Abstract
Idiopathic pulmonary fibrosis (IPF) is characterized by the relentless expansion of fibroblasts depositing type I collagen within the alveolar wall and obliterating the alveolar airspace. MicroRNA (miR)-29 is a potent regulator of collagen expression. In IPF, miR-29 levels are low, whereas type I collagen expression is high. However, the mechanism for suppression of miR-29 and increased type I collagen expression in IPF remains unclear. Here we show that when IPF fibroblasts are seeded on polymerized type I collagen, miR-29c levels are suppressed and type I collagen expression is high. In contrast, miR-29c is high and type I collagen expression is low in control fibroblasts. We demonstrate that the mechanism for suppression of miR-29 during IPF fibroblast interaction with polymerized collagen involves inappropriately low protein phosphatase (PP) 2A function, leading to histone deacetylase (HDA) C4 phosphorylation and decreased nuclear translocation of HDAC4. We demonstrate that overexpression of HDAC4 in IPF fibroblasts restored miR-29c levels and decreased type I collagen expression, whereas knocking down HDAC4 in control fibroblasts suppressed miR-29c levels and increased type I collagen expression. Our data indicate that IPF fibroblast interaction with polymerized type I collagen results in an aberrant PP2A/HDAC4 axis, which suppresses miR-29, causing a pathologic increase in type I collagen expression.
Keywords: IPF fibroblasts, type I collagen, PP2A, HDAC4, miR-29
Clinical Relevance
Our work offers vital insight in type I collagen regulation in idiopathic pulmonary fibrosis fibroblasts and can potentially offer multiple therapeutic targets in the future.
Idiopathic pulmonary fibrosis (IPF) is a prevalent and progressive fibrotic lung disease that does not respond to therapy (1–5). IPF is characterized by a continuous expansion of the fibroblast population; fibroblasts deposit type I collagen within the alveolar wall, causing scarred nonfunctional airspaces, progressive hypoxia, and death by asphyxiation (5–11). As the fibrosis evolves, there is a contiguous spread of the process from affected alveoli into adjacent anatomically normal gas exchange units, resulting in an uninterrupted reticular network of fibrotic tissue (12).
MicroRNA (miR)-29 is a potent regulator of type I collagen expression. Human miR-29 has three members—miR-29a, miR-29b, and miR-29c—that target COL1A1 and COL1A2 messenger RNA (mRNA) and decrease type I collagen expression (13). Prior work has determined that miR-29 levels are low in experimental models of pulmonary fibrosis and in IPF, whereas type I collagen expression is high (14, 15). This suggests an important role of miR-29 in regulating the excessive production of collagen in IPF. However, the mechanism leading to suppression of miR-29 in IPF remains unclear.
Our prior studies indicate that IPF lung fibroblasts manifest a distinct pathological phenotype characterized by an aberrant integrin signaling in response to interaction with polymerized type I collagen, a major component of the IPF fibrotic matrix (16–18). A seminal study by Heino and colleagues demonstrated that when normal fibroblasts interact with polymerized collagen, α2β1 integrin binds to collagen resulting in activation of protein phosphatase (PP2) A phosphatase (19). In contrast, when IPF fibroblasts interact with polymerized type I collagen, they fail to appropriately activate PP2A due to aberrant α2β1 integrin function, resulting in activation of proliferation signaling pathways (18).
Although the complete mechanism by which low PP2A activity promotes acquisition of the pathologic IPF fibroblast phenotype remains to be elucidated, recent studies have shown that PP2A regulates histone deacetylase (HDA) C4, a class II histone deacetylase whose function has been linked to TGF-β–mediated differentiation of normal fibroblasts (20). HDAC4 regulates gene expression by undergoing nucleocytoplasmic shuttling in response to environmental cues (21, 22). Class II HDACs contain an N-terminal regulatory domain that is subject to phosphorylation, and nucleocytoplasmic shuttling of HDAC4 is controlled by its phosphorylation state (21). Dephosphorylation by PP2A stabilizes HDAC4 and promotes its nuclear accumulation, whereas phosphorylated HDAC4 is located in the cytoplasm and is prone to degradation (21–24). Within the nucleus, HDAC4 represses transcription when tethered to a promoter. It does so by forming a complex with tissue-specific transcription factors, repressing their function and thereby regulating cell phenotype (22, 25–29).
A recent report has linked HDAC4 function to regulation of miR-29 (30). Because we have found that the α2β1 integrin/PP2A axis is abnormal in IPF fibroblasts during their interaction with polymerized type I collagen, we hypothesized that alterations in HDAC4 function may be responsible for suppression of miR-29 in IPF. Here we report that, in response to IPF fibroblast interaction with polymerized type I collagen, low PP2A function results in HDAC4 hyperphosphorylation and decreased HDAC4 nuclear import. We demonstrate that low levels of nuclear HDAC4 result in suppression of miR-29 and increased type I collagen expression. We have discovered that the mechanism involves HDAC4 regulation of miR-29. We have found that knockdown of HDAC4 suppresses miR-29 levels, whereas overexpression of HDAC4 increases miR-29 expression. Our data indicate that during IPF fibroblast interaction with polymerized collagen, decreased nuclear HDAC4 levels suppress miR-29c transcription, thereby activating type I collagen expression.
Materials and Methods
Study Approval
Deidentified patient samples were obtained under a waiver of informed consent from the University of Minnesota Institutional Review Board.
Primary Cell Lines
Eleven primary mesenchymal cell lines were established from patients with IPF. All patients fulfilled the criteria for the diagnosis of IPF as established by the American Thoracic Society and the European Respiratory Society (8). Diagnosis of IPF was confirmed by microscopic analysis of lung tissue, which demonstrated the characteristic morphological findings of usual interstitial pneumonia. Cell lines were derived from lungs removed at the time of transplantation or death as described (31). Patient controls were selected to be similar in age to patients with IPF with nonfibrotic lung disorders. Ten nonfibrotic primary control adult human lung fibroblast lines were used. These lines were established from lung tissue uninvolved by the primary disease process: adenocarcinoma (n = 4), squamous cell carcinoma (n = 1), carcinoid tumor (n = 2), fibrosarcoma (n = 1), leimyosarcoma (n = 1), or bronchiectasis (n = 1). Primary lung mesenchymal cell lines were generated by explant culture and/or mechanical/chemical dispersion and maintained in high-glucose DMEM containing 10% FCS. Cells were used from passages 3, 4, and 5.
