Abstract
Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is implicated in vascular endothelial function. Vascular endothelial function is a potent regulator of arterial stiffness, an independent risk factor for cardiovascular disease. However, it is unknown whether LOX-1 is associated with arterial stiffness. Plasma concentrations of soluble LOX-1 (sLOX-1) and brachial-ankle pulse wave velocity (baPWV, an index of arterial stiffness) were measured in 143 individuals between 51 and 83 years of age. Plasma sLOX-1 concentration was correlated with baPWV (r = 0.288, p = 0.0005). In stepwise regression analysis, plasma sLOX-1 concentration was associated with baPWV, after adjusting for age; body mass index; blood pressure; heart rate; blood levels of cholesterol, triglycerides, glucose, hemoglobin A1c, and insulin; sex; and use of antihypertensives, lipid-lowering agents, and other medications (R2 = 0.575, p<0.0001). Multiple logistic regression demonstrated that plasma sLOX-1 concentration was independently associated with elevated baPWV (≥14.0 m/s; odds ratio, 1.01; 95% confidence interval, 1.00–1.03; p = 0.03). These results suggest that LOX-1 is associated with arterial stiffness.
Keywords: age, arterial stiffness, lectin-like oxidized low-density lipoprotein receptor-1, pulse wave velocity
Introduction
Central arteries such as the aorta and the carotid artery have elastic properties that buffer the pulsations in blood pressure and blood flow via wall extension. Arterial stiffness increases with age.(1) Increased arterial stiffness (i.e., decreased buffering function) is a well-established cardiovascular risk factor.(2) Structural factors such as elastin and collagen content in the arterial media are implicated in arterial stiffness. In addition, functional factors (e.g., endothelial function and sympathetic nervous system activity) play a role in regulation of arterial stiffness. Increased oxidative stress and decreased anti-oxidant capacity are functional factors for arterial stiffening. Brinkley et al.(3) reported that plasma oxidized low-density lipoprotein (oxLDL) levels are independently associated with arterial stiffness. However, the mechanisms underlying the oxLDL-related arterial stiffening have not been fully elucidated.
Lectin-like oxLDL receptor-1 (LOX-1) is the major receptor for oxLDL on the surface of endothelial cells.(4) Circulating levels of soluble LOX-1 (sLOX-1) have been investigated as a predictor of coronary and peripheral artery disease.(5,6) Animal studies have suggested that upregulation of LOX-1 reduces endothelium-dependent vasodilation via decreased nitric oxide (NO) availability.(7,8) In addition, a human study demonstrated that circulating levels of sLOX-1 are correlated with endothelial NO synthase (NOS) activity.(9) NO is a potent regulator of arterial stiffness.(10,11) However, it is unknown whether LOX-1 is related to arterial stiffness. The aim of this study was to investigate whether plasma concentrations of sLOX-1 are associated with arterial stiffness in middle-aged and older individuals.
Materials and Methods
Participants
Measurements were performed in 154 individuals. Smokers and premenopausal women were excluded from the analysis because both smoking and menstrual cycle affect arterial stiffness.(12,13) The remaining 143 individuals, between the ages of 51 and 83 years, were analysed. Subjects were divided into two groups based on brachial-ankle pulse wave velocity (baPWV), an index of arterial stiffness: low (<14.0 m/s) or high (≥14.0 m/s, Table 1).(14,15) Subjects refrained from alcohol consumption and intense physical activity starting the day before testing, and caffeine consumption on the day of testing.
Table 1.
