Abstract
Background. Although human immunodeficiency virus (HIV)–negative men having sex with men (MSM) bear a substantial burden of human papillomavirus (HPV)–associated disease, prospective studies of genital HPV infection in this population are scarce.
Methods. HPV genotyping was conducted on genital samples from men (aged 18–70 years) from Brazil, Mexico, or the United States who provided specimens at 6-month intervals for up to 4 years. Eligibility criteria included no history of genital warts or HIV infection. Evaluable specimens were collected from 564 MSM and 3029 men having sex with women (MSW). Incidence and clearance estimates with 95% confidence intervals were calculated.
Results. The 12-month cumulative incidence of genital HPV was high in both MSM (25%; 95% confidence interval, 21%–30%) and MSW (21%; 20%–23%). After stratifying by city, MSM and MSW incidence rates were comparable, with 3 exceptions where MSM had higher incidence in ≥1 city: the group of quadrivalent vaccine types, HPV-45, and HPV-11. Median times to HPV-16 clearance were also comparable, with point estimates of >6 months for both MSM and MSW.
Conclusions. Unlike with many other sexually transmitted infections, genital HPV natural history may be similar in HIV-negative MSM and MSW. Study periods of ≤6 months, however, may not be long enough to accurately measure the persistence of these infections in men.
Keywords: HPV, men, incidence, clearance, duration, epidemiology, MSM, bisexual men, heterosexual men
The incidence of genital warts has been increasing in many western countries since the 1970s [1]. Sexually transmitted human papillomavirus (HPV) infection, usually with types 6 or 11, causes these condylomas, which incur significant morbid effects and substantial economic costs [2, 3]. In addition, genital HPV-16 infection is a major cause of penile squamous cell carcinoma, which, though rare, has either a declining, stable, or increasing incidence in recent decades, depending on country [4–6]. Like cervical cancer, the incidence of penile carcinoma is higher in developing than in developed countries [7].
The burden of some HPV-associated anogenital diseases, but not all, is higher among men having sex with men (MSM) than among men having sex with women (MSW). For example, the incidence of anal cancer, almost all of which is caused by HPV, is many times higher in MSM than in MSW [8]. On the other hand, genital condylomas may have a similar prevalence among these populations [9].
Although anogenital HPV infection is thought to be the most common sexually transmitted infection (STI), there are few published data comparing genital HPV infection among human immunodeficiency virus (HIV)–negative MSM and MSW [10, 11]; however, there is a substantial amount of data from the US STI surveillance system indicating that MSM are more likely than MSW to report a prior diagnosis of certain STIs, such as HIV, syphilis, gonorrhea, human herpesvirus, and hepatitis B [12]. The prevalence of HPV infection in the anal canal is much higher in MSM than in MSW [13], but studies of genital HPV prevalence comparing MSM and MSW have found little difference or even a higher prevalence of genital HPV among MSW than among MSM [10, 14]. Among HIV-infected men, the prevalence of genital HPV is also higher in MSW than in MSM [15]. To our knowledge, however, no prospective studies have examined the incidence and clearance of genital HPV among HIV-negative MSW and MSM.
Our objective was to estimate and compare the incidence of genital HPV infections and the duration of incident infections among HIV-negative MSM and MSW. We also examined the incidence of HPV in MSM stratified into men having sex only with men (MSOM) and men having sex with both women and men (MSWM).
MATERIALS AND METHODS
Men were recruited in São Paulo, Brazil, Cuernavaca, Mexico, and Tampa, Florida from June 2005 to September 2009 for the prospective HPV Infection in Men (HIM) study. Inclusion criteria included an age of 18–70 years, no plans to relocate during the 4-year study, no self-reported history of penile or anal cancer or genital warts, and no current STI, including HIV infection. Additional details of the study design have been described elsewhere [16, 17].
Men were recruited in São Paulo from the general population through advertisements and from a genitourinary clinic that also tests for HIV and STIs. Men who went to the clinic because of STI symptoms or for treatment were excluded. In Cuernavaca, men were recruited through a health plan and from factories and the military. Men in Tampa were recruited from a university campus and the general public. MSM were not targeted for recruitment. All participants consented to the study and received a nominal incentive for participation. The study was approved by human subjects committees at each study site.
Study Protocol
A total of 4123 men enrolled in the HIM study. Follow-up occurred at 6-month intervals for a total of 4 years. A total of 72 men acknowledged HIV infection after enrollment and were removed from further analysis. Of the remaining 4051 men, 3661 (90.4%) returned for at least the first 6-month follow-up visit.
Men completed an 88-item computer-assisted self-interview at enrollment, written in the region's primary language (Portuguese, Spanish, or English). The interview elicited information about participant demographics, substance use, and sexual behaviors. At each follow-up visit, a similar self-interview elicited information about the participant's substance use and sexual behavior since the prior visit.
At each visit, a study clinician examined the men for STI symptoms, including warts and lesions (men found to have genital warts were retained in the study). For HPV sampling, the clinician used a saline-wetted swab to sweep 360° around the coronal sulcus and glans penis, and if present, a retracted prepuce. A second swab was used to sample the entire surface of the penile shaft, and a third was used to sample the scrotum. Finally, the clinician used a separate swab to sample the anal canal. Each swab was placed in a vial of transport media (STM; Qiagen) and stored at −80°C. For detection of genital HPV, swab samples from the coronal sulcus/glans, shaft, and scrotum were combined. First-catch urine and blood were collected to test for Chlamydia trachomatis (Chlamydia LCx [Abbott Laboratories] and COBAS Amplicor CT/NG Test [Roche Diagnostics]) and herpes simplex virus 2 antibodies (HerpeSelect 2 ELISA IgG; Focus Diagnostics), respectively, at the enrollment visit and annually thereafter.
HPV Analyses
Genital specimens were analyzed for HPV DNA as described elsewhere [16]. No anal specimens were included in the current analysis. Briefly, DNA was extracted using the QIAamp Media MDx Kit (Qiagen). The polymerase chain reaction consensus primer system (PGMY 09/11) was used to amplify a fragment of the HPV L1 gene [18]. HPV genotyping was conducted on all genital specimens, using DNA probes labeled with biotin to detect 36 HPV types (including subtypes) [19]. Accuracy and potential contamination were assessed using nontemplate negative controls and CaSki DNA-positive controls. Mean β-globin positivity at enrollment and all follow-up visits was >97%. Men must have had ≥2 β-globin–positive specimens to be included in the analysis. Of 3661 men, 2 men had only 1 β-globin positive specimen over all visits, and another 5 men were excluded based on age eligibility, leaving a total of 3654 men available for analysis.
