Abstract
Airway diseases are associated with abnormal circadian rhythms of lung function, reflected in daily changes of airway caliber, airway resistance, respiratory symptoms, and abnormal immune-inflammatory responses. Circadian rhythms are generated at the cellular level by an autoregulatory feedback loop of interlocked transcription factors collectively referred to as clock genes. The molecular clock is altered by cigarette smoke, LPS, and bacterial and viral infections in mouse and human lungs and in patients with chronic airway diseases. Stress-mediated post-translational modification of molecular clock proteins, brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1) and PERIOD 2, is associated with a reduction in the activity/level of the deacetylase sirtuin 1 (SIRT1). Similarly, the levels of the nuclear receptor REV-ERBα and retinoic acid receptor–related orphan receptor α (ROR α), critical regulators of Bmal1 expression, are altered by environmental stresses. Molecular clock dysfunction is implicated in immune and inflammatory responses, DNA damage response, and cellular senescence. The molecular clock in the lung also regulates the timing of glucocorticoid sensitivity and phasic responsiveness to inflammation. Herein, we review our current understanding of clock-controlled cellular and molecular functions in the lungs, the impact of clock dysfunction in chronic airway disease, and the response of the pulmonary clock to different environmental perturbations. Furthermore, we discuss the evidence for candidate signaling pathways, such as the SIRT1–BMAL1–REV-ERBα axis, as novel targets for chronopharmacological management of chronic airway diseases.
Keywords: sirtuin 1, Bmal1, REV-ERBα, asthma, chronic obstructive pulmonary disease
Clinical Relevance
This Translational Review highlights our current understanding of circadian clock–coupled cellular and molecular functions in the lungs as they relate to lung pathophysiology. This review explicates the role of the molecular clock in oxidative/carbonyl stress, inflammation, cellular senescence, and exacerbations associated with a decline in lung function in chronic airway diseases. It also communicates our current understanding of how clock-controlled cellular and molecular functions in the lung are influenced by environmental stresses and the process of inflammaging, which may lead to identification of specific molecular clock pathways as novel targets for chronopharmacotherapy in airway disease management.
Patients with chronic obstructive pulmonary disease (COPD) and asthma develop more frequent and severe exacerbations, with an increased rate of emergency room visits and hospitalization, mostly at night and in the early morning hours (1, 2). Exacerbation in patients with COPD/asthma, with increased lung inflammation and deterioration of the disease state, is associated with a rapid decline in lung function. Air pollutants, cigarette smoke (CS), and respiratory viral (influenza and rhinoviruses) and bacterial infections can lead to exacerbations of COPD/asthma, with the most severe effects appearing in the early morning hours and affecting lung function (1, 2). Hence, there is a connection between circadian decline in lung function and exacerbations of COPD/asthma.
Key Terminologies/Glossaries
Circadian timing system: the internal timing system that regulates daily rhythms of physiology and behavior.
Circadian molecular clock: key molecules involved in regulating circadian rhythms.
Circadian disruption: disruption of daily rhythm of the timing system and/or molecular clock.
Clock dysfunction: changes in circadian timing or amplitude of molecular clock affecting downstream clock-controlled output genes.
The circadian timing system in mammals drives daily circadian rhythms of physiology and behavior (3). Circadian rhythms are generated at the cellular level by an autoregulatory feedback loop of interlocked transcription factors collectively referred to as clock genes. Apart from the central clock, located within the suprachiasmatic nucleus of the basal hypothalamus, peripheral tissues, including the lung, liver, heart, and kidney, are also comprised of cell autonomous oscillators (3). Circadian disruption refers to a disruption of daily rhythm of the timing system and/or the molecular clock. The core clock gene brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1):CLOCK activator complex regulates expression of period (Per) 1–3 and cryptochrome (Cry) 1–2 genes. PER and CRY form heterodimers, are phosphorylated by casein kinases, and translocate back to the nucleus, where they repress their own transcription by blocking the transcriptional activity of the BMAL1:CLOCK complex (3). In addition to the core loop, the clock genes/nuclear receptors, REV-ERBα and retinoic acid receptor–related orphan receptor α (ROR α), provide stability to the oscillator and regulate the timing and amplitude of Bmal1 expression. Any significant change in the circadian timing or amplitude of molecular clock gene expression that can affect downstream clock-controlled output genes or cellular processes can be referred to as clock dysfunction. Clock gene expression in the lungs was first detected by Oishi and colleagues (4) and, later, Gibbs and colleagues (5) reported molecular clock function in bronchial epithelial cells. To date, there are few studies examining the impact of clock dysfunction in pulmonary physiology and pathology. Recently, we and others have shown that circadian molecular clock dysfunction has a profound influence on pulmonary function and lung pathophysiology (5–14).
