Abstract
Objective
To explore the efficacy of local oxytocin for the treatment of post-menopausal vaginal atrophy.
Design
Double-blinded randomised controlled trial.
Setting
Healthy post-menopausal women in Stockholm, Sweden.
Participants
Sixty four post-menopausal women between February and June 2012 at the Karolinska University Hospital Huddinge/Sweden.
Main outcome measures
The efficacy of oxytocin for treatment of vaginal atrophy after seven weeks and cytological evaluation.
Results
The percentage of superficial cells in the vaginal smears and the maturation values were significantly increased after seven weeks of treatment with vagitocin 400 IU (p = 0.0288 and p = 0.0002, respectively). The vaginal pH decreased significantly after seven weeks of treatment with vagitocin 100 IU (p = 0.02). The scores of vaginal atrophy, according to the histological evaluation, were significantly reduced after administration of vagitocin 100 IU (p = 0.03). The thickness of the endometrium did not differ between the treatment and placebo groups after seven weeks of treatment. The symptom experienced as the most bothersome was significantly reduced after seven weeks of treatment in the women receiving vagitocin 400 IU compared to women in the placebo group (p = 0.0089).
Conclusions
Treatment with intravaginally applied oxytocin could be an alternative to local estrogen treatment in women with post-menopausal vaginal atrophy.
Keywords: Estrogen, oxytocin, post-menopausal, vaginal atrophy, vagitocin
Introduction
Menopause occurs as a result of decreased ovarian function and a consequent reduction in estrogen production for 12 months or more. The average menopausal age is around 51 years in the Western world.1 Reduced circulating estrogen concentrations adversely affect the elasticity and the collagen synthesis in the vulvovaginal tissue and the growth of the vaginal epithelial lining, resulting in vaginal atrophy in post-menopausal women. Objective signs of vaginal atrophy are petechiae, friability, vaginal discharge, the presence of a dry and pale vaginal mucosa and loss of the rugae of the vaginal wall.2 Cytological examination of the mucosa reveals a reduction in the percentage of mature cells (superficial cells) and an increase in primitive cell types, such as parabasal cells. These cytological changes in the vaginal epithelium can be quantified by the maturation index (MI) that expresses the percentage of the different cell types occurring in the cytological sample.3,4 The changes can also be measured by the vaginal maturation value (VMV), which is calculated according to the following formula: 0 times the percentage of parabasal cells, + 0.5 times the percentage of intermediate cells, and +1 times the percentage of superficial cells.5 In addition, intravaginal pH increases as a consequence of a less functional epithelium.6,7
The subjective symptoms of vaginal atrophy, such as vaginal dryness, irritation/itching, dysuria, dyspareunia and post-coital bleeding, usually start in the late perimenopausal period and worsen over time as vaginal atrophy progresses.2,8 About 40% of post-menopausal women experience severe symptoms with a subsequent reduction in quality of life and sexual performance.8 These symptoms do not resolve spontaneously and often require treatment, yet only 20–25% of women seek medical advice.2,8 Women might experience symptoms of vaginal atrophy without any objective signs of atrophy, and, conversely, women may have clear atrophic signs without any symptoms.1
Some women may also experience symptoms from the urinary tract, such as urgency, an increased micturition frequency and recurrent urinary tract infections.1,2 Symptoms of urinary incontinence during menopause have been described; however, not all studies show an association.9,10
The most commonly used therapies for atrophic vaginal and urogenital symptoms are local and systemic estrogen treatments. Estrogen can be applied locally as a cream, a slow releasing vaginal ring, or in tablet form.11 Systemic estrogen can be administered in the form of tablets, patches or transdermal gel.1,12
Systemic estrogen treatment is associated with an increased risk of severe side effects, such as endometrial cancer (if not used with progestogen) and breast cancer13 and thromboembolic disease.14 On the other hand some studies show that estrogen may have protective effects against cardiovascular disease.15
Oxytocin is a nonapeptide hormone, which is synthesised in the hypothalamus and secreted through the posterior pituitary gland to systemic circulation.16 The most well-known function of oxytocin is to promote labour and milk ejection. Oxytocin also acts as a neurotransmitter in the brain, where it influences several physiological and behavioural functions. In addition, it is produced locally in many peripheral organs and in certain types of epithelial and endothelial cells and often exerts growth promoting effects.17
In previous studies, local intravaginal application of oxytocin significantly increased the growth of the mucosal epithelium and improved the appearance of the vaginal mucous membrane.18
The aim of this study was to explore the efficacy of local oxytocin for the treatment of post-menopausal vaginal atrophy.
Methods
This double-blind, dose–response, placebo-controlled study was conducted between February and June 2012 at the Karolinska University Hospital Huddinge/Sweden. Ethical approval was obtained from the Regional Ethical Review Board in Stockholm, Sweden (2011/1978-31/2). The study was also approved by the Swedish Medical Product Agency (LVFS 2011:19, 2012-02-01). Written and verbal information were given to all participants before they provided written consent to participate in the study.
Patient recruitment process
Post-menopausal women were recruited through advertising in the local newspaper. Eligible participants were healthy women without serious medical illnesses, without previous, concurrent, or suspected malignant diseases, and without allergies to any ingredient of the trial product, and who were four years post-menopausal (normal or artificial). The women suffered from symptoms of vaginal atrophy and did not use estrogen treatments (systemic or topical) during the three months prior to the trial.
