Abstract
Purpose
To evaluate the efficacy of anti-vascular endothelial growth factor (VEGF) monotherapy for large submacular hemorrhage (SMH) secondary to neovascular age-related macular degeneration (nAMD).
Methods
A total of 49 treatment-naive patients (49 eyes) with large SMH (more than five disc areas (DAs)) secondary to nAMD were retrospectively included. All patients were treated with an initial series of 3 monthly intravitreal anti-VEGF injections, followed by as-needed injections. At the 12-month follow-up, changes in best-corrected visual acuity (BCVA), hemorrhage area, central foveal thickness, and development of vitreous hemorrhage after treatment were evaluated.
Results
The mean SMH area was 13.9±8.8 disk areas (DAs) and mean symptom duration was 7.25±5.9 days at baseline. The mean number of injections was 4.49±1.61. Twelve months after treatment, the mean BCVA significantly improved from 1.14±0.61 logarithm of the minimum angle of resolution (logMAR; 20/276, Snellen equivalent) to 0.82±0.53 logMAR (20/132; P=0.002). Twenty-four eyes (49%) showed improvement of more than three lines of BCVA at 12 months after treatment. Baseline BCVA (odds ratio (OR), 5.119; 95% confidence interval (CI), 1.993–9.545; P=0.004), duration of symptoms (OR, 0.727; 95% CI, 0.332–0.952; P=0.024), hemorrhage area (OR, 0.892; 95% CI, 0.721–0.965; P=0.011), and baseline central foveal thickness (OR, 0.881; 95% CI, 0.722–0.945; P=0.032) were significantly associated with good visual acuity 12 months after treatment.
Conclusions
Intravitreal anti-VEGF monotherapy is a valuable treatment option for large SMH secondary to nAMD.
Introduction
Submacular hemorrhage (SMH) secondary to neovascular age-related macular degeneration (nAMD) may cause retinal damage through various mechanisms.1, 2, 3 In cases of large SMH secondary to nAMD, visual prognosis is particularly poor.2, 4, 5 At the 3-year follow-up, visual outcomes in such cases have been reported to be 20/1700, and patients lost, on average, 3.5 lines of visual acuity.1
Various treatments for SMH, including pneumatic displacement with expandable gas (sulfur hexafluoride (SF6) or octafluoropropane (C3F8)) injection,6, 7 application of recombinant tissue plasminogen activator (rTPA) as an adjuvant,8, 9 and vitrectomy combined with pneumatic displacement and rTPA have been considered.10 However, the effects of these treatment modalities have generally been limited and there are no reports of efficacy in cases with large SMH. Currently, there is no standard-of-care recommendation for large SMH associated with nAMD.
Recently, intravitreal anti-vascular endothelial growth factor (VEGF) injections have been considered as a treatment option for SMH. Encouraging results for intravitreal anti-VEGF monotherapy have been reported in patients with SMH related to nAMD.11, 12, 13 However, to date, only a few clinical studies with small study populations have investigated the efficacy of anti-VEGF treatments for large SMH associated with nAMD. In this study, we evaluate the efficacy and safety of intravitreal anti-VEGF injection therapy for large SMH secondary to nAMD.
Materials and methods
We conducted a computerized search for and medical record review of patients who were newly diagnosed with SMH secondary to nAMD and treated with intravitreal anti-VEGF injections from July 2011 to August 2013. All patients were examined and treated at the Retina Center of Kim's Eye Hospital, Konyang University College of Medicine. This study was approved by the Institutional Review Board of Kim's Eye Hospital, Konyang University College of Medicine (IRB number: A-2014-023), and adhered to the tenets of the Declaration of Helsinki.
Subjects
Patients were included if they met all of the following criteria: (1) age >50 years; (2) loss of vision due to SMH involving the fovea, and hemorrhage >5 DAs (as defined in the Macular Photocoagulation Study)14; (3) confirmation of nAMD by fundoscopy and spectral-domain optical coherence tomography (SD-OCT; Spectral OCT/SLO; OTI Ophthalmic Technologies Inc., Miami, FL, USA), fluorescein angiography (FA), and indocyanine angiography (ICGA), performed using a confocal laser scanning system (Spectralis HRA+OCT; Heidelberg Engineering) at the first visit; (4) treatment naivety; (5) treatment with anti-VEGF injections (ranibizumab or bevacizumab); and (6) a minimum follow-up period of 12 months.
Some of the patients with SMH secondary to nAMD from our previous study12, 13 were also included in the current study. However, the data from the previous study were re-evaluated using new criteria and included in the study. We did not define maximum hemorrhage area or symptom duration as an inclusion or exclusion criterion. The distinction between typical nAMD and polypoidal choroidal vasculopathy (PCV) was made using ICGA. Only patients who had a branching vascular network and/or polypoidal shaped choroidal vascular lesions on ICGA were considered to have PCV. In case of thick SMH interfering with identification of underlying pathologies, the diagnosis was based on the results of repeated ICGA following three loading anti-VEGF injection treatments.