Statistical Analyses
A specimen was considered positive for any genital HPV if it was positive for ≥1 of 36 genotypes. Specimens were labeled as high risk if ≥1 of 13 types were detected (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) [20], regardless of the presence of other genotypes. Similarly, specimens were labeled as low risk if any of the remaining 23 types in the linear array were detected, regardless of the presence of high-risk types; thus, incidence estimates for high- and low-risk groups overlap.
Men enrolled in the study were classified as MSM, MSW, or men having no sex [21] solely based on their answers to >20 questions about recent and lifetime penetrative sexual behavior (vaginal, anal, and oral sex). Recent sexual behavior was assessed by questions about behavior in the prior 3 or 6 months or since the prior study visit. A man was classified as MSW if he acknowledged sex with only women at all study visits. A man was classified as MSM if he acknowledged sex with men at any study visit. A man was classified as having no sex if he reported no sexual behavior during his lifetime. Of 3654 men, a total of 3029 (82.9%) participants were classified as MSW, 564 (15.4%) as MSM, and 60 (1.6%) as men having no sex, with 1 man classified as missing owing to incomplete survey results. Analyses were limited to men reporting sex; thus, the analysis included 3593 men. In a secondary analysis, we stratified the MSM group into MSOM during the lifetime (n = 93) and MSWM at baseline or during study follow-up visits (n = 471). A 2-sided χ2 test was used to assess differences in characteristics between MSM and MSW who returned for the first 6-month follow-up visit and those who did not return.
An incident infection was defined as the presence of a type-specific infection at any follow-up visit when that type was absent at enrollment. Person-months were calculated as the time from specimen collection at enrollment to the date of first HPV detection or last visit. The calculation of 95% confidence intervals (CIs) for the incidence rate was based on the number of incident events and followed a Poisson distribution. For incidence calculations, the unit of analysis was the person. Incidence rate ratios along with their 95% CIs were calculated for MSOM and MSWM.
The investigation of clearance used the infection as the unit of analysis. Incident HPV infections were used to determine type-specific clearance, which was defined as 2 consecutive HPV-negative results after testing positive for that type. Because a participant could have ≥1 infection, a robust sandwich estimator was used for the covariance matrix to account for within-subject correlation of infections [22].
The cumulative risk of genital HPV acquisition and clearance was estimated with the Kaplan–Meier method. To investigate potential heterogeneity by age and city for both MSM and MSW, stratified Kaplan–Meier curves were estimated for high-risk types, low-risk types, HPV-16, and HPV-6. The log-rank test was used to compare the difference in median time to clearance and cumulative incidence between groups. Data were analyzed using R (R Foundation for Statistical Computing) and SAS 9.3 (SAS Institute) software.
RESULTS
The median follow-up time for the men was 40.4 months. Among those who enrolled in the study, retention at 6 months was 95% and 90% for MSM and MSW, respectively. Among MSM, there were no differences between men who returned for the first 6-month follow-up visit and those who did not. MSW who did not return for the visit were more likely to be aged 18–30 years (13%) rather than 45–70 years (7%; P < .001) and more likely to be single, never married rather than married (P < .001). Nonreturning MSW were also more likely to be current smokers (15%) than former smokers (7%; P < .001), more likely to be from Tampa rather than São Paulo (P < .001), and more likely to be circumcised (P < .001) (data not shown).
Among men who returned for ≥1 follow-up visit, MSM and MSW differed on most characteristics, but not all (Table 1). For example, 43% of MSM were aged 18–30 years versus 48% of MSW (P = .01). A majority of MSM (62%) were from São Paulo; however, the median number of sexual partners (both male and female) was 1 for both MSM and MSW. In a subanalysis of MSM, similar proportions of MSOM and MSWM were from São Paulo (62% and 61%, respectively); however, 31% of MSWM and only 1% of MSOM reported a primary sexual relationship >5 years in duration (P < .001).
Table 1.
Enrollment Characteristics of Men Who Returned for the First 6-Month Follow-up Visit in São Paulo, Cuernavaca, and Tampa in the HIM Study, 2005–2012a
| Variable | MSM (n = 564) |
Total MSW (n = 3029)c | ||
|---|---|---|---|---|
| MSOM (n = 93)b | MSWM (n = 471)b | Totalc | ||
| Age, y | ||||
| 18–30 | 56 (60) | 189 (40) | 245 (43) | 1456 (48) |
| 31–44 | 33 (35) | 222 (47) | 255 (45) | 1167 (39) |
| 45–70 | 4 (4) | 60 (13) | 64 (11) | 406 (13) |
| Median (range) | 29 (18–55) | 33 (18–69) | 32 (18–69) | 31 (18–70) |
| City | ||||
| São Paulo | 58 (62) | 289 (61) | 347 (62) | 942 (31) |
| Cuernavaca | 13 (14) | 111 (24) | 124 (22) | 1064 (35) |
| Tampa | 22 (24) | 71 (15) | 93 (16) | 1023 (34) |
| Race | ||||
| White | 64 (69) | 245 (52) | 309 (55) | 1298 (43) |
| Black | 12 (13) | 94 (20) | 106 (19) | 446 (15) |
| Asian/Pacific Islander | 1 (1) | 2 (<1) | 3 (1) | 88 (3) |
| American Indian | 3 (3) | 13 (3) | 16 (3) | 56 (2) |
| Mestizo/other | 13 (14) | 108 (23) | 121 (21) | 1088 (36) |
| Declined to answer | 0 (0) | 9 (2) | 9 (2) | 53 (2) |
| Ethnicity | ||||
| Hispanic | 34 (37) | 198 (42) | 232 (41) | 1417 (47) |
| Non-Hispanic | 56 (60) | 267 (57) | 323 (57) | 1579 (52) |