Antibodies
Phosphorylated and HDAC4 antibodies were obtained from Cell Signaling (Danvers, MA). Collagen I antibodies were obtained from AbD Serotec (Raleigh, NC) and Southern Biotech (Birmingham, AL). PP2Ac monoclonal antibody was obtained from Millipore (Temecula, CA).
Western Blot Analysis
Western blot analysis was performed on cell lysates as described (16–18).
Quantitative RT-PCR
Quantitative RT-PCR was performed as described (32). The primer sequences were as follows: COL1A2 forward: 5′TGCCTAGCAACATGCCAATC; COL1A2 reverse: 5′TGAGCAGCAAAGTTCCCACC; HDAC4 forward: 5′TCCAGATGGACTTTCTGGCCG; HDAC4 reverse: 5′GCTGGGCATGTGGTTCACG; miR-29c: 5′UAGCACCAUUUGAAAUCGGUUA.
Gain of miR-29c Function
Gain of miR-29c function was performed as described (32).
Adenoviral Vectors for HDAC4 and PP2Ac Overexpression
Adenoviral vectors containing wild-type HDAC4 (Ad-HDAC4), wild-type PP2Ac (Ad-PP2Ac), and control (Ad-GFP) constructs were amplified to high titer according to the manufacturer’s instructions (abm, Richmond, BC, Canada). Cells were infected with adenoviral vectors at a multiplicity of infection of 1:20.
Lentiviral Vector for HDAC4 Knockdown
Lentivirus plasmids (HDAC4 V2LHS_239051 Mature antisense: ACAATGAAGAAATGGTTTC) were obtained from Thermo Scientific (Waltham, MA). Viral supernatants were harvested 48 hours after transfection, filtered through a 0.45-μm pore size polyvinylidene fluoride filter, aliquoted, and frozen at −80°C. IPF fibroblasts were plated in six-well dishes and incubated overnight. Virus was added to cells in DMEM–10% FBS with polybrene (final concentration, 8 μg/ml). The plate was centrifuged (1,200 × g; 25°C) for 1 hour and then incubated for 16 hours (37°C, 5% CO2). The virus was removed, 2 ml fresh medium per well was added, and incubation continued for 48 hours.
Immunohistochemistry
Immunohistochemistry was performed on 4-μm–sectioned, paraffin-embedded lung tissue specimens as described using a monoclonal antibody to HDAC4 (1:100) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and a biotinylated horse anti-mouse secondary antibody (1:500) (18).
Statistical Analysis
Comparisons of data among experiments were performed with the unipolar unpaired or paired Student’s t test. Experiments were independently replicated a minimum of three times. Data are expressed as mean ± SD. P < 0.05 was considered significant.
Results
Type I Collagen Expression Is High and miR-29c Is Low in IPF Fibroblasts
A key pathological feature of the IPF fibroblast phenotype is increased synthesis and deposition of type I collagen, leading to expansion of the alveolar wall and obliteration of the alveolar airspace (16–18, 33). Previous work indicates that miR-29, which regulates the expression of a variety of extracellular matrix components including type I collagen, is suppressed in IPF (15). This implicates a vital role for miR-29 in the excessive collagen production characteristic of IPF. However, the mechanism for suppression of miR-29 in IPF remains unclear. Because IPF fibroblasts are the key effector cell of the fibrotic response, synthesizing and depositing type I collagen, we began our experiments by quantifying miR-29 and type I collagen levels when IPF fibroblasts interact with polymerized collagen, which is a major component of the IPF fibrotic matrix. When IPF fibroblasts were seeded on polymerized collagen matrices, they expressed increased levels of collagen I and lower levels of miR-29 compared with control fibroblasts (Figure 1A). We next examined whether the increased expression of type I collagen in IPF fibroblasts was influenced by their interaction with the polymerized collagen matrix. To address this issue, we seeded IPF fibroblasts on polymerized collagen matrices or tissue culture plastic and examined collagen I and miR-29 expression. IPF fibroblasts seeded on polymerized collagen matrices expressed higher levels of collagen I and lower levels of miR-29 compared with when they were seeded on tissue culture plastic (Figure 1B). This suggested that IPF fibroblast interaction with the polymerized type I collagen matrix results in increased collagen I expression due to aberrant regulation of miR-29 expression.
Figure 1.
Type I collagen expression is high and microRNA (miR)-29c is low in idiopathic pulmonary fibrosis (IPF) fibroblasts. (A) Primary IPF (n = 6) and control human lung fibroblasts (n = 4) were seeded on polymerized type I collagen matrices for 4 hours. Left panel: Type I collagen protein expression was quantified by Western blot analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is shown as a loading control. Right panel: miR-29c levels were quantified by quantitative RT-PCR (qRT-PCR) and are shown as the ratio of miR-29 to U6-2 small nuclear RNA (RNU) expression. (B) IPF fibroblasts (n = 3) were plated on tissue culture plastic (TC) or polymerized type I collagen matrices (PC). Left panel: Type I collagen expression was quantified by Western analysis. GAPDH is shown as a loading control. Right panel: miR-29c levels were quantified by qRT-PCR and are shown as the ratio of miR-29 to RNU expression.