Characteristics of subjects
| baPWV<14.0 m/s (n = 32) | baPWV≥14.0 m/s (n = 111) | p value | |
|---|---|---|---|
| sLOX-1 (ng/L) | 144 ± 11 | 211 ± 11 | 0.002 |
| baPWV (m/s) | 12.8 ± 0.1 | 17.2 ± 0.2 | <0.0001 |
| Sex (male/female) | 7/25 | 28/83 | 0.70 |
| Use of antihypertensive medication (no/yes) | 29/3 | 75/36 | 0.01 |
| Use of lipid-lowering agent (no/yes) | 31/1 | 94/17 | 0.07 |
| Use of other medications (no/yes) | 23/9 | 84/27 | 0.66 |
| Age (years) | 62.8 ± 1.2 | 69.7 ± 0.5 | <0.0001 |
| Body mass index (kg/m2) | 22.8 ± 0.5 | 22.7 ± 0.3 | 0.75 |
| Systolic blood pressure (mmHg) | 121 ± 2 | 141 ± 1 | <0.0001 |
| Diastolic blood pressure (mmHg) | 73 ± 1 | 79 ± 1 | 0.0002 |
| Heart rate (bpm) | 59 ± 1 | 62 ± 1 | 0.05 |
| LDL (mg/dl) | 138 ± 5 | 125 ± 3 | 0.02 |
| HDL (mg/dl) | 70 ± 3 | 67 ± 2 | 0.48 |
| Triglycerides (mg/dl) | 96 ± 7 | 108 ± 5 | 0.19 |
| Glucose (mg/dl) | 97 ± 2 | 101 ± 2 | 0.28 |
| Hemoglobin A1c (%) | 5.7 ± 0.1 | 5.9 ± 0.1 | 0.29 |
| Insulin (µU/dl) | 4.9 ± 0.4 | 6.3 ± 0.5 | 0.15 |
Values are means ± SE. baPWV, brachial-ankle pulse wave velocity; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; sLOX-1, soluble lectin-like oxidized low-density lipoprotein receptor-1.
This study was approved by the Ethics Committee of Ryutsu Keizai University (Approval Number 1) and complied with the principles of the Helsinki Declaration. All participants gave their written informed consent prior to study participation.
BaPWV assessment
After a resting period of at least 20 min, baPWV was measured as in our previous studies.(16,17) Briefly, brachial and post-tibial artery pressure waveforms were obtained simultaneously in duplicate with cuffs connected to an air plethysmographic sensor and an oscillometric pressure sensor (formPWV/ABI; Omron Colin, Tokyo, Japan). The distance traveled by the pulse wave from the heart to the brachial recording site (Distance A) and that from the heart to the post-tibial recording site (Distance B) were calculated based on each subject’s height. The time it took from when the pulse wave reached the brachial recording site to when it reached the post-tibial recording site (T) was determined based on the time delay between the brachial and post-tibial ’foot’ waveforms. BaPWV was calculated as the difference between Distance A and B divided by T. Our representative day-to-day coefficient of variation (CV) for baPWV was 2.2 ± 1.3%.(16)
Blood chemical analysis
Blood samples were collected in the morning after an overnight fast. Plasma sLOX-1 concentrations were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) with two specific monoclonal antibodies against sLOX-1, with recombinant sLOX-1 as the assay standard. In our laboratory, intra-assay and inter-assay CVs for blood sLOX-1 concentration were 1.6–5.2% and 3.9–7.7%, respectively.(5)
Serum concentrations of low- and high-density lipoprotein cholesterol (LDL and HDL, respectively) and triglycerides were measured by using commercial enzymatic kits (Cholestest®LDL; Sekisui Medical, Tokyo, Japan: Determiner L HDL-C or MetaboLead HDL-C; Kyowa Medex, Tokyo, Japan: and Determiner C-TG; Kyowa Medex) and an autoanalyzer (JCA-BM 8060; JEOL, Tokyo, Japan). Plasma glucose concentrations and haemoglobin A1c were determined by using a enzymatic kit (L-type Wako Glu2; Wako Pure Chem. Ind., Ltd., Osaka, Japan), a latex agglutination kit (Determiner L HbA1c, Kyowa Medex), and an autoanalyzer (JCA-BM 9130; JEOL). Serum insulin levels were assessed by an electrochemiluminescence immunoassay by using an analyzer (E170; Roche Diagnostics K.K., Tokyo, Japan) and the designated regents.