| Declined to answer | 3 (3) | 6 (1) | 9 (2) | 33 (1) |
| Duration of primary sexual relationship, y | ||||
| No current relationship | 55 (59) | 137 (29) | 192 (34) | 611 (20) |
| <1 | 20 (22) | 82 (17) | 102 (18) | 523 (17) |
| 1–5 | 13 (14) | 84 (18) | 97 (17) | 678 (22) |
| >5 | 1 (1) | 148 (31) | 149 (26) | 989 (33) |
| Declined to answer | 4 (4) | 20 (4) | 24 (4) | 288 (8) |
| Circumcision status | ||||
| Prepuce present | 67 (72) | 346 (73) | 413 (73) | 1868 (62) |
| No prepuce present | 26 (28) | 125 (27) | 151 (27) | 1161 (38) |
| Cigarette smoking status | ||||
| Never smoker | 68 (73) | 235 (50) | 303 (54) | 1770 (58) |
| Former smoker | 6 (6) | 95 (20) | 101 (18) | 591 (20) |
| Current smoker | 19 (20) | 140 (30) | 159 (28) | 657 (22) |
| Declined to answer | 0 (0) | 1 (<1) | 1 (<1) | 11 (<1) |
| Alcoholic drinks in last 1 mo, No. | ||||
| 0–30 | 63 (68) | 319 (68) | 382 (68) | 2097 (69) |
| 31–60 | 10 (11) | 59 (13) | 69 (12) | 337 (11) |
| >60 | 14 (15) | 70 (15) | 84 (15) | 463 (15) |
| Declined to answer | 6 (6) | 23 (5) | 29 (5) | 132 (4) |
| Male anal sex partners in last 3 mo, No. | ||||
| 0 | 19 (20) | 307 (65) | 326 (58) | 2997 (99) |
| 1 | 27 (29) | 63 (13) | 90 (16) | 0 (0) |
| ≥2 | 41 (44) | 78 (17) | 119 (21) | 0 (0) |
| Declined to answer | 6 (6) | 23 (5) | 29 (5) | 32 (1) |
| Median; mean | 1; 2 | 0; 2 | 0; 2 | 0; 0 |
| Female sex partners in last 6 mo, No. | ||||
| 0 | 90 (97) | 181 (38) | 271 (48) | 768 (25) |
| 1 | 0 (0) | 128 (27) | 128 (23) | 1368 (45) |
| ≥2 | 0 (0) | 140 (30) | 140 (25) | 791 (26) |
| Declined to answer | 3 (3) | 22 (5) | 25 (4) | 102 (3) |
| Median; mean | 0; 0 | 1; 1 | 0; 1 | 1; 1 |
| Total male and/or female sex partners in last 6 mo, No. | ||||
| 0 | 19 (20) | 94 (20) | 113 (20) | 763 (25) |
| 1 | 27 (29) | 137 (29) | 164 (29) | 1357 (45) |
| ≥2 | 41 (44) | 206 (44) | 247 (44) | 787 (26) |
| Declined to answer | 6 (6) | 34 (7) | 40 (7) | 122 (4) |
| Median; mean | 1; 2 | 1; 3 | 1; 3 | 1; 1 |
| Insertive anal sex acts in last 6 mo, No.d | ||||
| 0 | 16 (17) | 129 (27) | 145 (26) | 1880 (62) |
| 1–5 | 11 (12) | 66 (14) | 77 (14) | 236 (8) |
| 6–10 | 5 (5) | 21 (4) | 26 (5) | 42 (1) |
| >10 | 12 (13) | 33 (7) | 45 (8) | 42 (1) |
| Declined to answer | 2 (2) | 8 (2) | 10 (2) | 31 (1) |
| Question not asked | 47 (51) | 214 (45) | 261 (46) | 798 (26) |
| Median; mean | 4; 9 | 0; 11 | 1; 11 | 0; 1 |
| Lifetime total male anal sex partners, No. | ||||
| 0–2 | 17 (18) | 249 (53) | 266 (47) | 2999 (99) |
| 3–9 | 24 (26) | 103 (22) | 127 (23) | 0 (0) |
| 10–19 | 16 (17) | 32 (7) | 48 (9) | 0 (0) |
| ≥20 | 28 (30) | 60 (13) | 88 (16) | 0 (0) |
| Declined to answer | 8 (9) | 27 (6) | 35 (6) | 30 (1) |
| Median; mean | 10; 43 | 2; 21 | 2; 25 | 0; 0 |
| Lifetime female sex partners, No. | ||||
| 0–2 | 87 (94) | 108 (23) | 195 (35) | 646 (21) |
| 3–9 | 0 (0) | 146 (31) | 146 (26) | 1044 (34) |
| 10–19 | 0 (0) | 78 (17) | 78 (14) | 533 (18) |
| ≥20 | 0 (0) | 101 (21) | 101 (18) | 640 (21) |
| Declined to answer | 6 (6) | 38 (8) | 44 (8) | 166 (5) |
| Median; mean | 0; 0 | 7; 17 | 5; 14 | 7; 16 |
| Anogenital warts (clinician report) | ||||
| Yes | 4 (4) | 21 (4) | 25 (4) | 168 (6) |
| No | 89 (96) | 450 (96) | 539 (96) | 2861 (94) |
| C. trachomatis infection | ||||
| Yes | 3 (3) | 4 (1) | 7 (1) | 51 (2) |
| No | 90 (97) | 466 (99) | 556 (99) | 2976 (98) |
| Response missing | 0 (0) | 1 (<1) | 1 (<1) | 2 (<1) |
| History of an HPV-vaccinated female sex partnerd,e | ||||
| Yes | 0 (0) | 44 (9) | 44 (8) | 455 (15) |
| No/don't know | 58 (62) | 274 (58) | 332 (59) | 1697 (56) |
| Declined to answer | 8 (9) | 8 (2) | 16 (1) | 65 (2) |
| Question not asked | 27 (29) | 145 (31) | 172 (31) | 812 (27) |
Abbreviations: C. trachomatis, Chlamydia trachomatis; HIM, HPV Infection in Men; HPV, human papillomavirus; MSM, men having sex with men; MSOM, men having sex only with men; MSW, men having sex with women; MSWM, men having sex with both women and men.
a Unless otherwise specified, data represent No. (%) of men.
b The χ2 P values for all variables by comparing MSOM and MSWM were <.05, except for race (P = .05), ethnicity (P = .40), circumcision (P = .78), alcoholic drinks in the last month (P = .91), total male and/or female sex partners in the last 6 months (P = .99), frequency of insertive anal sex in the last 6 months (P = .07), anogenital warts (P = .95), and C. trachomatis infection (P = .06). Responses that were refusals or missing were not included in hypothesis testing.
c The χ2 P values for all variables by comparing MSM and MSW were ≤.01, except for ethnicity (P = .02), alcoholic drinks in the last month (P = .71), anogenital warts (P = .28), and C. trachomatis infection (P = .45). Responses that were refusals or missing were not included in hypothesis testing.
d Data were collected in a subset of the full sample.
e Men's self-reports from all study visits. Data were collected in a subset of the full sample.
Within each city, the incidence rate per 1000 persons-months among MSM was modestly higher than that among MSW, although 95% CIs generally overlapped (Table 2). There were 3 exceptions in which higher incidence was observed among MSM than among MSW in ≥1 city: the group of quadrivalent vaccine types, HPV-45, and HPV-11. For example, in Cuernavaca, the incidence of quadrivalent HPV vaccine types was 9.1/1000 person-months (95% CI, 6.2–12.9) among MSM and 5.2/1000 person-months (4.5–6.1) among MSW. HPV-16 was one of the most commonly acquired types, with incidence ranging from a low of 2.2/1000 person-months (95% CI, 1.8–2.7) among MSW in Cuernavaca to a high of 6.3/1000 person-months (3.6–10.2) among MSM in Tampa.
