Abstract
We assessed the effect of multi-species probiotic mixture on the changes in fecal microbiota and irritable bowel syndrome (IBS) symptoms. Eighty-one IBS patients were randomly assigned to receive either probiotic mixture (n = 39; containing Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium breve, B. actis, B. longum, and Streptococcus thermophilus) or placebo (n = 42) for 4 weeks. A questionnaire regarding general symptom relief was administered. The change in total symptom scores (sum of 10 IBS symptoms) and subtotal scores in 4 domains (pain, constipation, diarrhea, and bloating/gas) were evaluated. The change in fecal flora was determined by quantitative real-time PCR. The concentration of probiotic strains significantly increased after ingestion in probiotics group (B. bifidum, p = 0.043; B. lactis, p<0.001; L. acidophilus, p = 0.016; L. rhamnosus, p<0.001). The proportion of patients with adequate symptom relief was higher in probiotics group than in placebo group (74.4% vs 61.9%, p = 0.230). The decrease in total symptom score over time was not significantly different between the groups (p = 0.703). Among subtotal scores of 4 IBS symptom domains, the time effect was significantly different for diarrhea-symptom score between the groups (p = 0.017). A 4-week administration of multi-species probiotic mixture significantly increased the fecal concentration of most probiotic strains and improved diarrhea-symptom scores in IBS patients.
Keywords: probiotics, irritable bowel syndrome, diarrhea, treatment, fecal microbiota
Introduction
Irritable bowel syndrome (IBS) is a very common gastrointestinal disease worldwide.(1) Although IBS does not reduce the patient’s life expectancy, it decreases the patient’s quality of life through repeated waxing and waning of the symptoms over a long period,(2) and increases the burden on medical services.(3) However, at present, no optimal treatment—more effective than other alternative treatment options—has been determined.
Probiotics, which are very safe and have few adverse effects, have been widely studied for the treatment of IBS. Recent meta-analysis suggested that probiotics might even be better than psychopharmacological interventions such as antispasmodics and antidepressants in terms of the improved therapeutic efficacy.(4) However, the results of different studies vary, and the overall efficacy of probiotics on IBS remains controversial. Moreover, it is difficult to interpret the results of systemic reviews and meta-analyses on this subject for the following reasons:(5) (i) patients with IBS are heterogeneous in their pathogeneses and symptoms; (ii) the methods that evaluated the responses to probiotics also varied with different studies; and (iii) the dose, strain, and combination of probiotics differed. When mixtures of several probiotics were compared with single strains of probiotics in the treatment of IBS, the systematic reviews and meta-analyses indicated that the multi-strain probiotics were more effective than single-strain probiotics.(6,7) Recently, a Korean study reported that a multi-species probiotic mixture containing Lactobacillus and Bifidobacterium (LacClean Gold-S®) is more effective than a placebo for the improvement of symptoms in IBS patients.(8) However, that study had several limitations. In particular, although a greater number of patients in the probiotics group experienced global relief of IBS symptoms, which was a dichotomous endpoint, as compared to patients in the placebo group, there were no significant differences in the change in each IBS symptom relative to the baseline between the 2 groups. In addition, among the 6 strains constituting the probiotic mixture, only 2 strains showed a significant increase in concentration in the fecal material after treatment in the probiotics group than in the placebo group: Lactobacillus rhamnosus (L. rhamnosus) and Streptococcus thermophilus (S. thermophilus). Considering that S. thermophilus is mainly used as a starter in probiotic production rather than as an effective probiotic in itself,(9) it is reasonable to conclude that the probiotic strain that successfully colonized in the intestines of subjects was L. rhamnosus only.
Thus, in the present study, we aimed to determine the effect of using a multi-species probiotic mixture on IBS symptoms in a larger number of subjects. First, we evaluated whether the administration of a multi-species probiotic mixture could increase the concentration of each probiotic strain in the subjects’ fecal material compared with control subjects. Second, we compared the change in IBS symptoms over time between the probiotics and placebo groups using both binary and continuous assessment tools.
