In a randomized, double-blind, placebo-controlled trial in human immunodeficiency virus (HIV)-negative persons, randomization to emtricitabine/tenofovir for HIV prevention was associated with modest but statistically significant decreases in bone mineral density that negatively correlated with intracellular tenofovir levels and tended to rebound following treatment discontinuation.
Keywords: bone, tenofovir, emtricitabine, PrEP, DXA
Abstract
Background. Daily preexposure prophylaxis (PrEP) with oral emtricitabine and tenofovir disoproxil fumarate (FTC/TDF) decreases the risk of human immunodeficiency virus (HIV) acquisition. Initiation of TDF decreases bone mineral density (BMD) in HIV-infected people. We report the effect of FTC/TDF on BMD in HIV-seronegative men who have sex with men and in transgender women.
Methods. Dual-energy X-ray absorptiometry was performed at baseline and 24-week intervals in a substudy of iPrEx, a randomized, double-blind, placebo-controlled trial of FTC/TDF PrEP. Plasma and intracellular tenofovir concentrations were measured in participants randomized to FTC/TDF.
Results. In 498 participants (247 FTC/TDF, 251 placebo), BMD in those randomized to FTC/TDF decreased modestly but statistically significantly by 24 weeks in the spine (net difference, −0.91% [95% confidence interval {CI}, −1.44% to −.38%]; P = .001) and hip (−0.61% [95% CI, −.96% to −.27%], P = .001). Changes within each subsequent 24-week interval were not statistically significant. Changes in BMD by week 24 correlated inversely with intracellular tenofovir diphosphate (TFV-DP), which was detected in 53% of those randomized to FTC/TDF. Net BMD loss by week 24 in participants with TFV-DP levels indicative of consistent dosing averaged −1.42% ± 29% and −0.85% ± 19% in the spine and hip, respectively (P < .001 vs placebo). Spine BMD tended to rebound following discontinuation of FTC/TDF. There were no differences in fractures (P = .62) or incidence of low BMD.
Conclusions. In HIV-uninfected persons, FTC/TDF PrEP was associated with small but statistically significant decreases in BMD by week 24 that inversely correlated with TFV-DP, with more stable BMD thereafter.
Clinical Trials Registration. NCT00458393.
(See the Editorial Commentary by Falutz on pages 581–3.)
Preexposure prophylaxis (PrEP) using an oral once-daily combination of the antiretroviral agent tenofovir disoproxil fumarate (TDF), alone or in combination with emtricitabine (FTC), decreases human immunodeficiency virus (HIV) acquisition in seronegative adult men and women [1–3]. In animal studies, TDF impairs bone mineralization and decreases bone mineral density (BMD) [4]. In randomized studies in HIV-infected people, initiation of antiretroviral therapy (ART) is associated with loss of BMD, with greater decreases observed with regimens containing TDF [5–9], and bone loss occurs when TDF replaces another drug in the regimen [10–14]. Thus, bone loss has emerged as a safety concern for PrEP containing TDF.
In other PrEP studies performed in HIV-seronegative men who have sex with men (MSM) in San Francisco [15] and heterosexual men and women in Botswana [16], TDF or FTC/TDF was associated with modest but statistically significant losses of BMD. In the current study, we measured changes in BMD both during and after randomized treatment with FTC/TDF in a large and diverse group of MSM and transgender women (TGW) enrolled in a multinational trial of PrEP. We also explored the quantitative relationship between bone loss and drug exposure.
METHODS
Study Design
Dual-energy X-ray absorptiometry (DXA) scans were performed in a subset of participants enrolled in the Preexposure Prophylaxis Initiative (iPrEx) study, in which HIV-seronegative MSM/TGW were randomized to receive either FTC/TDF or placebo [1]. The only additional eligibility criteria for the DXA substudy were weight <120 kg and no use of oral or inhaled glucocorticoids. Enrollment was offered to all eligible iPrEx participants in Chiang Mai, Thailand; San Francisco, California; and Cape Town, South Africa; and to participants in Lima, Peru and Rio de Janeiro, Brazil who enrolled in iPrEx after DXA scanning became available at those sites. Substudy participants reviewed and signed a separate consent form. DXA scanning began in July 2008. Enrollment in iPrEx ended in December 2009, and randomized treatment ended in August 2010.
DXA Scanning
Scans of the lumbar spine (L1–L4) and left hip were performed at baseline (within 1 week of enrollment) and 24-week intervals during randomized treatment. At treatment discontinuation visits, DXA scanning was performed for participants who had not had a scan in the previous 8 weeks (“stop” scan). A follow-up DXA scan was scheduled 24 weeks after discontinuation of study medication (“poststop”).