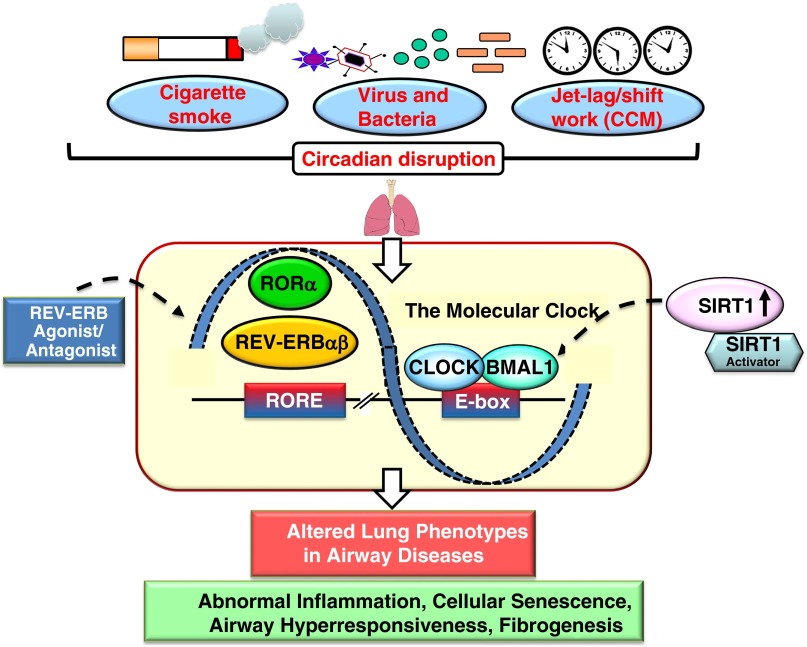
Environmental factors, such as air pollutants, xenobiotic detoxification, CS-induced oxidative/carbonyl stress, shift work, jet lag, and pathogens (bacteria/virus), can disturb molecular clock function in the lungs (Table 1). This perturbation of circadian clock function may be responsible for altered cellular and molecular functions in the lung during the pathogenesis and exacerbations of chronic airway diseases (Figure 1). Recently, a National Heart, Lung, and Blood Institute workshop (http://www.nhlbi.nih.gov/research/reports/2014-circadian-clock-lung-health) on “circadian clock at the interface of lung health and disease” carefully considered ongoing research in this area and provided recommendations moving forward in three overarching frameworks: (1) circadian-coupled mechanisms of lung disease pathogenesis; (2) circadian-based phenotypes of lung disease risk and stratification; and (3) circadian-based interventions for lung disease as future research opportunities in this emerging area. The field of chronopharmacology is tasked with developing and testing compounds that alter the timing and amplitude of clock or clock-dependent gene expression for the express purpose of treating disease (15). The scope of this review is to consolidate the recent findings in light of emerging knowledge regarding circadian clock–controlled cellular and molecular functions in the lung at baseline and in response to various environmental stressors. We have focused our discourse on the molecules that play an important role in regulating the circadian clock–coupled lung cellular and molecular functions implicated in the pathogenesis and exacerbations of chronic airway diseases that may also be promising targets for chronopharmacological intervention.
Table 1.