Patients were excluded from the study if any of the following criteria were met: (1) treatment modality aside from anti-VEGF injections, including pneumatic displacement, rTPA injection, or vitrectomy; (2) evidence of end-stage AMD, such as central geographic atrophy or disciform scarring at baseline; (3) evidence of retinal arterial macroaneurysm; (4) high myopia (more than the spherical equivalent of 6 diopters); (5) other secondary choroidal neovascularization (CNV); (6) other ocular disease that can affect visual acuity; or (7) previous vitreoretinal surgery.
Outcome measures
Primary outcomes were changes in mean best-corrected visual acuity (BCVA) at 3, 6, 9, and 12 months from baseline. The proportion of patients gaining more than three lines of vision was also evaluated at 3, 6, 9, and 12 months. For statistical analyses, Snellen BCVA measurements were converted to logarithm of the minimum angle of resolution (logMAR) values. Secondary outcomes included symptom duration, change in mean central foveal thickness from baseline, change in mean hemorrhage area, and development of vitreous hemorrhage after treatment.
Central foveal thickness that may reflect subfoveal hemorrhage thickness, was manually measured using the built-in OCT system software, and calculated as the distance between the internal limiting membrane and Bruch's membrane on fovea-centered SD-OCT images. Owing to the possibility of inaccurate measurements, when the SMH area extended to mid-periphery or further or exceeded a size of 25 DAs, we used a maximum threshold value of 25 DAs. In addition, central foveal thickness exceeding 1500 μm could not be measured accurately on SD-OCT; thus, 1500 μm of central foveal thickness was set as a threshold value.
The number of patients who developed vitreous hemorrhage following treatment (defined as hemorrhage developing within 3 days of injection) was also examined, along with the occurrence of treatment-related ocular and systemic adverse events.
Treatment and follow-up
Intravitreal injections of ranibizumab (0.5 mg/0.05 ml Lucentis; Genentech Inc., South San Francisco, CA, USA) or bevacizumab (1.25 mg/0.05 ml Avastin; Genentech Inc.) were administered to all patients following the same treatment and retreatment protocols. After performing three initial monthly loading injections, retreatment for each patient was performed as needed, based on the presence of any of the following: (1) visual deterioration of more than two lines (>0.2 logMAR) in comparison with the BCVA of previous visit; (2) evidence of persistent fluid or hemorrhage involving the macula on OCT at least 1 month after the previous injection; (3) newly developed macular hemorrhage; or (4) evidence of an active nAMD lesion on FA, ICGA, or OCT.
Follow-up examinations, including BCVA measurement, fundus photography, and SD-OCT, were performed 3, 6, 9, and 12 months after the initial treatment. Additional FA, ICGA, and SD-OCT examinations were performed whenever nAMD recurrence was suspected.
When breakthrough vitreous hemorrhage developed after treatment, anti-VEGF injection therapy was stopped and pars plana vitrectomy was considered. For mild vitreous hemorrhage (some vitreous hemorrhage was present, but posterior pole and macula were visible via indirect ophthalmoscopy), the decision to perform vitrectomy was made following discussion with the patient. In cases of severe vitreous hemorrhage in which no retinal detail could be seen posterior to the equator, vitrectomy was performed if the patient did not strongly refuse the surgery.
Statistical analyses
SPSS software (version 13.0, SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Frequencies were compared between groups by using χ2- or Fischer's exact test. Comparative statistical analyses were performed using unpaired t-tests. To investigate baseline clinical characteristics and to determine which treatments were associated with visual acuity improvement of ≥3 lines, a multivariate logistic regression analysis was performed. Forward and backward stepwise regression analyses were also performed using the likelihood-ratio model. The change in the likelihood-ratio statistic was used for variable selection, which was based on the maximum partial likelihood estimates for the covariate. All tests were two-sided and a P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics
During the study period, nAMD was newly diagnosed in a total of 969 patients at our institution. A total of 109 patients (11.2%) initially presented with SMH, and 70 patients (7.2%) presented with large SMH. Of the 70 eyes with large SMH, 21 were excluded for the following reasons: treatment with other modalities, including pneumatic displacement, rTPA, or vitrectomy (9 eyes), and loss to follow-up within 12 months (15 eyes). As a result, 49 eyes of 49 patients (29 men, 20 women) were included in the analysis.