Participants in San Francisco and Rio de Janeiro were scanned on Lunar Prodigy (Madison, Wisconsin) instruments; those in Cape Town and Chiang Mai were scanned on Hologic Discovery (Waltham, Massachusetts) devices, and those in Lima were scanned on a Hologic Explorer. All sites followed manufacturer-recommended calibration and maintenance procedures. A study-specific spine phantom was circulated for scanning at all sites. Each site's calibration and maintenance records were reviewed to assure consistency throughout the study. Scans were analyzed locally following a standardized protocol and reviewed centrally. With very few exceptions, all scans were analyzed by the same person at each DXA facility. BMD results were categorized using classification systems put forth by the International Society for Clinical Densitometry (ISCD) [17], which defines a z score ≤ −2.0 as “below the expected range for age,” and the World Health Organization (WHO) [18], which defines low BMD or osteopenia as a t score < −1.0 but > −2.5, and osteoporosis as a t score ≤ −2.5.
Other Data
Participants provided information on exercise and other factors associated with bone health by questionnaire. Data on use of hormones, dietary supplements, and recreational drugs, as well as gender identification, came from the parent study. Information on fractures in the entire iPrEx cohort during the full course of randomized treatment was obtained from adverse event reports.
Plasma and Intracellular Tenofovir and Emtricitabine Measurements
In all DXA substudy participants assigned to FTC/TDF, tenofovir (TFV) and FTC concentrations at weeks 24, 48, and 72 were measured in stored plasma. Intracellular TFV diphosphate (TFV-DP) concentrations at week 24 were measured in viably cryopreserved peripheral blood mononuclear cells [1, 19].
Data Analysis
Target enrollment in the DXA substudy was 500, which we estimated would provide 99% power to detect a treatment difference in change in BMD of 0.4% with a standard deviation (SD) of 1%, assuming a 10% dropout rate. Baseline data are mean ± SD. The primary analysis compared groups by randomized assignment. Baseline characteristics were compared by an unequal-variance t test for continuous variables and the Fisher exact test for categorical variables. The Fisher exact test was also used to compare categorical variables during follow-up (eg, percentage with ≥5% bone loss). Baseline BMD z scores were compared to their expected distribution by a 1-sample t test.
Percentage of change in BMD was calculated relative to baseline. Changes in BMD in randomized assignment and groups defined by drug detection during treatment and after discontinuation are reported as mean ± standard error. Net differences (percentage of change in FTC/TDF minus percentage of change in placebo) are reported as mean (95% confidence interval [CI]). The average change by time-point with treatment and drug detection was fit using a normal mixed-effects model [20] fit by maximum likelihood allowing for treatment by week interaction and an unstructured covariance. The association between TFV-DP levels and change in BMD at week 24 was explored using cubic splines for TFV-DP level by linear regression. “Poststop” analysis (after discontinuation of study medication) compared the difference between randomized groups from baseline and the “stop” to the “poststop” scans using a t test with unequal variances. Results in seroconverters were censored beginning at the first visit with laboratory evidence of HIV infection. Hip data were excluded for 2 participants (both FTC/TDF) because hip implants compromised image quality. In 21 participants (11 FTC/TDF, 10 placebo), the right hip was inadvertently scanned at baseline; in these cases the right hip was to be scanned at all subsequent visits. All P values are 2-sided.
RESULTS
Baseline Characteristics
Of the 2499 individuals who enrolled in the parent study, 503 enrolled in the DXA substudy. Data in 5 participants were subsequently excluded when retrospective analysis of HIV RNA revealed that they were HIV-infected at enrollment. Thus, the total analyzed population numbered 498 (247 FTC/TDF, 251 placebo). There were no statistically significant differences between randomized groups in demographic, anthropometric, or lifestyle characteristics, use of medications that affect BMD, the proportion who self-identified as transgender, or BMD in the spine or hip (Table 1). Approximately half of participants were aged <25 years. Three percent reported use of hormonal products containing estrogen and/or a progestational agent or androgen suppressor. Use of these agents was stable through the study.
Table 1.
