Abstract
Antibodies against reduction modifiable protein (anti-Rmp Abs) can block complement-dependent killing of Neisseria gonorrhoeae by otherwise bactericidal Abs. An anti-lipooligosaccharide bactericidal monoclonal Ab (mAb) 2C7, a gonococcal vaccine candidate Ab, attenuates vaginal colonization by gonococci in BALB/c mice. Here we show that anti-Rmp Abs block the efficacy of mAb 2C7 in mice in a dose-dependent manner. Anti-Rmp Abs also counteract 2C7-mediated enhancement of C3 deposition on gonococci in vivo. The mouse model will prove useful to study how blocking Abs influence the efficacy of gonococcal vaccines. Preexisting anti-Rmp Abs will be an important consideration in evaluating the efficacy of gonococcal vaccine candidates.
Keywords: Neisseria gonorrhoeae, vaccine, blocking antibody
The emergence of multidrug-resistant Neisseria gonorrhoeae in different parts of the world raises the possibility that we may soon witness an era of untreatable gonorrhea [1]. There is an urgent need to develop safe and effective vaccines against gonorrhea. Development of antigonococcal vaccines has been challenging for several reasons, including antigenic and phase variability of surface molecules that are targets for antibodies (Abs) that either are bactericidal or block critical functions, such as adhesion or invasion [2]. Further, the correlates of protection against gonorrhea are not well defined. We showed recently that Abs directed against a lipooligosaccharide (LOS) epitope defined by reactivity with monoclonal Ab (mAb) 2C7 (and therefore referred to as the 2C7 epitope) decreased the duration of infection and bacterial burden over time in the mouse vaginal colonization model of gonorrhea [3] and represents a promising gonococcal vaccine candidate.
Abs directed against certain bacterial targets that can block killing by other Abs that are bactericidal are commonly referred to as “blocking” Abs. A well-characterized target for blocking Abs against N. gonorrhoeae is reduction modifiable protein (Rmp; also referred to as protein III) [4]. Depletion of anti-Rmp Ab from the serum of an individual convalescing from disseminated gonococcal infection restored complement-dependent killing of the infecting strain [5]. Murine anti-Rmp mAbs block killing of N. gonorrhoeae by anti-PorB and anti-LOS mAbs [6]. Female commercial sex workers in Nairobi, Kenya, who possessed anti-Rmp Ab in their sera were 3.4-fold (adjusted odds ratio) more likely than women without anti-Rmp Ab to contract gonorrhea from their male sex partners [7]. These data all suggest that blocking Abs are important considerations in vaccine development because they may mitigate the efficacy of vaccine Ab. We sought to determine whether anti-Rmp Ab affected the ability of mAb 2C7 to attenuate experimental gonococcal vaginal colonization of mice.
MATERIALS AND METHODS
Ethics Statement
Phlebotomy of normal human volunteers was approved by the University of Massachusetts Medical School (UMMS) Institutional Review Board. Use of animals in this study was in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and was approved by the Institutional Animal Care and Use Committee at the UMMS.
N. gonorrhoeae Strains
Strains FA1090 (PorB.1B) and 15253 (PorB.1A) are both susceptible to the bactericidal activity of mAb 2C7 [8].
mAb
mAb 2C7 (murine isotype immunoglobulin G 3λ [IgG3λ]) [9] was affinity purified as described previously [3].
Expression of Recombinant Rmp (rRmp) and Purification and Characterization of Anti-Rmp Ab
Detailed methods are provided in Supplementary Figure 1.
Immunization of Mice
Female BALB/c mice (5–6 weeks old) were immunized with 10 µg of rRmp in 200 µL of normal saline emulsified with 5 µL (molecular weight, 78.00 g/mol) of alum (aluminum hydroxide gel; catalog number A8222, Sigma) intraperitoneally, followed by booster doses at 3 and 6 weeks. Immune Abs were characterized by Western blot and quantitated by enzyme-linked immunosorbent assay (ELISA).