All patients were South Korean and the mean subject age was 68.2±8.6 (range, 51 to 85) years. Mean symptom duration was 7.25±5.9 days (median, 6 days; range, 1 to 30 days). Mean SMH area was 13.9±8.8 DAs (median, 10 DA; range, 5.0–25.0 DAs). Thirty-five eyes (71.4%) were treated with ranibizumab and 14 eyes (28.6%) were treated with bevacizumab. The subjects received an average of 4.49±1.61 injections over the 12-month study period. Mean baseline BCVA was 1.14±0.61 logMAR (Snellen equivalent=20/276; range, hand movement to 20/30). Typical nAMD was diagnosed in 20 eyes (40.8%), and PCV was diagnosed in 27 eyes (55.1%); the condition in two eyes (4.1%) was categorized as unclassified because a differential diagnosis could not be performed despite repeated ICGA (Table 1).
Table 1. Baseline characteristics of patients with large submacular hemorrhage secondary to neovascular age-related macular degeneration.
| Total (n=49) | |
|---|---|
| Age (years) | 68.2±8.6 (range, 51–85) |
| Sex, n (%) | |
| Male | 29 (59.2%) |
| Female | 20 (40.8%) |
| Anticoagulant medication, n (%) | 10 (20.4%) |
| Duration of symptom (days) | 7.25±5.9 (range, 1 to 30) |
| Submacular hemorrhage area (DA)a | 13.9±8.8 (range, 5 to 20) |
| Mean baseline BCVA (logMAR) | 1.14±0.61 (range, HM to 20/30) (20/276) |
| Baseline BCVA (logMAR), n (%) | |
| <0.54 (20/70) | 11 (22.4%) |
| 0.54 (20/70) to 1.0 (20/200) | 18 (36.8%) |
| >1.0 (20/200) | 20 (40.8%) |
| Mean baseline central foveal thickness (μm)b | 554±246 (range, 225 to 1500) |
| Subtype, n (%) | |
| Typical nAMD | 20 (40.8%) |
| PCV | 27 (55.1%) |
| Unclassifiedc | 2 (4.1%) |
| Baseline subfoveal PED, n (%) | 26 (53.1%) |
| Vitreous hemorrhage during treatment, n (%) | 10 (20.4%) |
| Mean number of injections | 4.49±1.61 |
Abbreviations: BCVA, best-corrected visual acuity (Snellen equivalent provided); CNV, choroidal neovascularization; DA, disc area; logMAR, logarithm of the minimum angle of resolution; HM, hand motion; nAMD, neovascular age-related macular degeneration; PCV, polypoidal choroidal vasculopathy; PED, pigment epithelial detachment; VEGF, vascular endothelial growth factor.
Data presented as means±SD, where applicable.
We considered 25 DAs as the threshold value for submacular hemorrhage area because measurements exceeding 25 DAs could be inaccurate.
We considered 1500 μm the threshold values because thickness of >1500 μm could not be measured accurately by optical coherence tomography.
Cases in which a definite diagnosis was not possible even after repeated indocyanine angiography following three loading anti-vascular endothelial growth factor injection treatments.
Visual outcome
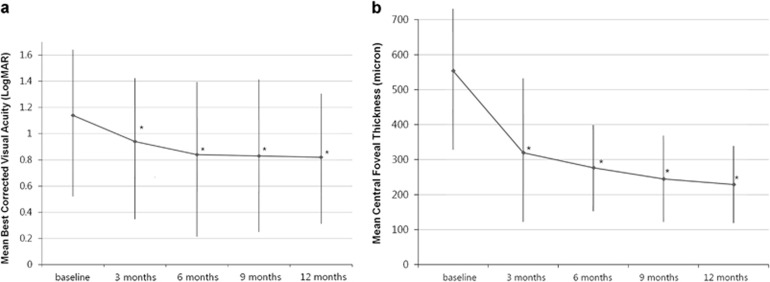
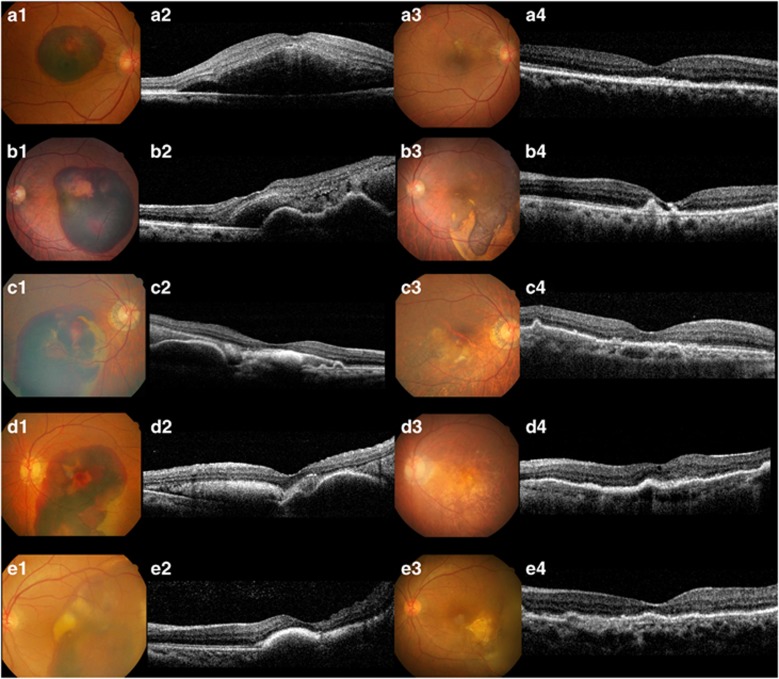
After treatment, overall BCVA significantly improved from 1.14±0.61 (20/276) at baseline to 0.94±0.58 (20/174, P=0.011) at 3 months, 0.84±0.55 (20/138, P=0.006) at 6 months, 0.83±0.59 (20/135, P=0.002) at 9 months, and 0.82±0.53 (20/132, P=0.002) at 12 months (Figure 1a). Figure 2 shows several cases of large SMH that were treated with anti-VEGF monotherapy.