Baseline Characteristics
| Characteristic | FTC/TDF | Placebo | P Value |
|---|---|---|---|
| No. | 247 | 251 | |
| Age, y | 28 ± 10 | 28 ± 10 | .58 |
| Age category, y | .22 | ||
| 18–24 | 46% | 51% | |
| 25–29 | 18% | 21% | |
| 30–39 | 22% | 15% | |
| ≥40 | 14% | 14% | |
| Race | .30 | ||
| Black/African American | 15% | 10% | |
| Asian | 20% | 20% | |
| White | 18% | 17% | |
| Mixed/other | 47% | 53% | |
| Hispanic | 50% | 54% | .37 |
| Height, cm | 170 ± 8 | 171 ± 7 | .41 |
| Weight, kg | 67.2 ± 12.6 | 68.1 ± 13.7 | .40 |
| Body mass index, kg/m2 | 23.1 ± 3.7 | 23.3 ± 3.9 | .65 |
| Total body fat, % | 19.7 ± 6.9 | 20.1 ± 6.5 | .46 |
| Total lean body mass, kg | 50.9 ± 7.0 | 51.4 ± 7.7 | .46 |
| Family history of osteoporosis | 7% | 10% | .19 |
| Transgender women | 11% | 10% | .99 |
| Use feminizing hormones | 2% | 5% | .14 |
| Weight-bearing exercise | 64% | 63% | .85 |
| Diet to lose weight in past 6 months | 9% | 11% | .45 |
| Smoke cigarettes | 44% | 42% | .65 |
| Drink alcohol | 81% | 80% | .74 |
| Use amphetamines | 3% | 3% | .99 |
| Use marijuana | 15% | 13% | .60 |
| Use cocaine | 6% | 6% | .85 |
| Use supplements containing calcium or vitamin D | 9% | 10% | .54 |
| Use topical corticosteroid | 3% | 6% | .14 |
| Spine (L1-L4) BMD, g/cm2 | 1.04 ± 0.17 | 1.04 ± 0.16 | .99 |
| Spine (L1-L4) z score | −0.58 ± 1.24 | −0.60 ± 1.19 | .90 |
| Spine (L1-L4) t score | −0.62 ± 1.22 | −0.62 ± 1.19 | .99 |
| Total hip BMD, g/cm2 | 1.02 ± 0.15 | 1.02 ± 0.15 | .72 |
| Total hip z score | −0.10 ± 0.99 | −0.14 ± 1.01 | .65 |
| Total hip t score | −0.06 ± 0.96 | −0.10 ± 0.98 | .64 |
Data are presented as mean ± SD or percentage. P values were determined by an unequal-variance t test for continuous variables and the Fisher exact test for categorical variables.
Abbreviations: BMD, bone mineral density; FTC/TDF, emtricitabine/tenofovir disoproxil fumarate; SD, standard deviation.
At baseline, average z and t scores for BMD were below zero for both the spine and hip (Table 1). When the distributions of baseline z scores were compared to the expected normal distribution around a mean of zero, they were statistically significantly shifted to the left (mean difference, −0.59 ± 1.2 [P < .001] for spine and −0.12 ± 1.0 [P = .011] for hip; Supplementary Figure 1). Using ISCD criteria [17], low BMD was seen in the spine and hip in 12% and 2% of participants, respectively. Using WHO cutpoints [18], 32% and 16% of participants had BMD t scores < −1.0 to > −2.5 in the spine and hip, respectively; and 5% and <1% had t scores ≤ −2.5 in the spine and hip, respectively. For subsequent analyses we report z scores, in accordance with the ISCD position that z scores are preferable to t scores in men aged <50 years [17]. Use of t scores produced similar results.
Changes in BMD During Randomized Treatment
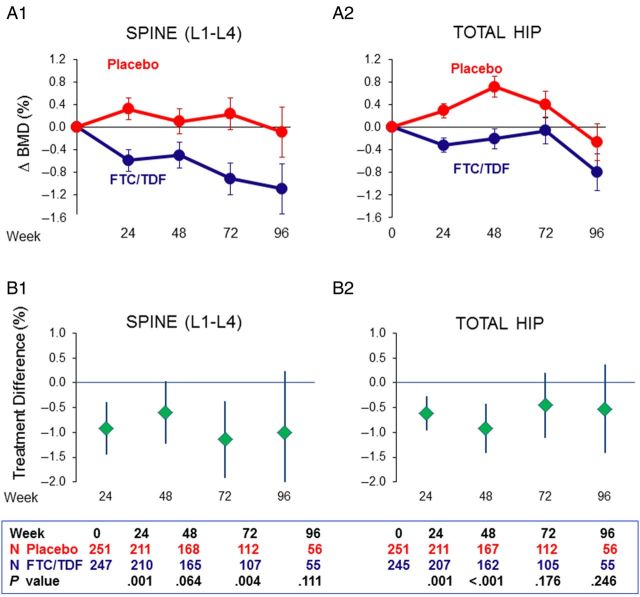
In the intent-to-treat analysis, spine BMD decreased in the FTC/TDF group after 24 weeks, with smaller further decreases to week 96 (Figure 1A1). No decreases were observed with placebo. Net differences were modest but consistently below zero, indicating greater BMD loss in the FTC/TDF group (week 24: −0.91% [95% CI, −1.44% to −.38%], P = .001; week 48: −0.60% [95% CI, −1.23% to .03%], P = .064; week 72: −1.14% [95% CI, −1.91% to −.37%], P = .004; week 96: −1.00% [95% CI, −2.24% to .23%], P = .111; Figure 1B1). After week 24, further net changes averaged −0.12% [95% CI, −.36% to .12%] for each additional 24-week interval and were not statistically significant (P = .28).