HDAC4 Protein Levels Are Decreased in IPF Fibroblasts
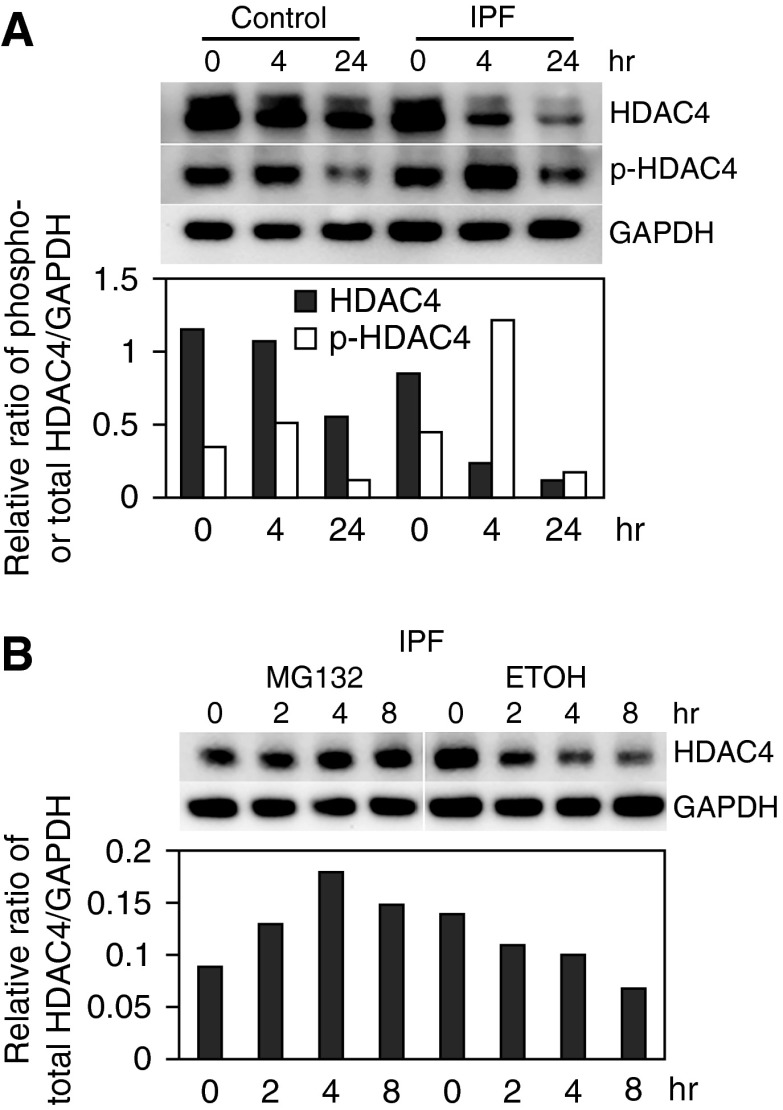
We have previously found that the α2β1 integrin/PP2A axis is abnormal during IPF fibroblast interaction with polymerized type I collagen and that this axis is a key regulator of the IPF fibroblast phenotype (18). Low α2β1 integrin expression results in a failure of PP2A to be appropriately activated when IPF fibroblasts interact with polymerized collagen (18). PP2A is a critical regulator of HDAC4 function (21). HDAC4 contains an N-terminal regulatory domain that is subject to phosphorylation. When phosphorylated, HDAC4 is susceptible to degradation by the proteasome. However, when dephosphorylated by PP2A, HDAC4 protein is stable (21–24). Importantly, HDAC4 has been linked to regulation of miR-29 expression (30). Because PP2A function is abnormal in IPF, we hypothesized that aberrant PP2A regulation of HDAC4 function may lead to suppression of miR-29. To begin to analyze HDAC4 function in IPF fibroblasts, we first examined HDAC4 expression in IPF fibroblasts seeded on polymerized type I collagen matrices as a function of time. Four hours after seeding cells on polymerized collagen, IPF fibroblast HDAC4 protein levels were decreased. By 24 hours, HDAC4 protein levels were markedly decreased (86% decrease). In contrast, although HDAC4 expression also decreased in control fibroblasts as a function of time, the level of decrease (47% decrease) was more modest (Figure 2A). We also quantified the level of phosphorylated HDAC4 in IPF and control fibroblasts seeded on polymerized collagen matrices as a function of time. We found that at the 4-hour time-point the level of phosphorylated HDAC4 had increased approximately 1.7-fold in IPF fibroblasts compared with a 47% increase in control fibroblasts. Phosphorylated HDAC4 is prone to degradation by the proteasome. Therefore, we next examined the effect of pretreating IPF fibroblasts with the proteasome inhibitor MG-132 on HDAC4 protein levels when seeded on polymerized collagen. We found that HDAC4 protein levels were relatively preserved in IPF fibroblasts treated with MG-132 compared with vehicle control (Figure 2B). Together, these data indicate that HDAC4 becomes phosphorylated and degraded when IPF fibroblasts interact with polymerized collagen.
Figure 2.
Histone deacetylase (HDAC) 4 protein levels are decreased in IPF fibroblasts. (A) IPF and control lung fibroblasts were seeded on polymerized type I collagen matrices as a function of time. Top panel: Phosphorylated HDAC4 (pHDAC4) and HDAC4 expression were examined by Western blot analysis. GAPDH is shown as a loading control. Bottom panel: pHDAC4 and HDAC4 levels were quantified by densitometry. (B) IPF fibroblasts were pretreated with the proteasome inhibitor MG132 (20 nM; 60 min) or vehicle control (ethanol [ETOH]) and seeded on polymerized collagen for 0, 2, 4, or 8 hours. HDAC4 protein levels were quantified by Western blot analysis. GAPDH is shown as a loading control. All experiments were repeated with three independent cell lines to confirm the findings.