Normal Human Serum
Normal human sera obtained from 13 healthy adult human volunteers were equally distributed into a pool, aliquoted, and stored at −70°C.
Serum Bactericidal Assays
Serum bactericidal assays were performed as previously described [8]. The final concentration of normal human sera used was 16.7%.
Mouse Challenge Studies
A schematic that illustrates the time line for procedures used initially for immunization with rRmp and then challenge with N. gonorrhoeae in the 17β-estradiol–treated mouse vaginal colonization model [10] is shown in Supplementary Figure 2. In passive transfer experiments, mice were administered anti-rRmp Ab intraperitoneally at a dose of 10 µg twice a day for 3 consecutive days, starting 2 days prior to infection.
Measurement of Serum and Vaginal Ab Concentrations
Serum anti-Rmp Ab concentrations and vaginal levels of anti-Rmp Ab and mAb 2C7 were measured by ELISA as described previously [3]. rRmp (1 µg/mL) or strain 15253 LOS (80 µg/mL) was used to coat microtiter wells.
Measurement of IgG and C3 Deposition on N. gonorrhoeae Recovered From Mouse Vaginal Secretions
Vaginal secretions from all mice from each group on each day were pooled to provide adequate numbers of bacteria for analysis. Bacteria were captured on microtiter wells, using a rabbit polyclonal Ab against neisserial lipoprotein H.8. Levels of C3 deposited on and IgG bound to captured bacteria were measured using goat anti-mouse C3 conjugated to horseradish peroxidase and goat anti-mouse IgG conjugated to alkaline phosphatase, respectively. The number of bacteria captured was determined with anti-H.8 mAb 2-8C-4-1. Detailed methods for this assay are provided in the Supplementary Materials.
Statistical Analyses
Experiments that compared clearance of N. gonorrhoeae in independent groups of mice estimated and tested 3 characteristics of the data: time to clearance (represented by Kaplan–Meier survival curves), longitudinal trends in the mean log10 number colony-forming units (CFU), and the cumulative number of CFU, expressed as the area under the curve (AUC), as described previously [3]. Correlations were performed with Spearman correlation tests.
RESULTS
Characterization of Blocking Activity of Polyclonal Mouse Anti-Rmp Ab Elicited by Immunization With rRmp
By week 7, the median anti-Rmp Ab level in the sera of mice immunized with rRmp was 43.14 µg/mL (range, 24.77–74.90 µg/mL). The specificity of anti-Rmp Ab was confirmed by Western blot (Supplementary Figure 1). This affinity-isolated polyclonal anti-Rmp Ab could block the baseline bactericidal activity of mAb 2C7 against strains FA1090 and 15253 in a dose-dependent manner (Supplementary Figure 3).
Active Immunization With rRmp and Passive Transfer of Anti-Rmp Ab Decreases the Efficacy of mAb 2C7 in the Mouse Vaginal Colonization Model
Prior to examining blocking of 2C7 function by anti-Rmp Ab in vivo, we first confirmed that passive transfer of mAb 2C7 reduced both the duration of infection (median times to clearance, 10 days for naive mice vs 5 days 2C7-treated mice; P = .018) and the burden of N. gonorrhoeae strain FA1090 infection throughout the period of infection, as measured by AUC analysis (P < .001), which was consistent with our previous work [3].
Two approaches were used to examine the effects of anti-Rmp Ab in vivo. One group of 10 mice was given anti-Rmp Ab (passive transfer), while a second group of 20 mice was actively immunized with rRmp; both groups were then administered mAb 2C7 and challenged with strain FA1090. The mAb 2C7–mediated clearance control for this experiment was a group of 15 naive mice that were given mAb 2C7 and challenged with strain FA1090 (no anti-Rmp Ab was present).
