Abstract
Bronchial asthma is a chronic inflammatory disease of the airways characterized by a marked infiltration of eosinophils at the site of inflammation. Eotaxins are potent chemoattractants for eosinophils and play important roles in pathogenesis of asthma. In the course of screening for eotaxin-3 inhibitors, we found that wogonin showed potent inhibitory activity of interleukin-4 (IL-4)-induced eotaxin-3 expression in BEAS-2B cells. In this study, we examined the effects of wogonin on IL-4/STAT6 signaling pathway and biological implication in a mouse model of asthma. Wogonin inhibited IL-4-induced activation and nuclear translocation of STAT6 which plays a key role in either the transcription of STAT6-response genes or Th2 cytokine-mediated inflammation. Oral administration of wogonin significantly reduced activation of STAT6 in the lung and the expression of eotaxin and RANTES in bronchoalveolar lavage fluids. Histological examination of lung tissue demonstrated that wogonin significantly inhibited allergen-induced eosinophilic inflammation. Administration of wogonin reduced the total IgE and ovalbumin-specific IgE levels compared with the ovalbumin-challenged group. All of these data demonstrated that wogonin could alleviate airway inflammation through inhibition of STAT6 activation induced by Th2 cytokines. Our finding implicates a potential therapeutic value of wogonin in the treatment of asthma through regulation of IL-4/STAT6 signaling pathway.
Keywords: wogonin, eotaxin-3, STAT6, airway inflammation, bronchial asthma
Introduction
Bronchial asthma is a chronic inflammatory disease of the airways, characterized by the accumulation of eosinophils, elevated IgE, mucus hypersecretion, and airway inflammation/hyper-responsiveness(1,2), for which T cells, in particular CD4+ T cells, orchestrated the inflammatory microenvironment through robust production of cytokines relevant to T helper type 2 (Th2) lymphocytes such as interleukin-4 (IL-4), IL-13, and IL-5, and contributed to the recruitment of eosinophils into the airways in response to cytokines.(3) During these pathogenesis, airway epithelial cells play a critical role in the development of airway inflammation through the polarization of allergen-induced Th2 lymphocyte and the production of Th2 cytokines,(4) including eotaxins and chemokine receptor-3 (CCR-3).(5)
Since the eotaxin expressions are very cell-type specific, eotaxin-1 (CCL11) is secreted by eosinophils, macrophages, lymphocytes and fibroblasts(6,7) and eotaxin-2 (CCL24) and eotaxin-3 (CCL26) are mainly released by epithelial and endothelial cells.(8,9) Airway epithelial cells are the major source of eotaxins and principally release high levels of eotaxin-3 when stimulated with IL-4 or IL-13.(10–12) The signaling pathway activated by IL-4 involves the recruitment of Janus family of tyrosine kinases, JAK1, JAK2, and JAK3 to the receptor subunits, receptor dimerization, and phosphorylation of tyrosine residues in the cytoplasmic domain of the IL-4Rα. Also, signal transducer and activator of transcription 6 (STAT6) is recruited to IL-4Rα upon IL-4Rα phosphorylation.(13,14) Upon IL-4 binding to the receptor complex, STAT6 becomes phosphorylated within minutes by receptor-associated JAK kinases(15) and the phosphorylated STAT6 homodimerizes and translocates into the nucleus, finally initiates gene transcription via specific DNA consensus motif.(16–18) Analyses of STAT6-deficient mice revealed that STAT6 is required for IL-4-mediated airway inflammations including eosinophilia, airway hyper-responsiveness, goblet cell hyperplasia, mucus secretion, and chemokine production.(19,20) The importance of activation of airway epithelium by IL-4 through the STAT6 signal transduction pathway was also shown by studies using knockout mice in which selective expression of IL-4Rα or STAT6 was reinstated in epithelial cells alone. (21,22)
In order to identify novel compounds that can inhibit IL-4/STAT6 signaling pathway implicated in bronchial asthma, in this study, we initially focused on the extracts of traditional herbal medicines using an eotaxin-3 promoter reporter and found that the extracts of Scutellariae radix, the dry root of Scutellaria baicalensis Georgi as a medicinal herb widely used for the treatment of various allergic and inflammatory diseases in East Asian countries, such as Korea, Japan, and China(23) significantly inhibited IL-4-induced eotaxin-3 transcriptional activity.
Materials and Methods
Flavonoids and reagents
Wogonin, oroxylin A, baicalein and baicalin were purchased from Alexis Biochemicals (San Diego, CA) and dissolved in dimethylsulfoxide (DMSO). Human recombinant IL-4 was purchased from R&D Systems (Minneapolis, MN). Anti-STAT6, anti-phosho-STAT6, anti-JAK1, anti-phosho-JAK1 antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Cell culture
BEAS-2B (human bronchial epithelial) cells and NCI-H292 (human lung mucoepidermoid) cells were grown in DMEM (Gibco BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Gibco) and 1% mixture of penicillin and streptomycin (Gibco). Cells were maintained in a humidified atmosphere of 5% CO2 at 37°C.