Role of the Molecular Clock in Lung Pathophysiology
| Agents and Models Used in Pulmonary Circadian Clock Studies | Major Outcomes/Findings | References |
|---|---|---|
| Lung circadian timing in C57BL/6J and PER2::Luc transgenic mice | Clara/Club cells are critical for maintaining clock function in lung tissue | 5 |
| Diurnal oscillations in the lung transcriptome in Wistar rats | Genes involved in the metabolism and transport of endogenous compounds and xenobiotics in clock control of lung pathophysiology | 7 |
| A repeated light-shifting regimen (simulated jet lag model) in C57BL/6J mice | Disturbing molecular clock function alters lung mechanics in a sexually dimorphic manner | 6 |
| Cigarette smoke exposure in Sprague-Dawley rats | Transcriptomic changes in lung clock gene expression | 8 |
| Cigarette smoke exposure in C57BL/6J and A/J mice | Suppression of clock gene, Nr1d1, which encodes Rev-erbα | 11 |
| Cigarette smoke exposure in C57BL/6J mice (COPD/emphysema model) | Circadian clock dysfunction is associated with increased lung inflammation via the SIRT1–BMAL1 pathway | 10 |
| Circadian clock regulates Nrf2-mediated antioxidant defense pathway by bleomycin in mice | Circadian clock as an endogenous regulatory mechanism controlling the rhythmic activity of the redox-sensitive Nrf2 in the lung | 9 |
| Circadian rhythm reprogramming by LPS in mice | Genome-wide expression of clock genes during LPS-induced lung inflammation | 13 |
| Host defense to bacterial infection and pulmonary inflammation by LPS in mice | Genetic ablation of Bmal1 in bronchiolar cells disrupts rhythmic Cxcl5 expression | 14 |
| Influenza A virus–dependent remodeling of pulmonary clock function in mouse model of COPD/emphysema | Chronic cigarette smoke exposure combined with influenza A virus infection altered the timing of clock gene expression, along with increased lung inflammation, and disrupted rhythms of lung function | 12 |
Definition of abbreviations: BMAL1, brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1; Clock, circadian locomotor output cycles protein kaput; COPD, chronic obstructive pulmonary disease; Cxcl5, chemokine (C-X-C) motif ligand 5; Luc, luciferase; Nrf2, nuclear factor (erythroid-derived 2)-like 2; Nr1d1, nuclear receptor subfamily 1, Group D, member 1; PER2, period 2; SIRT1, sirtuin 1.
Figure 1.
Impact of environmental agents on molecular clock function in the lung. Various environmental stressors, including cigarette smoke (oxidative/carbonyl stress), viral and bacterial infections, and chronic circadian misalignment (CCM) (chronic jet lag) alter molecular clock functions in the lungs. Circadian disruption of the daily timing system can affect the rhythmic expression of clock output genes in the lung that may influence lung pathophysiology. Activation of sirtuin 1 (SIRT1) and REV-ERBα by pharmacological activators/agonists may have beneficial effects against altered lung phenotypes caused by molecular clock dysfunction in chronic airway diseases. BMAL1, brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1; Clock, circadian locomotor output cycles protein kaput; E-box, enhancer box; RORα, retinoic acid receptor–related orphan receptor α; RORE, retinoic acid receptor–related orphan receptor response element.
Molecular Clock Dysfunction and Immune-Inflammatory Responses
Studies from animal models highlight the extent to which the molecular clock regulates fundamental aspects of the immune-inflammatory response, including Toll-like receptor 9 signaling and CCL2 expression (16). The clock protein and heme receptor, REV-ERBα, has been shown to attenuate the activation of IL-6 expression (17). It has been shown that REV-ERBα binds to nuclear factor kappa B (NF-κB) (RelA/p65) and can activate NF-κB–dependent transcription. Transcription factors activator protein 1 (AP-1) and NF-κB share unique sequences that overlap consensus sequences from Rev-erb α promoters, suggesting a role for REV-ERBα in oxidative stress and/or inflammation (18).