Materials and Methods
Patients
Patients who were diagnosed with IBS based on the Rome III criteria were consecutively enrolled. Patients aged 19–75 years who did not have any organic disease—as confirmed by common blood counts, biochemistry tests, and colonoscopy—during the screening period were included. Patients who had lactose intolerance, severe systemic illnesses, and a history of psychiatric disorder were excluded. Moreover, patients who took drugs during the study period that might influence the evaluation of treatment efficacy were excluded. All patients provided informed consent and this study was approved by the institutional review board of Seoul National University Bundang Hospital (IRB number: B-0905/076-001).
Study design
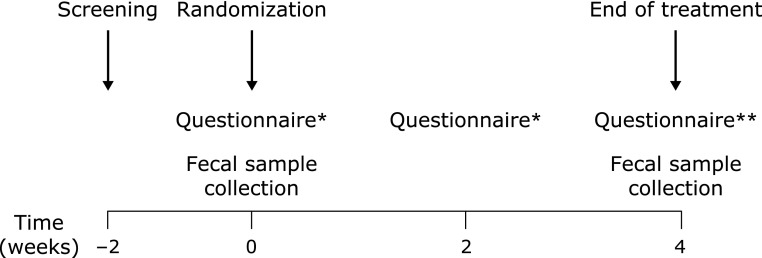
Fig. 1 is a flow diagram illustrating the study design. In patients who fulfilled the inclusion criteria, the baseline (week 0) stool form, stool frequency, and visual analogue scale (VAS) score (from 0 to 10) for 10 symptoms associated with IBS were recorded. The 10 symptoms encompass the 4 major domains representing the physical symptoms of IBS: pain (abdominal pain, abdominal discomfort), constipation (hard/lumpy stools, stool straining, incomplete evacuation), diarrhea (loose/watery stools, mucus in stools, bowel urgency), and bloating/gas (bloating, passage of gas).(10) Four subtypes of IBS were also classified according to the predominant stool pattern.(11) After fecal samples were collected, patients were randomized to receive either a multi-species probiotic mixture or placebo. Randomization was performed by a computer-generated table in blocks of 4. All patients and investigators, except for the study coordinator, remained blinded to the randomization process until study completion.
Fig. 1.
A flow diagram illustrating the study design. *Questionnaire on stool form/frequency and VAS scores for 10 IBS symptoms. **Questionnaire on stool form/frequency, VAS scores for 10 IBS symptoms, and whether any relief in the general symptoms was achieved.
Patients visited the clinic to obtain their investigational products and to undergo an assessment for compliance, symptoms, and safety at 2 weeks and 4 weeks after the first administration. At these visits, patients submitted self-administered questionnaires on stool form/frequency and VAS scores for each IBS symptom. At 4 weeks, we administered a questionnaire on whether any relief in the general symptoms was achieved, and collected a follow-up fecal sample.
According to the Rome III Committee recommendations,(12) we used 2 types of measurement: binary endpoints addressing the construct of relief and an integrative symptom questionnaire that addressed the change in the severity of each IBS symptom. The primary outcome was the proportion of patients who reported adequate relief of overall IBS symptoms over 4 weeks of treatment. The secondary outcomes were as follows: the change in the fecal microbiota, as determined by quantitative real-time polymerase chain reaction (PCR); the change of stool form/frequency; total symptom scores, which represented the sum of 10 IBS symptom scores; and subtotal scores of the 4 IBS symptom domains during the study period.
Study medication
LacClean Gold-S® (Cell Biotech, Co., Ltd., Gimpo, Korea) included 6 strains of probiotics: Bifidobacterium bifidum (KCTC 12199BP), B. lactis (KCTC11904BP), B. longum (KCTC 12200BP), Lactobacillus acidophilus (KCTC 11906BP), L. rhamnosus (KCTC 12202BP), and Streptococcus thermophilus (KCTC 11870BP). A total of 5 × 109 viable cells in a lyophilized powder form were included in a capsule and constituted 13.1% (w/w) of the total weight (500 mg/capsule). The other ingredients in the capsules included maltodextrin (50%, w/w), corn starch (35.9%, w/w), and silicon dioxide (1%, w/w). Two capsules were administered orally, daily, for 4 weeks. The placebo powder had almost the same contents as the active medication, although the bacteria were replaced with maltodextrin.