Statistical analysis
Results are given as means ± SE. The unpaired t test or chi-squared test was used to detect differences between groups, as appropriate. Relationships between two variables were investigated using Pearson’s simple correlation analysis. Stepwise and logistic regression were used to identify independent predictors. P values <0.05 were considered statistically significant.
Results
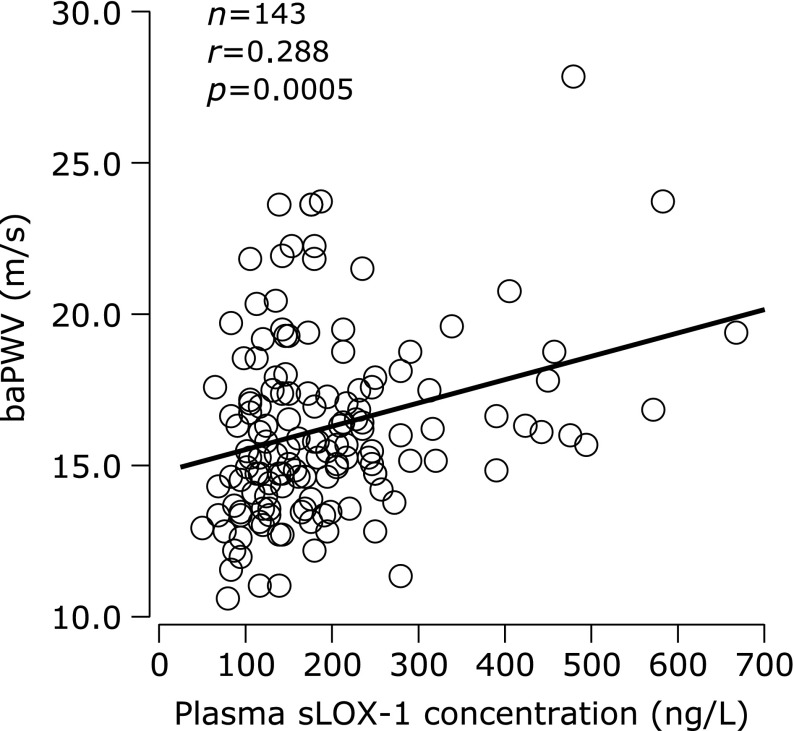
BaPWV was correlated with plasma sLOX-1 concentration (Fig. 1), age (r = 0.466, p<0.0001), systolic blood pressure (SBP; r = 0.615, p<0.0001), diastolic blood pressure (DBP; r = 0.402, p<0.0001), heart rate (HR; r = 0.238, p = 0.004), plasma glucose concentration (r = 0.274, p = 0.0009), and hemoglobin A1c (r = 0.261, p = 0.001). BaPWV was not correlated with body mass index (BMI) and serum concentrations of LDL, HDL, triglycerides, and insulin. Plasma sLOX-1 concentration, age, SBP, and HR were identified as independent predictors of baPWV in the stepwise regression analysis that included sex (male = 0, female = 1); use of antihypertensives, lipid-lowering agents, and other medications (yes = 0, no = 1); age; BMI; SBP; DBP; HR; and blood levels of sLOX-1, LDL, HDL, triglycerides, glucose, hemoglobin A1c, and insulin (Table 2).
Fig. 1.
Correlation between plasma concentration of soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1) and brachial-ankle pulse wave velocity (baPWV).
Table 2.