Table 2.
Incidence Rates for Type-Specific Genital HPV Infection Among MSM and MSW by City in the HIM Study, 2005–2012a
| HPV Typec | Incidence Rate per 1000 Person-Months (95% CI)b |
|||||
|---|---|---|---|---|---|---|
| MSM (n = 564) |
MSW (n = 3029) |
|||||
| São Paulo (n = 347) | Cuernavaca (n = 124 | Tampa (n = 93) | São Paulo (n = 942) | Cuernavaca (n = 1064) | Tampa (n = 1023) | |
| Any HPV | 49.1 (40.6–58.9) | 26.9 (19.3–36.5) | 36.9 (26.1–50.6) | 37.7 (33.4–42.5) | 18.7(16.5–21.1) | 30.7 (27.6–34.1) |
| 6, 11, 16 or 18 | 11.4 (9.3–13.9) | 9.1 (6.2–12.9) | 12.5 (8.2–18.3) | 8.5 (7.4–9.7) | 5.2 (4.5–6.1) | 8.1 (7.0–9.2) |
| High risk | 22.2 (18.6–26.2) | 13.7 (9.6–18.9) | 20.2 (13.7–28.7) | 19.2 (17.2–21.5) | 10.9 (9.6–12.3) | 19.2 (17.2–21.3) |
| 16 | 4.4 (3.3–5.8) | 4.1 (2.5–6.4) | 6.3 (3.6–10.2) | 3.6 (2.9–4.3) | 2.2 (1.8–2.7) | 4.3 (3.6–5.1) |
| 18 | 2.3 (1.6–3.3) | 1.4 (.5–2.8) | 2.0 (.7–4.4) | 1.6 (1.2–2.1) | .6 (.4–.9) | 1.9 (1.5–2.4) |
| 31 | 1.1 (.6–1.7) | 1.3 (.5–2.8) | 1.3 (.4–3.3) | 1.2 (.9–1.6) | .8 (.6–1.1) | 1.4 (1.0–1.9) |
| 33 | .6 (.2–1.1) | .2 (.0–1.0) | 1.7 (.5–3.9) | .5 (.3–.8) | .1 (.0–.3) | .3 (.1–.5) |
| 39 | 1.5 (.9–2.3) | 2.0 (1.0–3.7) | 1.6 (.5–3.8) | 1.9 (1.5–2.4) | 1.5 (1.1–1.9) | 2.7 (2.2–3.4) |
| 45 | 2.9 (2.1–4.0) | 1.3 (.5–2.8) | 4.2 (2.2–7.3) | 1.3 (1.0–1.8) | .7 (.5–1.0) | 1.4 (1.0–1.9) |
| 51 | 3.9 (2.9–5.1) | 1.4 (.6–2.9) | 4.7 (2.0–8.0) | 4.2 (3.5–5.0) | 1.8 (1.4–2.3) | 4.7 (3.9–5.5) |
| 52 | 4.0 (3.0–5.3) | 1.9 (.8–3.5) | 1.7 (.6–4.0) | 2.6 (2.1–3.2) | 1.7 (1.3–2.1) | 2.6 (2.0–3.2) |
| 58 | 1.9 (1.2–2.8) | 1.4 (.5–2.8) | 1.0 (.2–2.9) | 1.8 (1.4–2.3) | .9 (.6–1.3) | 1.2 (.9–1.7) |
| 59 | 3.5 (2.6–4.7) | 2.7 (1.4–4.6) | 3.8 (1.8–6.9) | 2.4 (1.9–3.0) | 2.0 (1.6–2.5) | 3.7 (3.1–4.5) |
| Low risk | 38.1 (31.8–45.3) | 20.5 (15.0–27.5) | 22.6 (15.9–31.4) | 31.0 (27.7–34.6) | 14.4 (12.7–16.2) | 22.6 (20.3–25.1) |
| 6 | 4.3 (3.2–5.6) | 3.4 (2.0–5.6) | 5.0 (2.7–8.4) | 3.2 (2.6–3.8) | 2.1 (1.7–2.6) | 2.9 (2.4–3.6) |
| 11 | 1.4 (.9–2.2) | .8 (.2–2.0) | 2.1 (.8–4.5) | .8 (.6–1.2) | .6 (.4–.9) | .4 (.2–.7) |
| 44 | 2.5 (1.8–3.5) | 1.6 (.7–3.1) | 2.0 (.7–4.3) | 1.6 (1.2–2.0) | .5 (.3–.8) | 1.5 (1.1–2.0) |
| 53 | 4.5 (3.4–5.9) | 2.4 (1.3–4.3) | 1.6 (.5–3.8) | 3.9 (3.2–4.6) | 2.1 (1.6–2.6) | 3.3 (2.7–4.1) |
| 54 | 2.6 (1.8–3.5) | 2.9 (1.6–4.8) | 2.3 (.9–4.8) | 2.5 (2.0–3.0) | 1.0 (.7–1.4) | 2.5 (2.0–3.1) |
| 61 | 4.8 (3.7–6.2) | 2.5 (1.3–4.3) | 3.0 (1.4–5.8) | 3.5 (2.9–4.2) | 1.1 (.8–1.5) | 1.9 (1.5–2.5) |
| 62 | 5.2 (3.9–6.7) | 1.4 (.5–2.8) | 4.5 (2.4–7.8) | 4.6 (3.9–5.4) | 1.8 (1.4–2.3) | 3.8 (3.2–4.6) |
| 66 | 3.7 (2.7–4.9) | 2.3 (1.1–4.0) | 2.5 (1.0–5.1) | 3.2 (2.6–3.8) | 1.3 (1.0–1.7) | 4.4 (3.6–5.2) |
| 70 | 2.6 (1.8–3.6) | .4 (.0–1.4) | 1.0 (.2–2.8) | 1.8 (1.3–2.2) | .7 (.4–1.0) | .8 (.5–1.2) |
| 81 | 3.1 (2.2–4.2) | 1.2 (.4–2.6) | 1.6 (.5–3.8) | 1.9 (1.5–2.4) | 1.0 (.7–1.4) | .8 (.5–1.1) |
| 83 | 2.3 (1.5–3.2) | .8 (.2–2.0) | .6 (.1–2.3) | 1.5 (1.2–2.0) | .8 (.6–1.1) | 1.3 (.9–1.7) |
| 84 | 6.5 (5.1–8.2) | 3.8 (2.3–6.0) | 5.9 (3.4–9.5) | 4.0 (3.4–4.8) | 1.9 (1.5–2.4) | 5.6 (4.8–6.5) |
| 89 | 6.2 (4.8–7.8) | 2.2 (1.1–3.9) | 3.9 (1.9–6.9) | 3.7 (3.0–4.4) | 2.2 (1.7–2.7) | 4.3 (3.6–5.1) |
Abbreviations: CI, confidence interval; HIM, HPV Infection in Men; HPV, human papillomavirus; MSM, men having sex with men; MSW, men having sex with women.
a The unit of analysis was the individual person.
b The number of infections and person-months for each group and HPV genotype are included in Supplementary Table 1.
c HPV genotypes shown are those in the 9-valent vaccine and those with any incidence rate >2.2/1000 person-months.