Plasmids, transient transfection and luciferase activity assay
The luciferase reporter construct containing the human eotaxin-3 gene promoter (Eotaxin-3-Luc) was described previously.(11) A DNA fragment containing the human eotaxin-3 promoter (970 bp) was amplified from genomic DNA with a primer set of 5'-AGT CAA GCT TCA TCA TGT GCT GCA AAT CAG G-3' (forward) and 5'-CTG ACT CGA GTC TGT TAG ATC TCT CAA ATG CC-3' (reverse). The PCR fragment was digested with XhoI and HindIII and cloned into pGL3-basic vector (Promega Corp., Madison, WI) to give pGL3-Eotaxin-3-Luc. BEAS-2B cells were transiently transfected with pGL3-Eotaxin-3-Luc and the internal control pCMV-β-gal in 24-well plate using PEI reagent. After 24 h transfection, cells were treated with 10 ng/ml of IL-4 for 24 h. Luciferase activities were quantified by using the Luciferase Assay System (Promega) according to the manufacturer’s protocol. All of the values were normalized with the β-galactosidase activities. All experiments were performed in triplicate and repeated at least three times.
Reverse Transcription and quantitative PCR
Total RNA was isolated from cells using RNAiso Plus reagent (Takara Bio Inc., Shiga, Japan) according to the manufacturer’s protocol. Reverse transcription was performed with 1 µg of pure RNA using M-MLV reverse transcriptase (Promega). The synthesized cDNA was amplified by PCR using specific primers. PCR products were visualized by electrophoresis on agarose gels with RedSafe (iNtRON) staining and analyzed using an ImageQuant LAS 4000 image analyzer (GE Healthcare Life Sciences, Piscataway, NJ). Quantitative RT-PCR was performed using an Applied Biosystems Prism 7900HT sequence detection system (Carlsbad, CA). Cyclophilin served as the housekeeping gene. All samples were analyzed in triplicate and expressed as the mean ± SD.
Measurement of eotaxin-3 release in culture medium
Eotaxin-3 protein levels were quantified in supernatants from BEAS-2B cells stimulated with IL-4 by using Quantikine human eotaxin-3 ELISA according to the manufacturer’s instructions (R&D Systems).
Western Blotting
Cells were lysed in lysis buffer containing 25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM EDTA, and protease inhibitor mixture (Complete, Roche Applied Science, Indianapolis, IN). Extracts were separated by SDS-PAGE followed by electro-transfer to polyvinylidene difluoride (PVDF) membranes and probed with polyclonal or monoclonal antisera, followed by horseradish peroxidase-conjugated anti-rabbit, anti-mouse IgG and visualized by chemiluminescence, according to the manufacturer’s instructions (Pierce, Rockford, IL).
Immunofluorescence
Cells seeded on chamber slides were fixed with 4% paraformaldehyde solution for 15 min. Cells were blocked with 5% bovine serum albumin for 1 h and incubated with anti-STAT6 antibody overnight at 4°C. Antibody-bound cells were detected by Alexa Fluor 546-conjugated secondary antibody (Invitrogen, Carlsbad, CA). Immunofluorescence images were obtained using a LSM-710 confocal microscope (Zeiss, Oberkochen, Germany).
Induction of allergic asthma model in mice
Six-week female BALB/c mice were purchased from Orient Bio (Seongnam, Korea). Animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Gachon University. Mice were maintained in the specific pathogen-free facility of CACU in Lee Gil Ya Cancer and Diabetes Institute of Gachon University. Mice were immunized on days 0 and 14 by intraperitoneal injection of 100 µg chicken ovalbumin (OVA, Sigma, St. Louis, MO) emulsified in 2 mg aluminium hydroxide adjuvant in 100 µL of PBS buffer. Mice were challenged through the airway with OVA (5%, w/v, in PBS) for 30 min using an ultrasonic nebulizer (NE-C28, Omron, Tokyo, Japan) on days 28, 29, and 30 after the initial sensitization. Mice were sacrificed 48 h after the last challenge (day 32) to characterize the suppressive effects of wogonin on airways of allergic asthma animals. The negative controls were sham-sensitized and challenged with PBS following the same protocol.
Lung tissue histology.
After BAL fluids were obtained, the lung tissue was fixed in 10% (v/v) neutral buffered formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin (H&E) solution, and examined for histological changes in light microscope.
Inflammatory cell counts in BAL fluids.
