Abstract
Aim
To present our experience of the use of stereotactic radiosurgery and proton beam therapy to treat posterior uveal melanoma over a 10 year period.
Methods and materials
Case notes of patients treated with stereotactic radiosurgery (SRS), or Proton beam therapy (PBT) for posterior uveal melanoma were reviewed. Data collected included visual acuity at presentation and final review, local control rates, globe retention and complications. We analysed post-operative visual outcomes and if visual outcomes varied with proximity to the optic nerve or fovea.
Results
191 patients were included in the study; 85 and 106 patients received Stereotactic radiosurgery and Proton beam therapy, respectively. Mean follow up period was 39 months in the SRS group and 34 months in the PBT group. Both treatments achieved excellent local control rates with eye retention in 98% of the SRS group and 95% in the PBT group. The stereotactic radiosurgery group showed a poorer visual prognosis with 65% losing more than 3 lines of Snellen acuity compared to 45% in the PBT group. 33% of the SRS group and 54% of proton beam patients had a visual acuity of 6/60 or better.
Conclusions
Stereotactic radiosurgery and proton beam therapy are effective treatments for larger choroidal melanomas or tumours unsuitable for plaque radiotherapy. Our results suggest that patients treated with proton beam therapy retain better vision post-operatively; however, possible confounding factors include age, tumour location and systemic co-morbidities. These factors as well as the patient's preference should be considered when deciding between these two therapies.
Introduction
The primary aim of treatment for uveal melanoma is tumour destruction. Secondary to this is preservation of the eye and retention or restoration of visual function. To this end, a number of treatment options exist including plaque brachytherapy,1, 2 proton beam irradiation3, 4 stereotactic radiosurgery,5 local resection6 and to a lesser extent transpupillary thermotherapy7 or photodynamic therapy8 as well as of course primary enucleation. The treatment modality used is dependent on several factors including size and location of the tumour, proximity to the optic disc or fovea, suitability for surgery and patient choice.
Since the results of the COMS study which suggested no difference in mortality for patients with medium-sized melanomas treated with either brachytherapy or enucleation,2 eye-conserving therapies have been used in the majority of patients. Treatment of larger tumours as well as tumours in close proximity to the optic nerve is more challenging as accurate placement of a plaque to cover the entire tumour can be technically difficult. Other eye sparing radiation treatments such as proton beam therapy (PBT) and stereotactic radiosurgery (SRS) do not suffer from these limitations and have been used for many years to treat these more challenging melanomas. As both of these treatments options are available at our national ocular oncology centre (in conjunction with the Douglas Cyclotron Unit at the Clatterbridge Oncology Centre), this study was designed to compare outcomes in our patient cohort over a 10-year period.
Methods and materials
Details of patients treated with SRS or PBT between 2001 and 2011 at the Sheffield ocular oncology service were retrieved from hospital records. Stereotactic radiosurgery has been performed at our institution for more than 20 years; the technique and our long-term results have been published previously.5 In brief, the treated eye is immobilised by means of retrobulbar anaesthesia with or without placement of stay-sutures in the horizontal rectus muscles. The stereotactic frame is applied to the patient's head and this allows localisation of the tumour volume following MRI scanning. In cases in which MRI was contraindicated, CT localisation was performed. All patients received a dose of 35 Gy to the 50% isodose delivered in a single session by means of a Leksell Gamma Knife.
Proton beam therapy for uveal melanoma has been performed in the UK for more than 25 years in conjunction with all 4 Ocular Oncology Centres in the UK and the methods have been described previously.4 In brief, all patients underwent insertion of tantalum markers performed under general anaesthesia at our institution. Custom-made software (Eyeplan 3.07) creates a model of the patient's eye and tumour using clinical measurements as well as measurements obtained from A and B scan ultrasonography and fundus photography. The planning software calculates the required maximum range and modulation of treatment, as well as the area and shape of the radiation field. An individual brass collimator is then machined which corresponds to the shape of the patient's tumour, with a safety margin of 2.5 mm. The patient is seated in a custom-built chair and the patient's head is guided by a mouthpiece and partial face mask. During the treatment, the patient voluntarily gazes at a red LED, positioned at an angle determined at the planning stage, to minimise radiation dose to critical eye tissue and eye lids. During PBT (proton beam therapy) all patients received a total dose of 58.4 Gy (53.1 Cobalt Gray equivalent) in four daily fractions.
A total of 191 patients treated for posterior (choroidal and ciliary body) uveal melanoma were identified. Cases which had failed previous radiotherapy or laser therapy as well as melanomas of the iris and other tumours were excluded. Diagnosis of a uveal melanoma was made on clinical grounds by means of slit-lamp examination, indirect ophthalmoscopy, standardised A and B scan ultrasonography, ultrasound biomicroscopy in the case of ciliary body lesions and fluorescein and indocyanine green angiography if required.
