Abstract
Introduction:
Electronic cigarettes (e-cigarettes) are designed to generate inhalable nicotine aerosol (vapor). When an e-cigarette user takes a puff, the nicotine solution is heated and the vapor is taken into lungs. Although no sidestream vapor is generated between puffs, some of the mainstream vapor is exhaled by e-cigarette user. The aim of this study was to evaluate the secondhand exposure to nicotine and other tobacco-related toxicants from e-cigarettes.
Materials and Methods:
We measured selected airborne markers of secondhand exposure: nicotine, aerosol particles (PM2.5), carbon monoxide, and volatile organic compounds (VOCs) in an exposure chamber. We generated e-cigarette vapor from 3 various brands of e-cigarette using a smoking machine and controlled exposure conditions. We also compared secondhand exposure with e-cigarette vapor and tobacco smoke generated by 5 dual users.
Results:
The study showed that e-cigarettes are a source of secondhand exposure to nicotine but not to combustion toxicants. The air concentrations of nicotine emitted by various brands of e-cigarettes ranged from 0.82 to 6.23 µg/m3. The average concentration of nicotine resulting from smoking tobacco cigarettes was 10 times higher than from e-cigarettes (31.60±6.91 vs. 3.32±2.49 µg/m3, respectively; p = .0081).
Conclusions:
Using an e-cigarette in indoor environments may involuntarily expose nonusers to nicotine but not to toxic tobacco-specific combustion products. More research is needed to evaluate health consequences of secondhand exposure to nicotine, especially among vulnerable populations, including children, pregnant women, and people with cardiovascular conditions.
INTRODUCTION
Passive smoking, also referred to as exposure to secondhand smoke (SHS), happens when a person inhales a mixture of toxic compounds released from burning cigarettes (California Environmental Protection Agency, 2005; Nelson, 2001; Wallace-Bell, 2003). Despite the comprehensive smoke-free regulations introduced in many countries, passive smoking remains a global health problem. It has been estimated that passive smoking causes more than six hundred thousand deaths every year around the world (Oberg, Jaakkola, Woodward, Peruga, & Prüss-Ustün, 2011). Current laws and regulations do not adequately protect vulnerable populations, including children, pregnant women, and those with preexisting health conditions, from exposure to SHS. Based on data from 192 countries, Oberg et al. (2011) estimated that 40% of children had been exposed globally to SHS. SHS (also referred to as environmental tobacco smoke, ETS) is comprised primarily of sidestream smoke released from burning cigarettes during puff breaks and smoke exhaled by smokers after each puff. While SHS may contain the same toxic substances as mainstream smoke, it contains higher concentrations of many toxic and carcinogenic compounds than mainstream smoke. Although toxicants released from burning cigarettes are diluted in the indoor air, passive smokers are often exposed to secondhand smoke for prolonged periods of time.
Electronic nicotine delivery systems (commonly referred as electronic cigarettes or e-cigarettes) are new consumer products designed to generate nicotine aerosol (vapor) without combustion of tobacco. A typical e-cigarette is composed of three essential parts: the battery, the heating element or atomizer, and a cartridge or tank that holds a nicotine solution. The product contains nicotine dissolved in propylene glycol, glycerin, or the mixture of the two. When an e-cigarette user takes a puff, the nicotine solution is heated and the vapor can be inhaled into lungs. E-cigarettes are designed to deliver nicotine without toxic constituents of tobacco or tobacco combustion toxicants and carcinogens. Studies have shown that vapor generated from e-cigarettes contains nicotine and that the devices might be effective in delivering nicotine to the body. There is also some evidence that the vapor may contain some toxic compounds like carbonyls, traces of nitrosamines, or particles of heavy metals (Bullen et al., 2010; Dawkins & Corcoran, 2013; Etter & Bullen, 2011; Goniewicz, Knysak, et al., 2013; Goniewicz, Kuma, Gawron, Knysak, & Kosmider, 2013; Trehy et al., 2011; Vansickel & Eissenberg, 2013; Vansickel, Cobb, Weaver, & Eissenberg, 2010; Williams, Villarreal, Bozhilov, Lin, & Talbot, 2013).
Analysis of global e-cigarette marketing indicates that the products are promoted to circumvent smoke-free policies and to reduce exposure to secondhand smoke (Grana & Ling, 2013). Although no sidestream vapor is generated from e-cigarettes between puffs, some of the vapor is exhaled by the user. A study by Schripp, Markewitz, Uhde, and Salthammer (2013) showed that ultrafine particles, volatile organic compounds (VOCs), and nicotine are released with exhaled vapor. McAuley, Hopke, Zhao, and Babaian (2012) investigated emissions and indoor air concentrations of common tobacco smoke by-products from four different vaporized nicotine solutions and found that they emitted traces of carbonyls, polyaromatic hydrocarbons, tobacco-specific nitrosamines, and glycols. There is limited evidence whether passive “vaping” exposes nonusers to nicotine. One study showed that 1-hr exposure to secondhand cigarette smoke and to exhaled “secondhand” e-cigarette vapors generated similar effects on serum cotinine levels (Flouris et al., 2013).