Women who fulfilled the criteria were invited for screening at the hospital. Blood samples were collected from each woman for the evaluation of follicle stimulating hormone (FSH) levels and 17β-estradiol. A gynaecological examination, including measurement of the endometrial thickness with ultrasound technique, was performed by the clinician. A vaginal smear was collected for cytological investigation and vaginal pH was measured. Vaginal biopsies were obtained in a subgroup of 24 women. Endometrial biopsies were taken in women whose uteruses were intact for the procedure.
Inclusion criteria
When the laboratory results were received women who had objective signs of vaginal atrophy had vaginal pH >5, endometrial thickness <4 mm as measured by ultrasound, body mass index (BMI) ≤30 kg/m2 and blood pressure <150/90 mmHg in connection with screening were included in the study and were randomised to treatment. The treatment was initiated immediately after the screening procedure was finalised.
Women with ≥5% superficial cells in the vaginal smears, plasma FSH levels <40 IU/L, 17β-estradiol levels ≥70 pmol/l or malignant changes in the endometrium were retroactively excluded from the study.
Study design
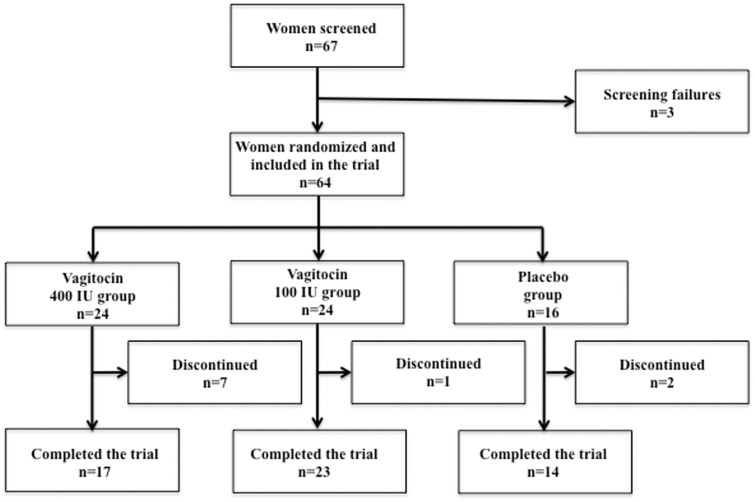
Altogether, 67 women were screened to take part in the study. Three screening failures were noted and only 64 women were randomised to treatment. Twenty-four women received vagitocin gel 400 IU, 24 received vagitocin gel 100 IU and 16 received placebo gel.
As the flowchart shows, some women discontinued the study. The reasons for this are given in Table 1. Note that some of them were screening failures, excluded retroactively from the study (Figure 1).
Table 1.
Women excluded or withdrawn from the study.
| Women excluded from the study | Vagitocin 400 IU n = 24 | Vagitocin 100 IU n = 24 | Placebo n = 16 | Total number |
|---|---|---|---|---|
| Superficial cells >5% at V1 | 3 | 1 | 0 | 4 |
| Abnormal endometrial biopsy | 1 | 0 | 0 | 1 |
| Withdrawal of consent | 2 | 0 | 0 | 2 |
| Adverse events | 1a | 1b | 1c | 3 |
| Total number of women who did not finalise the study | 7 | 2 | 1 | 10 |
Note: The table shows the number of randomised women in the different treatment groups who did not finalise the study because they (1) did not fulfil the inclusion criteria, (2) withdrew their consent or (3) experienced adverse events.
Headache, bExperience of illness, cPalpitations.
Figure 1.
Study flowchart.
The gel
The gel was intravaginally administered once daily during a seven-week period. The vagitocin (400 IU and 100 IU) and placebo gels were prepared by Recipharm AB (Stockholm, Sweden). Oxytocin was provided by Grindex (Riga, Latvia). The gel was based on hypromellose and pH was adjusted to 3.75. Benzoic acid was used as a preservative. The placebo gel was identical to the active gel, except for the absence of oxytocin.
The randomisation and blinding procedure
Active and placebo gels were prefilled in 1 ml syringes. The syringes containing oxytocin or placebo were packed in boxes. Coding and randomisation were performed in a parallel group design by Recipharm AB, Stockholm, Sweden.
The code was blinded to the hospital staff. The active substance and placebo were of identical appearance and with similar packaging and labelling. Only the patient identification and randomisation numbers were written on the label, which was placed on the box containing the prefilled syringes. The treatment code was broken once all assessments were performed and all the data were entered into the database.
Administration of the gel
The principal investigator administered the first dose of the gel to the participating women (V1). The women were then taught how to self-administer the gel at home and were instructed to administer it in the evening after going to bed.
Study protocol
The included women visited the hospital three times: once at the screening/randomisation visit (V1) and once after two and seven weeks of treatment (V2, V3, respectively). The following investigations were performed during the hospital visits:
The clinician performed a gynaecological examination at V1, V2 and V3, and the level of vaginal atrophy was graded according to the scale described below.
The thickness of the endometrial mucosa was measured by ultrasound at V1 and V3 according to the technique described below, and, if possible, endometrial biopsies were collected as described below.
Vaginal smears to determine MI and MV were collected at V1, V2 and V3 according to the technique described below.