Low PP2A Activity in IPF Fibroblasts Results in HDAC4 Hyperphosphorylation and Decreases Its Nuclear Localization
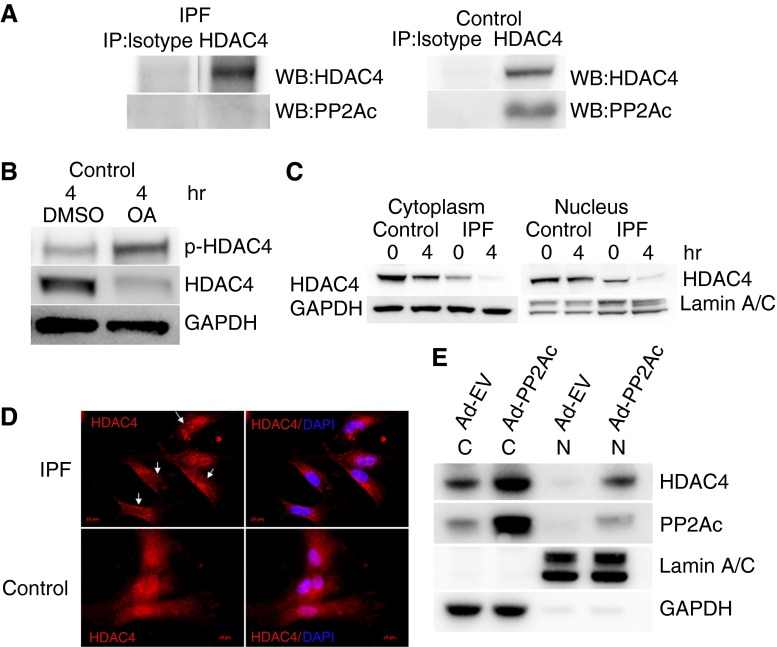
We have found that phospho-HDAC4 levels are high in response to IPF fibroblast interaction with polymerized collagen, whereas PP2A function is low (18). Because PP2A binds and dephosphorylates HDAC4, thereby promoting HDAC4 nuclear import (21), we next sought to determine whether we could detect a physical association of PP2A with HDAC4 when control or IPF fibroblasts were seeded on polymerized collagen matrices for up to 4 hours. Although a PP2A/HDAC4 complex could be readily detected in control fibroblasts, this complex was below the limits of detection in IPF fibroblasts (Figure 3A). Furthermore, when we pretreated control fibroblasts with the PP2A inhibitor okadaic acid, the level of phosphorylated HDAC4 increased sharply (Figure 3B). Taken together, these data suggest that, when IPF fibroblasts interact with polymerized type I collagen, low PP2A phosphatase function leads to increased levels of phosphorylated HDAC4.
Figure 3.
Low protein phosphatase (PP) 2A in IPF fibroblasts results in HDAC4 hyperphosphorylation and decreases its nuclear localization. (A) IPF and control fibroblasts were plated on type I polymerized collagen for 4 hours. Immunoprecipitation of HDAC4 was performed, and samples were analyzed for association with PP2Ac. Immunoprecipitation with isotype antibody was used as a control. (B) Control lung fibroblasts were pretreated with the PP2A inhibitor okadaic acid (OA) (10 nM; 60 min) or DMSO as a control. The cells were then seeded on type I polymerized collagen matrices and phosphorylated, and HDAC4 protein expression was examined by Western blot analysis as a function of time. GAPDH is shown as a loading control. (C) IPF and control fibroblasts were seeded on polymerized collagen for 4 hours. The cells were lysed, and nuclear and cytoplasmic fractions were analyzed for HDAC4 expression by Western blot analysis. Lamin A/C is shown as a nuclear loading control; GAPDH is shown as a cytoplasmic loading control. (D) IPF and control fibroblasts were seeded on polymerized type I collagen for 4 hours. The cells were stained with HDAC4 antibody conjugated with Cy-3. 4′6-Diamidino-2-phenylindole (DAPI) indicates nuclear staining. Arrows point to four IPF fibroblasts with low nuclear HDAC4 expression. (E) PP2Ac was overexpressed in IPF fibroblasts using an adenoviral vector containing a wild-type PP2Ac construct (Ad-PP2Ac). Cells infected with empty vector served as control (Ad-EV). The cells were seeded on polymerized collagen for 4 hours and then lysed. Nuclear (N) and cytoplasmic (C) fractions were analyzed for PP2Ac and HDAC4 expression by Western blot analysis. Lamin A/C is shown as a nuclear loading control; GAPDH is shown as a cytoplasmic loading control.
HDAC4 regulates gene expression by undergoing nucleocytoplasmic shuttling in response to external cues. Nucleocytoplasmic shuttling of HDAC4 is controlled by its phosphorylation state (21). Dephosphorylation by PP2A stabilizes HDAC4 and promotes its nuclear accumulation, whereas phosphorylated HDAC4 resides in the cytoplasm, where it is subject to proteasomal degradation (21, 22). Therefore, we next examined HDAC4 localization in IPF and control fibroblasts seeded on polymerized collagen matrices. We found that nuclear HDAC4 levels were markedly decreased in IPF fibroblasts compared with control (Figure 3C). In addition, cytoplasmic HDAC4 levels were decreased. After 4 hours on polymerized collagen, nuclear HDAC4 levels in IPF fibroblasts were at the lower limits of detection. Consistent with this, immunocytochemistry demonstrated that HDAC4 was predominantly cytoplasmic in IPF fibroblasts seeded on polymerized collagen matrices (Figure 3D). In contrast, HDAC4 was found in the nucleus and cytoplasm in control fibroblasts (Figures 3C and 3D). To directly test the role of PP2Ac in regulating HDAC4 localization in IPF fibroblasts, we used an adenoviral vector to overexpress PP2A in IPF fibroblasts. In accord with a direct causal role, PP2Ac gain-of-function in IPF fibroblasts augmented nuclear HDAC4 levels (Figure 3E). Thus, our data indicate that, when IPF fibroblasts interact with polymerized collagen, aberrantly low PP2A function results in HDAC4 phosphorylation and decreased levels of HDAC4 in the nucleus and cytoplasm.