As the popularity of e-cigarettes increases, it is becoming important to further investigate patterns and levels of passive exposure to nicotine and other toxicants from e-cigarettes. The present study explores various factors that might contribute to emission of chemicals from e-cigarettes. It also aims to compare the passive exposure to nicotine, particulates, carbon monoxide (CO), and VOCs from electronic and tobacco cigarettes.
MATERIALS AND METHODS
Study Protocols
We conducted two studies to assess emissions from e-cigarettes. The first study (Study 1) was designed to evaluate major factors that might affect exposure patterns. We generated vapor from three different models of e-cigarettes and released the vapor into an experimental exposure chamber. The aim of the second study (Study 2) was to compare emissions from e-cigarettes and cigarette smoke generated by experienced users of both products. Both studies are described in details below.
Study With Machine-Generated Vapors (Study 1)
Study 1 consisted of 12 experiments (Table 1; Experiments 1–12) conducted in an exposure chamber, each one lasting 2hr. During the first hour, background levels of all analyzed markers were taken. During the second hour, vapor from e-cigarettes was generated using a smoking machine and released into the exposure chamber. We measured 1-hr average concentrations of nicotine, aerosol particles (PM2.5), CO, and selected VOCs. We also monitored changes in PM2.5 and CO levels over 2hr.
Table 1.
Changes in Nicotine, Aerosol Particles (PM2.5), and Carbon Monoxide (CO) Air Concentration Inside Exposure Chamber After Use of E-Cigarette
| Experiment | Exposure level | Ventilation | E-cigarette brand | Nicotine (µg/m3) | PM2.5 (µg/m3) | CO (ppm vol/vol) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | E-cigarette | Baseline | E-cigarette | Baseline | E-cigarette | |||||
| Experiments with smoking- machine (Study 1) | ||||||||||
| 1 | Low | High | EC1 | BLD | 0.82 | 12.7 | 33.0 | 1 | 1 | |
| 2 | Low | High | EC2 | BLD | 1.13 | 85.0 | 80.0 | 1 | 1 | |
| 3 | Low | High | EC3 | BLD | 3.42 | 25.3 | 29.0 | 1 | 1 | |
| 4 | Low | Low | EC1 | BLD | 0.88 | 33.0 | 43.7 | 1 | 1 | |
| 5 | Low | Low | EC2 | BLD | 2.52 | 59.7 | 63.0 | 2 | 2 | |
| 6 | Low | Low | EC3 | BLD | 2.38 | 12.0 | 15.0 | 2 | 2 | |
| 7 | High | High | EC1 | BLD | 0.92 | 11.3 | 15.3 | 2 | 2 | |
| 8 | High | High | EC2 | BLD | 2.45 | 71.3 | 85.0 | 2 | 2 | |
| 9 | High | High | EC3 | BLD | 4.52 | 50.7 | 64.3 | 2 | 2 | |
| 10 | High | Low | EC1 | BLD | 0.95 | 12.0 | 15.0 | 1 | 1 | |
| 11 | High | Low | EC2 | BLD | 4.15 | 17.3 | 23.3 | 2 | 2 | |
| 12 | High | Low | EC3 | BLD | 6.23 | 6.6 | 69.7 | 1 | 1 | |
| Average (Experiments 1–12) | BLD | 2.53±1.75a | 33.1±26.9 | 44.7±26.4a | 1.50±0.52 | 1.50±0.52 | ||||
| Nicotine (µg/m3) | PM2.5 (µg/m3) | CO (ppm vol/vol) | ||||||||
| Baseline | E-cigarette | Tobacco cigarette | Baseline | E-cigarette | Tobacco cigarette | Baseline | E-cigarette | Tobacco cigarette | ||
| Experiments with dual product users (Study 2) | ||||||||||
| 13 | Smoker 1 | BLD | 0.65 | 26.9 | 80.0 | 63.3 | 690.0 | 1 | 1 | 3 |
| 14 | Smoker 2 | BLD | 0.85 | 38.1 | 8.0 | 123.0 | 726.0 | 2 | 2 | 5 |
| 15 | Smoker 3 | BLD | 5.02 | 25.6 | 13.3 | 91.3 | 661.3 | 2 | 2 | 3 |
| 16 | Smoker 4 | BLD | 3.87 | 25.6 | 44.0 | 208.3 | 802.0 | 1 | 1 | 4 |
| 17 | Smoker 5 | BLD | 6.23 | 41.8 | 16.7 | 272.7 | 1217.3 | 1 | 1 | 4 |
| Average (Experiments 13–17) | BLD | 3.32±2.49a,b | 31.6±7.8 | 32.4±30.0 | 151.7±86.8a,b | 819.3±228.6 | 1.40±0.55 | 1.40±0.55b | 3.80±0.84 | |
Note. BLD = below limit of detection (0.22 µg/m3).
aSignificant difference with baseline (p < .05).
bSignificant difference with tobacco cigarette (p < .05).