Vaginal pH was measured at V1, V2 and V3 with the technique described below.
Vaginal biopsies were taken in a subgroup of women at V1 and V3, as described below, and the biopsies were evaluated according to the protocol described below.
At V1, V2 and V3, each woman reported the intensity of her subjective symptoms according to the scale described below, including that which was considered most bothersome.
Plasma concentrations of FSH and 17β-estradiol
Plasma levels of FSH and 17β-estradiol were measured using well-established radioimmunoassay techniques at the hospital laboratory.
Clinician’s evaluation of the vaginal mucosa
The overall state of the vaginal mucosa was graded by the clinician (based on the level of mucosal dryness, discolour, blanching, rugosity, petechiae and friability) on a scale from 0–3 (0 = normal, 1 = mild, 2 = moderate, and 3 = severe).
Determination of endometrial thickness and collection of endometrial biopsies
Vaginal ultrasound examination was performed using the LOGIQ™ P6 ultrasound system from General Electric Company (Little Chalfont, Buckinghamshire, United Kingdom).
An endometrial biopsy was obtained (whenever practical) using Endorette® endometrial suction curette (Medscand Endorette, E 0020, Medscand Medical AB, Sweden). All the endometrial histology samples were analysed at Aleris Medilab, Täby, Sweden.
Cytological assessment of vaginal atrophy
Two smears were obtained, one from each lateral vaginal wall, by gentle scratching with the flat, round end of an Ayre’s spatula. The samples were placed on a glass slide and immediately immersed in alcohol for fixation. Microscopic evaluations of all samples were performed at Labmedicin, Malmö, Sweden, in a blinded manner and by the same cytologist to prevent bias and inter-rater variability. Six different fields of the vaginal smears were examined. The percentages of superficial, intermediate and parabasal cells were calculated.
Vaginal pH
Intravaginal pH was assessed using a pH indicator, ECPH601PLUS (Thermo Fisher Scientific). The accuracy of the technique was ± 0.01 units. Vaginal fluid was collected before the vaginal smears were obtained and was applied to the pH indicator for measurement of pH by the staff.
Histological evaluation of vaginal atrophy
The biopsy sampling was performed under local anaesthesia using Xylocain® (Astra Zeneca, 10 mg/ml injection). The tissue samples were collected from 2 cm inside the vaginal introitus, using a 6 mm skin punch from Miltex (Rietheim-Weilheim, Germany).
Microscopic evaluations of all biopsies were performed in a blinded manner at Aleris Medilab, Täby, Sweden by the same pathologist to prevent bias and inter-rater variability. Further details of this evaluation technique have been published elsewhere.18
Patient's self-assessment of symptoms
Each participating woman graded the intensity of the subjective symptoms of vaginal atrophy, which were dryness, irritation/itching, dysuria and dyspareunia on a scale from 0–3 (0 = normal, 1= mild, 2 = moderate, and 3 = severe).
Each woman reported the intensity of her subjective symptoms, according to the scale described below, and also that which was considered most bothersome.
Statistical analyses
All data were analysed per protocol. The data were presented as mean ± SD or mean ± SEM.
Two instruments were used to test the differences within and between groups: The Wilcoxon signed-rank test was used to determine whether the change from baseline (V1) to the end of treatment (V3) within each group was equal to zero and the Wilcoxon rank-sum was used to compare the change from V1–V3 between each of the active dose levels and placebo. The results were considered statistically significant when p values were ≤ 0.05. Fisher's exact test was used to calculate differences in the occurrence of the most bothersome symptom.
Results
Demographic data for the participants in the different treatment groups are shown in Table 2.
Table 2.
Demographic data for participants.
| Treatment | Vagitocin 400 IU n = 24 | Vagitocin 100 IU n = 24 | Placebo n = 16 |
|---|---|---|---|
| Age | 61.1 ± 5.3 | 62.0 ± 5.7 | 63.2 ± 5.8 |
| BMI | 23.6 ± 3.2 | 23.1 ± 2.4 | 24.2 ± 2.6 |
| Hysterectomy | n = 5 (20.8%) | n = 3 (12.5%) | n = 2 (12.5%) |
BMI: body mass index.
Note. The table shows age (years), BMI (kg/m2), and the number of hysterectomies (yes) in the groups of women receiving vagitocin 400 IU, 100 IU and placebo.
Clinician's evaluations of the vaginal mucosal health
The vaginal mucosa was judged by the clinicians as atrophic when the study began (V1) and as less atrophic in all the treatment groups after seven weeks of treatment (V3).
The mean scores of vaginal atrophy for the vagitocin 400 IU group were 2.00 ± 0.70 at (V1) and 1.05 ± 0.82 at (V3). For the vagitocin 100 IU group, the scores were 2.17 ± 0.49 at (Vl) and 1.47 ± 0.73 at (V3). In the placebo group, the corresponding values were 2.29 ± 0.47 at (V1) and 1.50 ± 0.65 at (V3).
The scores of vaginal atrophy decreased significantly in all groups (p = 0.0001, 0.0005, 0.0039, respectively). Despite the greater effect in the vagitocin 400 IU group, the effect was not significantly different versus placebo.
Endometrial thickness as measured by ultrasound
Endometrial thickness was measured by ultrasound before (V1) and after seven weeks of treatment (V3).