The impact of molecular clock dysfunction, particularly as it relates to lung pathophysiology and inflammatory responses, has been investigated in mice bearing loss-of-function clock gene mutations. A dominant-negative mutation of the CLOCK protein (ClockΔ19) showed altered temporal Nrf2 activity in the lungs complemented by reduced glutathione levels, increased protein oxidation, and a spontaneous fibrotic-like phenotype (9). Earlier reports also show that toxin-mediated activation of the aryl hydrocarbon receptor (Ahr) signaling pathway was modulated in a CLOCK-dependent circadian manner (19). CLOCK mutant mice, which are deficient in CLOCK transcriptional activity, lack rhythmic Ahr gene expression. The messenger RNA levels of Ahr in the lungs of clock mutant mice remained lower compared with wild-type mice (19). Behaviorally arrhythmic Bmal1−/− mice show signs of advanced aging and underlying pathologies, correlated with increased levels of reactive oxygen species and inflammation (20).
Several studies have shown that bacterial infection alters the timing and amplitude of clock gene expression (17, 21). It has been shown that the Toll-like receptor 4 pathway in peritoneal macrophages is regulated by the clock, providing a mechanism whereby the timing system may drive rhythmic variation in immune response (22). A recent study identified a regulatory mechanism, whereby the lung epithelial clock and glucocorticoid hormones control daily variation of pulmonary inflammatory responses to bacterial infection (14). Another report suggests that endotoxemia-induced lung inflammation reorganizes circadian rhythms of leukocytes in a Bmal1-dependent manner (13). Similarly, rhythms of pulmonary function define time-dependent sensitivity to steroids and β2-agonists in patients with nocturnal asthma and patients with asthma who smoke (23). Hence, it is possible that the mechanism that couples the molecular circadian clock and bronchiolar glucocorticoid receptor to pulmonary innate immunity and inflammatory responses plays an important role during COPD exacerbations and respiratory infections.
We have recently shown that respiratory infection (influenza A) can produce molecular clock dysfunction in the lungs and increase mortality in Bmal1 knockout mice, suggesting that altered clock function dampens the immune response. This assertion is supported by data showing that the response to influenza A virus infection is exacerbated in mice previously exposed to CS, suggesting that molecular clock dysfunction due to environmental CS dampens the immune response to respiratory infection via a clock-dependent mechanism (12). Although we observe direct effects of infection and CS on clock function, inflammatory responses, and mortality, it is unclear whether these effects are related to clock dysfunction–dependent or other cellular programming in lung tissue and during the course of exacerbations in chronic airway diseases.
Molecular clock dysfunction is known to affect pulmonary physiology and injurious responses (6, 10, 14). It has been reported that chronic jet lag, a well established form of circadian disruption of the daily timing system, also alters clock gene expression and pulmonary function in the lungs (6). We have shown that environmental CS exposure also causes circadian disruption, producing molecular clock dysfunction and enhanced inflammatory responses in the lungs (10). Abundance of clock proteins, such as BMAL1, PER2, and REV-ERBα, are reduced in peripheral blood mononuclear cells, sputum cells and lung tissues associated with inflammatory responses from smokers and patients with COPD when compared to nonsmokers (24). Hence, it is likely that the molecular clock dysfunction can augment lung inflammatory responses to environmental stressors/agents.
Molecular Clock Dysfunction, DNA Damage/Repair, and Stress-Induced Premature Senescence
Recent studies have shown that aging differentially affects re-entrainment rates of central and peripheral oscillators (25). Circadian disruption has particularly adverse effects on the elderly population. This may have implications in aged populations/patients with chronic airway diseases who are susceptible to develop more frequent exacerbations. Thus, molecular clock dysfunction may cause and/or enhance stress-induced premature senescence (SIPS).
Environmental CS, which we have established causes molecular clock dysfunction in the lungs, also causes DNA damage and impairs double-strand break (DSB) repair. Both DNA damage and impaired DSB repair are aggravated in COPD, a disease characterized by accelerated and premature aging of the lung. Persistent DNA damage induces SIPS and a senescence-associated secretory phenotype (SASP). Thus, DNA damage response (DDR) may cause an inflammatory phenotype in senescent cells. The DSB is the most dramatic form of DNA damage, and is repaired predominantly by nonhomologous end joining (NHEJ) in response to stress. The molecular clock plays an important role in determining the strengths of cellular responses to DNA damage, including repair. Mice deficient in Bmal1 exhibit early signs of aging (20), possibly via augmented DNA damage, impaired DNA repair (nonhomologous end joining), cellular senescence, and inflammatory responses. Apart from regulation of gene transcription, circadian clock proteins also interact with other factors, including Ku70 (protein encoded by the gene XRCC6), Ku80 (protein encoded by the gene XRCC5), and ataxia–telangiectasia mutated involved in DNA damage checkpoints after genotoxic stress, thereby mediating DNA damage/repair responses (26). BMAL1 negatively regulates prosenescent gene, p21, which is required for cell cycle arrest upon DNA damage (27). However, it remains unclear whether CS-mediated molecular clock dysfunction (i.e., altered BMAL1 and REV-ERBα expression) may enhance the susceptibility to DNA damage leading to lung cellular senescence and premature aging, particularly during the pathogenesis of COPD.