Analysis of fecal microbiota
Fecal samples were collected from patients before and after treatment using a sterile container, which was then brought to the laboratory in a frozen condition and stored at −80°C until analysis. We measured the quantity of the 6 probiotic strains that were included in the probiotic mixture. Moreover, as the ratio of Firmicutes to Bacteroidetes in fecal samples of patients with IBS is reportedly increased as compared with healthy controls,(13,14) the quantity of these 2 bacterial phyla were assessed. The methods of preparation of bacterial genomic DNA from the fecal samples and the quantitative real-time PCR tests were performed, as described previously.(8) The primer sets used in quantitative real-time PCR are shown in Table 1.
Table 1.
Oligonucleotides used for quantitative real-time polymerase chain reaction assays
| Target bacteria | Primer sets | Primer sequences (5'-3') | References |
|---|---|---|---|
| B. bifidum | BiBIF-1 | CCACATGATCGCATGTGATTG | (18) |
| BiBIF-2 | CCGAAGGCTTGCTCCCAAA | ||
| B. lactis | BlactF | CCCTTTCCACGGGTCCC | (19) |
| BlactR | AAGGGAAACCGTGTCTCCAC | ||
| B.longum | BlonF | CAGTTGATCGCATGGTCTT | (20) |
| BlonR | TACCCGTCGAAGCCAC | ||
| L. acidophilus | F-acid-IS | GAAAGAGCCCAAACCAAGTGATT | (21) |
| R-acid-IS | CTTCCCAGATAATTCAACTATCGCTTA | ||
| L. rhamnosus | LU-5 | CTAGCGGGTGCGACTTTGTT | (22) |
| RhaII | GCGATGCGAATTTCTATTAT | ||
| S. thermophilus | ST-F | ACGGAATGTACTTGAGTTTC | (23) |
| ST-R | TTTGGCCTTTCGACCTAAC | ||
| Bacteriodetes | Bact934F | GGARCATGTGGTTTAATTCGATGAT | (24) |
| Bact1060R | AGCTGACGACAACCATGCAG | ||
| Firmicutes | Firm934F | GGAGYATGTGGTTTAATTCGAAGCA | (24) |
| Firm1060R | AGCTGACGACAACCATGCAC |
Statistical analysis
SPSS for Windows (ver. 18.0; SPSS, Chicago, IL) was used for statistical analysis. Continuous variables were analyzed using Student’s t tests and categorical variables were analyzed using Chi-square tests or Fisher’s exact tests. To assess the quantitative changes of fecal microbiota before and after the study period in both groups, paired t tests were performed. To evaluate the change in the stool form/frequency, total symptom score, and subtotal score in the 4 IBS symptom domains over time, and to compare the score between the groups, we used a general linear model with repeated-measures analysis of variance. Results were considered statistically significant when the p values were <0.05.
Results
Between October 2009 and May 2012, a total of 103 patients were screened. Eight patients did not meet the inclusion criteria and 14 dropped out; finally, 81 patients (placebo group: 42, probiotics group: 39) completed the study. Among these patients, 40 in the placebo group and 38 in the probiotics group submitted both pre- and post-study fecal samples (96.3%). There was no significant difference in the baseline characteristics between the 2 groups (Table 2). IBS with diarrhea was the most common subtype of IBS in both groups.
Table 2.