After seven weeks of treatment, the endometrial thickness was significantly decreased in the vagitocin 100 IU group (p = 0.0147), but not in the vagitocin 400 IU or the placebo groups. However, no significant difference in the endometrial thickness was observed between the vagitocin 100 IU group and the placebo group (Table 3).
Table 3.
Endometrial thickness in mm.
| Treatment | Vagitocin 400 IU n = 19 | Vagitocin 100 IU n = 13 | Placebo n = 14 |
|---|---|---|---|
| V1 | 1.2 ± 0.8 | 1.3 ± 0.7 | 1.5 ± 1.1 |
| V3 | 1.1 ± 0.6 | 0.9 ± 0.5 | 1.1 ± 0.5 |
| V3-V1 | −0.1 ± 0.8 | −0.4 ± 0.6 | −0.5 ± 1.1 |
| p | 0.9898 | 0.0147 | 0.2588 |
Note: The table shows the thickness of the endometrial mucosa (mm) at screening (V1) and after seven weeks of treatment (V3). In addition, the difference between the values obtained between V3–V1 and the levels of significance are given.
Cytological assessment of vaginal smears
Superficial cells
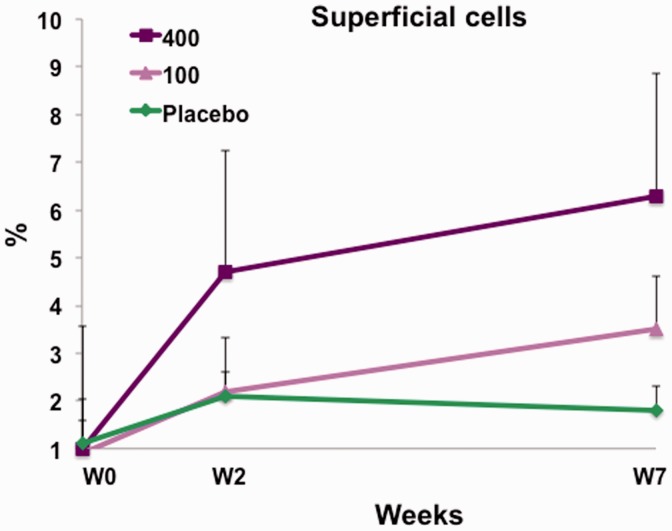
Intravaginal treatment with vagitocin 400 IU for seven weeks (from V1 to V3) increased the percentage of superficial cells in the vaginal smears from 1.03 ± 1.42% to 6.34 ± 1.88%. This increase was significant (p = 0.0288). A slight increase in the percentage of the superficial cells from 0.93 ± 0.83% to 3.54 ± 6.29% was also seen in response to the seven-week treatment with vagitocin 100 IU (p = 0.0749). No effect was observed in the placebo group (1.14 ± 1.08% to 1.85 ± 2.46%) (p = 0.33) (Figure 2).
Figure 2.
The percentage of superficial cells at weeks 0, 2, and 7 for vagitocin 400 IU (purple), vagitocin 100 IU (pink), and placebo (green). The increase in the percentage of the superficial cells from weeks 0–7 in the vagitocin 400 IU group was significant (p = 0.0288).
Maturation value
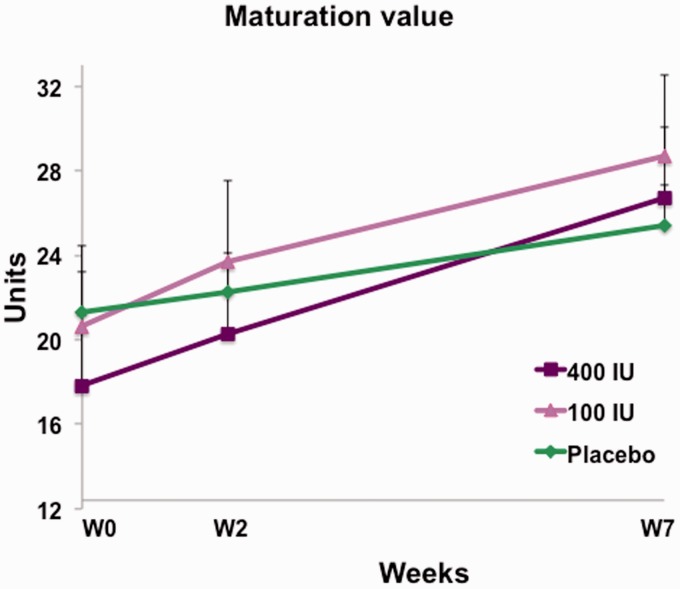
Treatment with vagitocin 400 IU for seven weeks (V1–V3) increased the maturation value significantly from 17.60 ± 21.04 to 26.62 ± 26.75 (p = 0.0002). The change in the maturation value in response to treatment with vagitocin 100 IU, from 20.64 ± 20.82 to 28.68 ± 23.35, was not significant (p = 0.1131). However, placebo treatment induced a statistically significant effect on maturation value from 21.31 ± 22.07 to 25.43 ± 23.13 (p = 0.0494) (Figure 3).
Figure 3.
The maturation value at weeks 0, 2, and 7 for vagitocin 400 IU (purple), vagitocin 100 IU (pink), and placebo (green). The increase in the maturation value from weeks 0–7 in the vagitocin 400 IU and placebo groups was significant (p = 0.0002 and p = 0.0494, respectively).
