | No |
"No" includes no specific mention of use of coaxial needle in case report.
RCC, renal cell carcinoma; N/A, not available.
CASE REPORT
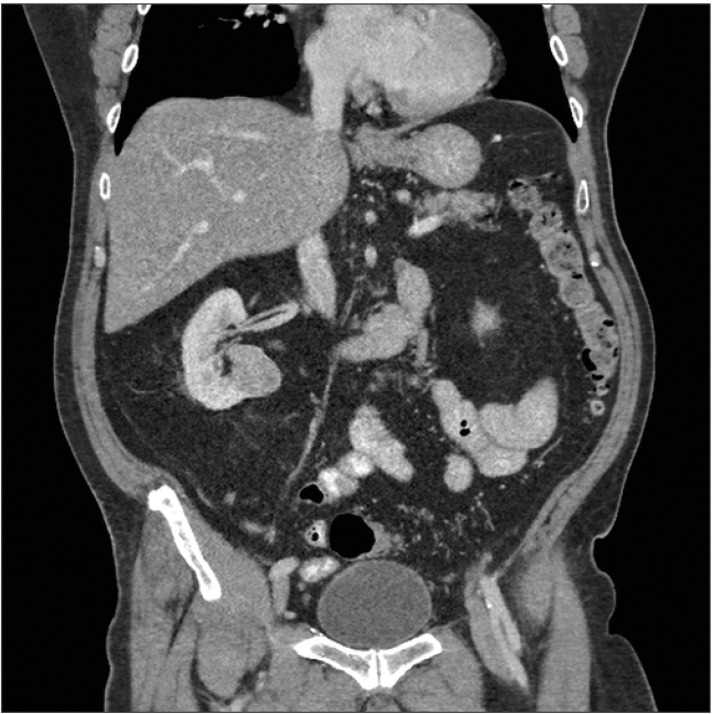
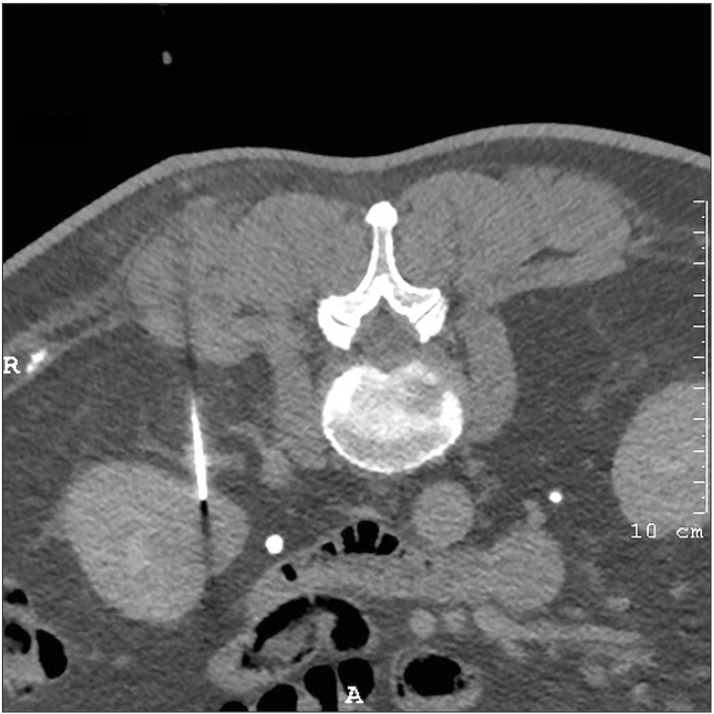
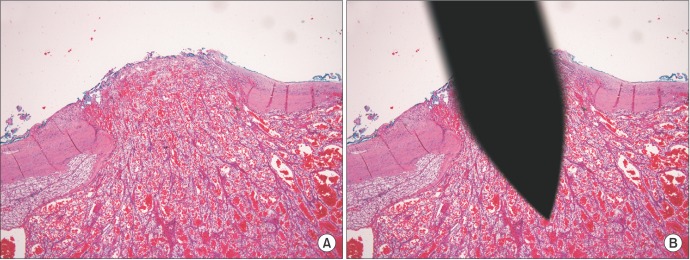
A 66-year-old man was incidentally found to have a 32-mm right lower pole renal mass on a computed tomography (CT) scan (Fig. 1). CT-guided needle biopsy of the renal mass (Fig. 2) revealed Fuhrman grade 2 clear cell RCC. Two samples were obtained by use of a 16-gauge Temno core biopsy needle (CareFusion, San Diego, CA, USA) and a 22-gauge Francine needle. One pass was made for each needle sampling. No coaxial needle was used. Within 2 months, the patient underwent right partial nephrectomy. Perinephric fat was sent along with the excised renal lesion for histopathological analysis. No specific feature of tumor seeding was seen intraoperatively. Histopathology revealed a well-circumscribed 30-mm clear cell RCC, predominantly Fuhrman grade 2 with focal areas of grade 3. There was an area where the capsule was interrupted that corresponded to a hemorrhagic area on the cortical surface (Fig. 3). A tumor deposit was also noted in the perinephric fat. These features suggested that the tumor deposit in the fat was likely due to tumor seeding rather than a metastasis and that the tumor seeding could have resulted from the needle biopsy. His TNM staging was pT3a NX MX, at least stage 3 disease (American Joint Committee on Cancer, 7th edition, 2010) and his Leibovich score was 5 (intermediate risk). Six months after the operation, there was no radiological evidence of tumour recurrence on a CT scan.
Fig. 1. Solid 32-mm right lower pole renal mass seen on computed tomography scan.