Figure 1.
Changes in mean best-corrected visual acuity (BCVA) and mean central foveal thickness in eyes with large submacular hemorrhage (SMH) secondary to neovascular age-related macular degeneration (nAMD) after treatment. The mean BCVA showed significant improvements during the follow-up period (a), and the mean central foveal thickness (b) also showed significant improvements during the follow-up period (*P<0.05).
Figure 2.
Several cases of anti-vascular endothelial growth factor (VEGF) monotherapy for large submacular hemorrhage (SMH) secondary to neovascular age-related macular degeneration (nAMD). a1, Color fundus photography (CFP) showing large SMH; best corrected visual acuity (BCVA) was 10/200. a2, Spectral-domain optical coherence tomography (SD-OCT) showing a thick SMH at the fovea. a3, CFP 12 months after treatment (total number of injections: 3). a4, SD-OCT at 12 months; BCVA recovered to 20/30. b1, Large SMH with a BCVA of 20/200. b2, SD-OCT at baseline showing subfoveal hemorrhage and pigment epithelial detachment (PED). b3, CFP at 12 months showing SMH resolution around the macular area (total number of injections: 5). b4, SD-OCT at 12 months; BCVA recovered to 20/70. c1, Large SMH 28 days after onset; BCVA was 5/200. c2, SD-OCT at baseline shows subfoveal hyperreflective material due to an organized blood clot. c3, CFP at 12 months showed resolved SMH (total number of injections: 4). c4, SD-OCT at 12 months; BCVA recovered to 20/50. d1, CFP showing extensive SMH extending to the mid-periphery with a symptom duration of 14 days; BCVA was finger counting. d2, SD-OCT at baseline showing some hyperreflective material due to an organized blood clot and PED. d3, CFP at 12 months showing complete resolution of SMH (total number of injections: 5). d4. SD-OCT at 12 months; BCVA recovered to 20/70. e1, CFP showing extensive SMH extending to the periphery. e2, SD-OCT at baseline showing a subfoveal organized blood clot; BCVA was 5/200. e3, CFP at 12 months showing much improved SMH (total number of injections: 4). e4, SD-OCT at 12 months; BCVA recovered to 20/30.
Subjects were divided into three groups based on the visual outcome at 12 months: patients with a BCVA decrease of more than three lines (≥0.3 logMAR units) were placed in the ‘worsened' group, whereas patients with a BCVA increase of ≥3 lines or more (≥0.3 logMAR units) were placed in the ‘improved' group; the remaining patients were placed in the ‘stable' group. The proportion of patients with improved BCVA was 55.1% (27 of 49 eyes) at 3 months, 53.0% (26 of 49 eyes) at 6 months, 51.0% (25 of 49 eyes) at 9 months, and 49.0% (24 of 49 eyes) at 12 months. The proportions of patients with stable and worsened BCVA at 12 months were 34.7% (27 of 49 eyes) and 16.3% (8 of 49 eyes), respectively (Figure 3).
Figure 3.
Percentages of study participants with improved (gain of ≥3 lines), stable (gain or loss of <3 lines), or worsened (loss of ≥3 lines) best-corrected visual acuity (BCVA) after anti-vascular endothelial growth factor (VEGF) monotherapy for large submacular hemorrhage (SMH).
Anatomical outcome
The mean central foveal thickness significantly improved from 554±246 μm at baseline to 320±221 μm (P=0.012) at 3 months, 227±184 μm (P=0.005) at 6 months, 245±145 μm (P<0.001) at 9 months, and 229±118 μm (P<0.001) at 12 months (Figure 1b).