Sirtuin 1 and Clock Protein Deacetylation during Inflammation and Cellular Senescence
Clock gene transcription factors are heavily influenced by post-translational modifications, such as acetylation and phosphorylation, that can affect both their activity and stability. sirtuin (SIRT) 1 mediates deacetylation of BMAL1 at Lys537 and PER2, thereby regulating the transcription of clock genes. SIRT1 level/activity shows daily variation in the lungs, correlated with rhythmic acetylation of BMAL1 and PER2. As mentioned previously, SIRT1 activity is decreased in human lung epithelial cells, macrophages, and lungs of mice exposed to CS, and in the lungs of patients with COPD (28). It is possible that oxidative/carbonyl stress (CS)–mediated reduction of SIRT1 level/activity leads to hyperacetylation of clock proteins and/or clock gene–associated histones, culminating in abnormal rhythms of proinflammatory and molecular clock gene expression. Indeed, we have shown that molecular clock dysfunction, along with SIRT1 reduction, contributes to abnormal inflammatory responses in smokers and patients with COPD (24). SIRT1 activation reduces CS-induced acetylation and degradation of BMAL1 in mouse lungs. Thus, targeting time-dependent pharmacological activation of SIRT1 may have considerable potential as a novel form of chronopharmacology in chronic airways disease (24).
SIRT1 is also implicated in regulation of senescence during DDR (28). SIRT1 regulates cellular senescence (SIPS and SASP) in response to CS stress by protecting genomic DNA against DSB and by modulating chromatin in lung cells. SIRT1 knockout mice exhibit not only senescence, possibly via DNA damage and impaired DNA repair, but also display augmented inflammatory responses (28). Hence, SIRT1 functions in DDR to promote DNA repair, and down-regulates inflammation by deacetylating specific histones, although the involvement of another sirtuin (SIRT6) cannot be ruled out. However, there is no information available regarding the influence of SIRT1 on clock protein expression and transcriptional activity as it relates to DNA damage/repair, as well as SIPS and SASP in lungs.
The Potential for Chronopharmacology in the Treatment of Chronic Airway Disease
Activated glucocorticoid receptor influences the molecular clock through binding of glucocorticoid response elements in Per gene promoter regions (29). The expression of clock genes is induced by dexamethasone and prednisone in bronchial epithelial cells, peripheral blood mononuclear cells, lymphocytes, and fibroblasts (29). Bronchodilators, including β2-adrenoreceptor agonists used in treatment of COPD, also induce Per gene expression in Beas-2B cells (30). These findings suggest that induction of clock-dependent output genes is mechanistically linked to the antiinflammatory effects of glucocorticoids and β2-adrenoreceptor agonists. Thus, compounds designed to increase the amplitude and normalize the phase of clock and clock-dependent gene expression in the lungs could have considerable promise for improving the inflammation in chronic airway diseases.
Clock-enhancing or clock-modifying molecules have been developed, and could prove effective as an alternative to steroid or β2-adrenoreceptor agonists as a novel chronopharmacological approach for respiratory disease (31). These small molecules have been shown to target various components of the clock, leading to altered timing and amplitude of clock and clock-dependent gene expression. Synthetic ligands for REV-ERBα (e.g., GSK4112, SR9011, SR9009) have also been developed, and may have the ability to alter lung clock function and/or improve respiratory function and inflammation in patients with asthma and COPD (32). A recent study showed that many of the most commonly used medications/drugs, such as Advair Diskus, Combivent, and ProAir HFA, target rhythmic genes in the lung and other peripheral tissues. Furthermore, it has been shown that most of the top-selling drugs on the market target the circadian clock (33). These data strengthen the argument for chronopharmacotherapy in lung diseases, and highlight the importance of considering time-of-day administration for the treatment and management of chronic airway diseases and their exacerbations. However, it remains to be seen if any or all of these chronopharmacological agents may be used to effectively treat inflammation, steroid resistance, and cellular senescence in chronic lung diseases.