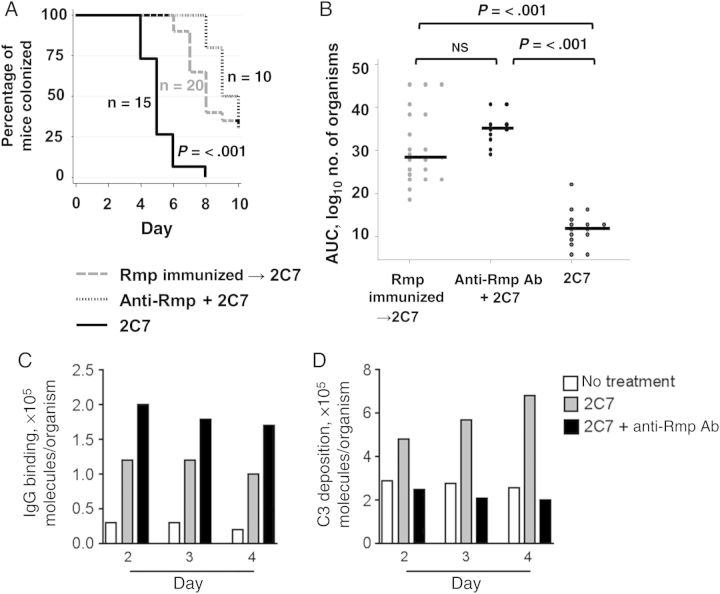
Anti-Rmp Ab, either passively transferred or elicited following immunization with rRmp, significantly delayed the clearance of bacteria by mAb 2C7 (median times to clearance, 5 days for animals without anti-Rmp Ab vs 9 days for animals that received anti-Rmp Ab and 8 days for animals immunized with rRmp; P < .001 for both comparisons; Figure 1A). Anti-Rmp Ab in both passive transfer and actively immunized groups was also associated with increased infectious loads (calculated as the mean log10 number of CFU throughout the period of infection) over time (P < .0001; data not shown) and cumulative number of CFU (expressed as the AUC; Figure 1B).
Figure 1.
The presence of antibodies against reduction modifiable protein (anti-Rmp Ab) decreases the efficacy of monoclonal Ab (mAb) 2C7 in clearing Neisseria gonorrhoeae in the mouse vaginal colonization model of gonorrhea. The ability of mAb 2C7 to clear FA1090 infection was examined in the following 3 groups of mice: (1) mice actively immunized with rRmp (n = 20; gray dashed line), (2) mice given polyclonal mouse anti-Rmp Abs intraperitoneally at a dose of 20 µg (10 µg twice daily) for 3 consecutive days (dotted line), and (3) naive mice (black solid line). A, Kaplan–Meier curves indicating time to clearance of FA1090. B, The mean area under the curve (AUC; log10 number of colony-forming units [CFU]) versus time was computed for each mouse; the AUC was compared among groups. C and D, Immunoglobulin G (IgG) binding to (C) and C3 deposition (D) on N. gonorrhoeae recovered directly ex vivo. Secretions from all mice from each group on each day were pooled to provide adequate numbers of bacteria for C3 and IgG measurements. Abbreviation: NS, not significant.
Anti-Rmp Ab Blocks C3 Deposition on Bacteria In Vivo Despite Increased Total IgG Binding
As shown in Figure 1C and as expected, gonococci recovered from mice treated with mAb 2C7 and anti-Rmp Ab were coated with more IgG (representing the sum total of 2C7, anti-Rmp, and endogenous mouse IgG bound to bacteria) than mice given 2C7 alone or untreated mice. Paradoxically (and consistent with the blocking effect of anti-Rmp Ab), the presence of anti-Rmp Ab reduced the number of C3 molecules/organism recovered directly ex vivo to levels similar to that seen in untreated mice (Figure 1D).