Baseline characteristics of patients
| Placebo group (n = 42) | Probiotics group (n = 39) | p value | |
|---|---|---|---|
| Male, n (%) | 24 (57.1) | 19 (48.7) | 0.448 |
| Mean age ± SD (years) | 58.8 ± 13.3 | 59.9 ± 11.1 | 0.712 |
| IBS subtype, n (%) | 0.548 | ||
| IBS-C | 6 (14.3) | 9 (23.1) | |
| IBS-D | 23 (54.8) | 16 (41.0) | |
| IBS-M | 9 (21.4) | 8 (20.5) | |
| IBS-U | 4 (9.5) | 6 (15.4) | |
| Stool form (BSFS) | 4.5 | 4.6 | 0.682 |
| Stool frequency/week | 8.8 | 7.9 | 0.489 |
| Total symptom score | 21.2 ± 12.3 | 22.1 ± 13.3 | 0.773 |
| Pain/discomfort score | 5.4 ± 3.5 | 4.9 ± 3.9 | 0.552 |
| Constipation score | 4.7 ± 4.9 | 4.8 ± 4.5 | 0.877 |
| Diarrhea score | 2.9 ± 4.0 | 5.5 ± 5.3 | 0.013 |
| Bloat/gas score | 8.3 ± 4.7 | 6.8 ± 4.8 | 0.155 |
BSFS, Bristol Stool Form Scale; IBS, irritable bowel syndrome; IBS-C, IBS with constipation; IBS-D, IBS with diarrhea; IBS-M, mixed IBS; IBS-U, un-subtyped IBS; SD, standard deviation.
Fig. 2 shows the quantitative changes in probiotic strains in fecal samples before and after the study period. There was no significant difference in the levels of probiotic strains in the placebo group. However, the concentrations of most of the probiotic strains significantly increased after ingestion in the probiotics group (B. bifidum, p = 0.043; B. lactis, p<0.001; L. acidophilus, p = 0.016; L. rhamnosus, p<0.001). There was no significant difference in the levels of Bacteroidetes and Firmicutes and in the Firmicutes/Bacteroidetes ratio before and after the study period in both groups (Fig. 3).
Fig. 2.
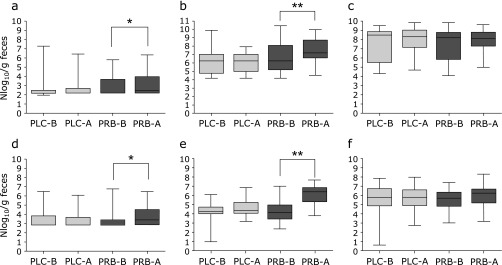
The quantitative changes in the probiotics in the fecal sample before and after the study period. (a) Bifidobacterium bifidum, (b) B. lactis, (c) B. longum, (d) Lactobacillus acidophilus, (e) L. rhamnosus, (f) Streptococcus thermophilus; PLC-B, placebo group before the study period; PLC-A, placebo group after the study period; PRB-B, probiotics group before the study period; PRB-A, probiotics group after the study period; *p<0.05, **p<0.001.
Fig. 3.
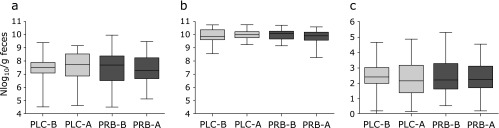
The quantitative changes in the levels of Bacteroidetes and Firmicutes in the fecal sample before and after study period. (a) Firmicutes, (b) Bacteroidetes, (c) Firmicutes/Bacteroidetes ratio; PLC-B, placebo group before the study period; PLC-A, placebo group after the study period; PRB-B, probiotics group before the study period; PRB-A, probiotics group after the study period.
The proportion of patients with adequate symptom relief was higher in the probiotics group than in the placebo group (Table 3); however, the difference was not statistically significant (74.4% vs 61.9%, p = 0.230). In addition, there was no significant difference in the proportion of patients with adequate symptom relief in both groups according to the IBS subtype.
Table 3.