Figure 1.
Changes from baseline in bone mineral density (BMD) during randomized treatment. Intent-to-treat results are presented as mean ± standard error percentage changes from baseline in BMD in the lumbar spine (A1) and total hip (A2) and net treatment differences (B1 and B2), calculated as percentage of change in the emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) group minus percentage of change in the placebo group, with error bars representing the 95% confidence interval. See “Results” section for data at each time-point.
In the total hip, BMD initially decreased in the FTC/TDF group and subsequently rebounded toward baseline by week 72 (Figure 1A2). Hip BMD tended to increase with placebo. Net treatment differences were modest but consistently below zero for FTC/TDF, with differences between groups statistically significant at weeks 24 and 48 (week 24: −0.61% [95% CI, −.96% to −.27%], P = .001; week 48: −0.92% [95% CI, −1.42% to −.41%], P < .001; week 72: −0.45% [95% CI, −1.11% to .21%], P = .176; week 96: −0.53% [95% CI, −1.42 to .37%], P = .246; Figure 1B2). After week 24, further net changes in hip BMD averaged −0.01% (95% CI, −.19% to .17%) for each additional 24 weeks (P = .92).
Comparison of the average slopes in the first 24 weeks to those after 24 weeks showed that the declines were significantly steeper in the initial 24 weeks (P = .02 for spine and P = .005 for hip), thus ruling out a constant decline over the follow-up period.
Categorical Analyses of BMD Results
There were no statistically significant differences between randomized groups in the percentage of participants who met ISCD criteria for low BMD for age at any time point (Supplementary Figure 2A). A statistically significantly greater proportion of participants randomized to FTC/TDF had a spine BMD loss ≥5% at week 24 (8% vs 2% in FTC/TDF and placebo, respectively; P = .003, Supplementary Figure 2B). Differences between groups were not statistically significant at any other time point. Analysis of changes in BMD by quartiles of baseline BMD z score demonstrated that participants who started in the lowermost quartile (Q1) did not experience greater BMD loss by 24 weeks than those in the uppermost quartile (Q4) in the hip (net treatment difference, −0.78% ± 0.34% in Q1 vs −0.79% ± 0.36% in Q4, Ptrend across quartiles = 0.87).There was a nonsignificant trend toward less loss of BMD in the spine in lower quartiles (−0.33% ± 0.53% in Q1 vs −1.31% ±0.55% in Q4, Ptrend across quartiles = 0.17).
Net Changes in BMD at and After Discontinuation of Randomized Treatment
The median duration of randomized treatment for DXA results was 61 weeks. Net differences from baseline to the “stop” scan showed modest but statistically significant decreases in BMD (Table 2). The median interval between the “stop” and “poststop” scans was 24 weeks (range, 10–88 weeks). Spine BMD tended to increase in the FTC/TDF group after discontinuation of randomized treatment, while remaining unchanged with placebo. However, the difference between groups did not achieve a level of statistical significance. Hip BMD trended upward in both groups with minimal treatment difference. With regard to overall changes from baseline to the “poststop” scan, net treatment differences remained negative for FTC/TDF in both the spine and hip (Table 2).
Table 2.
Percentage of Change in Bone Mineral Density From Before to After Discontinuation of Randomized Treatment
| Change | TDF/FTC, No. | Placebo, No. | TDF/FTC, Mean ± SE | Placebo, Mean ± SE | Net Difference, Mean (95% CI) | P Valuea |
|---|---|---|---|---|---|---|
| Baseline to stop | ||||||
| Spine (L1–L4) | 214 | 216 | −0.76 ± 0.27 | 0.08 ± 0.22 | −0.84 (−1.51 to −.16) | .016 |
| Total hip | 212 | 216 | −0.42 ± 0.16 | 0.32 ± 0.16 | −0.74 (−1.19 to −.29) | .001 |
| Stop to poststop | ||||||
| Spine (L1–L4) | 167 | 165 | 0.64 ± 0.27 | 0.07 ± 0.22 | 0.59 (−.10 to 1.28) | .096 |
| Total hip | 166 | 165 | 0.33 ± 0.18 | 0.18 ± 0.15 | 0.15 (−.31 to .60) | .523 |
| Baseline to poststop | ||||||
| Spine (L1–L4) | 167 | 165 | −0.46 ± 0.33 | −0.01 ± 0.28 | −0.45 (−1.30 to .30) | .294 |
| Total hip | 166 | 165 | −0.26 ± 0.24 | 0.49 ± 0.21 | −0.76 (−1.39 to −.13) | .019 |
Data are mean ± SE percentage changes in bone mineral density; treatment difference is mean percentage change in TDF/FTC minus percentage change in placebo ± SE; P values by t test. Stop visit is final visit on randomized treatment; poststop visit was a median of 24 weeks after stop visit. Includes only participants with at least 1 on-treatment DXA.
