Sir,
Choroidal neovascularisation (CNV) is a well-known complication of angioid streaks (AS). It affects 42–86% of patients and if untreated can result in significant vision loss.1 Treatment options have included laser photocoagulation and photodynamic therapy;1, 2 with the advent of anti-vascular endothelial growth factor (VEGF) therapies, off-label anti-VEGF has become the treatment of choice and both bevacizumab and ranibizumab have been shown to be effective.1, 2, 3 We describe the off-label use of 2 mg intravitreal aflibercept in two patients as primary treatment for AS-associated CNV (AS-CNV).
Case report
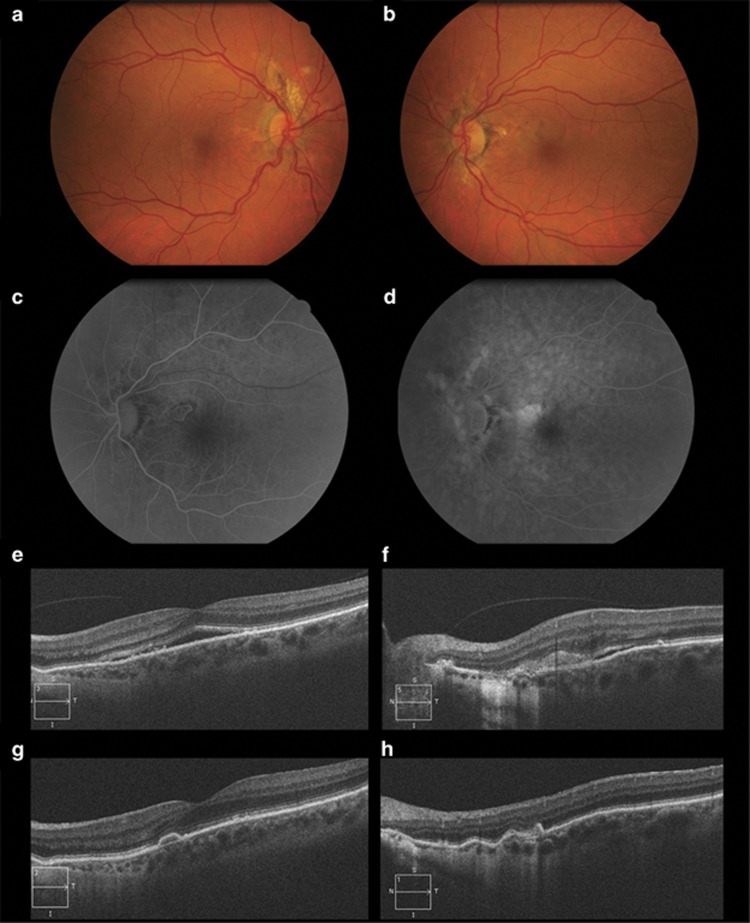
The first is a 57-year-old woman with recent left eye (OS) distortion. Visual acuities (VA) were 20/20 right and 20/25 left. Fundoscopy showed bilateral AS and a classic CNV OS (Figures 1a and b). Fluorescein angiography (FA) and spectral-domain optical coherence tomography (SD-OCT) confirmed the diagnosis (Figures 1c–f) and she was started on a pro re nata (PRN) off-label aflibercept regimen. VA was 20/20 after a single injection and at 12-months follow-up there were no signs of recurrence (Figures 1g and h).
Figure 1.
Colour fundus photographs of the first patient, whose sister was known to be affected by AS and pseudoxanthoma elasticum, show bilateral AS, OD (a) and OS (b), along with an extrafoveal greyish area of CNV, with associated subretinal haemorrhages in OS. Early-phase fundus fluorescein angiography demonstrates a well-demarcated lesion (c) with late leakage (d) in OS, compatible with a classic CNV. Spectral-domain optical coherence tomography reveals a neurosensory detachment involving the fovea (e) and a hyperreflective lesion with subretinal fluid above the retinal pigment epithelium in the cross-section through the lesion. After treatment, there was resolution of the foveal subretinal fluid (f) with scarring of the CNV (g).