Electronic Cigarettes
We studied three different models of e-cigarettes selected from the popular brands in Poland: (a) Colinss Age with Camel High atomized cartridge (cartomizer) (Colins Poland; EC1); (b) Dekang 510 Pen with SGC Regular cartridge (Ecigars Polska; EC2); and (c) Mild M201 Pen with Marlboro cartridge (Mild Poland; EC3). Although all cartridges were labeled as containing 18mg of nicotine, our previous study showed that they differed in nicotine levels: Colinss Camel contained 11mg, SGC Regular contained 18mg, and Mild Marlboro contained 19mg of the drug (Goniewicz, Kuma, et al., 2013). All products were purchased from online stores or shopping mall kiosks, and e-cigarettes batteries were charged for 24hr before the experiments.
Exposure Chamber
A 39-m3 laboratory room (3.4×4.1×2.8 m) was equipped as an exposure chamber. The chamber had plain acrylic painted walls and tiled floor, with no windows, carpets, linings, or curtains inside. It was equipped with a regulated exhaust, ventilation system, and two fans for mixing the indoor air. Inside the chamber, there was a sampling station equipped with pumps and monitors, a smoking machine for generating e-cigarette vapors (see Generation of Vapors From E-Cigarettes section), and two chairs. The sampling station was located 1 m from a smoking machine and 10cm above the level of e-cigarettes.
The air exchange rates were determined before each experiment using a ventilation marker (methane) released into the exposure chamber according to the method described previously (Czogala & Goniewicz, 2005). The ventilation rate during the study varied from 1.37 (low) to 12.6 (high) air changes per hour (see also Supplementary Materials). Before each experiment, all surfaces inside the chamber were decontaminated by wiping with 10% aqueous solution of ethanol and intensive ventilation. Only one person, who operated the smoking machine and sampling station, was allowed inside the exposure chamber during Study 1.
Generation of Vapors From E-Cigarettes
In order to generate vapors from the e-cigarettes, a smoking machine was placed in the exposure chamber. We used an automatic single-channel piston-operated smoking machine Palaczbot (Technical University of Lodz) designed to generate vapor from e-cigarettes (Goniewicz, Knysak, et al., 2013; Goniewicz, Kuma, et al., 2013). In all experiments, the vapors from e-cigarettes were generated using the following puffing conditions: puff volume of 70ml, puff duration of 1.8 s, and intervals between puffs of 10 s. Two doses of vapor (see Generation of Vapors From E-Cigarettes section) were released into the exposure chamber with 30-min interval.
Vapors were generated from each of the three e-cigarettes under two variants of ventilation (intensive vs. restricted) and two variants of emission pattern (high vs. low) (3 brands × 2 variants of ventilation × 2 variants of emission). Ventilation of the exposure chamber was controlled during each experiment and adjusted by operating the exhaust. During the experiments with intensive ventilation, exhaust from the exposure chamber was fully opened, while it was partly closed during the experiments with restricted ventilation.
In order to modify exposure patterns, vapors from e-cigarettes were generated using 7 or 15 puffs, for low and high exposure, respectively. The rationale for using two levels of exposure (low vs. high) was to examine various doses of nicotine released with secondhand vapor. Although studies have shown that e-cigarette vapors contain significant amounts of nicotine, there are some controversy as to whether this nicotine is effectively absorbed in the lungs (Zhang, Sumner, & Chen, 2013). If there is little absorption, vapor exhaled by e-cigarette users might contain high levels of the drug. We assumed that if an e-cigarette user takes 15 puffs, and no nicotine is absorbed, then the entire amount of nicotine would be exhaled. If e-cigarettes effectively deliver nicotine to the bloodstream, exhaled vapors will contain only some of nicotine inhaled by the user. By releasing 7 puffs, we simulated the scenario in which approximately half of the nicotine from 15 puffs is absorbed and the balance is exhaled.
Analytical Procedures
Nicotine was measured using gas chromatography with nitrogen–phosphorus detector following active sampling on XAD-4 sorption tubes (SKC Inc.) according to the National Institute of Occupational Safety and Health reference method 2551 (National Institute of Occupational Safety and Health, 2003) with a detection limit of 0.22 μg/m3. Aerosol particles (PM2.5) were measured continuously with a SidePak AM510 Personal Aerosol Monitor. CO was also measured continuously with a Q-Trak Indoor Air Quality 8550 monitor (both instruments from TSI Inc.). The Sidepak was used with a calibration factor setting of 0.32, suitable for secondhand smoke (Jiang et al., 2011; Klepeis, Ott, & Switzer, 2007). VOCs were analyzed using gas chromatography with mass spectrometry following active sampling on Anasorb CSC sorption tubes (SKC Inc.) according to the Occupational Safety and Hazards Agency reference method (Occupational Safety and Hazards Agency, 2000). The method allowed us to measure 11 compounds: benzene, toluene, chlorobenzene, ethylbenzene, m,p-xylene, o-xylene, styrene, naphthalene, 1,2-dichlorobenzene, 1,3-dichlorobenzene, and 1,4-dichlorobenzene. Each monitor was calibrated according to manufacturer’s recommendations, and all analytical procedures were validated and described in details in the Supplementary Materials.