Mice were sacrificed with an overdose of 50 mg/kg of phenobarbital 48 h after the last challenge, and tracheotomies were performed. After ice-cold PBS was instilled into the lungs, BAL fluid was obtained by two aspirations via tracheal cannulation. The BAL fluids were centrifuged and supernatants were collected and stored at −70°C prior to use. Total inflammatory cell numbers were assessed by counting cells in at least five squares of a hemocytometer, after exclusion of dead cells staining with trypan blue. One hundred microliters of BAL fluids were loaded onto a slide and centrifuged to fix the cells onto the slide using a cytospin machine. Cell pellets were suspended in 0.5 ml of PBS, and 100 µl of each solution was spun onto a slide. After slides were dried, cells were fixed and stained using Diff-Quick stain reagent (Dade Behring, Marburg, Germany) according to manufacturer’s instructions.
Analysis of serum and BAL fluids.
The levels of total IgE and OVA-specific IgE in serum were measured using a mouse anti-IgE ELISA kit (Shibayagi Co., Gunma, Japan) and a mouse anti-OVA IgE ELISA kit (Alpha Diagnostic Intl., San Antonio, TX), respectively. The levels of cytokines, including IL-4, eotaxin, and RANTES in BAL fluids were measured using ELISA kits according to manufacturer’s instructions (R&D Systems).
Statistical analysis
Results in bar graphs are presented as means ± SEMs and are representative of three independent experiments. Statistical analysis was performed using the Student’s t test, and p values of less than 0.05 were considered statistically significant.
Results
Screening of flavone compounds possessing inhibitory action of exotain-3 expression among Scutellariae radix extracts
Through the initial screening of methanol extracts of medicinal plants using IL-4-induced eotaxin-3 reporter assay system, we found that the four kinds of extracts of Scutellariae radix, the bioactive components of Scutellariae radix have been known to be flavones and the major constituents of Scutellaria baicalensis are wogonin, oroxylin A, baicalein, and bacalin (Fig. 1A),(24,25) showed potent inhibitory effects on IL-4-induced eotaxin-3 transcriptional activity. In order to identify active compounds in the Scutellariae radix, four flavone compounds were assayed for their IL-4-induced eotaxin-3 transcriptional activities, respectively. Human bronchial epithelial (BEAS-2B) cells were transfected with eotaxin-3 promoter reporter construct and stimulated with IL-4 for 24 h. As shown Fig. 1B and C, only wogonin significantly inhibited IL-4-induced eotaxin-3 transcriptional activity and mRNA expression, while oroxylin A moderately inhibited eotaxin-3 transcriptional activity and mRNA expression, whereas baicalein and baicalin had little effect.
Fig. 1.
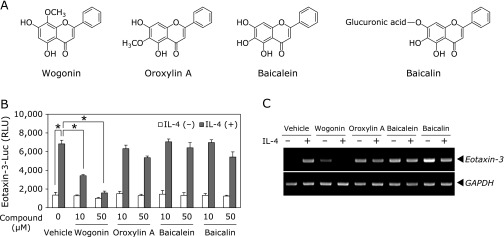
Effects of four major flavones found in Scutellaria baicalensis Georgi on eotaxin-3 expression. (A) Chemical structures of wogonin, oroxylin A, baicalein, and baicalin. (B) BEAS-2B cells transfected with eotaxin-3 promoter reporter were treated with the indicated concentrations of different flavones for 4 h. Luciferase activity was measured after 24 h of IL-4 stimulation. Data shown are the mean ± SD of three separate experiments. *p<0.05. (C) BEAS-2B cells were treated with 50 µM of each flavone and stimulated with IL-4 for 8 h. Total RNA was isolated and levels of eotaxin-3 mRNA was analyzed by RT-PCR.
Wogonin inhibited IL-4-induced eotaxin-3 expressions and their releases in human bronchial epithelial cells
Prior to the investigation into the bioactive potential of wogonin, we first checked the cytotoxic effects of wogonin in BEAS-2B cells by means of MTT assay and found that wogonin at concentrations lower than 100 µM had little cytotoxic effect on the cells (data not shown). In order to confirm the inhibitory effect of wogonin on IL-4-induced eotaxin-3 expression in BEAS-2B cells, the transcriptional activity, mRNA expression, and protein expression of eotaxin-3 were analyzed. As shown in Fig. 2A, eotaxin-3 promoter activity was increased up to 4-fold in BEAS-2B cells treated with IL-4 as compared with untreated cells. IL-4-induced activation of eotaxin-3 promoter was inhibited by wogonin. In addition, wogonin reduced IL-4-induced eotaxin-3 mRNA levels in a dose-dependent manner (Fig. 2B). Consistent with these results, eotaxin-3 protein levels were also inhibited by wogonin. As shown in Fig. 2C, stimulation of IL-4 for 24 h caused a significant increase in eotaxin-3 secretion (245 ± 28 pg/ml) in BEAS-2B cells. Treatment with 3 µM of wogonin significantly reduced the IL-4-stimulated eotaxin-3 protein secretion (24 ± 9 pg/ml) at 24 h. Under the same culture conditions with ELISA assay, wogonin suppressed IL-4-induced eotaxin-3 mRNA expression (Fig. 2D).
Fig. 2.