The 12-month cumulative incidence of high-risk types among MSM was also comparable across cities (0.25; 95% CI, .21–.30 for all MSM; log-rank P = .08), whereas MSM in São Paulo acquired low-risk types more quickly than in Tampa or Cuernavaca (Figure 1). There were statistically significant differences in acquisition by city for groups of high- and low-risk types. Most notably, for low-risk types among MSW, the 12-month cumulative incidence rates were 39%, 21%, and 27% for São Paulo, Cuernavaca, and Tampa, respectively (P < .001). There was no difference in acquisition of HPV-16 and HPV-6 by city among MSM (Supplementary Figure 1). Within the larger sample of MSW, point estimates for the acquisition of HPV-16 and HPV-6 were almost identical. For example, for HPV-16, the 12-month cumulative incidence rates were 5%, 4%, and 5% for São Paulo, Cuernavaca, and Tampa, respectively, and the respective incidence rates for HPV-6 were 5%, 4%, and 4%.
Figure 1.
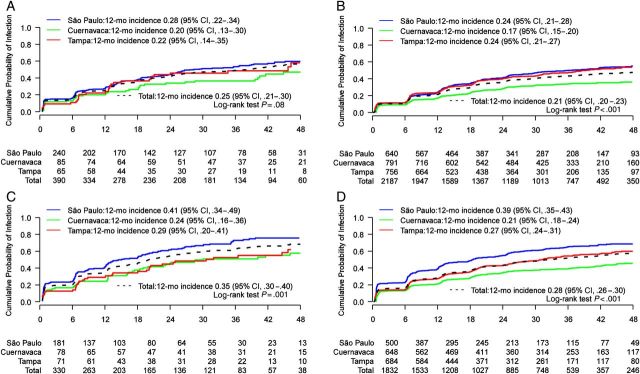
Cumulative incidence by city of genital human papillomavirus with number of men at risk at each 6-month study visit. A, Men having sex with men (MSM): high-risk types. B, Men having sex with women (MSW): high-risk types. C, MSM: low-risk types. D, MSW: low-risk types. Values on the x-axis denote months of follow-up. Abbreviation: CI, confidence interval.
Little difference in the 12-month cumulative incidence was observed by age among MSM or MSW (Figure 2). Point estimates for HPV-16 and HPV-6 among MSW, though significantly different, were comparable (eg, 6%, 4%, and 5% for HPV-16 among men aged 18–30, 31–44, and 45–70 years, respectively). There was no difference in the 12-month cumulative incidence rates for high- and low-risk HPV groups by age among MSM or MSW (Supplementary Figure 2).
Figure 2.
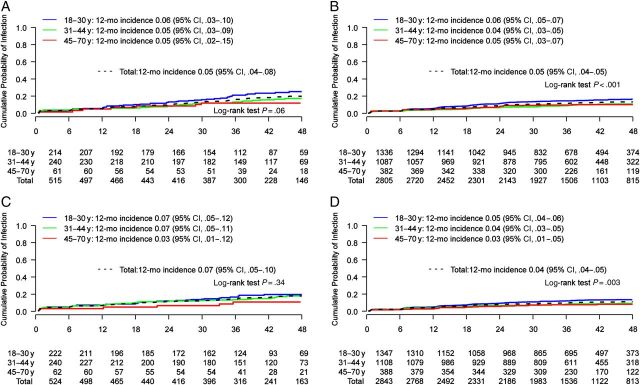
Cumulative incidence by age of genital human papillomavirus (HPV) with number of men at risk at each 6-month study visit. A, Men having sex with men (MSM): HPV 16. B, Men having sex with women (MSW): HPV 16. C, MSM: HPV 6. D, MSW: HPV 6. Values on the x-axis denote months of follow-up. Abbreviation: CI, confidence interval.
There were no notable differences between MSM and MSW in the median months to clearance after stratifying by city (Table 3). For example, the median duration to clearance among São Paulo men for HPV-16 genital HPV was 6.1 months (95% CI, 5.9–7.8) among MSM and 6.4 months (95% CI, 6.0–7.4) among MSW. Clearance rates for HPV-6 were also comparable among MSM and MSW in each city. When we stratified MSM by MSOM and MSWM, the incidence rates in both groups were generally comparable with incidence rate ratios indicating a higher incidence for MSOM only for the groups of any HPV and high-risk HPV and the single type HPV-45 (Table 4). The incidence was higher among MSWM than among MSOM only for HPV-44.
Table 3.