Study With Human-Generated Vapors and Smoke (Study 2)
Subjects
We recruited five volunteers (all male; average age 37.6±16.0; body mass index 23.4±2.1; nicotine dependence by Fagerström Test for Nicotine Dependence 5.8±2.9), who were dual users of e-cigarettes and conventional tobacco cigarettes. The subjects reported using e-cigarettes on average 14±7 times a day for at least 8 months (12.0±4.2) and additionally smoking on average 11±6 cigarettes per day for at least 5 years (18.2±14.1). Two subjects reported using M201 pen-style e-cigarette (18mg/ml; Mild brand), two others used eGo model (16mg/ml; Janty brand), and one used M401 model (18mg/ml; Nicore brand, Atina Poland). Three volunteers smoked L&M Blue Label brand of cigarettes (ISO yields/cigarette: nicotine 0.6mg; tar 8mg; CO 9mg), and two smoked Marlboro Gold brand (nicotine 0.5mg; tar 7mg; CO 7mg). All volunteers who participated in experiments were not given any money, gifts, or other economic incentives. Study 2 protocol was reviewed and approved by the Institutional Review Board at the Medical University of Silesia, Poland.
Emission of E-Cigarettes Vapors and Tobacco Smoke
Study 2 comprised five experiments (Table 1; Experiments 13–17), each lasting for 3hr. After background measures were taken for 1hr, a volunteer entered the room. Each volunteer used ad libitum their own e-cigarette twice for 5min with a 30-min interval. Then, the room was decontaminated as described above and ventilated for 5min. In the last hour, each subject smoked ad libitum entire tobacco cigarettes of their own brand. As with e-cigarettes, volunteers smoked two cigarettes lighting the second cigarette 30min after the first. One-hour average concentrations of nicotine, aerosol particles (PM2.5), CO, and VOCs were determined as described above (baseline, e-cigarette, and tobacco cigarette). PM2.5 and CO levels were also monitored continuously over 3hr of each experiment. Only two persons were allowed in the exposure chamber during Study 2: volunteer and operator of the sampling station.
Statistical Analysis
We compared average concentrations of each airborne marker using a nonparametric Mann–Whitney test. For both studies, we assessed the differences between baseline measures and each test condition (e-cigarette and tobacco cigarette). For Study 2, we also assessed differences in average indoor concentrations of each marker between electronic and tobacco cigarettes. For all tests, Statistica 10.0 software (StatSoft Inc.) was used. The significance level was established as p < .05.
RESULTS
Secondhand Exposure to Nicotine From E-Cigarettes
Study 1
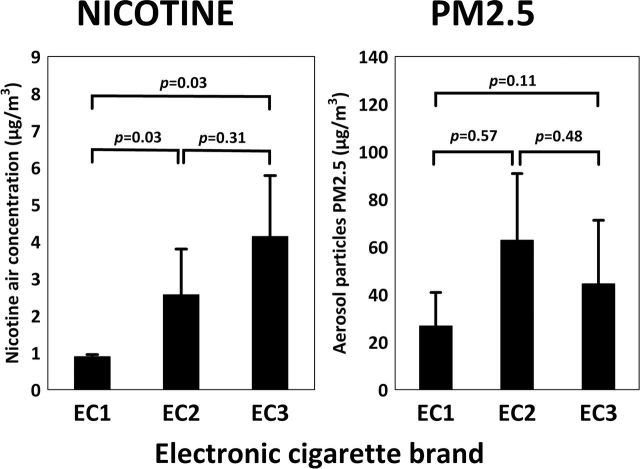
Nicotine was detected in the air during all experiments where e-cigarette vapor was generated with the smoking machine and released into the exposure chamber. Mean 1-hr concentration of nicotine was 2.51±1.68 µg/m3 and ranged from 0.82 to 6.23 µg/m3. Comparison of average indoor air nicotine concentrations in the exposure chamber from three e-cigarette brands are presented in Figure 1. Changes between baseline values and an average nicotine concentration after emission of machine-generated vapors from e-cigarettes are presented in Table 1.
Figure 1.
Effect of e-cigarette brand on nicotine (left) and aerosol particle (right) concentration in the air inside exposure chamber.