Intravaginal pH
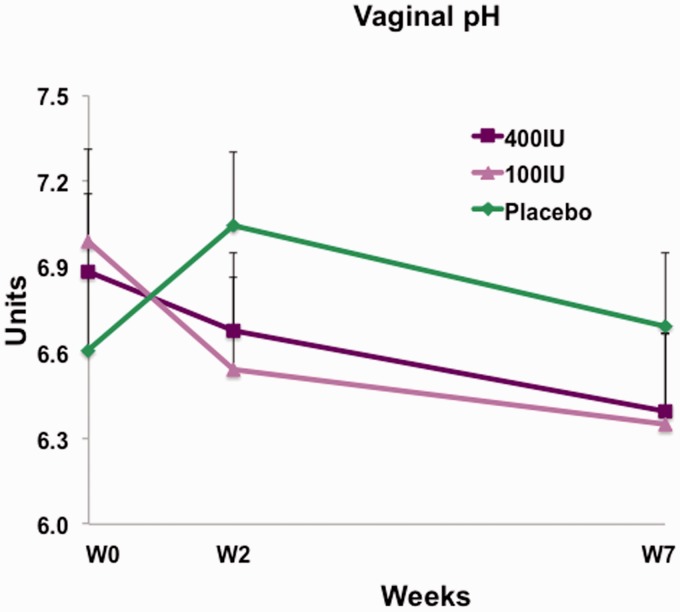
The decrease in intravaginal pH from 6.99 ± 1.18 to 6.34 ± 1.50 that was caused by treatment with vagitocin 100 IU for seven weeks (V1 to V3) was statistically significant (p = 0.0245).
The higher dose of vagitocin (400 IU) decreased intravaginal pH from 6.88 ± 1.30 to 6.39 ± 1.70 during the seven-week treatment period. However, this effect was not statistically significant (p = 0.1938). The placebo treatment did not decrease vaginal pH, which was 6.61 ± 1.30 before treatment and 6.69 ± 1.34 after treatment (p = 0.8521) (Figure 4).
Figure 4.
Vaginal pH at weeks 0, 2, and 7 for vagitocin 400 IU (purple), vagitocin 100 IU (pink), and placebo (green). The decrease in the pH from weeks 0–7 in the vagitocin 100 IU group was significant (p = 0.024).
Histological evaluations of vaginal biopsies in a subgroup of women
Vaginal biopsies were collected before (V1) and after seven weeks of treatment (V3) in 10 women in the vagitocin 400 IU group, in 12 women in the vagitocin 100 IU group and in eight women in the placebo group.
Before treatment, all the vaginal biopsies showed atrophic changes. After the seven-week treatment period (V3), the atrophy scores had significantly decreased from 1.83 ± 0.93 to 0.75 ± 0.97 in the vagitocin 100 IU group (p = 0.0313). In the vagitocin 400 IU group, the scores of atrophy decreased from 1.40 ± 1.17 to 0.80 ± 1.03. This effect was not significant (p = 0.250). No significant effect was caused by placebo treatment; the scores of atrophy were 1.50 ± 1.06 and 1.12 ± 0.64 before (V1) and after (V3) treatment, respectively, (p = 0.50).
Patients' subjective symptoms
Dryness, irritation/itching, dysuria and sum of symptoms
All women reported the presence of one or more subjective symptoms of vaginal atrophy (dryness, irritation/itching, and dysuria) before the start of treatment. The symptom scores, including the sum of the symptoms, decreased significantly over the seven-week treatment period, particularly in the vagitocin 400 IU and 100 IU groups; however, a decrease was also seen in the placebo group (Table 4). No significant difference between the treatment and placebo groups was observed.
Table 4.
Scores of subjective symptoms of vaginal atrophy.
| Symptoms | Vagitocin 400 IU n = 17 |
Vagitocin 100 IU n = 23 |
Placebo n = 14 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | V3 | V3-V1 | p | V1 | V3 | V3-V1 | p | V1 | V3 | V3-V1 | p | |
| Dryness | 2.5 ± 0.6 | 0.9 ± 1.2 | −1.5 ± 1.3 | 0.0007 | 2.6 ± 0.5 | 1.4 ± 1.1 | −1.2 ± 0.9 | 0.000 | 2.6 ± 0.5 | 1.1 ± 0.9 | −1.4 ± 0.9 | 0.0005 |
| Irritation/ Itching | 1.9 ± 0.9 | 0.6 ± 1.1 | −1.3 ± 1.2 | 0.001 | 1.8 ± 1.1 | 1.0±1.2 | −0.8 ± 1.0 | 0.0012 | 1.4 ± 1.1 | 0.7 ± 0.8 | −0.7 ± 0.9 | 0.016 |
| Dysuria | 0.3 ± 0.6 | 0.0 ± 0.0 | − 0.3 ± 0.6 | 0.1250 | 0.1 ± 0.5 | 0.0 ± 0.0 | −0.1 ± 0.5 | 0.50 | 0.1 ± 0.4 | 0.1 ± 0.5 | 0.0 ± 0.7 | 1.000 |
| Sum | 4.7 ± 1.7 | 1.5 ± 2.2 | −3.1 ± 2.5 | 0.0003 | 4.5 ± 1.3 | 2.4 ± 2.0 | −2.1 ± 1.8 | 0.000 | 4.1 ± 1.4 | 2.0 ± 1.7 | −2.1 ± 1.6 | 0.0013 |
Note: The table shows the scores (mean ± SD) of the vaginal atrophy symptoms (dryness, itching/irritation, and dysuria) and the sum of the symptoms at screening (V1) and after seven weeks of treatment (V3). In addition, the differences between the values obtained between V3–V1 and the levels of significance are given.