Stepwise regression analysis to identify independent predictors of arterial stiffness (brachial-ankle pulse wave velocity)
| Independent predictors (n = 143, R2 = 0.575, p<0.0001) | ||||
|---|---|---|---|---|
| Regression coefficient | SE | β | p value | |
| Constant | –1427.5 | 254.5 | <0.001 | |
| Systolic blood pressure (mmHg) | 9.9 | 1.0 | 0.557 | <0.001 |
| Age (years) | 14.8 | 2.9 | 0.299 | <0.001 |
| Heart rate (bpm) | 10.1 | 2.2 | 0.248 | <0.001 |
| sLOX-1 (ng/L) | 0.3 | 0.1 | 0.120 | 0.04 |
Excluded variables: sex (male = 0, female = 1), use of antihypertensive medication (yes = 0, no = 1), use of lipid-lowering agent (yes = 0, no = 1), use of other medications (yes = 0, no = 1), body mass index (kg/m2), diastolic blood pressure (mmHg), low-density lipoprotein cholesterol (mg/dl), high-density lipoprotein cholesterol (mg/dl), triglycerides (mg/dl), glucose (mg/dl), hemoglobin A1c (%), insulin (µU/dl). sLOX-1, soluble lectin-like oxidized low-density lipoprotein receptor-1.
Plasma sLOX-1 concentrations, proportion of subjects on antihypertensive medications, age, SBP, and DBP were higher in the high baPWV group than in the low baPWV group (Table 1). Serum LDL concentrations were lower in the high baPWV group compared to the low baPWV group. Multiple logistic regression analysis demonstrated that plasma sLOX-1 concentration, age, SBP, and HR were associated with baPWV, independent of other factors (Table 3).
Table 3.
Odds ratios for increased arterial stiffness (brachial-ankle pulse wave velocity ≥14.0 m/s)
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| Odds ratio | (95% CI) | p value | Odds ratio | (95% CI) | p value | ||
| sLOX-1 | 1.01 | (1.00–1.02) | 0.004 | 1.01 | (1.00–1.03) | 0.03 | |
| Sex | 1.20 | (0.47–3.09) | 0.69 | 6.27 | (0.62–63.44) | 0.12 | |
| Antihypertensive use | 4.64 | (1.32–16.25) | 0.01 | 4.69 | (0.45–49.10) | 0.19 | |
| Lipid-lowering agent use | 5.61 | (0.72–43.87) | 0.10 | 5.84 | (0.08–416.44) | 0.41 | |
| Other medication use | 0.92 | (0.40–2.08) | 0.83 | 0.49 | (0.10–2.42) | 0.37 | |
| Age | 1.25 | (1.14–1.36) | <0.0001 | 1.31 | (1.07–1.60) | 0.009 | |
| Body mass index | 0.98 | (0.85–1.13) | 0.75 | 0.81 | (0.58–1.13) | 0.21 | |
| Systolic blood pressure | 1.13 | (1.08–1.19) | <0.0001 | 1.40 | (1.16–1.69) | 0.0005 | |
| Diastolic blood pressure | 1.10 | (1.04–1.16) | 0.0005 | 0.87 | (0.72–1.04) | 0.12 | |
| Heart rate | 1.05 | (1.00–1.11) | 0.05 | 1.18 | (1.03–1.34) | 0.01 | |
| LDL | 0.98 | (0.97–1.00) | 0.02 | 1.00 | (0.96–1.03) | 0.77 | |
| HDL | 0.99 | (0.97–1.01) | 0.48 | 1.00 | (0.94–1.05) | 0.86 | |
| Triglycerides | 1.00 | (0.99–1.02) | 0.19 | 1.00 | (0.97–1.02) | 0.88 | |
| Glucose | 1.02 | (0.99–1.05) | 0.29 | 0.92 | (0.82–1.03) | 0.16 | |
| Hemoglobin A1c | 1.56 | (0.68–3.58) | 0.29 | 10.69 | (0.41–278.39) | 0.15 | |
| Insulin | 1.11 | (0.96–1.29) | 0.14 | 1.22 | (0.80–1.88) | 0.36 | |
CI, confidence interval; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; sLOX-1, soluble lectin-like oxidized low-density lipoprotein receptor-1.