Median Months to Clearance for Incident Type-Specific Genital HPV Infection Among MSM and MSW by City in the HIM Study, 2005–2012a
| HPV typec | Duration to Clearance, Median (95% CI), mob |
|||||
|---|---|---|---|---|---|---|
| MSM (n = 564) |
MSW (n = 3029) |
|||||
| São Paulo (n = 347) | Cuernavaca (n = 124) | Tampa (n = 93) | São Paulo (n = 942) | Cuernavaca (n = 1064) | Tampa (n = 1023) | |
| Any HPV | 7.1 (6.7–7.6) | 9.4 (7.6–11.8) | 6.3 (6.2–6.7) | 7.8 (7.3–8.1) | 11.3 (9.4–12.0) | 6.7 (6.6–7.0) |
| 6, 11, 16 or 18 | 6.4 (6.1–7.2) | 9.8 (6.2–14.4) | 8.0 (6.4–12.0) | 6.9 (6.4–7.8) | 7.9 (6.4–11.3) | 7.0 (6.5–8.3) |
| High risk | 6.9 (6.6–8.0) | 11.8 (6.7–14.7) | 6.5 (6.2–7.1) | 7.6 (7.1–8.4) | 9.6 (7.6–11.9) | 6.8 (6.5–7.2) |
| 16 | 6.1 (5.9–7.8) | 6.3 (5.7–16.4) | 11.3 (7.1–22.5) | 6.4 (6.0–7.4) | 6.4 (6.1–7.9) | 8.1 (6.8–12.2) |
| 18 | 6.3 (5.8–14.0) | 14.0 (11.8–NE) | 9.2 (6.0–NE) | 6.3 (5.9–7.4) | 11.3 (6.0–31.4) | 11.7 (6.0–18.1) |
| 31 | 6.2 (6.0–11.8) | 9.4 (6.2–17.4) | 12.1 (6.0–30.1) | 8.0 (6.8–11.1) | 6.3 (6.0–10.2) | 7.4 (6.5–13.6) |
| 33 | 11.9 (6.9–14.7) | 5.7 (5.0–NE) | 12.2 (5.7–NE) | 8.3 (6.9–11.2) | 14.3 (6.2–29.1) | 6.2 (6.2–6.5) |
| 39 | 6.7 (5.7–8.1) | 16.5 (6.7–NE) | 6.5 (5.7–NE) | 7.2 (6.1–17.9) | 13.5 (6.6–30.3) | 6.4 (6.0–6.7) |
| 45 | 9.5 (5.6–NE) | 7.4 (NE–NE) | 6.0 (5.7–NE) | 8.9 (5.8–12.3) | 6.0 (5.0–NE) | 6.0 (5.8–6.5) |
| 51 | 16.4 (6.2–23.1) | 6.1 (NE–NE) | 6.9 (NE–NE) | 13.5 (6.5–24.3) | 11.3 (5.7–NE) | 8.1 (6.2–12.9) |
| 52 | 6.8 (5.8–18.7) | 6.3 (5.1–NE) | 6.2 (5.5–NE) | 12.0 (6.9–18.0) | 17.7 (10.5–26.3) | 7.3 (6.4–11.8) |
| 58 | 11.9 (6.4–18.1) | 5.5 (NE–NE) | 6.1 (5.5–NE) | 9.1 (6.9–18.1) | 6.9 (5.8–35.0) | 7.1 (6.0–12.0) |
| 59 | 6.6 (6.1–7.8) | 6.4 (5.7–NE) | 6.0 (5.6–6.1) | 9.7 (6.2–13.6) | 7.8 (5.9–16.1) | 6.3 (6.1–6.9) |
| Low risk | 7.2 (6.7–7.8) | 8.6 (7.4–11.5) | 6.2 (6.2–6.7) | 7.8 (7.2–8.2) | 11.7 (9.6–13.4) | 6.7 (6.6–7.0) |
| 6 | 6.4 (6.1–11.3) | 9.8 (5.7–NE) | 6.1 (5.7–NE) | 7.1 (6.2–11.7) | 8.1 (6.0–15.6) | 6.4 (6.2–7.0) |
| 11 | 6.8 (6.0–8.3) | 12.0 (5.7–NE) | 6.3 (5.5–NE) | 8.0 (6.4–12.5) | 13.1 (6.8–24.3) | 6.9 (6.2–11.9) |
| 44 | 6.7 (6.0–8.3) | 17.5 (6.0–NE) | 6.5 (5.7–7.9) | 7.1 (6.2–12.6) | 30.2 (14.9–48.1) | 10.6 (6.2–12.2) |
| 53 | 6.9 (6.0–17.2) | 7.6 (5.4–17.7) | 6.9 (6.5–NE) | 11.7 (9.5–17.4) | 13.8 (6.2–31.4) | 6.3 (6.0–10.5) |
| 54 | 6.4 (5.9–8.1) | 6.1 (5.6–11.8) | 6.2 (6.0–7.1) | 6.7 (6.0–8.1) | 9.3 (6.2–20.7) | 6.8 (6.2–7.8) |
| 61 | 7.1 (6.4–12.5) | 7.8 (5.9–NE) | 6.7 (6.0–NE) | 8.5 (7.0–17.0) | 30.2 (14.9–48.1) | 7.7 (6.5–12.0) |
| 70 | 6.4 (6.2–19.9) | 8.6 (5.3–NE) | 6.0 (5.7–NE) | 7.2 (6.2–12.9) | 11.9 (7.1–17.7) | 6.6 (6.1–12.2) |
| 81 | 8.2 (6.0–11.8) | 7.0 (5.5–NE) | 6.2 (5.7–NE) | 8.1 (6.8–12.0) | 7.7 (6.1–23.2) | 6.6 (6.2–23.5) |
| 83 | 11.0 (6.5–17.8) | 7.6 (5.7–NE) | 12.3 (6.0–NE) | 9.5 (6.9–12.9) | 11.7 (6.2–18.9) | 6.2 (6.0–7.4) |
| 84 | 7.1 (6.3–11.0) | 11.8 (6.0–20.9) | 6.2 (6.0–12.2) | 7.6 (6.4–11.8) | 28.3 (12.7–36.4) | 7.6 (6.4–12.8) |
Abbreviations: CI, confidence interval; HIM, HPV Infection in Men; HPV, human papillomavirus; MSM, men having sex with men; MSW, men having sex with women; NE, not estimable.
a The unit of analysis was the infection.
b Number of new infections and cleared infections for each group and HPV genotype are included in Supplementary Table 2.
c HPV genotypes shown are those in the 9-valent vaccine and those with any incidence rate >2.2/1000 person-months. Types 62, 66, and 89 are not shown because of unstable point estimates for ≥1 city.
Table 4.