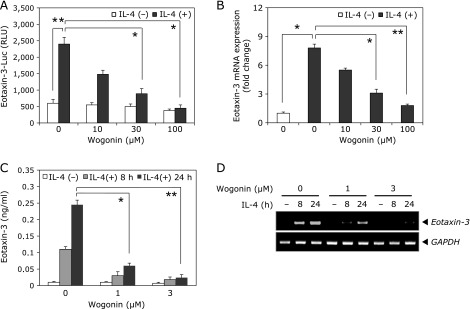
Effects of wogonin on IL-4-induced eotaxin-3 expression in human bronchial epithelial cells. (A) BEAS-2B cells transfected with eotaxin-3 promoter reporter were treated with the indicated concentrations of wogonin for 4 h. Luciferase activity was measured after 24 h of IL-4 stimulation. (B) BEAS-2B cells were pretreated with wogonin for 4 h and then stimulated with IL-4 for another 8 h. Levels of eotaxin-3 mRNA was analyzed by quantitative RT-PCR. (C) BEAS-2B cells were pretreated with wogonin for 4 h and then stimulated with IL-4 for 8 or 24 h. The protein levels of eotaxin-3 in culture supernatants were determined by ELISA. (D) Culture conditions were identical to those in (C). Total RNA was isolated and levels of eotaxin-3 mRNA was analyzed by RT-PCR. Data shown are the mean ± SD of three separate experiments. *p<0.05, **p<0.01.
Wogonin inhibited IL-4-induced phosphorylation and nuclear translocation of STAT6 in human bronchial epithelial cells
Next, we test whether the inhibition of IL-4-induced eotaxin-3 promoter activation and mRNA expression by wogonin was due to the suppression of activation of STAT6. In earlier studies, IL-4-induced transcriptional regulation of eotaxin-3 expression is mainly mediated by STAT6 in various cell types.(11,26) We performed Western blotting analysis for STAT6 phosphorylation in the presence and absence of 30 µM of wogonin. The stimulation of IL-4 induced phosphorylation of STAT6 in BEAS-2B (Fig. 3A) and NCI-H292 (Fig. 3B) cells was observed within 5 min. Treatment of wogonin significantly suppressed the IL-4-induced phosphorylation of STAT6. Wogonin also inhibited the phosphorylation of JAK1, an upstream kinase of STAT6. Total levels of STAT6 and JAK1 were consistent with the treatment of wogonin. Upon phosphorylation, STAT6 dimerizes and translocates to the nucleus where it can bind DNA and transcription of target gene.(13) Therefore we examined whether wogonin interfered with the nuclear translocation of STAT6. To investigate whether wogonin would inhibit the nuclear localization of STAT6 in response to IL-4, BEAS-2B cells were pretreated with 30 µM of wogonin for 4 h and then stimulated with 50 ng/ml of IL-4 for 30 min, after which each cellular compartment was separated and analyzed by Western blotting. As shown in Fig. 3C, the nuclear lysates from cells stimulated with IL-4 contained substantial amount of phospho-STAT6 that was not present in the nuclear lysates from un-stimulated cells. Pretreatment with wogonin before stimulation with IL-4 significantly reduced the amount of nuclear phospho-STAT6. To confirm cellular localization of STAT6, confocal microscopy was performed in BEAS-2B cells. STAT6 was found in cytoplasm in the absence of IL-4 treatment. Upon IL-4 stimulation, STAT6 was predominantly translocated into the nucleus, however wogonin significantly blocked the IL-4-induced nuclear translocation of STAT6 in BEAS-2B cells (Fig. 3D). These results suggested that wogonin inhibits IL-4-induced eotaxin-3 expression through the activation and nuclear translocation of STAT6 which plays a critical role in signaling by the Th2 cytokine IL-4.
Fig. 3.
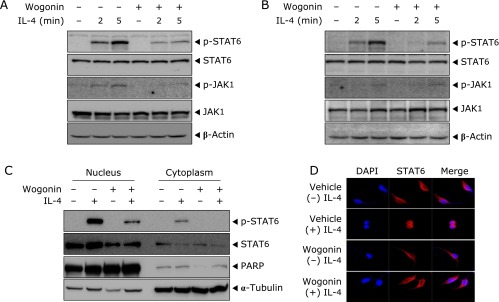
Effects of wogonin on IL-4-induced phosphorylation and nuclear translocation of STAT6 in human bronchial epithelial cells. BEAS-2B (A) and NCI-H292 (B) cells were treated with wogonin and stimulated with IL-4 for 2 or 5 min. Phospho-STAT6, phospho-JAK1 and β-actin were assessed by Western blotting analysis. (C) BEAS-2B cells were treated with wogonin and stimulated with IL-4 for 30 min. Nuclear and cytosolic proteins were extracted from cells. STAT6 was detected by Western blotting analysis using anti-phospho-STAT6 and anti-STAT6 antibodies. (D) Culture conditions were identical to those in (C). Cells were fixed in 4% PFA, and then stained with anti-STAT6 antibody followed by Alexa Fluor goat anti-rabbit IgG. The data are representative of at least three experiments.