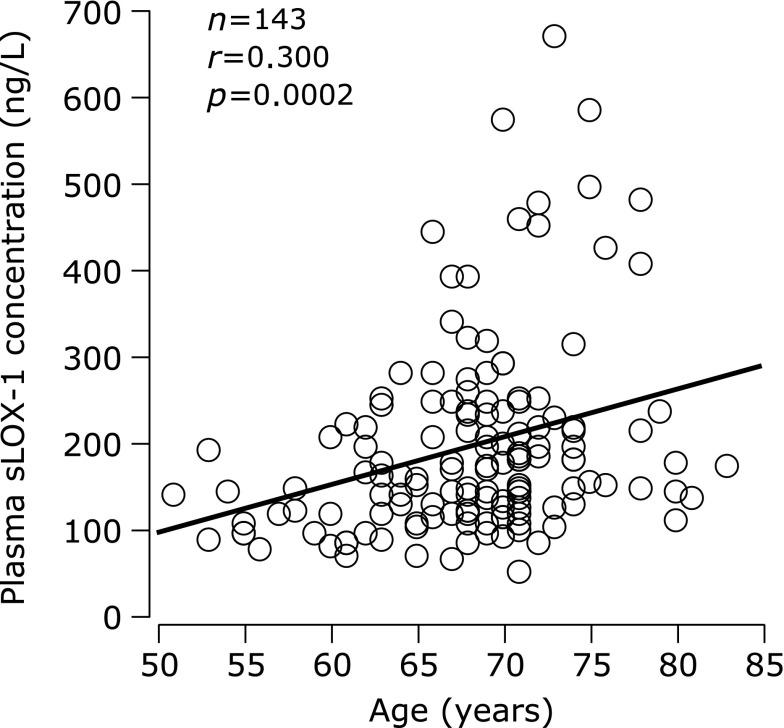
Age was correlated with plasma sLOX-1 levels (Fig. 2). Correlation coefficients between age and plasma sLOX-1 concentration were comparable between the low (r = 0.232, p = 0.20) and high (r = 0.229, p = 0. 01) baPWV groups although the coefficient in the low baPWV group did not reach statistical significance because of the small sample size.
Fig. 2.
Correlation between age and plasma concentration of soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1).
Discussion
First, plasma sLOX-1 concentration was correlated with baPWV. In stepwise regression analysis, plasma sLOX-1 concentration was associated with baPWV, after adjusting for sex; use of antihypertensives, lipid-lowering agents, and other medications; age; BMI; blood pressure; HR; and blood levels of cholesterol, triglycerides, glucose, hemoglobin A1c, and insulin. Second, plasma concentrations of sLOX-1 were higher in the high baPWV group than in the low baPWV group. Multiple logistic regression analysis demonstrated that plasma sLOX-1 concentration was independently associated with elevated baPWV. These results suggest that LOX-1 is associated with arterial stiffness in middle-aged and older individuals.
This study chose 14.0 m/s as cutoff value to divide subjects into the low or high baPWV group. Munakata et al.(14) reported that higher baPWV (≥14.0 m/s) was an independent risk factor for microalbuminuria after two years in 321 hypertensive patients. Logistic regression analysis in 10,828 healthy subjects showed that baPWV >14.0 m/s was independently associated with higher risk based on the Framingham score and the presence of atherosclerotic cardiovascular disease.(15) On the other hand, recent studies have proposed a higher cutoff baPWV value. Turin et al.(18) demonstrated that risk of all-cause mortality was higher in the highest baPWV group (≥17.0 m/s) relative to the lowest baPWV group (<14.0 m/s) in 2,642 individuals without a history of cardiovascular disease. Miyano et al.(19) documented that baPWV ≥18.6 m/s was associated with an increased risk of three-year total cardiovascular mortality in 530 normotensive and hypertensive subjects. Based on these and other previous studies, guidelines for non-invasive vascular function testing by the Japanese Circulatory Society in 2013 defined baPWV of 14.0 m/s as indicative of cardiovascular risk, which was associated with recommendations for lifestyle modification, and 18.0 m/s as a marker of organ dysfunction. When the present subjects were categorized according to a baPWV cutoff of 18.0 m/s, an independent association between plasma sLOX-1 concentration and baPWV was not detected (data not shown). In a previous study by Kataoka et al.,(20) carotid arterial endothelial cells covering early atherosclerotic lesions were more frequently positive for LOX-1 expression (71.4%) relative to those in advanced atherosclerotic lesions (33.3%). LOX-1 may be implicated in the early stage of arterial stiffening rather than its more advanced stages.