Dyspareunia
Before the start of the trial, a subgroup of women (n = 33) who were sexually active reported dyspareunia (10 women in the vagitocin 400 IU group, 15 women in the vagitocin 100 IU group and eight women in the placebo group).
Treatment with vagitocin 100 IU reduced the scores of dyspareunia from 2.80 ± 0.41 at screening (V1) to 1.45 ± 1.29 after seven weeks of treatment (V3). This decrease was significant (p = 0.0156). In the vagitocin 400 IU group, the scores were 2.10 ± 0.73 before and 0.88 ± 1.26 after seven weeks of treatment; this decrease was almost significant (p = 0.0625). Placebo treatment did not significantly influence the scores of dyspareunia: 2.75 ± 0.46 to 1.66 ± 1.00 (p = 0.1250).
Most bothersome symptom
The women were asked to name which symptom they experienced as most bothersome before treatment and after two and seven weeks of treatment. For a symptom to be labelled as most bothersome, it had to be scored as moderate or severe (scores 2–3) (Table 5). Nine women out of 17 in the vagitocin 400 IU group did not experience the most bothersome symptom after seven weeks of treatment; the corresponding figures were 14 and 13 in the placebo group.
Table 5.
Most bothersome symptom.
| Treatment | Visit | Yes | No |
|---|---|---|---|
| Vagitocin 400 IU | V1 | 17 | 0 |
| V2 | 15 | 2 | |
| V3 | 8 | 9 | |
| Vagitocin 100 IU | V1 | 23 | 0 |
| V2 | 20 | 3 | |
| V3 | 20 | 3 | |
| Placebo | V1 | 14 | 0 |
| V2 | 14 | 0 | |
| V3 | 13 | 1 |
Note: The table shows the number of women experiencing the most bothersome symptom (Yes or No) at screening (V1) and after two and seven weeks of treatment (V2 and V3) for the vagitocin 400 IU, vagitocin 100 IU, and placebo groups.
The difference in the decrease of the number of women experiencing the most bothersome symptom between the start of treatment and after the seven-week treatment period was highly significant when the results in the vagitocin 400 IU and placebo groups were compared (p = 0.0089).
Discussion
We demonstrated that local vaginal application of vagitocin for seven weeks improved some expressions of post-menopausal vaginal atrophy. The percentage of superficial cells and the maturation value increased significantly after treatment with vagitocin 400 IU. Vaginal pH and the scores of vaginal atrophy were significantly reduced after treatment with vagitocin 100 IU. The experience of the most bothersome symptom was strongly reduced by vagitocin 400 IU. This effect was significant versus placebo.
Treatment with vagitocin improved the appearance of the atrophic mucosa according to the clinician’s investigation. These results confirmed the finding of a previous study, in which oxytocin applied intravaginally for seven days significantly improved post-menopausal vaginal atrophy.18
New findings in the present study are that treatment with vagitocin 400 IU induced a significant increase in the percentage of superficial cells and that the maturation value was significantly increased. These findings demonstrate that local treatment with vagitocin 400 IU stimulates the maturation of cells in the vaginal epithelium. Similar results, such as an increase in the percentage of superficial cells and in the maturation value, were found in a previous study wherein oxytocin 600 IU treatment was administered for 12 weeks (to be published).
Interestingly, the vaginal pH decreased particularly in response to vagitocin 100 IU, whereas the effect of vagitocin 400 IU was less clear. As discussed below, the less clear effect in the vagitocin 400 IU group may be due to lower amount of individuals in this group. The decrease in the pH indicates the presence of a more functional vaginal epithelium with production of acid metabolites.
The histological evaluation of the vaginal biopsies showed that vagitocin improved the condition of the vaginal mucosa, particularly at the lower dose (100 IU). It is important to recognise that the histological evaluation was not only based on the presence of superficial cells, but also on other expressions of proliferation and maturity such as the total number of cell layers in the vaginal epithelium.18 In a previous paper an increase in the number of cell layers of the mucosal epithelium, from 2–3 to 10–12, was observed in response to one week of intravaginal treatment with oxytocin 600 IU.18
Oxytocin in a concentration of 1 µmol stimulated the growth of human vaginal epithelial cells in vitro at a rate similar to estrogen (Uvnäs-Moberg and Sjögren, manuscript to be submitted). Oxytocin has been shown to stimulate the growth of several types of epithelial and endothelial cells.17 These effects were induced by activation of oxytocin binding G-protein linked receptors on the cell surface. A similar mechanism should be activated in the vaginal epithelial cells. Oxytocin may also stimulate growth by other mechanisms (e.g. by increasing the circulation of the vaginal mucosa and, thereby, the transport of oxygen and nutrients to the tissues).17,19
A very important finding in the present study was that intravaginal application of oxytocin in the doses used did not stimulate the growth of the endometrium. This is in contrast to intravaginally applied estrogen, which, after absorption into the circulation, may stimulate endometrial growth.20
Subjective symptoms related to post-menopausal vaginal atrophy, such as vaginal dryness and irritation/itching, were slightly improved after seven weeks of treatment with vagitocin. In contrast, vagitocin significantly decreased the symptom of dyspareunia in the subgroup of women who were sexually active during the study.