Wogonin suppressed OVA-induced phosphorylation of STAT6 and expression of Th2 cytokines of BAL fluids
In order to determine the therapeutic effect of wogonin on airway inflammatory reactions, an OVA-induced airway inflammation model in mice was employed. Airway inflammation was induced in BALB/c mice by intraperitoneal injection OVA followed by challenges with aerosolized OVA on days 28, 29 and 30, respectively (Fig. 4A). Wogonin was administered by oral gavage before each OVA challenge. Treatment of wogonin significantly decreased levels of phosphorylated STAT6 in the lung, by which we examined IL-4 and chemokine expression in BAL fluids by ELISA 48 h after the last challenge. OVA-sensitized and challenged mice induced significant elevation of IL-4 (Fig. 4B) and Th2 inflammation-related chemokines such as eotaxin (Fig. 4C) and RANTES (Fig. 4D) in BAL fluids (vs control group, *p<0.05), compared with control BAL fluids. However, treatment with wogonin significantly decreased the levels of IL-4, eotaxin, and RANTES (vs OVA group; *p<0.05), compared to OVA-challenge group with a dose-dependent manner. Dexamethasone (4 mg/kg) also caused a significant reduction in IL-4, eotaxin, and RANTES in BALF (vs OVA group, *p<0.05). These results suggested that wogonin attenuates the OVA-induced Th2 cytokines and chemokines expression by inhibiting STAT6 activation (Fig. 4E). As a negative control, mice were treated with PBS. Based on the knowledge that STAT6 plays a key role in allergic inflammation of lung by inducing the transcription of various inflammatory mediators, we hypothesized that wogonin would attenuate airway inflammatory reactions by suppressing STAT6 and Th2 signaling pathway activation. To address this issue, we examined the activation status of STAT6 in the lung tissue using antibodies against phosphorylated STAT6 and STAT6 by Western blotting. As shown in Fig. 4E, the total amount of STAT6 was comparable in lung tissue of control, OVA-sensitized and challenged mice. In contrast, significantly increased levels of phosphorylated STAT6 were found in the lung tissue of OVA-sensitized and challenged mice, indicating that the Th2 signaling pathway was activated in the lung of OVA-sensitized and challenged mice.
Fig. 4.
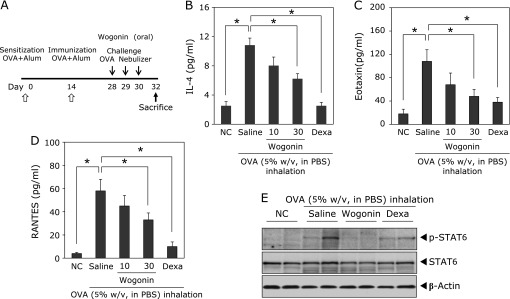
Effects of wogonin on OVA-induced STAT6 activation and Th2 chemokine expression. (A) Experimental protocol for development of allergic asthma and treatment with wogonin using a murine model. Levels of IL-4 (B), eotaxin (C) and RANTES (D) in BAL fluids were analyzed by ELISA. BAL fluids were collected 48 h after the last OVA challenge. NC, normal control mice; OVA, OVA-sensitized and challenged mice, Wogonin, OVA-sensitized and challenged mice treated with wogonin (10 and 30 mg/kg); Dexa, OVA-sensitized and challenged mice treated with dexamethasone (4 mg/kg). The values are expressed as the means ± SEM (n = 6 per group). *p<0.05. (E) Western blotting of protein extract of lung tissues isolated from mice 48 h after the last OVA challenge. Activation of STAT6 was detected by Western blotting analysis using anti-phospho-STAT6 and anti-STAT6 antibodies.
Wogonin attenuated OVA-induced airway inflammation and serum IgE secretion
To examine the effect of wogonin on the recruitment of inflammatory cells into airway, inflammatory cells were counted in BAL fluids. We analyzed the cellular composition of the BAL fluids of mice 48 h after the last OVA challenge. Inflammatory cell levels (i.e., total cells, eosinophils, macrophages and lymphocytes) in BAL fluid were significantly elevated in OVA-challenged mice in BAL fluids (vs control group, *p<0.05), compared with control BAL fluids as shown in Fig. 5A. However, treatment of wogonin (30 mg/kg) significantly attenuated OVA challenge-induced recruitment of eosinophils, macrophages and lymphocytes (vs OVA group, *p<0.05), compared to OVA-challenge group. The observed reduction in the recruitment of inflammatory cells into airway correlated with the histological changes in lung tissues. The inflammatory cells infiltration into peribronchial and perivascular areas was observed in OVA-challenged mice as compared with PBS controls using H&E staining. Treatment with wogonin (30 mg/kg) markedly reduced the magnitude of inflammatory cells infiltration into the peribronchial and perivascular connective tissues as compared with OVA group (Fig. 5B). These results indicate that treatment with wogonin efficiently inhibits the infiltration of inflammatory cells and attenuates airway inflammation. To investigate the effect of wogonin on serum IgE levels, total IgE and OVA-specific IgE levels were analyzed by a sandwich ELISA. As shown in Fig. 5C and D, OVA-challenged mice showed higher levels of serum IgE and OVA-specific IgE (vs NC group, *p<0.05) compared to normal PBS control mice. However, a significant reduction in total IgE and OVA-specific IgE was observed in mice treated with wogonin at dose-dependent manner (vs OVA group; *p<0.05). Additionally, the protection of wogonin at the dose of 30 mg/kg was comparable with that of dexamethasone treatment at 4 mg/kg.