HDAC4 Regulates Type I Collagen and miR-29 Expression
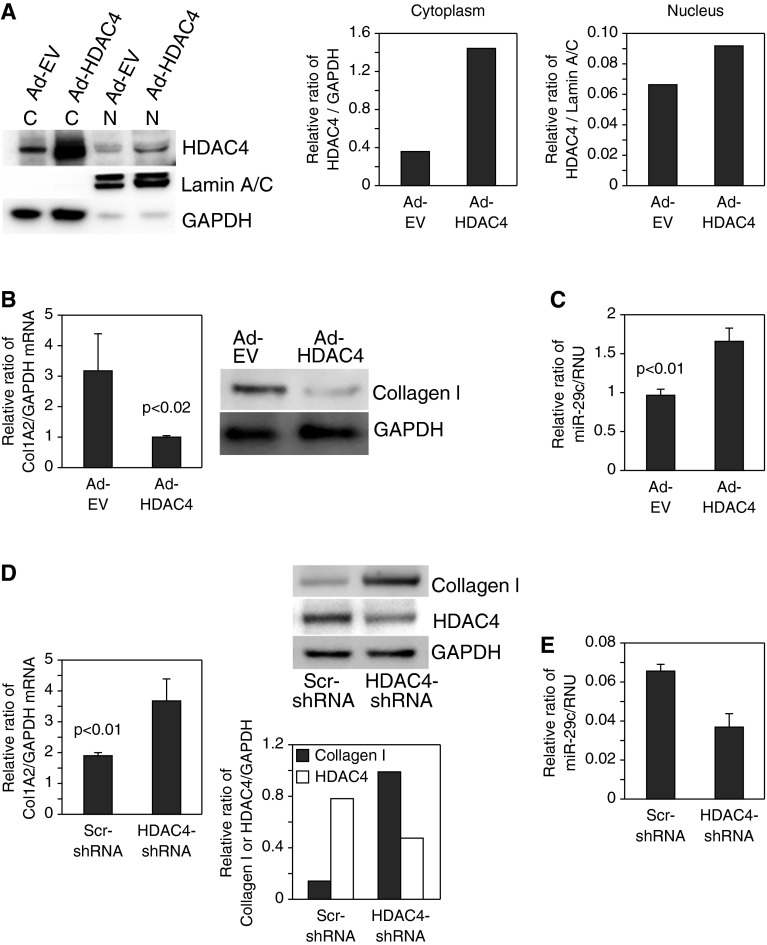
We next examined the role of HDAC4 in regulating type I collagen expression in our system. To do this, we performed HDAC4 gain-of-function experiments in IPF fibroblasts. Overexpression of HDAC4 increased nuclear and cytoplasmic HDAC4 protein levels (Figure 4A). Importantly, we found that overexpression of HDAC4 in IPF fibroblasts markedly decreased type I collagen mRNA and protein expression (Figure 4B) while increasing miR-29 expression (Figure 4C). In contrast, knockdown of HDAC4 by short hairpin RNA (shRNA) in control fibroblasts decreased HDAC4 expression by 40% and substantially increased type I collagen mRNA and protein expression (Figure 4D) while decreasing miR-29 expression (Figure 4E). These data suggest that HDAC4 regulates collagen 1 expression via miR-29.
Figure 4.
HDAC4 regulates type I collagen and miR-29 expression. (A–C) HDAC4 was overexpressed in IPF fibroblasts using an adenoviral vector containing a wild-type HDAC4 construct (Ad-HDAC4). Controls consisted of cells expressing empty vector (Ad-EV). (A) Cells were seeded on polymerized type I collagen matrices for 4 hours. Cells were lysed and nuclear (N) and cytoplasmic (C) fractions were analyzed for HDAC4 protein levels by Western analysis (left panel). Nuclear and cytoplasmic HDAC4 levels were quantified by densitometric analysis (middle and right panels). (B) COL1A2 messenger RNA (mRNA) (left panel) and collagen I protein (right panel) expression were examined by qRT-PCR and Western blot analysis, respectively. GAPDH is shown as a loading control. (C) miR-29c levels were quantified by qRT-PCR. (D and E) HDAC4 was knocked down in control fibroblasts using a lentiviral vector containing HDAC4 short hairpin RNA (shRNA). Cells infected with lentiviral vector containing scrambled shRNA (Scr-shRNA) were used as a control. The cells were plated on polymerized type I collagen for 4 hours. (D) COL1A2 mRNA (left panel) and collagen I and HDAC4 protein levels (top right panel) were examined by qRT-PCR and Western blot analysis, respectively. GAPDH is shown as a loading control. Collagen I and HDAC4 protein levels were quantified by densitometric analysis (bottom right panel). (E) miR-29c levels were quantified by qRT-PCR.
The PP2A/HDAC4 Axis Regulates Collagen I Expression via miR-29
We have shown that PP2A gain-of-function increases HDAC4 levels in IPF fibroblasts (Figure 3E) and that high levels of HDAC4 decrease collagen I expression (Figure 4B). We next sought to analyze the role of the PP2A/HDAC4 axis in regulating collagen I and miR-29 expression. We found that PP2A gain-of-function in IPF fibroblasts decreased collagen I mRNA expression while increasing miR-29 expression (Figure 5A, lane 3 and Figure 5B, lane 3). To ascertain whether PP2A-mediated increase in HDAC4 is responsible for the changes in collagen I and miR-29 expression, we knocked down HDAC4 in IPF fibroblasts in which PP2A had been overexpressed. We found that knockdown of HDAC4 in IPF fibroblasts overexpressing PP2A resulted in increased collagen I expression and suppression of miR-29 expression (Figure 5A, lane 4 and Figure 5B, lane 4). These data indicate that PP2A regulates collagen I and miR-29 expression via HDAC4 and strongly suggest that in IPF fibroblasts, low PP2A function leads to decreased HDAC4 levels. Low HDAC4 function in turn suppresses miR-29, which leads to an increase in collagen I expression.
Figure 5.
PP2A/HDAC4 axis regulates type I collagen expression via miR-29. (A and B) HDAC4 was knocked down by HDAC4 shRNA in IPF fibroblasts in which PP2Ac was overexpressed using an adenoviral vector (Ad-PP2Ac/HDAC4-shRNA). Controls consisted of IPF fibroblasts in which PP2A was overexpressed and treated with scrambled shRNA (Ad-PP2Ac/Scr-shRNA), cells infected with empty vector (control for PP2A) and treated with HDAC4-shRNA (Ad-EV/HDAC4-shRNA), and cells infected with empty vector and treated with scrambled shRNA (Ad-EV/Scr-shRNA). qRT-PCR was done to assess mRNA levels of Col1A1 (A) and miR-29c (B). (C and D) Control fibroblasts in which HDAC4 had been knocked down were infected with a lentiviral vector containing a miR-29c construct to overexpress miR-29 (HDAC4-shRNA/miR-29 overexpression [OE]). Controls consisted of control fibroblasts in which HDAC4 had been knocked down and then treated with empty vector (HDAC4-shRNA/EV), control fibroblasts treated with scrambled shRNA in which miR-29c had been overexpressed (Scr-shRNA/miR-29 OE), and control fibroblasts treated with scrambled shRNA and empty vector (Scr-shRNA/EV). The cells were seeded on polymerized type I collagen matrices for 4 hours. (C) Left panel: To confirm HDAC4 knockdown, HDAC4 expression was quantified by Western blot analysis. Shown is the ratio of HDAC4 to GAPDH. Right panel: To confirm miR-29 overexpression, miR-29c expression was quantified by qRT-PCR. Shown is the ratio of miR-29 to RNU. EV, empty vector. (D) Type I collagen expression was assessed by Western blot analysis. GAPDH is shown as a loading control (top panel). Collagen I protein expression was quantified by densitometric analysis (bottom panel).