Fig. 2. Computed tomography-guided biopsy of the right renal mass.
Fig. 3. (A) Tongue of tumor tissue extending into the perinephric fat through an interruption on the renal capsule. (B) Superimposed 16-gauge Temno core biopsy needle (CareFusion, San Diego, CA, USA) over the site of interruption on the renal capsule. H&E stain, ×4.
DISCUSSION
Aside from the potential for false-negative results, a key risk of renal mass biopsy is seeding of the biopsy tract with malignant cells. Several factors in theory could affect the risk of biopsy tract seeding, such as needle size, the number of needle passes, and the use of a coaxial needle. Biopsy tract seeding has been reported in renal mass biopsies using needles as fine as 23-gauge and as large as 14-gauge [2,3,4]. Theoretically, a larger-bore needle would increase the risk of seeding owing to an increased area of defect on the surface of the tumor and an increased circumference or surface area of the needle. However, because of the scarcity of cases, it is difficult at this stage to accurately determine a relationship between needle size and the risk of seeding. It is also difficult because of underreporting to associate the risk of needle tract seeding with the number of needle passes through a tumor.
Use of a coaxial needle allows multiple passes through the renal mass with only one pass through the surrounding normal tissue. This theoretically reduces the risk of needle tract seeding into normal tissue and potentially reduces patient discomfort as well [5]. Although it is interesting to note that a coaxial needle was not used in any of the currently reported cases of needle tract seeding after renal mass biopsy (Table 1), there are just too few cases to establish a firm relationship between the risk of biopsy tract seeding and the use of a coaxial needle. Visualization of larger coaxial needles on ultrasound or CT may be easier than with smaller biopsy needles [5], and this may improve accuracy.
Histological evidence of biopsy tract seeding may not always be found after definitive surgery to remove the renal mass. Seeding into excised perinephric tissues can be found soon after surgery but seeding into surrounding muscle, fascia, and skin may only be apparent months, or even years, after surgery. As was seen with this case, the biopsy needle traversed skin, subcutaneous tissue, multiple muscle and fascia layers, and perinephric fat before reaching the renal lesion (Fig. 2). Thus, the tumor could theoretically seed into one or more of these tissues; seeding as superficial as the subcutaneous tissue has been reported (Table 1). This delayed presentation may increase the risk of adverse outcomes such as further metastasis and poorer prognosis. Time to presentation or diagnosis of tumor seeding after renal mass biopsy has ranged from 24 days to 84 months in previously reported cases where tumor seeding was not found on the initial histopathological analysis (Table 1).
In conclusion, a common feature in all reported cases of needle tract seeding from a renal mass biopsy is that a coaxial needle was not used. However, because of the paucity of cases, there is currently no satisfactory association between the risk of needle tract seeding and needle size or the number of needle passes. It is important to consider that histopathological evidence of needle tract seeding may not be apparent in all cases, especially if seeding occurred beyond the excised tissues.
ACKNOWLEDGMENTS
We would like to acknowledge Dr. Bibiana Tie from the Fremantle Hospital Department of Anatomical Pathology for her contribution to the production and discussion of the histological images.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Smith EH. Complications of percutaneous abdominal fine-needle biopsy: review. Radiology. 1991;178:253–258. doi: 10.1148/radiology.178.1.1984314. [DOI] [PubMed] [Google Scholar]
- 2.Shenoy PD, Lakhkar BN, Ghosh MK, Patil UD. Cutaneous seeding of renal carcinoma by Chiba needle aspiration biopsy: case report. Acta Radiol. 1991;32:50–52. [PubMed] [Google Scholar]
- 3.Kiser GC, Totonchy M, Barry JM. Needle tract seeding after percutaneous renal adenocarcinoma aspiration. J Urol. 1986;136:1292–1293. doi: 10.1016/s0022-5347(17)45318-3. [DOI] [PubMed] [Google Scholar]
- 4.Abe M, Saitoh M. Selective renal tumour biopsy under ultrasonic guidance. Br J Urol. 1992;70:7–11. doi: 10.1111/j.1464-410x.1992.tb15654.x. [DOI] [PubMed] [Google Scholar]
- 5.Volpe A, Kachura JR, Geddie WR, Evans AJ, Gharajeh A, Saravanan A, et al. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol. 2007;178:379–386. doi: 10.1016/j.juro.2007.03.131. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons RP, Bush WH, Jr, Burnett LL. Needle tract seeding following aspiration of renal cell carcinoma. J Urol. 1977;118:865–867. doi: 10.1016/s0022-5347(17)58226-9. [DOI] [PubMed] [Google Scholar]
- 7.Auvert J, Abbou CC, Lavarenne V. Needle tract seeding following puncture of renal oncocytoma. Prog Clin Biol Res. 1982;100:597–598. [PubMed] [Google Scholar]
- 8.Wehle MJ, Grabstald H. Contraindications to needle aspiration of a solid renal mass: tumor dissemination by renal needle aspiration. J Urol. 1986;136:446–448. doi: 10.1016/s0022-5347(17)44900-7. [DOI] [PubMed] [Google Scholar]
- 9.Giorgadze T, Qureshi F, Aulicino M, Jacques SM. Retroperitoneal recurrence of a stage 1 renal cell carcinoma four years following core biopsy and fine needle aspiration: possible needle tract seeding. Diagn Cytopathol. 2013;41:470–472. doi: 10.1002/dc.22815. [DOI] [PubMed] [Google Scholar]
- 10.Mullins JK, Rodriguez R. Renal cell carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J. 2013;7:E176–E179. doi: 10.5489/cuaj.499. [DOI] [PMC free article] [PubMed] [Google Scholar]