Study 2
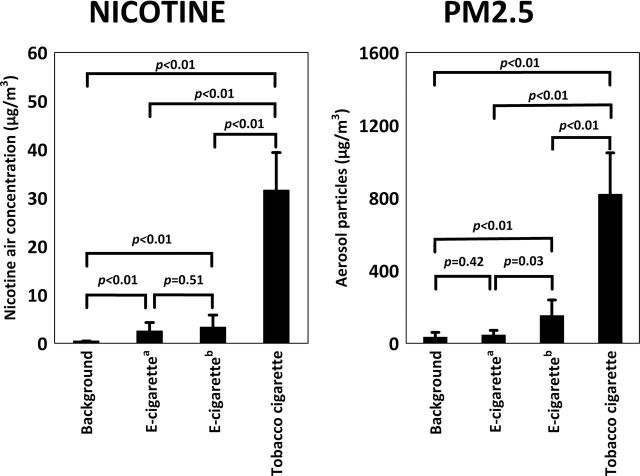
Figure 2 shows baseline concentrations of nicotine and 1-hr medium concentrations after using e-cigarettes or after smoking tobacco cigarettes by volunteers. The average concentration of nicotine resulting from smoking tobacco cigarettes was 10 times higher than from e-cigarettes (31.60±6.91 vs. 3.32±2.49 µg/m3, respectively; p = .0081).
Figure 2.
Comparison of indoor air nicotine (left) and aerosol particle (right) concentrations released from e-cigarette with background values and tobacco cigarette smoking.Note. aVapor generated with smoking machine (Study 1); bVapor exhaled by users (Study 2).
Secondhand Exposure to PM2.5 From E-Cigarettes
Study 1
Aerosol particles were detected in the air during all experiments with vapor generated with the smoking machine and released into the exposure chamber. Mean concentration of PM2.5 was 33.1±26.9 μg/m3 and ranged from 6.6 to 85.0 μg/m3. Comparison of average indoor air PM2.5 levels in exposure chamber from three e-cigarette brands are presented on Figure 1. Changes between baseline values and mean PM2.5 levels after emission of machine-generated vapors from e-cigarettes are presented in Table 1.
Study 2
Figure 2 shows baseline concentrations of PM2.5 and 1-hr mean concentrations after using e-cigarettes or after smoking tobacco cigarettes by volunteers. The mean concentration of PM2.5 resulting from smoking tobacco cigarettes was 7 times higher than from e-cigarettes (819.3±228.6 vs. 151.7±86.8 μg/m3, respectively; p = .0081). Figure 3 shows changes in PM2.5 concentration in the exposure chamber during one of the experiments in Study 2 (Experiment 15; see Table 1).
Figure 3.
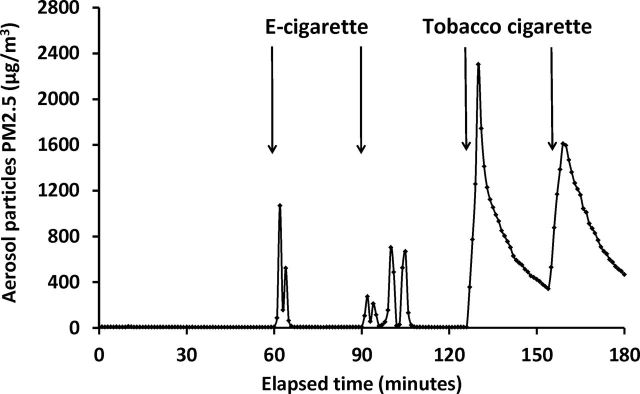
Changes of aerosol particle PM2.5 concentrations during experiment of e-cigarette use and tobacco cigarette smoking in exposure chamber.
Secondhand Exposure to CO From E-Cigarette
Studies 1 and 2
There were no changes in CO concentration after using e-cigarettes in both studies (p > .05). However smoking of two tobacco cigarettes in Study 2 increased CO concentration in the exposure chamber on average by 2 to 3 ppm (vol/vol) (Table 1; p < .05).
Secondhand Exposure to VOCs From E-Cigarettes
Study 1
During the study with machine-generated e-cigarette vapor, only toluene was detected in the exposure chamber. No statistical difference was found between average toluene concentration after release of e-cigarette vapor and baseline values (6.63±0.21 vs. 4.15±2.69 µg/m3, respectively; p = .1582).
Study 2
As with Study 1, toluene was the only VOC detected in the exposure chamber, and the use of e-cigarette did not change the concentration of toluene (3.79±2.16 vs. 4.09±2.12 µg/m3, respectively; p = .8513). Smoking two tobacco cigarettes increased the concentration of four compounds: toluene, ethylbenzene, m,p-xylene, and o-xylene (p < .05). For toluene, the average concentration after smoking tobacco cigarettes was 3.5-fold higher than after using e-cigarettes (14.75±6.02 vs. 4.15±2.69 µg/m3, respectively; p < .05). The average concentrations of ethylbenzene, m,p-xylene, and o-xylene after smoking tobacco cigarettes were 1.17±1.44, 1.94±1.14, and 0.48±0.95 µg/m3, respectively; p < .05).