To directly test whether the regulation of collagen I by HDAC4 operates through its effect on miR-29c, we conducted a rescue experiment where we overexpressed miR-29c in control fibroblasts in which HDAC4 had been knocked down. Knockdown of HDAC4 by shRNA decreased HDAC4 expression by 45% (Figure 5C, left panel). Overexpression of miR-29c increased miR-29 levels by 65% (Figure 5C, right panel). We found that knockdown of HDAC4 increased collagen I expression (Figure 5D, lane 2), whereas overexpression of miR-29c suppressed collagen I expression (Figure 5D, lane 3). When the miR-29c precursor was ectopically expressed in control fibroblasts in which HDAC4 had been knocked down, the previously observed increase in type I collagen was ablated (Figure 5D, lane 4). This places miR-29 suppression of type I collagen downstream of HDAC4. Taken together, these data support a model in which the failure of IPF fibroblasts to appropriately activate PP2A when interacting with the polymerized collagen matrix leads to decreased nuclear HDAC4 levels. Depleted nuclear HDAC4 in turn suppresses miR-29 expression and subsequently activates type I collagen expression.
IPF Fibroblastic Foci Contain a Paucity of HDAC4 Immunoreactive Cells
To examine the in vivo relevance of our findings, we analyzed HDAC4 expression in IPF lung tissue specimens by immunohistochemistry. We found a paucity of HDAC4 immunoreactive cells within IPF fibroblastic foci (Figure 6A). In contrast, numerous HDAC4 immunoreactive cells were interspersed within the walls of anatomically normal alveolar structures in control lung tissue. Importantly, many cells demonstrated nuclear HDAC4 staining (Figure 6C). As controls for HDAC4 immunohistochemistry, no immunoreactivity was present in IPF or control lung tissue stained with secondary antibody only (Figures 6B and 6D, respectively). Thus, HDAC4 levels are low in IPF fibroblasts populating the IPF fibrotic reticulum.
Figure 6.
IPF fibroblastic foci contain a paucity of HDAC4 immunoreactive cells. IPF (A and B) and control (C and D) human lung tissue specimens (n = 3 each) were analyzed for HDAC4 immunoreactivity by immunohistochemistry. (A) Representative image of immunohistochemistry of IPF lung tissue demonstrating a paucity of HDAC4-expressing cells in the IPF fibrotic reticulum. (B) Negative control: IPF lung tissue stained with secondary antibody only. (C) Numerous HDAC4-expressing cells were detected in alveolar structures of anatomically normal control lung tissue. Arrows point to HDAC4 immunoreactive cells. Inset: High-power image of a cell displaying nuclear HDAC4 immunoreactivity. (D) Negative control: control lung tissue stained with secondary antibody only. Scale bar, 50 μm.
Discussion
Fibroblasts derived from the lungs of patients with IPF display a distinct pathologic phenotype, including increased expression of type I collagen. Prior studies have demonstrated that miR-29, a key regulator of collagen expression, is abnormally suppressed in IPF (reviewed in Reference 15). However, the mechanism for this aberrant suppression of miR-29 in IPF remained unclear. In this report, we demonstrate that, in response to IPF fibroblast interaction with polymerized type I collagen, an aberrant PP2A/HDAC4 axis leads to hyperphosphorylation of HDAC4 and reduced HDAC4 nuclear import, resulting in suppression of miR-29 and activation of type I collagen expression. These data suggest an important role for the PP2A/HDAC4/miR-29 axis in regulating the excessive collagen production that is a hallmark feature of IPF.
We have recently discovered that, in response to fibroblast residence on decellularized IPF fibrotic matrices, miR-29 expression is suppressed, causing increased type I collagen expression (32). These findings indicate that fibroblasts manifest a pathologic feed-forward circuit in which residence on the fibrotic matrix promotes the production of excessive amounts of type I collagen, driving progressive fibrosis. However, the mechanism by which the fibrotic matrix confers pathologic suppression of miR-29 and abnormally high expression of type I collagen remained unclear. Here we show that culture of IPF fibroblasts on polymerized type I collagen matrices mirrored the response of culture on decellularized IPF matrices in terms of suppression of miR-29 levels and augmentation of collagen expression. Thus, aberrant control of miR-29–type I collagen feedback by the IPF fibroblast was recapitulated on polymerized type I collagen matrices. This suggests that IPF fibroblast interaction with type I collagen in the IPF fibrotic matrix is a major determinant regulating the pathologic suppression of miR-29 in IPF.
The IPF extracellular matrix is rich in cross-linked type I collagen, which is mechanically altered, displaying increased stiffness. Recent studies indicate that the fibrotic collagen-rich matrix can activate fibroblasts and drive fibrosis (32, 34–38). Importantly, studies in cancer indicate that a cross-linked type I collagen matrix alters integrin signaling and promotes tumor progression (39–41). α2β1 integrin is a major collagen receptor that cells utilize when adhering to collagen. Interestingly, many cancer daughter cells display abnormally low levels of α2β1 integrin, and this has been linked to cancer progression and metastasis, suggesting that aberrant α2β1 integrin/type I collagen interaction promotes acquisition of a pathologic phenotype (42–46). In further support of this concept, a recent study demonstrated that fibroblasts cultured for a prolonged period on matrices with altered mechanical properties displayed an activated phenotype that persisted even when the cells were returned to pliable matrices (47). This suggests that a fibrotic, noncompliant matrix is capable of “reprogramming” fibroblasts such that they manifest a durable pathologic phenotype. The mechanism by which a fibrotic type I collagen matrix “reprograms” fibroblasts remains to be elucidated.