Fig. 5.
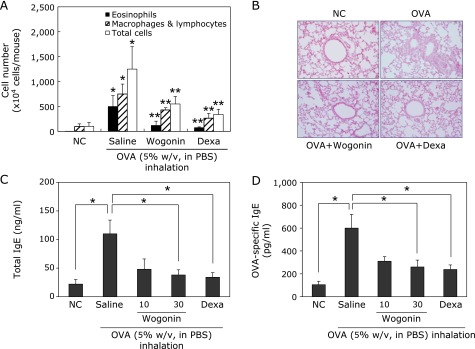
Effects of wogonin on OVA-induced airway inflammation. (A) BAL fluids were collected 48 h after the last OVA challenge of mice. Cells were isolated by cyto-spinning and stained with Diff-Quick. Cell numbers were assessed by counting cells directly under a light microscope in at least five squares of a hemocytometer, after excluding dead cells staining with trypan blue. NC, normal control mice; OVA, OVA-sensitized and challenged mice, Wogonin, OVA-sensitized and challenged mice treated with wogonin (30 mg/kg); Dexa, OVA-sensitized and challenged mice treated with dexamethasone (4 mg/kg). The values are expressed as the means ± SEM (n = 6 per group). *Significant difference from NC, p<0.05, and **significant difference from OVA p<0.05. (B) Histological examination of lung tissue was performed 48 h after the last OVA challenge. Lung tissues were fixed and stained with H&E solution. (C, D) Samples of serum were collected 48 h after the last OVA challenge. Levels of total IgE (C) and OVA-specific IgE (D) in serum were analyzed by ELISA. *p<0.05.
Discussion
In this study, we have found that wogonin could inhibit IL-4-induced STAT6 activation in human bronchial epithelial cells and suppressed OVA-induced STAT6 activation in mouse model of bronchial asthma. These results provide the first evidence that a bioactive flavone, wogonin can be used as a treatment of Th2-mediated inflammatory diseases such as bronchial asthma. As Th2 cytokines have pivotal roles in allergen-mediated allergic airway responses(27,28) and STAT6 transcription factor have been shown to be essential in Th2 differentiation,(29,30) we examined the effects of wogonin on IL-4-induced eotaxin-3 expression and STAT6 activation and on OVA-induced chemotactic chemokines such as eotaxin and RANTES and IgE production which are STAT6-dependent target genes.(16) Our results demonstrated that wogonin efficiently attenuates the expression of IL-4/STAT-dependent inflammatory genes via inhibition of the activation and nuclear translocation of STAT6 (Fig. 6).
Fig. 6.
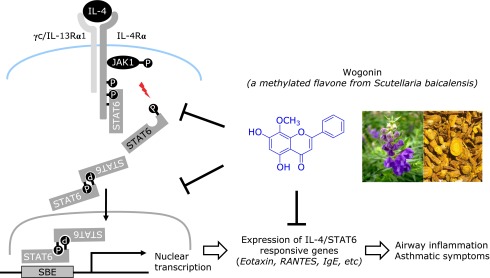
Schematic diagram of IL-4 signal transduction pathways linked to Th2-type airway inflammation and anti-inflammatory action of wogonin. IL-4 binds to IL-4Rα, the functional receptor subunit of both the type I receptor (IL-4Rα and γC) and type II receptors (IL-4Rα and IL-13Rα1). Once IL-4 binds to its cognate receptor, the associated JAK kinases are activated and phosphorylate conserved tyrosine residues on the receptor via an SH2 domain allowing the JAKs to phosphorylate STAT6. Phosphorylated STAT6 dimerizes, translocates to the nucleus, and binds to the promoters of the IL-4 responsive genes, such as those associated with Th2 cell differentiation, airway inflammation, airway hyper-responsiveness and epithelial mucous metaplasia. Wogonin inhibits IL-4-induced phosphorylation of JAK1/STAT6 thereby blocks translocation of activated STAT6 into nucleus that is a pivotal role in Th2-mediated airway inflammatory responses.