The correlation coefficient between baPWV and plasma sLOX-1 concentration was lower than between baPWV and age, SBP, and HR. First, there seems to be difference in role of LOX-1 between early and advanced stages of atherosclerosis.(20) This difference may be associated with the lower correlation coefficient. Unfortunately, the sample size in the low baPWV group was insufficient to test this hypothesis. It is an important next step. Second, LOX-1 is possibly associated with baPWV via NO and other factors although SBP and HR directly participate in regulation of baPWV. There is an unequivocal interaction between blood pressure and arterial stiffness; elevated blood pressure increases arterial stiffness and vice versa. Progressive increases in HR caused by pacing were accompanied by progressive increases in elastic arterial stiffness.(21) Finally, plasma sLOX-1 concentrations were used as a surrogate marker of LOX-1 expression in the vascular endothelium although age, SBP and HR were directly measured. However, this study demonstrated that plasma sLOX-1 concentration was associated with baPWV, independent of other factors. This is an important step to elucidate the LOX-1 pathway of arterial stiffening.
Incorporation of oxLDL into the vascular wall via LOX-1 initiates and promotes further atherosclerosis, leading to reduction of NO-related vasorelaxation. Xu et al.(8) demonstrated that acetylcholine (ACh)-mediated vasodilation and endothelial NOS protein expression in apolipoprotein-E knockout mice were lower than those in wild-type mice, but restored by anti-LOX-1 antibodies. Eichhorn et al.(7) reported that ACh-induced vasorelaxation and gene expression of endothelial NOS were lower in LOX-1 overexpressing mice on a high-fat diet compared to wild-type mice on high-fat diet. These previous studies have used animal models. However, expression of LOX-1 was observed also in the human carotid artery.(20) In addition, Yavuzer et al.(9) demonstrated that circulating levels of sLOX-1 are correlated with endothelial NOS activity. The present results imply that uptake of oxLDL through LOX-1 may be involved in the process of arterial stiffening.
To the best of our knowledge, this is the first study showing a correlation between circulating sLOX-1 levels and age. LOX-1 is upregulated by inflammatory stimuli(22) and advanced glycation end products(23) as well as oxLDL. These factors are induced and enhanced by aging.(24,25) It is possible that these factors increase expression of LOX-1 in endothelial cells of aged subjects, resulting in the positive correlation between plasma concentrations of sLOX-1 and age. However, it is unclear whether there is an independent relationship between LOX-1 levels and age. This study is an initial step in exploring the effects of aging on LOX-1.
The values of blood sLOX-1 level vary among studies. A review by Pirillo and Catapano(26) showed that patients with acute coronary syndrome reveal very high plasma and serum sLOX-1 concentrations. In addition, there are some variations in ng/L order of plasma sLOX-1 concentrations even in subjects without acute coronary syndrome. Kobayashi et al.(27) reported that median values of plasma sLOX-1 concentrations for myocardial infarction patients with ST-elevation, those without ST-elevation, and patients with or without stable coronary artery disease were 241.0, 147.3, and 64.3 ng/L. Brinkley et al.(28) demonstrated that mean values of plasma sLOX-1 concentrations for normal-weight, overweight, and obese postmenopausal women were approximately 30, 40, and 55 ng/L. These studies measured plasma sLOX-1 concentrations using different sets of two monoclonal antibodies that were different from the antibodies used in this study. Besides, methods to determine protein concentrations of the assay standard influence the assay results. The monoclonal antibodies and the methods to determine protein concentrations may be related to the differences in values of plasma sLOX-1 concentrations between our study and the previous studies.