The indications for SRS or PBT were tumours that were considered too large (thickness greater than 6.5 mm or basal diameter greater than 16 mm) for Ruthenium plaque brachytherapy or were situated less than 2.5 mm from the optic disc. Other indications included patient preference and in the case of SRS (which is performed under local anaesthesia) unsuitability for general anaesthesia. Stereotactic radiosurgery was performed at the National Centre for Stereotactic Radiosurgery, Sheffield whilst proton beam radiotherapy was performed at the Douglas Cyclotron Unit in Clatterbridge.
We recorded and analysed clinical variables such as age, tumour dimensions, distance to the optic nerve and fovea. Snellen visual acuity was documented at presentation and at each clinic attendance and significant visual loss was defined as a loss of 3 or more lines of Snellen acuity. Local control rates, patient survival, eye retention and complications requiring additional therapy were also recorded.
Results
191 patients were included in the study of whom 85 (57 males, 28 females) had stereotactic radiosurgery and 106 (63 males, 43 females) had proton beam therapy. There was a significant difference between the two groups with regard to age (t-test, P value=0.002). Patients in the SRS group had a mean age of 63 years (median 64, range 17 to 87 years) compared to a mean of 57 years in the PBT group (median 59, range 24–82). The left eye was affected in 48 patients and the right eye in 37 of the SRS group compared to 56 left eye and 50 right eyes in the PBR group. Maximum basal diameter of the tumours was greater in the PBT group but this did not reach statistical significance (t-test P-value=0.09) with a mean diameter of 11.2 mm (median 11, range 3.6–20.8 mm) in the PBT group compared to 9.6 mm (median 9.8, range 3.6–17.6 mm) in the SRS group. Similarly, thickness was also slightly greater in the PBT group (mean 4.3 mm, median 4mm, range 1–11.6 mm) compared to the SRS group (mean 3.9 mm, median 3.4, range 0.7–8.7 mm). This was also statistically insignificant (t-test P-value=0.14).
Mean distance of the tumour to the optic disc was 2.2 mm in the SRS group (median 0mm, range 0–18 mm) compared to a mean of 2.9 mm in the PBT group (median 2 mm, range 0–15 mm). This was not statistically significant (P-value=0.19). Mean follow up in the SRS group was 39 months (median 27, range 6 to 124 months) compared to a mean of 34 months in the PBT group (median 29, range 7 to 95).
At last review 33% of the SRS group and 54% of patients treated with proton beam therapy had a visual acuity of 6/60 or better (Table 1). For the purposes of this study we defined significant visual loss as a loss of 3 or more lines of Snellen acuity. In the follow up period, 65% of the stereotactic radiosurgery group lost more than 3 Snellen chart lines of visual acuity compared to 45% in the proton beam therapy group (Table 1). As expected the risk of severe visual loss was related to the proximity of the tumour to the optic disc or fovea. Patients whose melanoma was touching the optic nerve head (Figures 1a and b) were significantly more likely to suffer severe visual loss when treated with stereotactic radiosurgery than those treated with proton beam therapy (P-value=0.008). No significant difference between the two therapies was found for tumours located more than 0.5 mm from the optic disc (P-value=0.695). Similarly, there was no difference in the risk of severe visual loss in patients with tumours situated beneath or touching the fovea (P-value=0.271). There was however a significant difference for those tumours located more than 3 mm from the fovea with patients in the PBT group suffering a lower rate of severe visual loss (P-value=0.04).
Table 1. Visual outcomes following treatment with Stereotactic radiosurgery or proton beam therapy. Significant visual loss defined as a loss of 3 or more lines of Snellen acuity.
| Visual outcome | Stereotactic radiosurgery | Proton beam therapy |
|---|---|---|
| Visual acuity ≥6/60 | 33% | 55% |
| Loss of ≥3 snellen Lines | 65% | 45% |
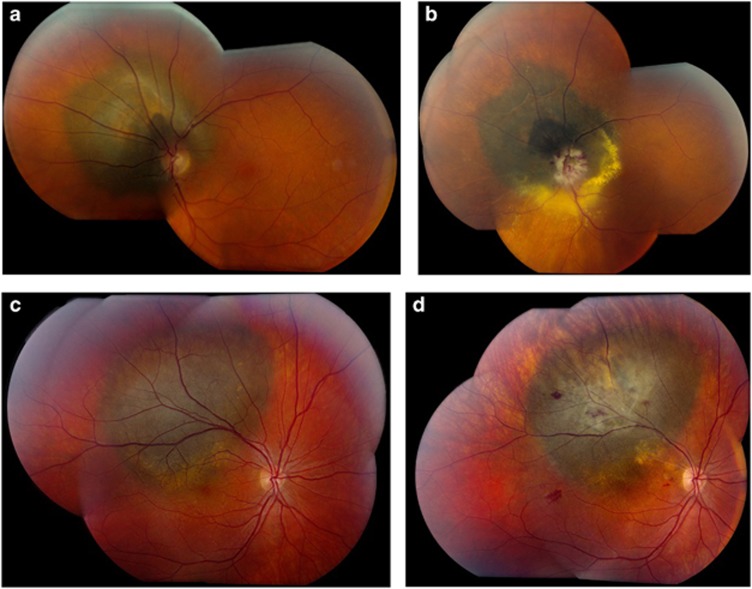
Figure 1.
