Abstract
Purpose:
To evaluate frequency of injections, visual and anatomical outcomes of neovascular age-related macular degeneration (nAMD) patients transitioned to intravitreal aflibercept after failure to extend treatment interval beyond 8 weeks with prior intravitreal bevacizumab or ranibizumab.
Methods:
Retrospective review of patients with nAMD switched to aflibercept following ≥6 prior intravitreal ranibizumab or bevacizumab injections at 4–8-week intervals. Three monthly aflibercept injections were given followed by a treat-and-extend dosing regimen.
Results:
Twenty-one eyes of 18 patients who had received a mean of 23.8±18.8 (mean±SD; range 6–62) prior ranibizumab or bevacizumab injections were included. Over a mean follow-up of 24 months after the transition, 9.2±2.9 (range 4–21) aflibercept injections were required. Interval between aflibercept injections increased to 57.3 days (range 35–133 days), as compared with 37±6.1 days (range 29–54 days) with the prior agents (P=0.01). Mean best-corrected visual acuity was preserved (0.42±0.31 vs 0.42±0.23 logMAR; P=0.2). Mean OCT central subfoveal thickness (292.1±83.2 μm to 283.6±78.6 μm; P=0.4) and mean macular volume (7.9±0.95 mm3 to 7.67±0.94 mm3; P=0.16) remained stable.
Conclusion:
Patients requiring treatment more frequently than every 8 weeks with ranibizumab and bevacizumab were transitioned to >8-week treatment interval with aflibercept while maintaining the anatomic and visual gains.
Introduction
The ‘treat-and-extend' dosing regimen for bevacizumab and ranibizumab in patients with neovascular age-related macular degeneration (nAMD) offers decreased dosing frequency compared with monthly or PRN regimens while potentially maintaining efficacy.1, 2 However, some patients are unable to maintain an extended exudation-free treatment interval.
Aflibercept (Eylea, Regeneron, Tarrytown, NY, USA) administered every 2 months following three monthly loading doses was non-inferior to monthly ranibizumab (Lucentis, Genentech, South San Francisco, CA, USA) in treatment-naive nAMD3 and monthly aflibercept is often effective in recalcitrant nAMD.4, 5, 6
We examined nAMD patients transitioned to aflibercept who were previously treated with a ‘treat-and-extend' protocol with either ranibizumab or bevacizumab (Avastin, Genentech, South San Francisco, CA, USA), and could not be extended exudation-free beyond an 8-week interval.
Case series
IRB-approved retrospective review of patients with nAMD (treated with ≥6 prior intravitreal 0.5 mg/0.05 ml ranibizumab or 1.25 mg/0.05 ml bevacizumab injections in previous 12 months) who required treatment on a 4–8-week interval to remain exudation free (on an OCT guided treat-and-extend protocol) and were switched to aflibercept 2.0 mg. We excluded eyes with idiopathic polypoidal choroidal vasculopathy, central serous retinopathy, anti-VEGF therapy <28 days prior, prior photodynamic therapy, significant subfoveal-fibrosis or large subretinal hemorrhage, prior triamcinolone (<6 months), intraocular surgery (<2 months), prior vitrectomy, active intraocular inflammation, vitreous hemorrhage, retinal pigment epithelium (RPE) tear, or best-corrected visual acuity (BCVA) <20/400.
The number of, and interval between injections, as well as the visual and anatomical outcomes were evaluated. Three monthly aflibercept injections were followed by treatment at a generally fixed interval of 8 weeks, further extended by 2-week intervals at the discretion of the treating physician based on persistent/recurrent intraretinal fluid (IRF)/subretinal fluid (SRF)/sub-RPE fluid, new hemorrhage/SRF/IRF on exam, increase in central subfoveal thickness (CFT) >100 μm and worsening vision by >1 Snellen line. Statistical analysis was performed using SPSS v.20.0 (IBM Corp., Armonk, NY, USA).