Abbreviations: CI, confidence interval; DXA, dual-energy X-ray absorptiometry; SE, standard error; TDF/FTC, emtricitabine/tenofovir disoproxil fumarate.
a P values that are statistically significant are highlighted in boldface.
Changes in BMD in Relation to Drug Detection
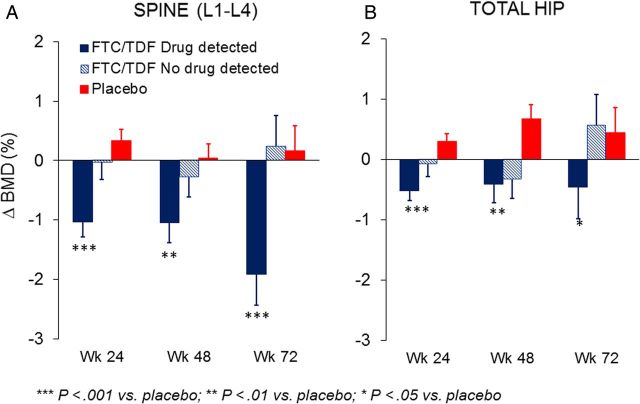
At week 24, intracellular TFV-DP was detected in only 53% of substudy participants randomized to FTC/TDF. TFV-DP levels exhibited a statistically significant inverse relationship with changes in BMD in both the spine and hip (P = .01; Figure 2). Exploration with cubic splines found a generally linear relationship with no evidence of a threshold effect. Among those with TFV-DP levels >16 (average, 43) fmol/106 peripheral blood mononuclear cells, which are indicative of consistent dosing [19], changes in BMD averaged −1.42% ± 0.29% and −0.85% ± 0.19% in the spine and hip, respectively (P < .001 vs placebo both cases). In plasma, TFV or FTC was detected in 57%, 48%, and 53% of those randomized to FTC/TDF at weeks 24, 48, and 72, respectively. Decreases in BMD were statistically significant vs placebo in those with detectable drug levels at each time-point (Figure 3). Adjustment for demographic, anthropometric, and lifestyle factors listed in Table 1 did not affect the results of either the intent-to-treat or drug-based analyses.
Figure 2.
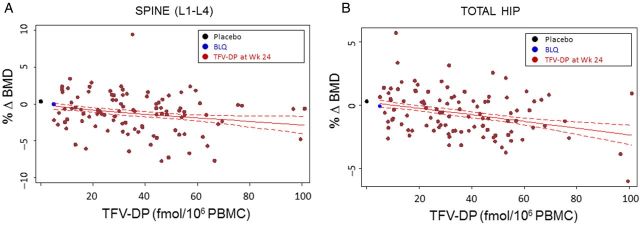
Association of changes in bone mineral density (BMD) at week 24 with intracellular tenofovir diphosphate (TFV-DP) levels measured at week 24. Among participants randomized to emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) who had detectable TFV-DP levels, there was a significant inverse association across the full range of TFV-DP concentrations measured in both the spine and hip (A and B, respectively; P = .01 in both) using cubic spline to model the mean change in BMD by TFV-DP level. The figures display the 95% confidence interval and mean values for participants randomized to placebo (black symbols: spine, 0.34% ± 0.19%; hip, 0.30% ± 0.12%) and those randomized to FTC/TDF with TFV-DP below the limit of quantitation (BLQ; blue symbols: spine, −0.03% ± 0.30%; hip, −0.08% ± 0.19%).
Figure 3.
Changes from baseline in bone mineral density (BMD) through week 72 during randomized treatment, based on detection of drug in plasma at each time-point. In plasma, tenofovir (TFV) or emtricitabine (FTC) was detected in 57%, 48%, and 53% of those randomized to FTC/tenofovir disoproxil fumarate (TDF) at weeks 24, 48, and 72, respectively. Decreases in BMD in the spine (A) and hip (B) were statistically significant vs placebo in the groups with detectable drug levels at each time-point (for drug detection vs placebo: ***P < .001; **P < .01; *P < .05).