This was a cross-sectional study. Not all possible confounders can be excluded by the study design. It is unclear whether plasma sLOX-1 concentrations correlate well with LOX-1 expression in the vascular endothelium. As pointed out in the Editorial by Brown et al.,(29) plasma sLOX-1 levels may be influenced by LOX-1—regulating enzymes, forces that release LOX-1 from the cell surface, sLOX-1 clearance, and other factors. Further studies are warranted to elucidate the role of LOX-1 in the stiffening of large arteries.
In conclusion, the present results suggest that LOX-1 is related to arterial stiffness in middle-aged and older individuals.
Acknowledgments
This work was supported by the Nakatomi Foundation, JSPS KAKENHI Grant Number 23650437, and the Uehara Memorial Foundation. We thank Ryugasaki City for supporting our study based on a partnership agreement between Ryugasaki City and Ryutsu Keizai University (Ryu Ryu Renkei).
Abbreviations
- ACh
acetylcholine
- baPWV
brachial-ankle pulse wave velocity
- BMI
body mass index
- CI
confidence interval
- CV
coefficient of variation
- DBP
diastolic blood pressure
- ELISA
enzyme-linked immunosorbent assay
- HDL
high-density lipoprotein cholesterol
- HR
heart rate
- LDL
low-density lipoprotein cholesterol
- LOX-1
lectin-like oxidized low-density lipoprotein receptor-1
- NO
nitric oxide
- NOS
nitric oxide synthase
- oxLDL
oxidized low-density lipoprotein
- SBP
systolic blood pressure
- sLOX-1
soluble lectin-like oxidized low-density lipoprotein receptor-1
- T
time it took from when the pulse wave reached the brachial recording site to when it reached the post-tibial recording site
References
- 1.Tomiyama H, Yamashina A, Arai T, et al. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis. 2003;166:303–309. doi: 10.1016/s0021-9150(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. 2012;60:556–562. doi: 10.1161/HYPERTENSIONAHA.112.194779. [DOI] [PubMed] [Google Scholar]
- 3.Brinkley TE, Nicklas BJ, Kanaya AM, et al. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension. 2009;53:846–852. doi: 10.1161/HYPERTENSIONAHA.108.127043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 5.Fukui M, Tanaka M, Senmaru T, et al. LOX-1 is a novel marker for peripheral artery disease in patients with type 2 diabetes. Metabolism. 2013;62:935–938. doi: 10.1016/j.metabol.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Inoue N, Okamura T, Kokubo Y, et al. LOX index, a novel predictive biochemical marker for coronary heart disease and stroke. Clin Chem. 2010;56:550–558. doi: 10.1373/clinchem.2009.140707. [DOI] [PubMed] [Google Scholar]
- 7.Eichhorn B, Muller G, Leuner A, Sawamura T, Ravens U, Morawietz H. Impaired vascular function in small resistance arteries of LOX-1 overexpressing mice on high-fat diet. Cardiovasc Res. 2009;82:493–502. doi: 10.1093/cvr/cvp089. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 9.Yavuzer S, Yavuzer H, Cengiz M, et al. Endothelial damage in white coat hypertension: role of lectin-like oxidized low-density lipoprotein-1. J Hum Hypertens. 2015;29:92–98. doi: 10.1038/jhh.2014.55. [DOI] [PubMed] [Google Scholar]
- 10.Sugawara J, Komine H, Hayashi K, et al. Effect of systemic nitric oxide synthase inhibition on arterial stiffness in humans. Hypertens Res. 2007;30:411–415. doi: 10.1291/hypres.30.411. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson IB, MacCallum H, Cockcroft JR, Webb DJ. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol. 2002;53:189–192. doi: 10.1046/j.1365-2125.2002.1528adoc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi K, Miyachi M, Seno N, et al. Variations in carotid arterial compliance during the menstrual cycle in young women. Exp Physiol. 2006;91:465–472. doi: 10.1113/expphysiol.2005.032011. [DOI] [PubMed] [Google Scholar]