DISCUSSION
Principal Findings
The key finding of this study is that e-cigarettes emit significant amounts of nicotine but do not emit significant amounts of CO and VOCs. We also found that the level of secondhand exposure to nicotine depends on the e-cigarette brand. However, the emissions of nicotine from e-cigarettes were significantly lower than those of tobacco cigarettes.
Strengths and Limitations of the Study
To our knowledge, this is one of the first studies to measure the concentrations of nicotine, PM2.5, CO, and VOCs emitted by e-cigarettes and to compare the emissions of electronic and conventional tobacco cigarettes in a conventionally ventilated, full-sized room. By comparing e-cigarette vapors generated with a smoking machine to those generated by experienced e-cigarette users in a controlled setting allowed us to control for potential factors that may affect exposure patterns.
Results from experiments with human subjects who used both electronic and tobacco cigarettes allowed us to compare the emissions and the potential exposures by the two products. One of the most important aspects of our study is that the e-cigarette vapors and tobacco smoke were generated by long-term dual users of the products, and we did not modified the way volunteers were typically using the products.
Our findings are supported by results from study by McAuley et al. (2012) who examined the chemical composition of freshly generated vapor collected in a small emission chamber and found that the total air emission concentrations for many pollutants from e-cigarettes were very low. Our study examined the potential effect of various e-cigarette brands on patterns of exposure, whereas McAuley et al. (2012) studied vapors generated from the same model of e-cigarette with varying nicotine solutions and found that the chemical composition of the vapors from different solutions differed in levels of nicotine and other chemicals. Our study showed that the level of exposure also differs between e-cigarette brands. These findings are also consisted with our previously reported data showing high variability in composition of freshly generated vapors among the products (Goniewicz, Kuma, et al., 2013). These findings should be taken into careful consideration when exposure to e-cigarette vapors is considered.
The study has several limitations. An important limitation of our study is that we measured a limited number of chemicals that might be contained within e-cigarette vapors. We reported previously that e-cigarette vapors contain significant levels of carbonyls, including toxic and carcinogenic formaldehyde, acetaldehyde, and acrolein (Goniewicz, Knysak, et al., 2013). These compounds were not measured in this study. Studies by Schripp, Markewitz, Uhde, and Salthammer (2013) and McAuley et al. (2012) found that there is a risk of exposure to carbonyls from e-cigarettes, although the levels of the compounds were lower than those in SHS. We did not investigate other significant factors affecting exposure to e-cigarette vapors, for example, room volume and number of e-cigarettes used simultaneously in a single room. The exposure chamber input air was not filtered during the experiments, and ventilation air exchange rates of exposure chamber were higher than residential rates (Yamamoto, Shendell, Winer, & Zhang, 2010). Finally, the study assessed concentrations of several markers in the air but not serum concentrations in people exposed to secondhand vapors. These airborne concentrations do not necessarily reflect the serum concentration and the impact on health of people exposed to these vapors.
Unanswered Questions and Future Research
This study did not test potential health effects associated with secondhand exposure to vapors from e-cigarettes. To date, there are few studies that have tested the acute effects of brief exposure to secondhand e-cigarette vapors. One study by Flouris et al. (2012) found that acute passive “vaping” of e-cigarettes did not influence complete blood count in human subjects. Another study by the same authors found that controlled 1-hr exposure to e-cigarette vapors did not significantly affect lung function in human subjects (Flouris et al., 2013). We found no publications on the cardiovascular effects of passive exposure to e-cigarette vapors or on the health effects of secondhand exposure to e-cigarette vapors among vulnerable population, including children, pregnant women, and people with cardiovascular conditions.
There is some discrepancy between our findings and results reported recently by Flouris et al. (2013) on secondhand exposure to nicotine. Our data suggest that secondhand exposure to nicotine from e-cigarettes is on average 10 times less than from tobacco smoke. However, Flouris et al. (2013) found that e-cigarettes and tobacco cigarette generated similar effects on serum cotinine levels after 1-hr passive exposure (2.4±0.9 vs. 2.6±0.6ng/ml, respectively; p < .001). Future research should look for correlation between indoor air levels of nicotine from e-cigarettes and its uptake by passive smokers to explain this discrepancy.
Future research should also study exposure patterns over extended periods of time and the potential health effects of long-term exposure to secondhand e-cigarette vapors. Data are also needed from the field studies conducted in homes and public places where e-cigarettes are in use. Moreover, this study only focused on nicotine and a limited number of chemicals released from e-cigarettes. Further research is needed to explore emission and exposure to other toxicants and carcinogens identified in e-cigarettes, for example, carbonyl compounds (Goniewicz, Knysak, et al., 2013).