(a): Fundus photograph of a 64 year-old female patient with a melanoma encircling 8 clock hours of the left optic disc before stereotactic radiosurgery. Visual acuity at presentation was 6/12. (b): Fundus photograph of the patient in figure 1a, 2 years post-stereotactic radiosurgery showing the melanoma to be in regression with oedema and haemorrhage involving the optic disc consistent with radiation optic neuropathy. Final visual acuity was counting fingers. (c): Fundus photograph of the right eye of a 28 year-old male patient with a juxtafoveal melanoma before proton beam therapy. Visual acuity at presentation was 6/12. (d): Fundus photograph of the patient in figure 1c, 2 years following proton beam therapy showing the melanoma to be in regression with scattered retinal haemorrhages and a small vascular occlusion consistent with radiation retinopathy. Visual acuity at this stage was 6/18.
Radiation retinopathy was the most frequent post-operative complication affecting 20 (24%) and 31 (30%) patients in the SRS and PBT group respectively. Similarly, optic neuropathy affected 23 (28%) and 14 (13%) patients in the stereotactic and proton beam group respectively. Nine patients (11%) in the SRS group developed rubeotic glaucoma of whom 7 were managed conservatively and 2 required enucleation. In the proton beam group 5 (5%) patients developed rubeotic glaucoma of whom 2 required enucleation. There were no cases of local tumour recurrence noted in the stereotactic group compared to 3 recurrences in the proton beam therapy group (2.8%), all of whom underwent secondary enucleation. Overall the eye retention rate was slightly better in the SRS group at 97.6% compared to 95.3% in the PBT group.
At the end of the study period overall survival was 84% in the SRS group and 87% in the PBT group with 14 deaths in each group of which 7 (50%) were confirmed to be due to metastatic disease in each treatment group.
Discussion
Proton beam therapy and stereotactic radiosurgery are established treatments for choroidal melanomas. These two treatment modalities are particularly suited to patients with large, posterior and/or juxtapapillary tumours for which treatment with brachytherapy may not be suitable. Proton beam therapy allows for precise and uniform dose distribution targeted at the tumours sparing surrounding healthy tissues. Proton beams deposit most of their energy in a defined range known as the Bragg peak area.
Stereotactic radiosurgery uses emission of high-dose gamma radiation concentrated over a small volume. Radiation energy is delivered to a well-defined area with little exposure to surrounding tissue. In this study patients treated with Stereotactic radiosurgery received a single dose of 35 Gy. This ability to direct radiation to a precise target is advantageous when treating tumours near the macula and optic disc. For the PBT group, treatment was in four daily fractions with a total dose of 58.4 Gy. There is no straightforward equivalence between the dose of radiation given in the two treatments as SRS is a single shot of radiation and PBT is fractionated hence a higher total dose. The variation in total radiation dose used for both treatments is unlikely to influence outcomes as SRS radiation can be directed to a precise and defined area whilst proton beam radiation is delivered to wider area.
Although these modalities aim to reduce exposure of healthy tissue to radiation, ocular radiotherapy is associated with complications which significantly impacts final visual acuity. The proportion of patients in our study with final visual acuity of 6/60 or better is comparable to other studies. In our study 33% of patients treated with stereotactic radiosurgery had a final visual acuity of 6/60 or better, compared to 14% in a recent study by Wackernagel et al.9 In contrast 54% of our patients treated with proton beam therapy retained a visual acuity of 6/60 or better. This compares to 48% of patients with visual acuity of 20/200 or better at 5 years post-treatment in a study by Gragoudas3 and 61% with acuity of 6/60 or better reported by Damato et al.4
Distance of the tumour from the optic disc or fovea is a recognised risk factor for poorer outcomes and tumours within 2 disc diameters of both the fovea and optic disc tend to have poorer visual outcomes.10 Our results confirmed these findings with 91% of cases in which the tumour touched or encircled the optic disc in the SRS group and 73% in the PBT group having a final visual acuity of 6/60 or less. This may be due to differences in the way radiation is delivered to the tumour between the two groups. However, there were a number of confounding factors. Firstly, tumours in the stereotactic group tended to be closer to the optic disc and involved a greater proportion of the disc margin than those patients in the PBT group despite there being no statistically significant difference between the 2 groups overall. In fact in the SRS group the tumour was touching the optic disc in 44 patients (52%) and was within 1 mm of the disc in a total of 55 patients (65%) compared to a total of 39 patients (37%) of patients in the proton group.