Proportion of patients who reported adequate symptom relief
| Placebo group (n = 42) | Probiotics group (n = 39) | p value | |
|---|---|---|---|
| Total, n (%) | 26 (61.9) | 29 (74.4) | 0.230 |
| IBS-C | 5 (83.3) | 6 (66.7) | 0.604 |
| IBS-D | 12 (52.2) | 13 (81.3) | 0.063 |
| IBS-M | 6 (66.7) | 5 (62.5) | 1.0 |
| IBS-U | 3 (75.0) | 5 (83.3) | 1.0 |
IBS-C, IBS with constipation; IBS-D, IBS with diarrhea; IBS-M, mixed IBS; IBS-U, un-subtyped IBS.
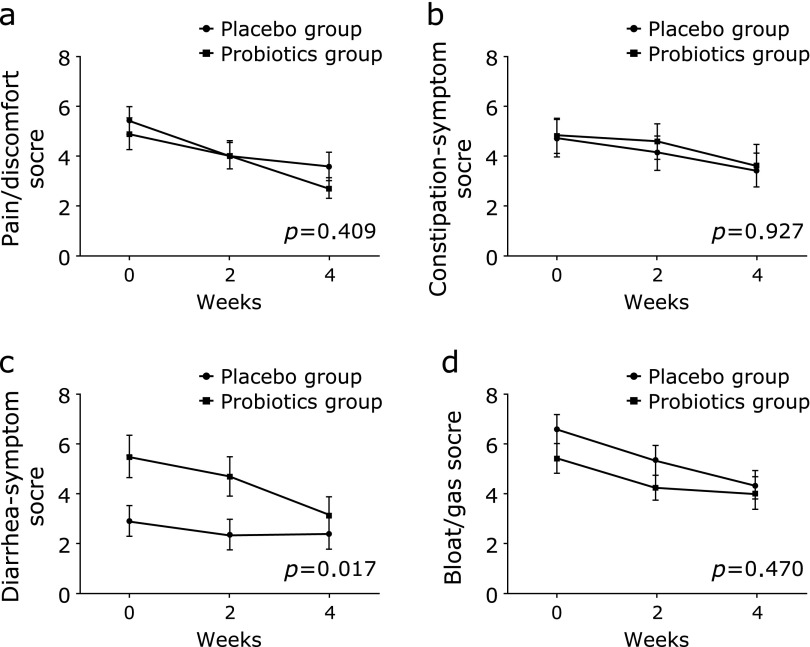
No time effect for stool form and frequency was noted in both groups (Fig. 4a and b). The repeated-measures analysis of variance showed a significant time effect for the total symptom score (p<0.001); however, the decrease in score over time did not significantly differ according to the group (p = 0.703) (Fig. 4c).
Fig. 4.
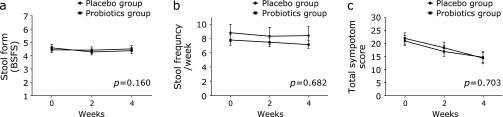
The change in stool form/frequency and total symptom score during the study period. (a) Stool form, (b) Stool frequency, (c) Total symptom score; Means and SE are shown. P values were calculated for time-group interactions by repeated-measures analysis of variance.
Fig. 5 shows the change of the subtotal score in the 4 IBS symptom domains during the study period. Similar to the total symptom score, each subtotal score of the 4 IBS symptom domains significantly decreased over time (pain/discomfort score, p<0.001; constipation-symptom score, p = 0.017; diarrhea-symptom score, p<0.001; bloating/gas score, p<0.001). However, the time effect showed a significant difference according to the groups only for the diarrhea-symptom score (p = 0.017).
Fig. 5.
The change of score in 4 IBS symptom domains during the study period. (a) Pain/discomfort score, (b) Constipation-relate symptom score, (c) Diarrhea-related symptom score, (d) Bloat/gas score; Means and SE are shown. P values were calculated for time-group interactions by repeated-measures analysis of variance.