Recent studies have begun to uncover the basic mechanism(s) by which α2β1/collagen interaction regulates cellular phenotype. Seminal work by Heino and colleagues discovered that when α2β1 integrin binds polymerized type I collagen, the PP2A phosphatase becomes activated (19). We have previously found that α2β1 integrin expression is low during IPF fibroblast interaction with polymerized type I collagen matrices and that this results in a failure for PP2A to be appropriately activated (18). Importantly, prior work demonstrated that PP2A is a critical regulator of HDAC4, a class II histone deactylase that has been shown to regulate normal fibroblast differentiation (19). HDAC4 function is controlled by its phosphorylation state (21). HDAC4 contains an N-terminal regulatory domain that is subject to phosphorylation. When phosphorylated, HDAC4 is retained in the cytoplasm, where it is prone to degradation by the proteasome. However, when dephosphorylated by PP2A, HDAC4 undergoes nuclear import. Within the nucleus, deacetylation of histones by HDACs results in chromatin compaction, which represses gene transcription, altering cell phenotype. Importantly, studies indicate that the matrix microenvironment can reprogram cells through epigenetic regulatory mechanisms, including histone modifications via HDACs, further supporting the concept that the collagen-rich IPF fibrotic matrix may regulate the IPF fibroblast phenotype via HDAC4.
Here we demonstrate that when IPF fibroblasts interact with polymerized collagen, low PP2A activity causes HDAC4 to be phosphorylated and degraded and HDAC4 protein levels are markedly depressed. This results in depleted nuclear HDAC4. Moreover, we have found that in IPF fibroblasts restoration of PP2A function increases nuclear HDAC4 levels and miR-29 expression while decreasing collagen I levels. Our studies demonstrate that HDAC4 functions downstream of PP2A in regulating miR-29 and collagen I expression. We have discovered that HDAC4 regulates miR-29 expression. We show that knockdown of HDAC4 suppresses miR-29 levels and promotes type I collagen expression, whereas overexpression of HDAC4 increases miR-29 expression while suppressing collagen expression. miR-29 is a potent post-translational inhibitor of type I collagen. Interestingly, a recent study involving mouse hepatic stellate cells also found a relationship between HDAC4 and miR-29 (30). However, in contrast to our findings, this study found that inhibition of HDAC4 resulted in up-regulation of miR-29. The reason for these discrepant results is not clear, but one possible explanation is that HDAC4 regulation of miR-29 is cell type and condition specific.
Our data support a model where IPF fibroblast interaction with the polymerized collagen matrix results in a failure of PP2A to be activated, which depletes nuclear HDAC4 levels. Decreased nuclear HDAC4 suppresses miR-29 expression, which in turn causes an increase in COL1A1 and COL1A2 translation and subsequently collagen I protein expression. To verify this, we performed a series of experiments. First, we demonstrated that overexpression of HDAC4 in IPF fibroblasts augments miR-29 expression while decreasing type I collagen protein levels. Next, we showed that knockdown of HDAC4 in control fibroblasts suppressed miR-29 levels and increased collagen expression. We went on to demonstrate that the increased collagen expression that resulted by knocking down HDAC4 can be reversed by gain-of-function of miR-29. These data strongly support the concept that, in response to interaction with polymerized type I collagen, the PP2AHDAC4 axis regulates the increased production of type I collagen, a hallmark feature of the IPF fibroblast phenotype, by suppressing miR-29 expression.
Precisely how HDAC4 regulates miR-29 expression is unclear. Prior work indicates that upon nuclear import, HDAC4 functions by binding to tissue-specific transcription factors and inhibiting their function (22, 25–29). Transcription factors can be positive or negative regulators of gene expression. If HDAC4 binds to a transcription factor that is a positive gene regulator, it will lead to inhibition of gene expression. In contrast, if HDAC4 binds to a transcription factor that is a negative regulator of gene expression, it will lead to activation of gene transcription. Thus, one possible mechanism by which HDAC4 regulates miR-29 involves HDAC4 binding and inhibiting a transcription factor that functions as a negative regulator of miR-29, possibly Sp1, which has been shown to bind to a regulatory element on the miR-29 gene and to repress its transcription (48). In this scenario, the repressive function of the transcription factor toward miR-29 is released, thereby increasing miR-29 gene transcription. We acknowledge a key limitation of our study because we were unable to identify the transcription factor responsible for regulating miR-29 expression. Our future studies will focus on identifying this elusive transcription factor, which will further strengthen our understanding of type I collagen regulation in IPF fibroblasts.
In summary, we demonstrate that during IPF fibroblast interaction with polymerized collagen, inappropriately low PP2A activity leads to aberrant HDAC4 function. This altered HDAC4 function is responsible for the pathologic decrease in miR-29c and acquisition of a hallmark feature of the IPF fibroblast: increased type I collagen protein translation and expression. Our data suggest that IPF fibroblasts have been epigenetically reprogrammed to respond pathologically when they interact with polymerized type I collagen matrices and that the α2β1 integrin/PP2A/HDAC4/miR-29c axis underlies this pathologic response.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute grants R01HL074882 and P01HL91775 (C.A.H.) and R01HL089249 (P.B.B.).