Twenty-one eyes of 18 patients (15 females, 3 males; aged 83.6±7.1 years (mean±SD)) who had received an average of 23.8±18.8 (range 4–62) prior ranibizumab or bevacizumab injections over the prior 28±20.5 months were included (Table 1). Ninteen of 21 eyes (90.5%) demonstrated clinical and OCT evidence of posterior vitreous detachment, defined as detachment of the posterior vitreous cortex over the entire posterior pole including the fovea and the optic disc, at time of the first aflibercept injection. Over a mean follow-up of 2 years, mean BCVA remained stable from 0.42±0.31 logMAR at baseline to 0.42±0.23 at 24 months follow-up (P=0.2; paired t-test) (Table 2). Seven eyes (33.3%) gained ≥3 Snellen lines BCVA, whereas 14 eyes (66.7%) had stable vision (<3 line change). The mean OCT CFT remained stable from 292.1±83.2 μm at baseline to 283.6±78.6 μm at 2-year follow-up (P=0.4; paired t-test; Table 2). Mean OCT macular volume was stable from 7.9±0.95 mm3 at baseline to 7.67±0.94 mm3 at 2-year follow-up (P=0.16; paired t-test; Table 2).
Table 1. Baseline patient demographics (n=18 patients).
| Characteristic | Mean value |
|---|---|
| Age | 83.6±7.1 years |
| Gender (M/F) | 3/15 |
| Current smoker | 2 (11.1%) |
| Former smoker | 8 (44.4%) |
| Known cardiovascular disease | 2 (11.1%) |
| Prior ranibizumab/bevacizumab injections | 23.8±18.8 |
Table 2. Visual and anatomic characteristics (n=21 eyes).
| Baseline following previous ranibizumab/bevacizumab | 24-month post transition to aflibercept | P-valuea | |
|---|---|---|---|
| Mean BCVA (logMAR) | 0.42±0.31 | 0.42±0.23 | 0.20 |
| Mean OCT CFT (microns) | 292.1±83.2 | 283.6±78.6 | 0.40 |
| Mean OCT MV (mm3) | 7.9±0.95 | 7.67±0.94 | 0.16 |
| Mean treatment interval (days) | 37.0 | 59.3 | 0.01 |
Abbreviations: BCVA, best-corrected visual acuity; CFT, central subfoveal thickness; MV, macular volume.
Paired t-test; data presented as mean±SD.
Mean number of aflibercept injections over the 2-year period was 9.2±2.9 (range 4–21). The maximum exudation free interval on aflibercept was 133 days, whereas the minimum was 35 days. Mean interval between aflibercept injections was 59.3 days, as compared with 37±6.1 days (range 29–54 days) with ranibizumab/bevacizumab before the transition (P=0.01; paired t-test; Table 2, Figure 1). No adverse ocular or systemic events were reported during the follow-up period.
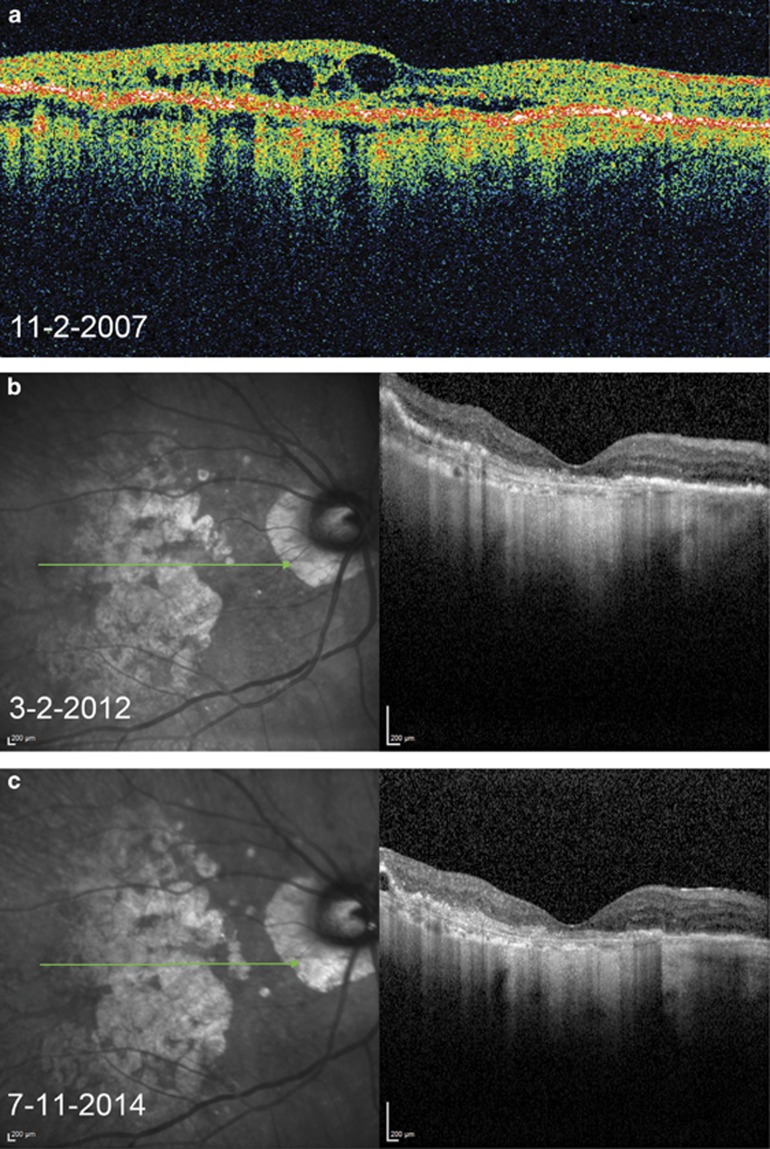
Figure 1.