Fractures
Among all 2499 participants in the parent study, there were 42 fractures in 38 participants, with no statistically significant differences between groups (1.7% FTC/TDF vs 1.4% placebo, P = .62). With the exception of 1 dental fracture 8 months after discontinuation of treatment, all fractures were judged to be trauma related. Eight participants with fractures were enrolled in the DXA substudy (5 FTC/TDF, 3 placebo). One had a baseline spine z score of −1.1. The z scores in spine and hip for all others with fractures were > −1.0. No participants who had fractures had BMD levels that met either ISCD criteria for low BMD or WHO criteria for osteoporosis at baseline or during the study.
DISCUSSION
We report a modest statistically significant loss of BMD in the spine and hip in the first 24 weeks in a multinational study of HIV-seronegative MSM/TGW randomized to receive FTC/TDF for HIV prevention. Among those randomized to FTC/TDF, the magnitude of BMD loss at week 24 was inversely proportional to intracellular levels of TFV-DP. At a median of 24 weeks after discontinuation of randomized treatment, there was a nonsignificant trend to a rebound in BMD in the spine.
Studies in ART-naive HIV-infected adults have typically shown statistically significant decreases in BMD over the first 6–12 months after ART initiation that average between 4% and 6% [5–7, 21] and subsequently appear to stabilize or partially reverse [5, 6, 22, 23]. To address the possibility that the apparently lesser effect of randomization to FTC/TDF for PrEP compared with combination ART for HIV may be attributed to variable adherence and reduced drug exposure, we examined changes in BMD in the context of plasma and intracellular drug levels. This analysis also allowed us to further quantify the effect of exposure to FTC/TDF. In the first 24 weeks, the period of most rapid decline in BMD, bone loss in the spine averaged −1.42% among those with intracellular TFV-DP levels indicative of consistent dosing [19]. This value is similar to the calculated differences in bone loss between regimens containing TDF and those that do not contain TDF in randomized ART initiation studies in HIV-infected individuals [5–7]. The relationship between TFV-DP levels and loss of BMD was linear over the entire range of intracellular drug levels, thus providing no evidence of a threshold effect in the range of concentrations observed. Bone loss among highly adherent PrEP users was still less than that observed after starting combination ART for HIV infection [5–7]. Dose-reduction strategies, such as nondaily dosing, may mitigate this effect further.
The clinical significance of an average BMD loss of 1.42% (spine) or 0.85% (hip) in healthy young and middle-aged persons is not known [24]. In secondary analyses to further probe risk of bone loss, we focused on participants who might be considered to be at greatest risk—those whose BMD was low at baseline or who experienced ≥5% BMD loss during treatment. The proportion of participants with z scores ≤ −2.0 did not differ between groups at any time-point. In addition, we saw no evidence that individuals with low BMD z scores at baseline were at greater risk for bone loss during treatment.
There was no statistically significant difference between groups in fracture rates in the current study or the aforementioned trials of oral TDF or FTC/TDF PrEP [3, 15]. However, the relatively short duration of follow-up and young age of most participants made it unlikely that we would see such differences. Reports of fractures in retrospective examination of large databases of HIV-infected patients have shown variable results. Although some studies have reported that TDF is associated with increased fracture risk [25–27], others have found decreased risk [28] or no association [29] with TDF. To date, the randomized treatment studies using TDF that have reported fracture rates in HIV-infected individuals have shown no difference based on assigned regimen, despite greater BMD loss with TDF [5, 7].
A higher-than-expected proportion of participants had evidence of low BMD at baseline, consistent with reports from other studies in seronegative MSM [15, 30, 31]. The t or z scores in all of these studies were based on normative data collected in healthy young men and, thus, we would expect average scores among seronegative men to be closer to zero. Although it is possible that the normative data do not accurately represent the current distribution of BMD among healthy young men, we also note that these same standards are used in multiple reports of a high prevalence of low BMD among HIV-infected men [32–36]. We saw no evidence that those with low BMD z scores at baseline were at greater risk for bone loss with FTC/TDF or bone fracture during the study, but we cannot exclude the possibility that the group with low BMD at baseline may be at greater risk for fragility fractures later in life, or that extended use of PrEP with FTC/TDF may exacerbate that risk.
The current study has several strengths, including a randomized placebo-controlled design and, for the first time, use of drug concentrations to characterize the relationship between drug exposure and BMD loss. Inclusion of a diverse international population of MSM/TGW no doubt introduced some variability, but nonetheless we were able to detect statistically significant changes between randomized groups. A longer period of follow-up is required to assess changes in BMD following discontinuation of study medication and determine whether full recovery can occur and to evaluate the effects of intermittent PrEP.