The mean hemorrhage area also significantly improved from 13.9±8.8 DAs at baseline to 2.5±1.8 DAs (P<0.001) at 3 months, 2.3±1.1 DAs (P<0.001) at 6 months, 1.5±0.7 DAs (P<0.001) at 9 months, and 1.2±0.6 DAs (P<0.001) at 12 months. Complete hemorrhage resolution after treatment was observed in 28 eyes (57.1%) at 3 months, 30 eyes (61.2%) at 6 months, 34 eyes (69.4%) at 9 months, and 39 eyes (79.6%) at 12 months.
Factors predictive of visual acuity improvement
Table 2 shows the relationship between BCVA improvement of ≥3 lines and various clinical factors at baseline, including BCVA at baseline, subtype (typical nAMD or PCV), symptom duration, baseline hemorrhage area, baseline central foveal thickness, presence of subfoveal pigment epithelial detachment (PED), number of injections, and development of vitreous hemorrhage. Univariate logistic regression analysis showed that good baseline BCVA, disease subtype (PCV), shorter duration of symptom, smaller hemorrhage area, and thinner baseline central foveal thickness were associated with good visual acuity at 12 months (Table 2). When baseline data were analyzed using multivariate logistic regression with forward and backward stepwise analysis, four factors were found to be significantly related to BCVA improvements of ≥3 lines at 12 months: baseline BCVA (odds ratio (OR), 5.119; 95% confidence interval (CI), 1.993–9.545; P=0.004), symptom duration (OR, 0.727; 95% CI, 0.332–0.952; P=0.024), hemorrhage area (OR, 0.892; 95% CI, 0.721–0.965; P=0.011), and baseline central foveal thickness (OR, 0.881; 95% CI, 0.722–0.945; P=0.032; Table 2).
Table 2. Logistic regression analysis to determine predictive factors of good visual acuity after intravitreal anti-vascular endothelial growth factor the treatment for large submacular hemorrhage secondary to neovascular age-related macular degeneration.
|
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Baseline BCVA (logMAR) | 7.022 (1.238–16.149) | 0.002 | 5.119 (1.993–9.545) | 0.004 |
| Diagnosis (typical nAMD or PCV)a | 10.451 (3.037–36.808) | 0.047 | 7.332 (4.221–18.423) | 0.076 |
| Duration of symptom | 0.883 (0.434–0.966) | 0.009 | 0.727 (0.332–0.952) | 0.024 |
| Hemorrhage area | 0.890 (0.644–0.956) | 0.006 | 0.892 (0.721–0.965) | 0.011 |
| Baseline foveal center thickness | 0.788 (0.601–0.992) | 0.011 | 0.881 (0.722–0.945) | 0.032 |
| Anticoagulation medication (yes or no)a | 0.557 (0.126–2.452) | 0.439 | ||
| Baseline subfoveal PED (yes or no)a | 0.911 (0.121–9.332) | 0.823 | ||
| Number of injections | 1.537 (0.724–3.263) | 0.263 | ||
| Occurrence of vitreous hemorrhage during treatment (yes or no)a | 0.156 (0.08–2.114) | 0.125 | ||
Abbreviations: BCVA, best-corrected visual acuity; CI, confidence interval; CNV, choroidal neovascularization; logMAR, logarithm of the minimum angle of resolution; nAMD, neovascular age-related macular degeneration; OR, odds ratio; PCV, polypoidal choroidal vasculopathy; PED, pigment epithelial detachment; VEGF, vascular endothelial growth factor.
Indicates categorical variable.
Vitreous hemorrhage during treatment and other complications
In 10 of 49 eyes (18.4%), vitreous hemorrhage developed after treatment. Six eyes (66.7%) hemorrhaged after the first anti-VEGF injection, three eyes hemorrhaged after the second injection, and one eye hemorrhaged after the third injection. Two of these ten patients developed minimal or mild vitreous hemorrhage (some vitreous hemorrhage was present, but posterior pole and macula were visible), while eight patients developed severe vitreous hemorrhage (no retinal detail seen posterior to the equator on ophthalmoscopic examination). Among patients with vitreous hemorrhage, the smallest hemorrhage area was 6.5 DAs. Every patient with >25 DAs of SMH area developed vitreous hemorrhage after anti-VEGF injection (six eyes).
Among baseline characteristics, mean hemorrhage area at baseline was significantly larger (19.82 DA; P<0.001), mean central subfoveal thickness was significantly greater (732 μm; P=0.032), and BCVA at baseline was significantly worse (1.74 logMAR; P=0.020) in the vitreous hemorrhage group than in the nonvitreous hemorrhage group (10.22 DA, 512 μm, and 1.22 logMAR, respectively). Subfoveal PED at baseline was more frequently found in the vitreous hemorrhage group (9 of 10 eyes (90%) vs 17 of 39 eyes (43.6%); P=0.009). Other baseline characteristics, including duration of symptom, subtype (typical nAMD or PCV), number of injections, and history of anticoagulation medication were not significantly different between groups.