Representative OCT images of a study patient. (a) Before initiation of anti-VEGF therapy with subretinal and intraretinal cystic fluid. (b) Stable resolution of fluid on 4-week ranibizumab treatment regimen, with return of fluid upon attempts to extend treatment interval beyond 4 weeks. (c) Maintenance of fluid resolution on 8-week aflibercept treatment regimen.
Discussion
Monthly anti-VEGF treatment imposes a significant burden on patients, caregivers, and the healthcare system, and compounds the risk of potential adverse ocular and systemic events. ‘Treat-and-extend' protocols allow for individual adjustment of the injection intervals. However, not all patients can be successfully extended to beyond a 4–8-week interval to remain exudation free. After transitioning to aflibercept, over a 2-year follow-up period, we found significantly fewer injections (9.2±2.9) were required over an increased treatment interval. Eyes required reinjection on average every 8+ weeks with aflibercept compared with every 5+ weeks with prior ‘treat-and-extend' ranibizumab or bevacizumab. The majority of eyes had stable to improved vision and stable OCT anatomic parameters.
Visual and anatomical improvements have been reported with aflibercept in previously recalcitrant nAMD eyes over 6-month follow-up.7 However, the maximum treatment interval between consecutive injections was limited to 56 days. Wykoff et al5 reported improved CFT with stable vision in a similar population, but also required monthly injections for most patients. Chang et al reported worsening of anatomic parameters in recalcitrant eyes switched to aflibercept with increased interval from 4 to 8 weeks over a 24-week follow period.4 A reduction of up to 3 injections per year has been reported following conversion to aflibercept at 1 year.8
Limitations of this current study include its retrospective nature, limited sample size, and a treat-and-extend protocol that may have induced physician bias.
In conclusion, we demonstrate the efficacy of aflibercept in maintaining anatomic and visual gains over a 24-month follow-up period while significantly extending the treatment interval in nAMD eyes previously treated with a ‘treat-and-extend' regimen.
Acknowledgments
This work was supported in part by a unrestricted grant from Research to Prevent Blindness, NY.
The authors declare no conflict of interest.
Footnotes
Meeting Presentation: Presented in part as a poster at the Association for Research in Vision and Ophthalmology Annual Meeting in May 2014
References
- Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010;117:2134–2140. doi: 10.1016/j.ophtha.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Shienbaum G, Gupta OP, Fecarotta C, Patel AH, Kaiser RS, Regillo CD. Bevacizumab for neovascular age-related macular degeneration using a treat-and-extend regimen: clinical and economic impact. Am J Ophthalmol. 2012;153:468–473. doi: 10.1016/j.ajo.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Chang AA, Li H, Broadhead GK, Hong T, Schlub TE, Wijeyakumar W, et al. Intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Ophthalmology. 2014;121:188–192. doi: 10.1016/j.ophtha.2013.08.035. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Brown DM, Maldonado ME, Croft DE. Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial) Br J Ophthalmol. 2014;98:951–955. doi: 10.1136/bjophthalmol-2013-304736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal DS, Gill MK, Sarezky D, Lyon AT, Mirza RG. Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye (Lond) 2014;28:895–899. doi: 10.1038/eye.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Marsiglia M, Mrejen S, Fung AT, Slakter J, Sorenson J, et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina. 2013;33:1605–1612. doi: 10.1097/IAE.0b013e31828e8551. [DOI] [PubMed] [Google Scholar]
- Messenger WB, Campbell JP, Faridi A, Shippey L, Bailey ST, Lauer AK, et al. Injection frequency and anatomic outcomes 1 year following conversion to aflibercept in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2014;98:1205–1207. doi: 10.1136/bjophthalmol-2013-304829. [DOI] [PubMed] [Google Scholar]