It remains unclear whether concentration of PM2.5 will be a suitable and reliable airborne marker to evaluate emission and exposure to secondhand vapors from e-cigarettes. Although some studies suggest that e-cigarette vapor and SHS have comparable aerosol particle size distribution and deposition patterns, we found that concentration of e-cigarette aerosol particles tends to decrease rapidly when diluted in the air. Figure 3 shows that there is a significant particle mass signal from e-cigarette vapor but that it dissipates much more rapidly than cigarette smoke. This may be due to the evaporation of the aerosol in addition to deposition on the surfaces and removal by ventilation. There is a need for developing an accurate methodology to assess e-cigarette vapor indoor concentrations. Finally, the vapor from e-cigarettes might be easily deposited on surfaces to form “thirdhand” e-cigarette vapor, and studies are needed to assess the deposition rate, potential formation of toxic derivatives, and human exposure.
Implications for Policy Makers
The study showed that e-cigarettes might involuntarily expose nonsmokers and people who do not use e-cigarettes to nicotine. In the past, secondhand exposure to nicotine has been primarily associated with exposure to ETS. E-cigarettes have created the new scenario under which bystanders might be exposed to low levels of nicotine but not to the other toxins found in tobacco smoke. It remains unclear whether exposure to low levels of nicotine indoors causes any harm to bystanders, including children, pregnant women, and person with cardiovascular conditions.
Besides nicotine, e-cigarette vapor contains significant amounts of propylene glycol and vegetable glycerin. Although both compounds are considered to be safe, there is lack of data on health risk associated with prolonged exposure to their vapors. Propylene glycol has been shown to cause upper airway irritation (Vardavas et al., 2011). Some volatile carbonyl compounds have been also identified in the vapor of e-cigarettes (Goniewicz, Knysak, et al., 2013). More research is needed about the health risk associated with exposure to toxic constituents of the vapors. The physicochemical changes may also occur after vapors are released into ambient air. It has been shown that such changes increase toxicity of tobacco smoke two- to four-fold (Schick & Glantz, 2006). These data are needed to inform regulators whether e-cigarettes should be included under smoke-free policies to protect nonusers from inhaling the toxicants.
E-cigarettes are promoted to circumvent smoke-free policies (Grana & Ling, 2013). Exempting e-cigarettes from smoke-free regulations, besides creating secondhand exposure to nicotine, might have additional implications for public health. It remains unclear whether observation of smokers using e-cigarettes, especially by young people, might reverse the denormalization of smoking behavior as a social norm. Cigarette smokers might use e-cigarettes as additional sources of nicotine in places with smoking bans. Data are needed to determine whether dual use of the products (e-cigarettes in addition to tobacco cigarettes) results in reinforcement of nicotine addiction.
SUPPLEMENTARY MATERIAL
Supplementary Material can be found online at http://www.ntr.oxfordjournals.org
FUNDING
This work was supported by the Ministry of Science and Higher Education of Poland (N N404 016939). The study sponsor had no involvement in the study design, collection, analysis, and interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication.
DECLARATION OF INTERESTS
MLG received research funding from Pfizer, manufacturer of stop smoking medication, and was funded by the UK Centre for Tobacco Control Studies (UKCTCS) during the study. AS received research funds and travel expenses from Chic Group Ltd., manufacturer of electronic cigarettes in Poland. Other authors declare no conflicts of interest.
Supplementary Material
REFERENCES
- Bullen C., McRobbie H., Thornley S., Glover M., Lin R., Laugesen M. (2010). Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomised cross-over trial. Tobacco Control, 19, 98–103. 10.1136/tc.2009.031567 [DOI] [PubMed] [Google Scholar]
- California Environmental Protection Agency. Air Resources Board. (2005). Proposed identification of environmental tobacco smoke as a toxic air contaminant. Part A. Exposure assessment. Sacramento, CA: California Environmental Protection Agency. [Google Scholar]
- Czogala J., Goniewicz M. L. (2005). The complex analytical method for assessment of passive smokers’ exposure to carbon monoxide. Journal of Analytical Toxicology, 29, 830–834. 10.1093/jat/29.8.830 [DOI] [PubMed] [Google Scholar]
- Dawkins L., Corcoran O. (2013). Acute electronic cigarette use: Nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl). Advance online publication. 10.1007/s00213-013-3249-8 [DOI] [PubMed] [Google Scholar]
- Etter J. F., Bullen C. (2011). Saliva cotinine levels in users of electronic cigarettes. European Respiratory Journal, 38, 1219–1220. 10.1183/09031936.00066011 [DOI] [PubMed] [Google Scholar]