Vitrectomy was performed for seven patients with severe vitreous hemorrhage (one patient refused surgery), and it was not performed for the two patients with mild vitreous hemorrhage; vitreous hemorrhage in the latter cleared without vitrectomy during the follow-up period. Three of seven patients underwent vitrectomy with silicone oil tamponade, whereas the others were treated without tamponade. No further hemorrhagic events occurred after vitreous surgery in any eye, but 5 of 10 eyes (50.0%) had very poor visual acuity (worse than 1.60 logMAR (5/200)) at 12 months. Three eyes developed a diffuse scar at the macula, and two eyes had diffuse retinal pigment epithelial atrophy.
Twelve months after treatment, mean BCVA was significantly worse in the vitreous hemorrhage group (1.31 logMAR (20/399) vs 0.71 logMAR (20/102) in the non-vitreous hemorrhage group; P=0.008). However, no difference was found with respect to the proportion of patients with BCVA improvement of ≥3 lines between the groups (4 of 10 eyes (40%) in the vitreous hemorrhage groups vs 20 of 39 eyes (40.8%) that did not develop vitreous hemorrhage; P=0.513).
No other complications associated with intravitreal anti-VEGF injections were observed, including endophthalmitis, traumatic lens injury, retinal detachment, or systemic adverse events.
Discussion
In the current study, BCVA significantly improved after anti-VEGF monotherapy in eyes with large SMH secondary to nAMD after 12 months of treatment. Even though all our patients had large SMH (mean, 13.9 DAs), 49% of the patients showed BCVA improvement of ≥3 lines at 12 months after treatment. In addition, 57.1% of the patients showed complete resolution of SMH after three anti-VEGF loading injection treatments. Baseline BCVA, hemorrhage area, symptom duration, and central foveal thickness at baseline were associated with good visual acuity at 12 months. To the best of our knowledge, this is the largest case series evaluating the efficacy of anti-VEGF monotherapy for large SMH to date.
Among the various treatment options for SMH associated with nAMD, including pneumatic displacement or surgery (pars plana vitrectomy), anti-VEGF agents might be the most important because only anti-VEGF can treat the underlying pathologic CNV. To date, encouraging results for intravitreal anti-VEGF monotherapy have also been reported in patients with SMH related to nAMD.11, 12, 13, 15 Compared with the patients in previous studies in which anti-VEGF monotherapy was used for SMH, our patients showed the largest baseline SMH area (mean, 7.8 to 8.3 DAs vs 13.9 DAs; when hemorrhage area was measured in mm2, it was recalculated as 1 DA=2.54 mm2).11, 12, 13, 15 However, the degree of BCVA improvement in the current study was similar or better than that observed in previous studies; 83.7% of patients showed improved or stable BCVA at 12 months after treatment compared to 69.5–94.4% in previous studies.11, 12, 13, 15 Furthermore, 49% of our patients showed BCVA improvement of more than three lines after treatment at 12 months compared with 34.7–64.8% in previous studies.11, 12, 13, 15
Surgical approaches, such as vitrectomy combined with gas tamponade or rTPA injection, for large SMH have also been evaluated in some reports. Although direct comparisons are difficult because of different SMH areas at baseline and follow-up periods, the visual outcomes observed this study are similar or even better than those for eyes treated surgically. For instance, in the Submacular Surgery Trial (SST), >40% of eyes exhibited lesion sizes larger than 16 DAs, and improvement in visual acuity by three lines or greater during 6 months was only achieved in 10% of eyes in the observation group and 11% of eyes in the surgery group.10 In another investigation of patients with relatively small SMH areas, Treumer et al16 reported that 12 of 26 eyes (46.1% mean baseline hemorrhage area=4.5 DA; mean follow-up time=17 months) had BCVA improvements of ≥3 lines of logMAR units after vitrectomy combined with SF6 gas tamponade, anti-VEGF treatment, and rTPA injections. Considering the possibility of surgical complications associated with vitrectomy, intravitreal anti-VEGF injections might be a more effective and less invasive treatment option for large SMH.