Discussion
In a previous study wherein a probiotic mixture—similar to that used in the present study—was used in IBS patients, the levels of only a few probiotic strains increased in the fecal samples of the probiotics group.(8) Hence, the authors could not easily explain the improvement of IBS symptoms in the probiotics group in relation to the change in fecal microbiota. In contrast, in the present study, we demonstrated that the 4-week administration of a multi-species probiotic mixture could successfully increase the concentration of each probiotic strain in the subjects’ intestines, and it is more effective than the placebo in attenuating diarrhea-related symptoms in IBS patients. However, the ratio of the Firmicutes/Bacteroidetes levels did not significantly differ before and after the study period in both groups. These results imply that the mechanisms involved in improving IBS symptoms after the ingestion of probiotics might involve more factors than just changes in the gut microbiota composition.(15)
The proportion of patients with adequate symptom relief was 12.5% higher in the probiotics group than in the placebo group. This finding is consistent with the fact that most treatments showed an additional benefit of approximately 10% in the relief of IBS symptoms as compared with the placebo.(16) However, we believe that our results failed to reach statistical significance because the rate of symptom relief in the placebo group in the present study was very high (61.9%), compared with that in previous studies (approximately 40%).(16) This finding confirms that a remarkably high placebo effect is a major obstacle in evaluating the true efficacy of probiotics on IBS treatment.(5) Hence, better study designs should be developed for clinical trials involving probiotics for IBS treatment.
When subgroup analyses were performed according to the IBS type, no significant difference was noted in the proportion of patients with adequate symptom relief between the probiotics and placebo groups. However, the difference in the proportion of patients with adequate symptom relief between the 2 groups was most remarkable in patients with diarrhea-type IBS (IBS-D) (81.3% vs 52.2%). Moreover, among the 4 IBS symptom domains, the degree of symptom relief over time was significantly higher in the probiotics group than in the placebo group in terms of the diarrhea-symptom score. In the previous study, which reported the effects of the same probiotic mixture used in the present study, although subgroup analysis was not performed according to the type of IBS due to a small sample size, >50% of patients had IBS-D.(8) Another Korean study on the use of multi-species probiotic mixtures, with a similar composition to that used in the present study, also confirmed the effect of probiotics in patients with IBS-D.(17) These results suggest that the effects of the multi-species probiotic mixture may primarily be greater in patients with diarrhea-dominant IBS.
In the present study, there was no time effect for stool form and frequency in the probiotics and placebo groups. In contrast, the total symptom score significantly decreased over time in both groups. These results suggest that when therapeutic interventions are attempted in patients with IBS, the therapeutic effects can be measured using the patients’ subjective responses, irrespective of any improvement in objective symptoms. These findings confirmed the importance of the psychological aspect in the perception of symptoms in IBS patients.
This study has several limitations. First, the study duration was short. We demonstrated that most probiotic strains could successfully colonize in patients’ intestines after a relatively short administration period. However, because we could not follow up the patients after the end of study, we could not determine whether the increased fecal concentration of probiotic strains would be maintained after stopping the probiotic administration. Similarly, we did not assess the duration for which the diarrhea-related symptoms in IBS patients would remain attenuated after stopping probiotic administration. Second, among 6 strains included in the multi-species probiotic mixture, the concentration of 2 strains did not increase in the fecal sample of the probiotics group. As we already mentioned in the introduction, because S. thermophilus is just a starter in probiotic production, no change in the fecal concentration of this strain is acceptable. However, we cannot fully explain the reason why the concentration of B. longum did not increase. Considering that this strain did not increase also in the previous study,(8) we just speculate that this specific strain might be relatively difficult to be settled down in human’s intestine after a short duration of ingestion. Third, although IBS decreases the patient’s quality of life, we did not analyze quality of life using validated tool. Finally, mean age of enrolled patients was close to 60 years old. Therefore, to determine whether we could apply the results of this study to younger IBS patients, further studies are needed.
In conclusion, the 4-week administration of a multi-species probiotic mixture significantly increased the fecal concentration of most probiotic strains and improved the diarrhea-symptom score in IBS patients.