Anti-Rmp Ab Levels Correlate Inversely With mAb 2C7 Efficacy
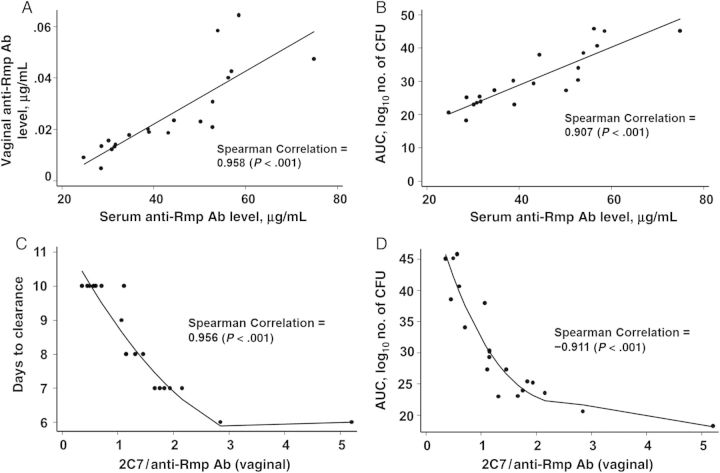
The heterogeneity in anti-Rmp Ab responses among the mice actively immunized with rRmp allowed us to correlate anti-Rmp Ab levels with clearance of bacteria by 2C7. Local concentration of Ab is an important determinant of its activity at mucosal surfaces. Vaginal and serum levels of anti-Rmp Ab correlated positively (Figure 2A). Serum anti-Rmp Ab levels correlated positively with the time to clearance of bacteria (Spearman correlation, 0.947; P < .001; data not shown) and the AUC (an indicator of cumulative number of CFU) for each mouse (Figure 2B). The importance of the relative concentrations of killing to blocking Ab at the local site of infection is illustrated in Figure 2C and 2D, where the ratio of vaginal 2C7 to anti-Rmp Ab correlated inversely with the time to clearance of infection and the AUC, respectively.
Figure 2.
Antibodies against reduction modifiable protein (anti-Rmp Ab) inhibit clearance of Neisseria gonorrhoeae by monoclonal Ab (mAb) 2C7 in mice in a dose-dependent manner: A, Vaginal anti-Rmp Ab levels correlate directly with serum anti-Rmp Ab levels in actively immunized mice. B, Serum anti-Rmp Ab levels correlate directly with mean areas under the curve (AUCs; log10 number of CFU). C and D, The ratio of mAb 2C7 to Rmp in vaginal secretions correlates inversely with the duration of colonization (C) and the mean AUC (D). The best-fitting line was applied to points in each graph. Results of Spearman correlation are indicated. Abbreviation: CFU, colony-forming units.
DISCUSSION
In this study, we provide in vivo evidence that anti-Rmp Ab can reduce the efficacy of a mAb directed against the 2C7 LOS epitope that is currently being developed as a vaccine candidate. Importantly, blocking is stoichiometric: levels of serum anti-Rmp Ab correlated directly with duration and burden of infection. The relative concentrations of protective (mAb 2C7) versus subversive (anti-Rmp) Ab at the local site of infection correlated inversely with the duration and burden of disease. Our data in mice, if they were extrapolated to humans, suggest that individuals who possess anti-Rmp Ab would likely require higher concentrations of protective Ab, such as anti-LOS Ab, to overcome the barrier against protection posed by blocking Ab. For gonococcal vaccines dependent on protective antibody, the demonstration of blocking in vivo underscores the need to consider that preexisting anti-Rmp Ab may be present, which may influence vaccine efficacy.
It is not clear whether complement-dependent killing (which predicts meningococcal vaccine efficacy [11]), opsonophagocytosis, or both are required for Ab-dependent clearance of gonococci in vivo. Abs that promote complement-dependent killing through insertion of the membrane attack complex are also likely to promote opsonophagocytosis because they enhance C3 fragment deposition and also engage Fc receptors. How blocking Ab prolongs infection in vivo may become evident once the correlates of protection against gonorrhea are defined. Nonetheless, our studies with protective anti-LOS Ab and subversive anti-Rmp Ab, used together, suggest that serum bactericidal activity correlates with vaccine Ab efficacy (or lack thereof).