In the current study, symptom duration, baseline BCVA, hemorrhage area, and central foveal thickness were associated with three lines of BCVA improvement after treatment. Our patients had a larger mean SMH area at baseline compared to those in previous studies; however, our results are comparable with those of previous studies with respect to SMH prognostic factors.1, 13, 17 In cases with expected poor prognosis at baseline, other treatment options (pneumatic displacement or rTPA administration) should also be considered over or in combination with anti-VEGF therapy. However, there are few studies evaluating the efficacy of pneumatic displacement and/or rTPA, particularly for large SMH. Moreover, the use, optimum dose, and efficacy of intravitreal rTPA remain controversial. It has been reported that rTPA has retinal toxicity18 and does not cross the intact rabbit retina.19 Several reports have shown that pneumatic displacement therapy for SMH is equally effective with and without the use of rTPA.9, 20 Our recent report showed no significant therapeutic difference between an anti-VEGF monotherapy group and anti-VEGF combined with pneumatic displacement group.21 To date, the efficacy of combination therapy has not been better than that of anti-VEGF monotherapy, and it is still unknown which modality is the most effective for treating large SMH. A randomized, controlled trial for various treatment options should be conducted in the future to elucidate the most effective treatment or combination modality for large SMH.
Vitreous hemorrhage after anti-VEGF treatment often requires reintervention with vitrectomy. The incidence of vitreous hemorrhage was 20.4% in the study. Our results show that baseline hemorrhage area and subfoveal PED were significantly related to the development of vitreous hemorrhage after treatment. More specifically, every patient with SMH larger than 25 DAs developed vitreous hemorrhage after anti-VEGF treatment. Even though our patients had a larger mean SMH area than did patients in any other study, the rate of vitreous hemorrhage was not much higher than those in other studies investigating anti-VEGF agents or pneumatic displacement, which were reported to be 15 to 22.2%.9, 22, 23, 24 Our results reveal that the final visual outcome was significantly worse in patients with vitreous hemorrhage than in patients without vitreous hemorrhage; however, worse visual prognosis may not be related to the vitreous hemorrhage itself, but might be related to the significantly worse BCVA at baseline in patients who developed vitreous hemorrhage. Therefore, considering our result that vitreous hemorrhage was not associated with the proportion of BCVA improvement of ≥3 lines after treatment, anti-VEGF therapy would not be contraindicated in patients with large SMH owing to the possibility of vitreous hemorrhage development.
Our study has several limitations, including its retrospective nature. First, the treatment modality for large SMH was not selected using established guidelines. The decision for each treatment modality was dependent on the discretion of each clinician, and because of the possibility of selection bias, we only included patients receiving anti-VEGF monotherapy and excluded those treated with pneumatic displacement and/or rTPA. Second, bevacizumab and ranibizumab were not strictly differentiated in the current study. However, the two agents have been shown to have therapeutic equivalence when injected using the same injection protocol.25
Despite its poor prognosis, there are no current standard-of-care recommendations for large SMH. The present study showed that anti-VEGF monotherapy is a valuable, minimally invasive treatment option for large SMH. Better baseline BCVA, shorter symptom duration, smaller hemorrhage area, and lesser central foveal thickness at baseline are predictive factors of a good visual prognosis after treatment. Future investigations comparing anti-VEGF monotherapy and other treatment modalities would help determine treatment strategies for large SMH associated with nAMD.
| Activity evaluation | ||||
| 1. The activity supported the learning objectives. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 2. The material was organized clearly for learning to occur. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 3. The content learned from this activity will impact my practice. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 4. The activity was presented objectively and free of commercial bias. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
The authors declare no conflict of interest.
References
- Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16:183–189. doi: 10.1097/00006982-199616030-00001. [DOI] [PubMed] [Google Scholar]
- Steel DH, Sandhu SS. Submacular haemorrhages associated with neovascular age-related macular degeneration. Br J Ophthalmol. 2011;95:1051–1057. doi: 10.1136/bjo.2010.182253. [DOI] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results Ophthalmology 20121191388–1398.22555112 [Google Scholar]