The strongest effect of vagitocin was exerted on the symptoms reported as most bothersome. After seven weeks of treatment with vagitocin 400 IU, 53% of the women who reported a most bothersome symptom at the start of the study no longer experienced the symptom. This effect was highly significant versus placebo.
The most common treatments for vaginal atrophy are various kinds of estrogen therapies systemic or local. Intravaginal estrogen therapy has a positive impact on the vaginal atrophic symptoms and signs and can restore normal vaginal pH. In addition, it can increase the number of superficial cells and the maturation value.21
Systemic estrogen replacement therapy has been associated with an increased risk of endometrial and breast cancer, coronary heart disease, stroke and thromboembolic disease.22 The risk of endometrial cancer is increased after long-term systemic estrogen use in post-menopausal women if not combined with progesterone treatment. A small increase of the incidence of breast cancer has been noted in some studies after systemic treatment with estrogen.23 Oral estrogen treatment has been reported to increase the frequency of cardiovascular and thromboembolic disease. The benefit or risk of estrogen therapy in the post-menopausal state depend on the duration of the treatment, age, physical condition and presence of certain genetic predispositions.24 On the other hand, the frequency of some types of cardiovascular disease seems to decrease following treatment with oral estrogen.
All types of vaginal estrogen preparations seem to be effective in improving symptoms related to vaginal atrophy, such as vaginal dryness, dyspareunia, irritation and itching. However, there are some differences in their effect profiles.25
Low-dose local estrogen treatment is associated with a lower risk of severe estrogen-linked side effects which has made this treatment more popular. Series of studies have shown that intravaginally applied estrogens may raise plasma estrogen levels.26 The local absorption of intravaginal estrogen varies greatly from one woman to another and it differs depending on the type and dose of estrogens used. Use of vaginal cream containing conjugated equine estrogens (CEE) is more frequently associated with uterine bleeding, perineal pain and breast tenderness when compared to low-dose vaginal tablets and estrogen containing vaginal rings. These effects of CEE resemble those of oral estrogens and probably reflect increased plasma levels.27 Consequently, a risk remains for a proliferative effect on the endometrium and stimulation of breast cancer cells.20 Regarding the use of low dose estrogen there are no conclusive data concerning the long-term safety.26
Despite its proliferative effect on the healthy epithelial and endothelial cells, oxytocin most often inhibits the growth of malignant cells.28 Interestingly, studies have shown that oxytocin has inhibitory effects on endometrial, ovarian and breast cancer cell growth.21,29,30
The inhibitory effects of oxytocin on cancer cells are mediated by the same oxytocin receptors that stimulate growth in normal cells. The effect of oxytocin in cancer cells, however, is mediated by a different intracellular pathway (cyclic AMP).21,29
Oxytocin may be a safer alternative for treatment of vaginal atrophy, as no stimulation of the endometrial growth was observed in the present study. Furthermore, oxytocin might be of value in women who have estrogen-dependent types of cancers, as oxytocin has not been shown to stimulate growth of breast cancer cells; rather, on the contrary.21
It is important to note that in the present study significant effects versus placebo were only achieved for the variable the most bothersome symptom. Using within-group statistical testing, however, significant effects within groups were obtained for oxytocin regarding all the physiological parameters. Significant effects versus placebo would have been obtained also between active treatment and placebo for these parameters had the study included more women and been of a longer duration. In addition, results from two other clinical studies support the fact that intravaginal treatment with oxytocin does indeed relieve signs of vaginal atrophy in post-menopausal women18 (Al-Saqi, Fianu Jonasson and Uvnäs Moberg, manuscript to be submitted). The lower dose of oxytocin (100 IU) seemed to be more efficient than the 400 IU dose regarding the effect on pH, histological evaluation and dyspareunia. This may be due to the fact that more patients were included in the 100 IU than in the 400 IU group. In spite of the randomisation procedure more women were excluded from the study in the 400 IU group than in the 100 IU group.
Conclusions
The present results indicate that intravaginal oxytocin induces important clinical effects in the context of menopausal disorders. It stimulates the growth of the cells in the vaginal epithelium, thereby restoring the atrophic vaginal mucosa by an effect exerted locally in the vagina. It profoundly decreases the experience of the most bothersome symptom.
As a non-estrogenic compound, oxytocin may be a future drug for treatment of vaginal atrophy, particularly in those women who cannot or do not wish to take estrogenic compounds.
Acknowledgment
This study was sponsored by Peptonic Medical, Sweden.
Trial registration
The trial is registered at ClinicalTrials.gov, number NCT 01987804.
Ethical approval
Regional Ethical Review Board/Stockholm, Sweden (2011/1978-31/2). Swedish Medical Product Agency (LVFS 2011:19, 2012-02-01).
Conflicts of interest
SHA has nothing to disclose. KUM is a board member, consultant and owns stock at Peptonic Medical. AFJ reports grants to cover the study from Peptonic Medical paid to Karolinska Institutet and she owns stock at Peptonic Medical.
Funding
Peptonic Medical, Sweden.