Prior in vitro studies suggested that blocking human IgG (the IgG fraction of human serum that contained anti-Rmp Ab) diverted C3 onto so-called nonbactericidal sites on the surface of gonococci [4], which may have led to ineffectual C5b-9 insertion. Paradoxically, blocking human IgG increased the amounts of C3 and C9 that associated with gonococci [4], which suggested that effete complement activation occurs in the presence of anti-Rmp Ab. By contrast, our data here show that the presence of affinity-purified murine anti-Rmp Ab counteracts the increase in C3 deposition mediated by mAb 2C7 in vivo in mice. The effects of anti-Rmp Ab on deposition of terminal complement components in vivo remains to be determined.
As early as 1894, Pfeiffer and Issaeff reported that animals given an excess of immune serum were sometimes more susceptible than naive animals to challenge organisms [12]. Subsequently, blocking in vitro has been described for N. meningitidis, Pseudomonas aeruginosa, Brucella species, and nontyphoidal Salmonella [13, 14]. Blocking may occur though different mechanisms. As an example, blocking Ab directed against the H.8 lipoprotein on meningococci limits complement activation and requires Fc glycan [15]; in contrast, blocking in vitro by gonococcal human anti-Rmp Ab requires F(ab)2 and promotes complement activation [4].
In conclusion, this study highlights the use of the BALB/c mouse model of gonococcal infection to examine the effects of blocking Ab upon the efficacy of a bactericidal vaccine Ab. This model will prove useful in predicting the efficacy of bactericidal Ab elicited by gonococcal vaccine in humans.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Nancy Nowak, for excellent technical assistance.
Financial support. This work was supported by the National Institutes of Allergy and Infectious Diseases, National Institutes of Health (grants AI084048 and AI032725 [to P. A. R.] and AI111728 and AI118161 [to S. R.]).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med 2012; 366:485–7. [DOI] [PubMed] [Google Scholar]
- 2.Jerse AE, Bash MC, Russell MW. Vaccines against gonorrhea: current status and future challenges. Vaccine 2014; 32:1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulati S, Zheng B, Reed GW, et al. Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection. PLoS Pathog 2013; 9:e1003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joiner KA, Scales R, Warren KA, Frank MM, Rice PA. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest 1985; 76:1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice PA, Vayo H, Tam M, Blake MS. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med 1986; 164:1735–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virji M, Heckels JE. Nonbactericidal antibodies against Neisseria gonorrhoeae: Evaluation of their blocking effect on bactericidal antibodies directed against outer membrane antigens. J Gen Microbiol 1988; 134:2703–11. [DOI] [PubMed] [Google Scholar]
- 7.Plummer FA, Chubb H, Simonsen JN, et al. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J Clin Invest 1993; 91:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulati S, Agarwal S, Vasudhev S, Rice PA, Ram S. Properdin is critical for antibody-dependent bactericidal activity against Neisseria gonorrhoeae that recruit C4b-binding protein. J Immunol 2012; 188:3416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati S, McQuillen DP, Mandrell RE, Jani DB, Rice PA. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids [published erratum appears in J Infect Dis 1997 Apr;175(4):1027]. J Infect Dis 1996; 174:1223–37. [DOI] [PubMed] [Google Scholar]
- 10.Jerse AE, Wu H, Packiam M, Vonck RA, Begum AA, Garvin LE. Estradiol-Treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front Microbiol 2011; 2:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969; 129:1307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeiffer F, Issaeff R. Uber die Specifishche der Bedeutung der Choleraimmunitat. Z Hyg Infecktionskr 1894; 17:355. [Google Scholar]
- 13.Lewis LA, Ram S. Meningococcal disease and the complement system. Virulence 2014; 5:98–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev 2010; 23:740–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray TD, Lewis LA, Gulati S, Rice PA, Ram S. Novel blocking human IgG directed against the pentapeptide repeat motifs of Neisseria meningitidis Lip/H.8 and Laz lipoproteins. J Immunol 2011; 186:4881–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.