The results of the current study provide no reason to modify the Centers for Disease Control and Prevention's recommendation that routine DXA monitoring during FTC/TDF for PrEP is not warranted unless there are additional risk factors for fracture [37]. Nonetheless, given the high prevalence of low BMD z scores in men in this and other studies [15, 30, 31], those beginning PrEP should be counseled on factors important for bone health, including limiting use of alcohol and tobacco, increasing weight-bearing exercise, and assuring adequate dietary intake of calcium and vitamin D [38].
In summary, we found small but statistically significant decreases in BMD in the hip and spine in a multinational study of seronegative MSM/TGW randomized to FTC/TDF for HIV prevention. We report for the first time a quantitative relationship between bone loss and intracellular TFV-DP after 24 weeks of treatment. Bone loss in the spine tended to reverse, albeit not completely, after discontinuation of treatment. These results demonstrate an effect of FTC/TDF that is independent of HIV infection or other ART. The relatively small bone loss associated with FTC/TDF PrEP is offset by the prevention of HIV infection, which requires combination ART that is associated with relatively greater loss of BMD.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors gratefully acknowledge the contributions of the following individuals: Lima: Lorena Vargas, Jorge Sanchez; Chiang Mai: Pongpun Saokhieo, San Francisco: Kerry Murphy, Hailey Gilmore, Sally Holland, Elizabeth Faber, John Duda; Cape Town: Linda Bewerunge, Elizabeth Batist, Christine Hoskin, Ben Brown; Rio de Janeiro: Carina Beppu-Yoshida, Marcellus Dias da Costa, Sergio Carlos Assis de Jesus Jr, Jose Roberto Grangeiro da Silva, Roberta Millan, Brenda Regina de Siqueira Hoagland, Nilo Martinez Fernandes, Lucilene da Silva Freitas, Beatriz Grinsztejn, Jose Pilotto; University of Colorado: Lane Bushman, Jia-Hua Zheng, Louis Anthony Guida, Brandon Kline; University of California, San Francisco/Gladstone: Pedro Goicochea, Jonathan Manzo, Robert Hance, Jeff McConnell, Patricia Defechereux, Vivian Levy, Malu Robles; DataFax/Net: Brian Postle; National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH): David Burns; Gilead: James Rooney. Most importantly, we are grateful to all the Preexposure Prophylaxis Initiative study volunteers who participated in the dual-energy X-ray absorptiometry substudy for their commitment to this project.
Financial support. This work was supported by the NIH (U01 AI64002, U01 AI84735) and the Bill & Melinda Gates Foundation. Study medication was provided by Gilead Sciences. Some infrastructure support at the University of California, San Francisco was provided by an award from the NIH/National Academy for Advancing Translational Science (UL1 TR000004).
Potential conflicts of interest. S. B. reports nonfinancial support from Gilead Sciences and other support from Gilead Sciences during the conduct of the study and personal fees from Clinical Care Options for serving as faculty for a program on preexposure prophylaxis, outside the submitted work. P. L. A. reports nonfinancial support from Gilead Sciences, outside the submitted work. M. S. reports personal fees from Gilead, outside the submitted work. R. M. G. reports personal fees from Siemens Healthcare, Merck, Clinical Care Options, and Medscape Education, outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 4.Van Rompay KK, Durand-Gasselin L, Brignolo LL, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother 2008; 52:3144–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191–201. [DOI] [PubMed] [Google Scholar]
- 6.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51:963–72. [DOI] [PubMed] [Google Scholar]
- 7.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203:1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedimo RJ, Drechsler H, Jain M, et al. The RADAR study: week 48 safety and efficacy of raltegravir combined with boosted darunavir compared to tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients. Impact on bone health. PLoS One 2014; 9:e106221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyle G, Hardy H, Farajallah A, McGrath S, Kaplita S, Ward D. Changes in bone mineral density after 96 weeks of treatment with atazanavir/ritonavir or lopinavir/ritonavir plus tenofovir DF/emtricitabine in treatment-naive patients with HIV-1 infection: the CASTLE body composition substudy. J Acquir Immune Defic Syndr 2015; 68:40–5. [DOI] [PubMed] [Google Scholar]
- 10.Moyle GJ, Sabin CA, Cartledge J, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS 2006; 20:2043–50. [DOI] [PubMed] [Google Scholar]
- 11.Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-lamivudine: a randomized, 96-week trial. Clin Infect Dis 2009; 49:1591–601. [DOI] [PubMed] [Google Scholar]