- Hasegawa T, Otani A, Sasahara M, Gotoh N, Ooto S, Tamura H, et al. Prognostic factors of vitreous hemorrhage secondary to exudative age-related macular degeneration. Am J Ophthalmol. 2010;149:322–329. doi: 10.1016/j.ajo.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990;109:33–37. doi: 10.1016/s0002-9394(14)75575-8. [DOI] [PubMed] [Google Scholar]
- Gopalakrishan M, Giridhar A, Bhat S, Saikumar SJ, Elias A, N S. Pneumatic displacement of submacular hemorrhage: safety, efficacy, and patient selection. Retina. 2007;27:329–334. doi: 10.1097/01.iae.0000231544.43093.40. [DOI] [PubMed] [Google Scholar]
- Averbukh E, Devenyi RG, Lam WC, Berger AR. Pneumatic displacement of submacular hemorrhage. Ophthalmology. 2000;107:2118–2119. doi: 10.1016/s0161-6420(00)00295-5. [DOI] [PubMed] [Google Scholar]
- Handwerger BA, Blodi BA, Chandra SR, Olsen TW, Stevens TS. Treatment of submacular hemorrhage with low-dose intravitreal tissue plasminogen activator injection and pneumatic displacement. Arch Ophthalmol. 2001;119:28–32. [PubMed] [Google Scholar]
- Mizutani T, Yasukawa T, Ito Y, Takase A, Hirano Y, Yoshida M, et al. Pneumatic displacement of submacular hemorrhage with or without tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2011;249:1153–1157. doi: 10.1007/s00417-011-1649-1. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB, Childs AL, Haller JA, Hawkins BS, Lewis H, et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology. 2004;111:1993–2006. doi: 10.1016/j.ophtha.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shienbaum G, Garcia Filho CA, Flynn HW, Nunes RP, Smiddy WE, Rosenfeld PJ. Management of submacular hemorrhage secondary to neovascular age-related macular degeneration with anti-vascular endothelial growth factor monotherapy. Am J Ophthalmol. 2013;155:1009–1013. doi: 10.1016/j.ajo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Koh KM, Kim HS, Lee TG, Kim CG, Kim JW. Anti-vascular endothelial growth factor monotherapy in the treatment of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;156:524–531. doi: 10.1016/j.ajo.2013.04.029. [DOI] [PubMed] [Google Scholar]
- Kim JH, Chang YS, Kim JW, Kim CG, Yoo SJ, Cho HJ. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology. 2014;121:926–935. doi: 10.1016/j.ophtha.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Bressler SB, Maguire MG, Bressler NM, Fine SL. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. The Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108:1442–1447. doi: 10.1001/archopht.1990.01070120090035. [DOI] [PubMed] [Google Scholar]
- McKibbin M, Papastefanou V, Matthews B, Cook H, Downey L. Ranibizumab monotherapy for sub-foveal haemorrhage secondary to choroidal neovascularisation in age-related macular degeneration. Eye (Lond) 2010;24:994–998. doi: 10.1038/eye.2009.271. [DOI] [PubMed] [Google Scholar]
- Treumer F, Roider J, Hillenkamp J. Long-term outcome of subretinal coapplication of rtPA and bevacizumab followed by repeated intravitreal anti-VEGF injections for neovascular AMD with submacular haemorrhage. Br J Ophthalmol. 2012;96:708–713. doi: 10.1136/bjophthalmol-2011-300655. [DOI] [PubMed] [Google Scholar]
- Iacono P, Parodi MB, Introini U, La Spina C, Varano M, Bandello F. Intravitreal ranibizumab for choroidal neovascularization with large submacular hemorrhage in age-related macular degeneration. Retina. 2014;34:281–287. doi: 10.1097/IAE.0b013e3182979e33. [DOI] [PubMed] [Google Scholar]
- Lüke M, Januschowski K, Warga M, Beutel J, Leitritz M, Gelisken F, et al. The retinal tolerance to bevacizumab in co-application with a recombinant tissue plasminogen activator. Br J Ophthalmol. 2007;91:1077–1082. doi: 10.1136/bjo.2006.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei M, Misono K, Lewis H. A study of the ability of tissue plasminogen activator to diffuse into the subretinal space after intravitreal injection in rabbits. Am J Ophthalmol. 1999;128:739–746. doi: 10.1016/s0002-9394(99)00239-1. [DOI] [PubMed] [Google Scholar]
- Fujikawa M, Sawada O, Miyake T, Kakinoki M, Sawada T, Kawamura H, et al. Comparison of pneumatic displacement for submacular hemorrhages with gas alone and gas plus tissue plasminogen activator. Retina. 2013;33:1908–1914. doi: 10.1097/IAE.0b013e318287d99d. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Koh KM, Kim JH, Kim HS, Han JI, Lew YJ, et al. Intravitreal ranibizumab injections with and without pneumatic displacement for treating submacular hemorrhage secondary to neovascular age-related macular degeneration. Retina. 2015;35:205–212. doi: 10.1097/IAE.0000000000000295. [DOI] [PubMed] [Google Scholar]
- Hassan AS, Johnson MW, Schneiderman TE, Regillo CD, Tornambe PE, Poliner LS, et al. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology. 1999;106:1900–1906. doi: 10.1016/S0161-6420(99)90399-8. [DOI] [PubMed] [Google Scholar]
- Hattenbach LO, Klais C, Koch FH, Gümbel HO. Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology. 2001;108:1485–1492. doi: 10.1016/s0161-6420(01)00648-0. [DOI] [PubMed] [Google Scholar]
- Wu TT, Kung YH, Hong MC. Vitreous hemorrhage complicating intravitreal tissue plasminogen activator and pneumatic displacement of submacular hemorrhage. Retina. 2011;31:2071–2077. doi: 10.1097/IAE.0b013e31822528c8. [DOI] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]