We used BEAS-2B, human bronchial epithelial cells and human eotaxin-3 promoter reporter construct which contains two canonical STAT6 binding elements (–693 and –89) to identify IL-4/STAT6 signaling inhibitors. Both STAT6 and NF-κB have been reported to regulate eotaxin-3 expression in various cell types.(31,32) However, previous studies showed that eotaxin-3 promoter activity was markedly induced following stimulation with either IL-4 or IL-13 in BEAS-2B cells. Addition of TNF-α had no additive effect on eotaxin-3 expression, whereas IL-4 and TNF-α significantly induced the eotaxin-1 expression in BEAS-2B cells.(26,33) All of these studies consistently demonstrated that STAT6 is critical for eotaxin-3 expression in airway epithelial cells.
The signal transduction pathway is activated by binding of IL-4 to the IL-4Rα and induces heterodimerization with γC or the IL-13Rα1. In cells of hematopoietic lineage, the type I receptor arises by recruitment of a common gamma chain (γC), whereas in non-hematopoietic cells, the type II receptor is formed by interaction of IL-4Rα with IL-13Rα1 instead of γC.(34) This dimerization activates the JAKs that initiate the phosphorylation cascade. IL-4Rα associates with JAK1 and γC with JAK3, whereas IL-13Rα1 interacts with either JAK2 or TYK2, but not JAK3. In non-hematopoietic cells such as bronchial epithelial cells, JAK1 or JAK3 can induce phosphorylation of STAT6. Unlike other members of the JAK family, the expression of JAK3 is highly restricted in hematopoietic cells.(35) We, therefore, investigated whether JAK1 or JAK2 can be involved in the IL-4-induced activation of STAT6 in BEAS-2B cells in the current study. In BEAS-2B cells, JAK1 activates IL-4-induced phosphorylation of STAT6. Non-specific JAK inhibitor (JAK inhibitor I) abolished IL-4-induced phosphorylation of STAT6, while JAK2 specific inhibitor, AG490 could not inhibit the IL-4-induced STAT6 phosphorylation in BEAS-2B cells (data not shown). These results indicate that JAK1-STAT6 is responsible for IL-4-mediated signaling in BEAS-2B cells. Wogonin efficiently suppressed IL-4-induced JAK1 and STAT6 activation in bronchial epithelial cells and attenuated OVA-induced airway inflammation. The data presented in Fig. 4E suggest that signaling via the IL-4/STAT6 axis is important for the inflammatory reaction in the airway in vivo. Furthermore, wogonin suppressed OVA-induced production of Th2 cytokine IL-4 and STAT6- dependent expression of IgE and chemokines.
Some natural product inhibitors of STAT6 signaling have been previously reported. TMC-264, a fungal secondary metabolite, selectively inhibited IL-4 signaling by interfering phosphorylation of STAT6.(36) Oxacyclododecindione inhibited IL-4-dependent signaling by blocking the binding of activated STAT6 to DNA binding site.(37) In addition, a synthetic STAT6 inhibitor, AS1517499 which synthesized on the basis of the structure of reported STAT6 inhibitor, was able to inhibit IL-13 secretion in cultured human bronchial smooth muscle as well as to decrease airway hyperactivity in BALB/c mice induced by albumin sensitization.(38)
Wogonin, a major ingredient of Scutellariae radix, is a natural product widely used for the treatment of various inflammatory diseases and allergic diseases.(23,39) To our knowledge, this is the first report to show that wogonin has the potency to inhibit the activation of JAK1 and STAT6, which should greatly contribute to the Th2-mediated inflammatory responses. Although increasing evidence has shown diverse anti-inflammatory actions in various in vitro and in vivo studies, our findings provide the evidence that wogonin could be a useful compound to control cell responses to IL-4-dependent JAK1/STAT6 signaling, deserving a potential therapeutic use for bronchial asthma.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0010487).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 3.Stokes K, LaMarche NM, Islam N, Wood A, Huang W, August A. Cutting Edge: STAT6 signaling in eosinophils is necessary for development of allergic airway inflammation. J Immunol. 2015;194:2477–2481. doi: 10.4049/jimmunol.1402096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Bai C, Li K, Adler KB, Wang X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir Med. 2008;102:949–955. doi: 10.1016/j.rmed.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Pease JE, Williams TJ. Eotaxin and asthma. Curr Opin Pharmacol. 2001;1:248–253. doi: 10.1016/s1471-4892(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Lilly CM, Nakamura H, Kesselman H, et al. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Invest. 1997;99:1767–1773. doi: 10.1172/JCI119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stellato C, Matsukura S, Fal A, et al. Differential regulation of epithelial-derived C-C chemokine expression by IL-4 and the glucocorticoid budesonide. J Immunol. 1999;163:5624–5632. [PubMed] [Google Scholar]