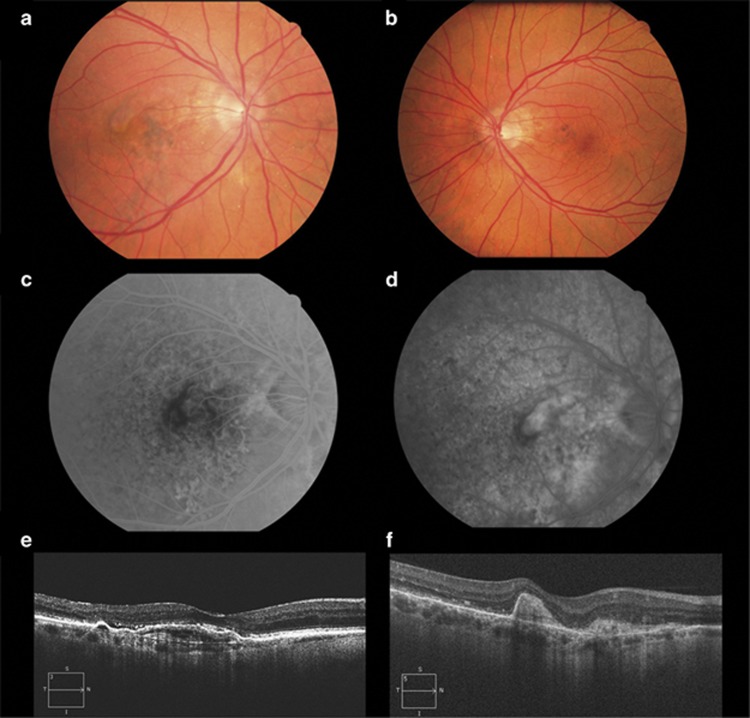
The second case relates to a 56-year-old male presenting with right eye (OD) reduced vision and known OS amblyopia. VA was 20/200 OD and 20/40 OS. Fundoscopy, SD-OCT and FA revealed bilateral AS (Figures 2a and b) and a predominantly classic CNV OD (Figures 2a and c–e). He was started on a 3+PRN off-label aflibercept regimen. VA increased to 20/50 after the loading dose and at 9 months follow-up there was no need for further intervention (Figure 2f).
Figure 2.
Colour fundus photographs show bilateral angioid streaks, OD (a) and OS (b) and a justafoveal choroidal neovascular membrane in OD (a). Fundus fluorescein angiography shows an early well-demarcated lesion (c) with late leakage (d) corresponding to a predominantly classic choroidal neovascularisation. Spectral-domain optical coherence tomography reveals a subfoveal pigment epithelium detachment with associated subretinal fluid (e) at baseline and resolution of the fluid and subretinal fibrosis after treatment (f). Diagnostic work-up to rule-out systemic associations was negative.
Comment
The advent of anti-VEGF agents to treat age-related macular degeneration has encouraged the use of these therapies for CNV of other causes.3 To the best of our knowledge this is the first report to describe the clinical benefit of aflibercept for AS-CNV. Moreover, as the one of the major difficulties in treating CNV secondary to AS is recurrence,2 it may be that aflibercept offers an additional benefit over other anti-VEGF agents as it has a higher affinity for VEGF-A as well as the ability to bind VEGF-B and placental growth factor, resulting in a more effective inhibition of the pathological angiogenic process.4, 5 Further longitudinal studies with a larger cohort of patients are required to verify this hypothesis.
Dr Vaz-Pereira has received consultant fees from Bayer and Novartis and has received travel grants from Bayer, Novartis, Alcon, Allergan and Alimera Sciences. Dr Collaço has received travel grants from Bayer. Dr De Salvo has received travel grants from Bayer and Heidelberg Engineering. Dr van Zeller declares no conflict of interest.
References
- Mimoun G, Tilleul J, Leys A, Coscas G, Soubrane G, Souied EH. Intravitreal ranibizumab for choroidal neovascularization in angioid streaks. Am J Ophthalmol. 2010;150:692–700.e1. doi: 10.1016/j.ajo.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Sawa M, Gomi F, Tsujikawa M, Sakaguchi H, Tano Y. Long-term results of intravitreal bevacizumab injection for choroidal neovascularization secondary to angioid streaks. Am J Ophthalmol. 2009;148:584–590.e2. doi: 10.1016/j.ajo.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Heier JS, Brown D, Ciulla T, Abraham P, Bankert JM, Chong S, et al. Ranibizumab for choroidal neovascularization secondary to causes other than age-related macular degeneration: a phase I clinical trial. Ophthalmology. 2011;118:111–118. doi: 10.1016/j.ophtha.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92:667–668. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]