Furthermore, in the stereotactic group, 29 patients (34%) had a tumour involving more than 3 clock hours of the optic disc (Figures 1a and b) compared to only 2 patients (2%) in the PBT group (Figures 1c and d). Secondly, one of the advantages of stereotactic radiosurgery is that it is performed under local anaesthesia without sedation and is performed in a single session. As a result, patients in the SRS group tended to be older with greater co-morbidities than those in the proton therapy group which could well have influenced the final visual outcome.
The secondary enucleation rates in our study are lower than in previous reports with 3.7% of patients in the proton beam group requiring secondary enucleation compared to 2.4% in the stereotactic group. This compares to secondary enucleation rates of 9% reported by Gragoudas,3 9.4% by Damato4 and almost 14% reported by Egger et al 10 years after proton beam radiotherapy.11 Wackernagel et al report an enucleation rate of 14% following stereotactic radiosurgery.12
In conclusion our results add to evidence of the efficacy of both stereotactic radiosurgery and proton beam therapy in the management of posterior uveal melanoma with comparable rates of local control and eye retention between the two groups. Comparing the visual outcome for these two treatment modalities, our results suggests patients treated with proton beam therapy retain better vision post-operatively however possible confounding factors in our study include age, tumour location and systemic co-morbidities. These factors as well as the patient's preference should be considered when deciding between these two therapies.
The authors declare no conflict of interest.
Footnotes
Presented as a poster at the North of England Ophthalmological Society Centenary Conference.
References
- Lommatzsch PK, Werschnik C, Schuster E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 2000;238:129–137. doi: 10.1007/pl00007880. [DOI] [PubMed] [Google Scholar]
- Singh AD, Kivela T. The collaborative ocular melanoma study. Ophthalmol Clin North Am. 2005;18 (1:129–142. doi: 10.1016/j.ohc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Lane AM. Uveal melanoma: proton beam irradiation. Ophthalmol Clin North Am. 2005;18 (1:111–118. doi: 10.1016/j.ohc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Damato B, Kacperek A, Chopra M, Campbell IR, Errington RD. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62 (5:1405–1411. doi: 10.1016/j.ijrobp.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Dinca EB, Yianni J, Rowe J, Radatz MW, Preotiuc-Pietro D, Rundle P, et al. Survival and complications following γ knife radiosurgery or enucleation for ocular melanoma: a 20-year experience. Acta Neurochir (Wien) 2012;154:605–610. doi: 10.1007/s00701-011-1252-6. [DOI] [PubMed] [Google Scholar]
- Peyman GA, Juarez CP, Diamond JG, Raichand M. Ten years experience with eye wall resection for uveal malignant melanomas. Ophthalmology. 1984;91 (12:1720–1725. doi: 10.1016/s0161-6420(84)34086-6. [DOI] [PubMed] [Google Scholar]
- Oosterhuis JA, Journée-de Korver HG, Keunen JE. Transpupillary thermotherapy: results in 50 patients with choroidal melanomas. Arch Ophthalmol. 1998;116 (2:157–162. doi: 10.1001/archopht.116.2.157. [DOI] [PubMed] [Google Scholar]
- Rundle P. Treatment of posterior uveal melanoma with multi-dose photodynamic therapy. Br J Ophthalmol. 2014;98 (4:494–497. doi: 10.1136/bjophthalmol-2013-304432. [DOI] [PubMed] [Google Scholar]
- Wackernagel W, Holl E, Tarmann L, Avian A, Schneider MR, Kapp K, Langmann G. Visual acuity after Gamma-knife radiosurgery of choroidal melanomas. Br J Ophthalmol. 2013;97:153–158. doi: 10.1136/bjophthalmol-2012-302399. [DOI] [PubMed] [Google Scholar]
- Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol. 2002;120:1665–1671. doi: 10.1001/archopht.120.12.1665. [DOI] [PubMed] [Google Scholar]
- Egger E, Zografos L, Schalenbourg A, Beati D, Böhringer T, Chamot L, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55 (4:867–880. doi: 10.1016/s0360-3016(02)04200-1. [DOI] [PubMed] [Google Scholar]
- Wackernagel W, Holl E, Tarmann L, Mayer C, Avian A, Schneider M, et al. Local tumour control and eye preservation after gamma-knife radiosurgery of choroidal melanomas. Br J Ophthalmol. 2014;98:218–223. doi: 10.1136/bjophthalmol-2013-304031. [DOI] [PubMed] [Google Scholar]