References
- 1.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet 1999; 353: 571–580. [DOI] [PubMed] [Google Scholar]
- 2.Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 2010; 13: 509–522. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson K, Risberg B, Heimer G. The vaginal epithelium in the postmenopause--cytology, histology and pH as methods of assessment. Maturitas 1995; 21: 51–556. [DOI] [PubMed] [Google Scholar]
- 4.Ballagh SA. Vaginal hormone therapy for urogenital and menopausal symptoms. Semin Reprod Med 2005; 23: 126–140. [DOI] [PubMed] [Google Scholar]
- 5.Meisels A. The maturation value. Acta Cytol 1967; 11: 249. [PubMed] [Google Scholar]
- 6.Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000; 61: 3090–3096. [PubMed] [Google Scholar]
- 7.Bergman A, Karram MM, Bhatia NN. Changes in urethral cytology following estrogen administration. Gynecol Obstet Inves 1990; 29: 211–213. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009; 16: 719–727. [DOI] [PubMed] [Google Scholar]
- 9.Wakavaiachi VM, Girao MJ, Sartori MG, et al. Changes in the lower urinary tract in continent women and in women with stress urinary incontinence, according to menopausal status. Int Urogynecol J Pel 2001; 12: 156–160. [DOI] [PubMed] [Google Scholar]
- 10.Legendre G, Fritel X, Ringa V, et al. [Urinary incontinence and menopause]. Prog Urol 2012; 22: 615–621. [DOI] [PubMed] [Google Scholar]
- 11.Krause M, Wheeler TL, 2nd, Snyder TE, et al. Local effects of vaginally administered estrogen therapy: a review. J Pelvic Med Surg 2009; 15: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sturdee DW. Hormone replacement therapy preparations. Br Menopause Soc 2000; 6: 34. [Google Scholar]
- 13.Sismondi P and Biglia N. Critical review of the article by Colditz et al. on ‘The use of estrogens and progestins and the risk of breast cancer in postmenopausal women' published in the N.E.J.M. 1995, 332: 1589. Pharmacol Res 1995; 32: 329–330. [DOI] [PubMed]
- 14.Varas-Lorenzo C, Garcia-Rodriguez LA, Cattaruzzi C, et al. Hormone replacement therapy and the risk of hospitalization for venous thromboembolism: a population-based study in southern Europe. Am J Epidemiol 1998; 147: 387–390. [DOI] [PubMed] [Google Scholar]
- 15.Manson JE. Postmenopausal hormone therapy and atherosclerotic disease. Am Heart J 1994; 128: 1337–1343. [DOI] [PubMed] [Google Scholar]
- 16.Lippert TH, Mueck AO, Seeger H, et al. Effects of oxytocin outside pregnancy. Horm Res 2003; 60: 262–271. [DOI] [PubMed] [Google Scholar]
- 17.Gutkowska J, Jankowski M, Mukaddam-Daher S, et al. Oxytocin is a cardiovascular hormone. Brazilian J Med Biol Res 2000; 33: 625–633. [DOI] [PubMed] [Google Scholar]
- 18.Jonasson AF, Edwall L, Uvnas-Moberg K. Topical oxytocin reverses vaginal atrophy in postmenopausal women: a double-blind randomized pilot study. Menopause Int 2011; 17: 120–125. [DOI] [PubMed] [Google Scholar]
- 19.Petersson M, Lundeberg T, Sohlstrom A, et al. Oxytocin increases the survival of musculocutaneous flaps. Naunyn Schmiedebergs Arch Pharmacol 1998; 357: 701–714. [DOI] [PubMed] [Google Scholar]
- 20.Lauritzen C. Clinical use of oestrogens and progestogens. Maturitas 1990; 12: 199–214. [DOI] [PubMed] [Google Scholar]
- 21.Cassoni P, Sapino A, Negro F, et al. Oxytocin inhibits proliferation of human breast cancer cell lines. Virchows Arch 1994; 425: 467–472. [DOI] [PubMed] [Google Scholar]
- 22.Shifren JL, Schiff I. Role of hormone therapy in the management of menopause. Obstet Gynecol 2010; 115: 839–855. [DOI] [PubMed] [Google Scholar]
- 23.Beral V, Reeves G, Bull D, et al. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer I 2011; 103: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krolik M, Milnerowicz H. The effect of using estrogens in the light of scientific research. Adv Clin Exp Med 2012; 21: 535–543. [PubMed] [Google Scholar]
- 25.Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause 2010; 17: 194–203. [DOI] [PubMed] [Google Scholar]
- 26.Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2014; 18: 1–14. [DOI] [PubMed] [Google Scholar]
- 27.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database System Rev 2006; 18: CD001500. [DOI] [PubMed] [Google Scholar]
- 28.Strunecka A, Hynie S, Klenerova V. Role of oxytocin/oxytocin receptor system in regulation of cell growth and neoplastic processes. Folia Biol 2009; 55: 159–165. [PubMed] [Google Scholar]
- 29.Cassoni P, Fulcheri E, Carcangiu ML, et al. Oxytocin receptors in human adenocarcinomas of the endometrium: presence and biological significance. J Pathol 2000; 190: 470–477. [DOI] [PubMed] [Google Scholar]
- 30.Morita T, Shibata K, Kikkawa F, et al. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. Int J Cancer 2004; 109: 525–532. [DOI] [PubMed] [Google Scholar]