Incidence Rates for Type-Specific Genital HPV Infection Among MSOM and MSWM in the HIM Study, 2005–2012a
| HPV Typeb | MSOM (n = 93) |
MSWM (n = 471) |
MSOM vs MSWM |
||||
|---|---|---|---|---|---|---|---|
| Incident Events, No. | Person- Months | Incidence Rate (95% CI)c | Incident Events, No. | Person- Months | Incidence Rate (95% CI)b | Incidence Rate Ratio (95% CI) | |
| Any HPV | 40 | 694 | 57.6 (41.2–78.4) | 155 | 4219 | 36.7 (31.2–43.0) | 1.6 (1.1–2.2) |
| 6, 11, 16, or 18 | 28 | 2077 | 13.5 (9.0–19.5) | 131 | 12 361 | 10.6 (8.9–12.6) | 1.3 (.8–1.9) |
| High risk | 44 | 1522 | 28.9 (21.0–38.8) | 158 | 8738 | 18.1 (15.4–21.1) | 1.6 (1.1–2.2) |
| 16 | 18 | 2861 | 6.3 (3.7–9.9) | 70 | 16 287 | 4.3 (3.4–5.4) | 1.5 (.9–2.5) |
| 18 | 7 | 3340 | 2.1 (.8–4.3) | 37 | 18 247 | 2.0 (1.4–2.8) | 1.0 (.5–2.3) |
| 31 | 3 | 3446 | .9 (.2–2.5) | 23 | 18 999 | 1.2 (.8–1.8) | .7 (.2–2.4) |
| 33 | 3 | 3394 | .9 (.2–2.6) | 11 | 19 350 | .6 (.3–1.0) | 1.6 (.4–5.6) |
| 39 | 9 | 3205 | 2.8 (1.3–5.3) | 27 | 18 505 | 1.5 (1.0–2.1) | 1.9 (.9–4.1) |
| 45 | 15 | 2972 | 5.0 (2.8–8.3) | 43 | 18 402 | 2.3 (1.7–3.1) | 2.2 (1.2–3.9) |
| 51 | 15 | 3124 | 4.8 (2.7–7.9) | 54 | 17 186 | 3.1 (2.4–4.1) | 1.5 (.9–2.7) |
| 52 | 9 | 3120 | 2.9 (1.3–5.5) | 57 | 17 521 | 3.3 (2.5–4.2) | .9 (.4–1.8) |
| 58 | 3 | 3418 | .9 (.2–2.6) | 32 | 18 181 | 1.8 (1.2–2.5) | .5 (.2–1.6) |
| 59 | 11 | 3085 | 3.6 (1.8–6.4) | 57 | 17 185 | 3.3 (2.5–4.3) | 1.1 (.6–2.0) |
| 68 | 9 | 3285 | 2.7 (1.3–5.2) | 33 | 18 158 | 1.8 (1.3–2.6) | 1.8 (.8–3.8) |
| Low risk | 37 | 1092 | 33.9 (23.8–46.7) | 173 | 6075 | 28.5 (24.4–33.1) | 1.2 (.8–1.7) |
| 6 | 16 | 2862 | 5.6 (3.2–9.1) | 66 | 16 688 | 4.0 (3.1–5.0) | 1.4 (.8–2.4) |
| 11 | 6 | 3178 | 1.9 (.7–4.1) | 24 | 18 734 | 1.3 (.8–1.9) | 1.5 (.6–3.6) |
| 44 | 2 | 3438 | .6 (.1–2.1) | 46 | 18 195 | 2.5 (1.9–3.4) | .2 (.1–0.9) |
| 53 | 12 | 3231 | 3.7 (1.9–6.5) | 60 | 16 878 | 3.6 (2.7–4.6) | 1.0 (.6–1.9) |
| 54 | 6 | 3229 | 1.9 (.7–4.0) | 50 | 17 966 | 2.8 (2.1–3.7) | .7 (.3–1.6) |
| 61 | 9 | 2973 | 3.0 (1.4–5.7) | 71 | 17 056 | 4.2 (3.3–5.3) | .7 (.4–1.5) |
| 62 | 8 | 3288 | 2.4 (1.1–4.8) | 72 | 16 367 | 4.4 (3.4–5.5) | .6 (.3–1.1) |
| 66 | 12 | 3058 | 3.9 (2.0–6.9) | 53 | 17 362 | 3.1 (2.3–4.0) | 1.2 (.7–2.3) |
| 70 | 9 | 3213 | 2.8 (1.3–5.3) | 30 | 18 349 | 1.6 (1.1–2.3) | 1.7 (.8–3.6) |
| 81 | 8 | 3125 | 2.6 (1.1–5.0) | 42 | 17 763 | 2.4 (1.7–3.2) | 1.1 (.5–2.3) |
| 84 | 18 | 2879 | 6.3 (3.7–9.9) | 89 | 15 753 | 5.6 (4.5–7.0) | 1.1 (.7–1.8) |
| 89 | 18 | 2921 | 6.2 (3.7–9.7) | 75 | 16 449 | 4.6 (3.6–5.7) | 1.4 (.8–2.3) |
Abbreviations: CI, confidence interval; HIM, HPV Infection in Men; HPV, human papillomavirus; MSOM, men having sex only with men; MSWM, men having sex with both women and men.
a The unit of analysis was the individual person.
b HPV genotypes shown are those in the 9-valent vaccine in addition to types with any incidence rate >2.2/1000 person-months and an incidence rate ratio excluding unity.
c Per 1000 person-months.
DISCUSSION
Although surveillance in the United States generally indicates increased burden among MSM for a number of STIs [12], we observed little difference in the burden of genital HPV among MSM and MSW recruited in 3 countries. However, acquisition was high overall, with 25% of MSM (95% CI, 21%–30%) and 21% of MSW (20%–23%) acquiring a high-risk type in the first 12 months of the study. Likewise, time to clearance differed little among the 2 groups of men, with the median duration of genital HPV among MSM and MSW generally >6 months, suggesting that measures of persistence of ≤6 months may not be useful to identify clinically relevant incident genital infection among men.
Giuliano et al [17] reported elsewhere that, for some groups and types of HPV, acquisition was higher overall in Brazilian men compared with men in the United States or Mexico. Because most MSM were recruited in Brazil, we stratified our analysis by country for comparison of MSM and MSW point estimates. Because stratifying affected the power of the study to observe differences between MSM and MSW and because most point estimates for incidence were higher among MSM, we may have underestimated differences among MSM and MSW; however, the differences are probably modest. Comparable incidence would be consistent with our observed median of 1 penetrative sexual partner in the 6 months before the baseline visit for both MSM and MSW.
Our findings of similar genital HPV burden among MSM and MSW across a wide age range reflect recent US surveillance data from 40 STI clinics indicating a similar burden of genital warts among MSM and MSW [9]. HPV-6 and HPV-11 are responsible for >90% of genital warts [23], and we observed comparable incidences of these types among MSM and MSW when stratified by city. Indeed, we also observed a comparable prevalence of clinician-diagnosed genital warts among MSM and MSW in this study's baseline data.
HPV-16 is a significant etiologic agent for both penile cancer and anal cancer [4, 24]. Our observation of a comparable incidence of genital HPV-16 in MSM and MSW is consistent with the lack of a known association between sexual orientation and penile cancer. In contrast, the annual incidence of anal cancer among HIV-negative MSM is 5–25 times greater than among MSW, even though prevalence of anal HPV-16 among MSM is <3 times greater than in MSW (ie, 6.3% and 2.2%, respectively) [8, 13]. Given these genital and anal data for MSM and MSW, perhaps anopenile sex is more likely to establish productive anal HPV infection leading to malignancy in the receptive partner, whereas HPV transmission to the anal canal of MSW may occur primarily through autoinoculation or anodigital sex and lead less often to productive infection [13, 25].
Overall, the duration of genital HPV in our study was comparable among MSM and MSW; however, we have observed an increased likelihood of HPV-16 and HPV-6 antibodies among MSM compared with MSW in the HIM study [26], which may influence clearance of infection [27]. Although it seems logical that these mechanisms might account for increased clearance of HPV-16 and HPV-6 among MSM, we found scant evidence for this hypothesis.