Acknowledgments
This study was funded by Cell Biotech, Co., Ltd., Korea.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–660. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 3.Nellesen D, Yee K, Chawla A, Lewis BE, Carson RT. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm. 2013;19:755–764. doi: 10.18553/jmcp.2013.19.9.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enck P, Junne F, Klosterhalfen S, Zipfel S, Martens U. Therapy options in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2010;22:1402–1411. doi: 10.1097/MEG.0b013e3283405a17. [DOI] [PubMed] [Google Scholar]
- 5.Rogers NJ, Mousa SA. The shortcomings of clinical trials assessing the efficacy of probiotics in irritable bowel syndrome. J Altern Complement Med. 2012;18:112–119. doi: 10.1089/acm.2011.0015. [DOI] [PubMed] [Google Scholar]
- 6.American College of Gastroenterology Task Force on Irritable Bowel Syndrome. Brandt LJ, Chey WD, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 7.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–1561. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 8.Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52–59. doi: 10.1111/jgh.12322. [DOI] [PubMed] [Google Scholar]
- 9.Delorme C. Safety assessment of dairy microorganisms: Streptococcus thermophilus. Int J Food Microbiol. 2008;126:274–277. doi: 10.1016/j.ijfoodmicro.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel BM, Bolus R, Agarwal N, et al. Measuring symptoms in the irritable bowel syndrome: development of a framework for clinical trials. Aliment Pharmacol Ther. 2010;32:1275–1291. doi: 10.1111/j.1365-2036.2010.04464.x. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Mangel AW, Fehnel SE, Drossman DA, Mayer EA, Talley NJ. Primary endpoints for irritable bowel syndrome trials: a review of performance of endpoints. Clin Gastroenterol Hepatol. 2007;5:534–540. doi: 10.1016/j.cgh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Rajilić-Stojanović M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery IB, O'Toole PW, Öhman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 15.Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39:1033–1042. doi: 10.1111/apt.12728. [DOI] [PubMed] [Google Scholar]
- 16.Enck P, Horing B, Weimer K, Klosterhalfen S. Placebo responses and placebo effects in functional bowel disorders. Eur J Gastroenterol Hepatol. 2012;24:1–8. doi: 10.1097/MEG.0b013e32834bb951. [DOI] [PubMed] [Google Scholar]
- 17.Ki Cha B, Mun Jung S, Hwan Choi C. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–227. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 18.Matsuki T, Watanabe K, Tanaka R, Oyaizu H. Rapid identification of human intestinal bifidobacteria by 16S rRNA-targeted species- and group-specific primers. FEMS Microbiol Lett. 1998;167:113–121. doi: 10.1111/j.1574-6968.1998.tb13216.x. [DOI] [PubMed] [Google Scholar]
- 19.Malinen E, Kassinen A, Rinttilä T, Palva A. Comparison of real-time PCR with SYBR Green I or 5'-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology. 2003;149 (Pt 1):269–277. doi: 10.1099/mic.0.25975-0. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 21.Haarman M, Knol J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2006;72:2359–2365. doi: 10.1128/AEM.72.4.2359-2365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Y, Kato N, Liu C, Matsumiya Y, Kato H, Watanabe K. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol Lett. 2000;187:167–173. doi: 10.1111/j.1574-6968.2000.tb09155.x. [DOI] [PubMed] [Google Scholar]
- 23.Tilsala-Timisjärvi A, Alatossava T. Development of oligonucleotide primers from the 16S-23S rRNA intergenic sequences for identifying different dairy and probiotic lactic acid bacteria by PCR. Int J Food Microbiol. 1997;35:49–56. doi: 10.1016/s0168-1605(97)88066-x. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Xia X, Tang R, Wang K. Real-time PCR quantification of the predominant bacterial divisions in the distal gut of Meishan and Landrace pigs. Anaerobe. 2008;14:224–228. doi: 10.1016/j.anaerobe.2008.04.001. [DOI] [PubMed] [Google Scholar]