Author Contributions: W.K., H.X., V.B., and J.K.: Study design, experimentation, and writing the manuscript. P.H., K.S., M. Peterson, M. Parker, and J.H.: Experimentation. P.B.B. and C.A.H.: Study design and writing the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0150OC on January 22, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Etzel RA, Parrish RG. Pulmonary fibrosis deaths in the United States, 1979–1991: an analysis of multiple-cause mortality data. Am J Respir Crit Care Med. 1996;153:1548–1552. doi: 10.1164/ajrccm.153.5.8630600. [DOI] [PubMed] [Google Scholar]
- 3.Noble PW. Idiopathic pulmonary fibrosis: natural history and prognosis. Clin Chest Med. 2006;27(1) Suppl 1:S11–S16. doi: 10.1016/j.ccm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Costabel U, Cordier JF, DoPico GA, DuBois RM, Lynch D, Lynch JP, III, Myers J, Panos R, Raghu G, et al. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment: International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 6.Basset F, Ferrans VJ, Soler P, Takemura T, Fukuda Y, Crystal RG. Intraluminal fibrosis in interstitial lung disorders. Am J Pathol. 1986;122:443–461. [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda Y, Ishizaki M, Masuda Y, Kimura G, Kawanami O, Masugi Y. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol. 1987;126:171–182. [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn C, III, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140:1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis: ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- 10.Noble PW, Homer RJ. Idiopathic pulmonary fibrosis: new insights into pathogenesis. Clin Chest Med. 2004;25:749–758. doi: 10.1016/j.ccm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 12.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med. 2006;174:654–658. doi: 10.1164/rccm.200602-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengupta S, Den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, Chen CJ, Hildesheim A, Sugden B, Ahlquist P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardosa WV, Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandit KV, Milosevic J, Kaminiski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, et al. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia H, Khalil W, Kahm J, Jessurun J, Kleidon J, Henke CA. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol. 2010;176:2626–2637. doi: 10.2353/ajpath.2010.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia H, Seeman J, Hong J, Hergert P, Bodem V, Jessurun J, Smith K, Nho R, Kahm J, Gaillard P, et al. Low α2β1 integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the β-catenin pathway. Am J Pathol. 2012;181:222–233. doi: 10.1016/j.ajpath.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivaska J, Nissinen L, Immonen N, Eriksson JE, Kahari VM, Heino J. Integrin α2β1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3β. Mol Cell Biol. 2002;22:1352–1359. doi: 10.1128/mcb.22.5.1352-1359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–L870. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paroni G, Cernotta N, Dello Russo C, Gallinari P, Pallaoro M, Foti C, Talamo F, Orsatti L, Steinkuhler C, Brancolini C. PP2A regulates HDAC4 nuclear import. Mol Biol Cell. 2008;19:655–667. doi: 10.1091/mbc.E07-06-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miska EA, Karlsson C, Langley E, Nielsen SJ, Pines J, Kouzarides T. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 1999;18:5099–5107. doi: 10.1093/emboj/18.18.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backs J, Worst BC, Lehmann LH, Patrick DM, Jebessa Z, Kreusser MM, Sun Q, Chen L, Heft C, Katus HA, et al. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J Cell Biol. 2011;195:403–415. doi: 10.1083/jcb.201105063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Dowling M, Yang XJ, Kao GD. Caspase-mediated specific cleavage of human histone deactylase 4. J Biol Chem. 2004;279:34537–34546. doi: 10.1074/jbc.M402475200. [DOI] [PubMed] [Google Scholar]
- 25.Cohen TJ, Barrientos T, Hartman ZC, Garvey SM, Cox GA, Yao TP. The deacetylase HDAC4 controls myocyte enhancing factor-2-dependent structural gene expression in response to neural activity. FASEB J. 2009;23:99–106. doi: 10.1096/fj.08-115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, et al. Histone deactylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang Q, Xiaobing Q, Yue Y, Wu H, Benda C, Lin J, Huang Z, Liu L, Xu Y, Bao X, et al. Class IIa histone deacetylases and MEF2 proteins regulate the mesenchymal-to-epithelial transition of somatic cell reprogramming. J Biol Chem. 2013;288:12022–12031. doi: 10.1074/jbc.M113.460766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mottet D, Pirotte S, Lamour V, Hagedorn M, Javerzat S, Bikfalvi A, Bellahcene A, Verdin E, Castronovo V. HDAC4 represses p21 (WAF/Cip1) expression in human cancer cells through a Sp1-dependent, p53-independent mechanism. Oncogene. 2009;28:242–256. doi: 10.1038/onc.2008.371. [DOI] [PubMed] [Google Scholar]
- 29.Wilson AJ, Byun DS, Nasser S, Murray LB, Ayyanar K, Arango D, Figueroa M, Melnick A, Kao GD, Augenlicht LH, et al. HDAC4 promotes growth of colon cancer cells via repression of p21. Mol Biol Cell. 2008;19:4062–4075. doi: 10.1091/mbc.E08-02-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannaerts I, Eysackers N, Onyema OO, Van Beneden K, Valente S, Mai A, Odenthal M, van Grunsven LA. Class II HDAC inhibition hampers hepatic stellate cell activation by induction of microRNA-29. PLoS One. 2013;8:E55786. doi: 10.1371/journal.pone.0055786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson O, Diebold D, Fan D, Peterson M, Nho RS, Bitterman PB, Henke CA. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS One. 2008;3:e3220. doi: 10.1371/journal.pone.0003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 34.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen KC, Sapinoro RE, Kottman RM, Kulkarni AA, Iismaa SE, Johnson GV, Thatcher TH, Phipps RP, Sime PJ. Transglutaminase 2 and its role in pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:699–707. doi: 10.1164/rccm.201101-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinkovic A, Liu F, Tschumperlin D. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2013;48:422–430. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox TR, Bird D, Baker AM, Barker HE, Ho MWY, Lang G, Erler JT. Lox-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez NE, Zhang Z, Madamanchi A, Boyd KL, O’Rear LD, Nashabi A, Li Z, Dupont WD, Zijlstra A, Zutter MM. The α2β1 integrin is a metastasis suppressor in mouse models and human cancer. J Clin Invest. 2011;121:226–237. doi: 10.1172/JCI42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grzesiak JJ, Bouvet M. The α2β1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br J Cancer. 2006;94:1311–1319. doi: 10.1038/sj.bjc.6603088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sottnik JL, Daignault-Newton S, Zhang X, Morrissey C, Hussain MH, Keller ET, Hall CL. Integrin α2β1 promotes prostate cancer skeletal metastasis. Clin Exp Metastasis. 2013;30:569–578. doi: 10.1007/s10585-012-9561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marthick JR, Dickinson JL. Emerging putative biomarkers: the role of alpha 2 and 6 integrins in susceptibility, treatment, and prognosis. Prostate Cancer. 2012;2012:298732. doi: 10.1155/2012/298732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirkland SC, Ying H. α2β1 integrin regulates lineage commitment in multipotent human colorectal cells. J Biol Chem. 2008;283:27612–27619. doi: 10.1074/jbc.M802932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balestrini JL, Chaudry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Wu L-C, Pang J, Santhanam R, Wu Y-Z, Hickley CJ, Yu J, Becker H, Maharry K, Radmacher MD, et al. SP1/NFkB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer Cell. 2010;17:333–347. doi: 10.1016/j.ccr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]