- 8.Ying S, Robinson DS, Meng Q, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–3516. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann N, Hogan SP, Mishra A, et al. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2000;165:5839–5846. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- 10.Banwell ME, Tolley NS, Williams TJ, Mitchell TJ. Regulation of human eotaxin-3/CCL26 expression: modulation by cytokines and glucocorticoids. Cytokine. 2002;17:317–323. doi: 10.1006/cyto.2002.1021. [DOI] [PubMed] [Google Scholar]
- 11.Hoeck J, Woisetschlager M. Activation of eotaxin-3/CCLl26 gene expression in human dermal fibroblasts is mediated by STAT6. J Immunol. 2001;167:3216–3222. doi: 10.4049/jimmunol.167.6.3216. [DOI] [PubMed] [Google Scholar]
- 12.Komiya A, Nagase H, Yamada H, et al. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003;225:91–100. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest. 2002;109:1279–1283. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 16.Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. 2011;50:87–96. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuperman DA, Schleimer RP. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Curr Mol Med. 2008;8:384–392. doi: 10.2174/156652408785161032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindler U, Wu P, Rothe M, Brasseur M, McKnight SL. Components of a Stat recognition code: evidence for two layers of molecular selectivity. Immunity. 1995;2:689–697. doi: 10.1016/1074-7613(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino A, Tsuji T, Matsuzaki J, et al. STAT6-mediated signaling in Th2-dependent allergic asthma: critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int Immunol. 2004;16:1497–1505. doi: 10.1093/intimm/dxh151. [DOI] [PubMed] [Google Scholar]
- 20.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly-Welch AE, Melo ME, Smith E, et al. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol. 2004;172:4545–4555. doi: 10.4049/jimmunol.172.7.4545. [DOI] [PubMed] [Google Scholar]
- 22.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 23.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Gao J, Sanchez-Medina A, Pendry BA, Hughes MJ, Webb GP, Corcoran O. Validation of a HPLC method for flavonoid biomarkers in skullcap (Scutellaria) and its use to illustrate wide variability in the quality of commercial tinctures. J Pharm Pharm Sci. 2008;11:77–87. doi: 10.18433/j39g6v. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Ye M, Xu M, Sun J, Wang B, Guo D. Characterization of flavonoids in the traditional Chinese herbal medicine-Huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:355–362. doi: 10.1016/j.jchromb.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard C, Durual S, Estienne M, Emami S, Vasseur S, Cuber JC. Eotaxin-3/CCL26 gene expression in intestinal epithelial cells is up-regulated by interleukin-4 and interleukin-13 via the signal transducer and activator of transcription 6. Int J Biochem Cell Biol. 2005;37:2559–2573. doi: 10.1016/j.biocel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 28.Del Prete GF, De Carli M, D'Elios MM, et al. Allergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disorders. Eur J Immunol. 1993;23:1445–1449. doi: 10.1002/eji.1830230707. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Tanaka T, Shi W, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 31.Rokudai A, Terui Y, Kuniyoshi R, et al. Differential regulation of eotaxin-1/CCL11 and eotaxin-3/CCL26 production by the TNF-alpha and IL-4 stimulated human lung fibroblast. Biol Pharm Bull. 2006;29:1102–1109. doi: 10.1248/bpb.29.1102. [DOI] [PubMed] [Google Scholar]
- 32.Matsukura S, Stellato C, Plitt JR, et al. Activation of eotaxin gene transcription by NF-kappa B and STAT6 in human airway epithelial cells. J Immunol. 1999;163:6876–6883. [PubMed] [Google Scholar]
- 33.Yuan Q, Campanella GS, Colvin RA, et al. Membrane-bound eotaxin-3 mediates eosinophil transepithelial migration in IL-4-stimulated epithelial cells. Eur J Immunol. 2006;36:2700–2714. doi: 10.1002/eji.200636112. [DOI] [PubMed] [Google Scholar]
- 34.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 35.Rane SG, Reddy EP. JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene. 1994;9:2415–2423. [PubMed] [Google Scholar]
- 36.Sakurai M, Nishio M, Yamamoto K, Okuda T, Kawano K, Ohnuki T. TMC-264, a novel inhibitor of STAT6 activation produced by Phoma sp. TC 1674. J Antibiot (Tokyo) 2003;56:513–519. doi: 10.7164/antibiotics.56.513. [DOI] [PubMed] [Google Scholar]
- 37.Erkel G, Belahmer H, Serwe A, et al. Oxacyclododecindione, a novel inhibitor of IL-4 signaling from Exserohilum rostratum. J Antibiot (Tokyo) 2008;61:285–290. doi: 10.1038/ja.2008.40. [DOI] [PubMed] [Google Scholar]
- 38.Chiba Y, Todoroki M, Nishida Y, Tanabe M, Misawa M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am J Respir Cell Mol Biol. 2009;41:516–524. doi: 10.1165/rcmb.2008-0163OC. [DOI] [PubMed] [Google Scholar]
- 39.Lin CC, Shieh DE. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Am J Chin Med. 1996;24:31–36. doi: 10.1142/S0192415X96000050. [DOI] [PubMed] [Google Scholar]