- Flouris A. D., Chorti M. S., Poulianiti K. P., Jamurtas A. Z., Kostikas K., Tzatzarakis M. N., Koutedakis Y. (2013). Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation Toxicology, 25, 91–101. 10.3109/08958378.2012.758197 [DOI] [PubMed] [Google Scholar]
- Flouris A. D., Poulianiti K. P., Chorti M. S., Jamurtas A. Z., Kouretas D., Owolabi E. O., Koutedakis Y. (2012). Accute effects of electronic and tobacco cigarette smoking on complete blood count. Food and Chemical Toxicology, 50, 3600–3603. 10.1016/j.fct.2012.07.025 [DOI] [PubMed] [Google Scholar]
- Goniewicz M. L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J., Benowitz N. (2013). Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control. Advance online publication. 10.1136/tobaccocontrol-2012-050859a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz M. L., Kuma T., Gawron M., Knysak J., Kosmider L. (2013). Nicotine levels in electronic cigarettes. Nicotine & Tobacco Research, 15, 158–166. 10.1093/ntr/nts103 [DOI] [PubMed] [Google Scholar]
- Grana R. A., Ling P. M. (2013). Electronic cigarettes marketing: A global tobacco control challenge. 2013 International Meeting of Society for Research on Nicotine and Tobacco, Boston, MA, March 13–16, 2013. PA17-4. [Google Scholar]
- Jiang R. T., Acevedo-Bolton V., Cheng K. C., Klepeis N. E., Ott W. R., Hildemann L. M. (2011). Determination of response of real-time SidePak AM510 monitor to secondhand smoke, other common indoor aerosols, and outdoor aerosol. Journal of Environmental Monitoring, 13, 1695–1702. 10.1039/c0em00732c [DOI] [PubMed] [Google Scholar]
- Klepeis N. E., Ott W. R., Switzer P. (2007). Real-time measurement of outdoor tobacco smoke particles. Journal of the Air & Waste Management Association, 57, 522–534. 10.3155/1047-3289.57.5.522 [DOI] [PubMed] [Google Scholar]
- McAuley T. R., Hopke P. K., Zhao J., Babaian S. (2012). Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhalation Toxicology, 24, 850–857. 10.3109/08958378.2012.724728 [DOI] [PubMed] [Google Scholar]
- National Institute of Occupational Safety and Health. (2003). Method 2551. Issue 1. Nicotine. Retrieved May 10, 2013, from www.cdc.gov/niosh/docs/2003-154/pdfs/2551.pdf
- Nelson E. (2001). The miseries of passive smoking. Human & Experimental Toxicology, 20, 61–83. 10.1191/096032701670538508 [DOI] [PubMed] [Google Scholar]
- Oberg M., Jaakkola M. S., Woodward A., Peruga A., Prüss-Ustün A. (2011). Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet, 377, 139–146. 10.1016/S0140-6736(10)61388-8 [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Hazards Agency. (2000). Method no. 07. Organic vapors Retrieved May 10, 2013, from www.osha.gov/dts/sltc/methods/organic/org007/org007.html
- Schick S., Glantz S. A. (2006). Sidestream cigarette smoke toxicity increases with aging and exposure duration. Tobacco Control, 15, 424–429. 10.1136/tc.2006.016162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schripp T., Markewitz D., Uhde E., Salthammer T. (2013). Does e-cigarette consumption cause passive vaping? Indoor Air, 23, 25–31. 10.1111/j.1600-0668.2012.00792.x [DOI] [PubMed] [Google Scholar]
- Trehy M. L., Ye W., Hadwiger M. E., Moore T. W., Allgire J. F., Woodruff J. T., Westenberger B. J. (2011). Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. Journal of Liquid Chromatography & Related Technologies, 34, 1442–1458. 10.1080/10826076.2011.572213 [Google Scholar]
- Vansickel A., Cobb C., Weaver M. F., Eissenberg T. E. (2010). A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology Biomarkers and Prevention, 19, 1945–1953. 10.1158/1055-9965.EPI-10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel A. R., Eissenberg T. (2013). Electronic cigarettes: Effective nicotine delivery after acute administration. Nicotine & Tobacco Research, 15, 267–70. 10.1093/ntr/ntr316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas C. I., Anagnostopoulos N., Kougias M., Evangelopoulou V., Connolly G. N., Behrakis P. K. (2011). Acute pulmonary effects of using an e-cigarette: Impact on respiratory flow resistance, impedance and exhaled nitric oxide. Chest, 141, 1400–1406. 10.1378/chest.11-2443 [DOI] [PubMed] [Google Scholar]
- Wallace-Bell M. (2003). The effects of passive smoking on adult and child health. Professional Nurse, 19, 217–219. [PubMed] [Google Scholar]
- Williams M., Villarreal A., Bozhilov K., Lin S., Talbot P. (2013). Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One, 8, e57987. 10.1371/journal.pone.0057987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Shendell D. G., Winer A. M., Zhang J. (2010). Residential air exchange rates in three major US metropolitan areas: Results from the Relationship Among Indoor, Outdoor, and Personal Air Study 1999–2001. Indoor Air, 20, 85–90. 10.1111/j.1600-0668. 2009.00622.x [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sumner W., Chen D. R. (2013). In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine & Tobacco Research, 15, 501–508. 10.1093/ntr/nts16 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.