- 13.Stefanadis C, Tsiamis E, Vlachopoulos C, et al. Unfavorable effect of smoking on the elastic properties of the human aorta. Circulation. 1997;95:31–38. doi: 10.1161/01.cir.95.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Munakata M, Miura Y, Yoshinaga K, J-TOPP study group Higher brachial-ankle pulse wave velocity as an independent risk factor for future microalbuminuria in patients with essential hypertension: the J-TOPP study. J Hypertens. 2009;27:1466–1471. doi: 10.1097/HJH.0b013e32832b4740. [DOI] [PubMed] [Google Scholar]
- 15.Yamashina A, Tomiyama H, Arai T, et al. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–622. doi: 10.1291/hypres.26.615. [DOI] [PubMed] [Google Scholar]
- 16.Otsuki T, Takanami Y, Aoi W, Kawai Y, Ichikawa H, Yoshikawa T. Arterial stiffness acutely decreases after whole-body vibration in humans. Acta Physiol (Oxf) 2008;194:189–194. doi: 10.1111/j.1748-1716.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 17.Otsuki T, Shimizu K, Iemitsu M, Kono I. Multicomponent supplement containing Chlorella decreases arterial stiffness in healthy young men. J Clin Biochem Nutr. 2013;53:166–169. doi: 10.3164/jcbn.13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turin TC, Kita Y, Rumana N, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: findings from the Takashima study, Japan. Hypertens Res. 2010;33:922–925. doi: 10.1038/hr.2010.103. [DOI] [PubMed] [Google Scholar]
- 19.Miyano I, Nishinaga M, Takata J, et al. Association between brachial-ankle pulse wave velocity and 3-year mortality in community-dwelling older adults. Hypertens Res. 2010;33:678–682. doi: 10.1038/hr.2010.56. [DOI] [PubMed] [Google Scholar]
- 20.Kataoka H, Kume N, Miyamoto S, et al. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 21.Mangoni AA, Mircoli L, Giannattasio C, Ferrari AU, Mancia G. Heart rate-dependence of arterial distensibility in vivo. J Hypertens. 1996;14:897–901. doi: 10.1097/00004872-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci. 2013;70:2859–2872. doi: 10.1007/s00018-012-1194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jono T, Miyazaki A, Nagai R, Sawamura T, Kitamura T, Horiuchi S. Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) serves as an endothelial receptor for advanced glycation end products (AGE) FEBS Lett. 2002;511:170–174. doi: 10.1016/s0014-5793(01)03325-7. [DOI] [PubMed] [Google Scholar]
- 24.Anuurad E, Enkhmaa B, Gungor Z, et al. Age as a modulator of inflammatory cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2011;31:2151–2156. doi: 10.1161/ATVBAHA.111.232348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai R, Shirakawa J, Fujiwara Y, et al. Detection of AGEs as markers for carbohydrate metabolism and protein denaturation. J Clin Biochem Nutr. 2014;55:1–6. doi: 10.3164/jcbn.13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirillo A, Catapano AL. Soluble lectin-like oxidized low density lipoprotein receptor-1 as a biochemical marker for atherosclerosis-related diseases. Dis Markers. 2013;35:413–418. doi: 10.1155/2013/716325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi N, Hata N, Kume N, et al. Soluble lectin-like oxidized low-density lipoprotein receptor-1 as an early biomarker for ST elevation myocardial infarction: time-dependent comparison with other biomarkers. Circ J. 2011;75:1433–1439. doi: 10.1253/circj.cj-10-0913. [DOI] [PubMed] [Google Scholar]
- 28.Brinkley TE, Kume N, Mitsuoka H, Phares DA, Hagberg JM. Elevated soluble lectin-like oxidized LDL receptor-1 (sLOX-1) levels in obese postmenopausal women. Obesity (Silver Spring) 2008;16:1454–1456. doi: 10.1038/oby.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown J, Nallamshetty S, Plutzky J. Intersecting vectors of basic science research and clinical medicine: LOX-1? Clin Chem. 2010;56:499–501. doi: 10.1373/clinchem.2009.142232. [DOI] [PubMed] [Google Scholar]