It is not possible to rule out HPV clearance and reinfection between study visits. Although the study had excellent retention and employed sensitive DNA detection, it is conceivable that differential dropout rates among MSW who were smokers and single, never married affected incidence and or clearance estimates. For example, it is possible that the observed MSW incidence was biased downward owing to the increased dropout rates of single, never married MSW compared with married MSW. On the other hand, since our age-stratified analysis indicated no difference in MSW incidence for groups of high-risk and low-risk types, it is unlikely MSW estimates were biased due to younger MSW (aged 18--30 years) leaving the study; however, HPV16 and HPV6 incidence was increased in MSW aged 18--30 years which could have led to decreased observed incidence for these single types in the full group of men. Furthermore, although recruitment included diverse sources of men, caution should be used in generalizing the results, particularly for MSOM because the sample size is limited; however, stratification of MSM revealed a substantial number of MSWM and allowed calculations of stable longitudinal estimates in this understudied group of men.
The incidence of HPV types 6, 11, 16, and 18 among MSW is not likely to be influenced by HPV vaccination in their female partners. In a subset of men asked questions about HPV vaccination, 21% of MSW reported vaccination in female sex partners; however, the prevalence of these HPV types among these MSW was not lower than that among those who reported no vaccinated partners.
In summary, we found comparable incidence of genital HPV-6 and HPV-16 in MSM and MSW and similar rates of clearance. These results are consistent with rates of penile disease in the 2 populations and comparable median numbers of sexual partners for these MSM and MSW.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Special thanks to the men who provided personal information and biological samples for the study. Thanks to the HIM study team in São Paulo, Cuernavaca, and Tampa, including Lenice Galan, Elimar Gomes, Elisa Brito, Filomena Cernicchiaro, Rubens Matsuo, Vera Souza, Ricardo Cintra, Ricardo Cunha, Birgit Fietzek, Raquel Hessel, Viviane Relvas, Fernanda Silva, Juliana Antunes, Graças Ribeiro, Roberta Bocalon, Rosária Otero, Rossana Terreri, Sandra Araujo, Meire Ishibashi, the CRT–DST/AIDS Nursing team, Aurelio Cruz, Pilar Hernandez, Griselda Diaz Garcia, Oscar Rojas Juarez, Rossane del Carmen Gonzales Sosa, Rene de Jesus Alvear Vazquez, Beibei Lu, Christine Gage, Kathy Eyring-Cabrera, Nadia Lambermont, Emily Jolles, Kayoko Kennedy, Kimberly Isaacs, Andrea Bobanic, Kyle Wolf, Anthony Bilotto, Abidemi Ajidahun, Michael Blackmer, Michael O'Keefe, and Bradley Sirak. Thanks to Digene Corp for donations of supplies.
Disclaimer. Publication and report contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health (NIH).
Financial support. This work was supported by the National Cancer Institute, NIH (grant R01 CA098803-01A1 to A. R. G.) and the National Institute of Allergy and Infectious Diseases (grant 5R21AI101417-3 to A. G. N.).
Potential conflicts of interest. L. L. V. and A. R. G. are on the speakers' bureau and advisory board for Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis 2013; 13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartwig S, Syrjänen S, Dominiak-Felden G, Brotons M, Castellsagué X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer 2012; 12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodhall SC, Jit M, Cai C, et al. Cost of treatment and QALYs lost due to genital warts: Data for the economic evaluation of HPV vaccines in the United Kingdom. Sex Transm Dis 2009; 36:515–21. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc H 2010; 46:S20–6. [DOI] [PubMed] [Google Scholar]
- 5.Gil-Prieto R, Ester PV, Alvaro-Meca A, Rodriguez MS, De Miguel AG. The burden of hospitalizations for anus and penis neoplasm in Spain (1997–2008). Hum Vaccin Immunother 2012; 8:201–7. [DOI] [PubMed] [Google Scholar]
- 6.Robinson D, Coupland V, Moller H. An analysis of temporal and generational trends in the incidence of anal and other HPV-related cancers in Southeast England. Br J Cancer 2009; 100:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleeker MCG, Heideman DAM, Snijders PJF, Horenblas S, Dillner J, Meijer C. Penile cancer: Epidemiology, pathogenesis and prevention. World J Urol 2009; 27:141–50. [DOI] [PubMed] [Google Scholar]
- 8.Daling JR, Weiss NS, Klopfenstein LL, Cochran LE, Chow WH, Daifuku R. Correlates of homosexual behavior and the incidence of anal cancer. JAMA 1982; 247:1988–90. [PubMed] [Google Scholar]
- 9.Llata E, Stenger M, Bernstein K, et al. Prevalence of genital warts among sexually transmitted disease clinic patients—Sexually Transmitted Disease Surveillance Network, United States, January 2010 to December 2011. Sex Transm Dis 2014; 41:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyitray AG, Carvalho da Silva RJ, Baggio ML, et al. The prevalence of genital HPV and factors associated with oncogenic HPV among men having sex with men and men having sex with women and men: the HIM study. Sex Transm Dis 2011; 38:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva RJ, Casseb J, Andreoli MA, Villa LL. Persistence and clearance of HPV from the penis of men infected and non-infected with HIV. J Med Virol 2011; 83:127–31. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control & Prevention. Sexually Transmitted Disease Surveillance 2013. Atlanta, GA: Centers for Disease Control and Prevention, 2014. [Google Scholar]
- 13.Nyitray AG, Carvalho da Silva RJ, Baggio ML, et al. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in Men (HIM) Study. J Infect Dis 2011; 203:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vriend HJ, Bogaards JA, van der Klis FRM, et al. Patterns of human papillomavirus DNA and antibody positivity in young males and females, suggesting a site-specific natural course of infection. PLoS ONE 2013; 8:e60696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darwich L, Cañadas MP, Videla S, et al. Prevalence, clearance, and incidence of human papillomavirus type-specific infection at the anal and penile site of HIV-infected men. Sex Transm Dis 2013; 40:611–8. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The Human Papillomavirus Infection in Men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev 2008; 17:2036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet 2011; 377:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol 2000; 38:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol 1998; 36:3020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—part B: biological agents. Lancet Oncol 2009; 10:321–2. [DOI] [PubMed] [Google Scholar]
- 21.Savin-Williams RC, Ream GL. Prevalence and stability of sexual orientation components during adolescence and young adulthood. Arch Sex Behav 2007; 36:385–94. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Wei L. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989; 84:1074–8. [Google Scholar]
- 23.Vandepapeliere P, Barrasso R, Meijer C, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: Infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis 2005; 192:2099–107. [DOI] [PubMed] [Google Scholar]
- 24.Van Poppel H, Watkin NA, Osanto S, et al. Penile cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi115–24. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis 2008; 14:888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu B, Viscidi RP, Wu Y, et al. Seroprevalence of human papillomavirus (HPV) type 6 and 16 vary by anatomic site of HPV infection in men. Cancer Epidemiol Biomarkers Prev 2012; 21:1542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syrjanen K. Mechanisms and predictors of high-risk human papillomavirus (HPV) clearance in the uterine cervix. Eur J Gynaecol Oncol 2007; 28:337–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