- 12.Martinez E, Arranz JA, Podzamczer D, et al. A simplification trial switching from nucleoside reverse transcriptase inhibitors to once-daily fixed-dose abacavir/lamivudine or tenofovir/emtricitabine in HIV-1-infected patients with virological suppression. J Acquir Immune Defic Syndr 2009; 51:290–7. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen TA, Jensen D, Tolstrup M, et al. Comparison of bone and renal effects in HIV-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. PLoS One 2012; 7:e32445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotter AG, Vrouenraets SM, Brady JJ, et al. Impact of switching from zidovudine to tenofovir disoproxil fumarate on bone mineral density and markers of bone metabolism in virologically suppressed HIV-1 infected patients; a substudy of the PREPARE study. J Clin Endocrinol Metab 2013; 98:1659–66. [DOI] [PubMed] [Google Scholar]
- 15.Liu AY, Vittinghoff E, Sellmeyer DE, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One 2011; 6:e23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One 2014; 9:e90111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewiecki EM, Gordon CM, Baim S, et al. International society for clinical densitometry 2007 adult and pediatric official positions. Bone 2008; 43:1115–21. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Prevention and management of osteoporosis: WHO technical report series 921. Geneva, Switzerland: WHO, 2003. [PubMed] [Google Scholar]
- 19.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 1997; 16:2349–80. [DOI] [PubMed] [Google Scholar]
- 21.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–61. [DOI] [PubMed] [Google Scholar]
- 22.Cassetti I, Madruga JV, Suleiman JM, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials 2007; 8:164–72. [DOI] [PubMed] [Google Scholar]
- 23.Moyle GJ, Stellbrink HJ, Compston J, et al. 96-week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. Antivir Ther 2013; 18:905–13. [DOI] [PubMed] [Google Scholar]
- 24.Carr A, Hoy J. Low bone mineral density with tenofovir: does statistically significant mean clinically significant? Clin Infect Dis 2010; 51:973–5. [DOI] [PubMed] [Google Scholar]
- 25.Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS 2012; 26:825–31. [DOI] [PubMed] [Google Scholar]
- 26.Horizon AA, Joseph RJ, Liao Q, Ross ST, Pakes GE. Characteristics of foot fractures in HIV-infected patients previously treated with tenofovir versus non-tenofovir-containing highly active antiretroviral therapy. HIV AIDS (Auckl) 2011; 3:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mary-Krause M, Viard JP, Ename-Mkoumazok B, et al. Prevalence of low bone mineral density in men and women infected with human immunodeficiency virus 1 and a proposal for screening strategy. J Clin Densitom 2012; 15:422–33. [DOI] [PubMed] [Google Scholar]
- 28.Mundy LM, Youk AO, McComsey GA, Bowlin SJ. Overall benefit of antiretroviral treatment on the risk of fracture in HIV: nested case-control analysis in a health-insured population. AIDS 2012; 26:1073–82. [DOI] [PubMed] [Google Scholar]
- 29.Yong MK, Elliott JH, Woolley IJ, Hoy JF. Low CD4 count is associated with an increased risk of fragility fracture in HIV-infected patients. J Acquir Immune Defic Syndr 2011; 57:205–10. [DOI] [PubMed] [Google Scholar]
- 30.Grijsen ML, Vrouenraets SM, Wit FW, et al. Low bone mineral density, regardless of HIV status, in men who have sex with men. J Infect Dis 2013; 207:386–91. [DOI] [PubMed] [Google Scholar]
- 31.Mulligan K, Harris DR, Emmanuel P, et al. Low bone mass in behaviorally HIV-infected young men on antiretroviral therapy: Adolescent Trials Network (ATN) study 021B. Clin Infect Dis 2012; 55:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cazanave C, Dupon M, Lavignolle-Aurillac V, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS 2008; 22:395–402. [DOI] [PubMed] [Google Scholar]
- 33.Bonjoch A, Figueras M, Estany C, et al. High prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort study. AIDS 2010; 24:2827–33. [DOI] [PubMed] [Google Scholar]
- 34.Sharma A, Flom PL, Weedon J, Klein RS. Prospective study of bone mineral density changes in aging men with or at risk for HIV infection. AIDS 2010; 24:2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calmy A, Fux CA, Norris R, et al. Low bone mineral density, renal dysfunction, and fracture risk in HIV infection: a cross-sectional study. J Infect Dis 2009; 200:1746–54. [DOI] [PubMed] [Google Scholar]
- 36.El-Maouche D, Xu X, Cofrancesco J, Jr, Dobs AS, Brown TT. Prevalence of low bone mineral density in a low-income inner-city population. J Bone Miner Res 2011; 26:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014: a clinical practice guideline. Washington, DC: Department of Health and Human Services, 2014:1–67. [Google Scholar]
- 38.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis 2010; 51:937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.