Conclusions and Future Directions
Environmental CS and other factors (bacterial and viral infections) lead to targeted molecular clock dysfunction in the lungs (Figure 1). The inflammatory response to CS appears to affect the peak phase and amplitude of clock gene expression through the activity of the deacetylase, SIRT1. CS reduces SIRT1 expression in the lungs, leading to changes in the acetylation and degradation of the clock proteins, BMAL1 and PER2. Furthermore, SIRT1 and BMAL1 levels are reduced in lungs from smokers and patients with COPD. SIRT1 activators and REV-ERB agonists, via the SIRT1–BMAL1–REV-ERBα axis, may be used to target the molecular clock in lung cells. This may attenuate lung inflammatory and prosenescence responses, and thereby improve the efficacy of glucocorticoids while also attenuating or even reversing DNA damage–initiated cellular senescence. However, there are considerable gaps in our knowledge on the role of the molecular clock in cellular and molecular mechanisms for controlling lung pathophysiological functions in response to environmental agents. A better understanding of the mechanism where molecular clock dysfunction contributes to the pathogenesis of chronic airway diseases could lead to new and effective avenues for treatment based on chronopharmacological agents.
Acknowledgments
Acknowledgments
The authors apologize to those authors whose excellent contribution in the field could not be cited here owing to space limitations.
Footnotes
This work was supported by National Institutes of Health grants 1R01HL097751, 1R01HL092842 (I.R.), ALA RG-266456 (H.Y.), and RG-305393 (I.K.S.).
Originally Published in Press as DOI: 10.1165/rcmb.2014-0476TR on May 4, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tsai CL, Brenner BE, Camargo CA., Jr Circadian-rhythm differences among emergency department patients with chronic obstructive pulmonary disease exacerbation. Chronobiol Int. 2007;24:699–713. doi: 10.1080/07420520701535753. [DOI] [PubMed] [Google Scholar]
- 2.Petty TL. Circadian variations in chronic asthma and chronic obstructive pulmonary disease. Am J Med. 1988;85:21–23. doi: 10.1016/0002-9343(88)90237-9. [DOI] [PubMed] [Google Scholar]
- 3.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between bmal1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun. 1998;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs JE, Beesley S, Plumb J, Singh D, Farrow S, Ray DW, Loudon AS. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology. 2009;150:268–276. doi: 10.1210/en.2008-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadden H, Soldin SJ, Massaro D. Circadian disruption alters mouse lung clock gene expression and lung mechanics. J Appl Physiol. 1985;2012:385–392. doi: 10.1152/japplphysiol.00244.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukumaran S, Jusko WJ, Dubois DC, Almon RR. Light–dark oscillations in the lung transcriptome: implications for lung homeostasis, repair, metabolism, disease, and drug action. J Appl Physiol. 2011;110:1732–1747. doi: 10.1152/japplphysiol.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebel S, Gerstmayer B, Kuhl P, Borlak J, Meurrens K, Muller T. The kinetics of transcriptomic changes induced by cigarette smoke in rat lungs reveals a specific program of defense, inflammation, and circadian clock gene expression. Toxicol Sci. 2006;93:422–431. doi: 10.1093/toxsci/kfl071. [DOI] [PubMed] [Google Scholar]
- 9.Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, et al. The circadian clock regulates rhythmic activation of the nrf2/glutathione–mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28:548–560. doi: 10.1101/gad.237081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014;28:176–194. doi: 10.1096/fj.13-232629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasu VT, Cross CE, Gohil K. Nr1d1, an important circadian pathway regulatory gene, is suppressed by cigarette smoke in murine lungs. Integr Cancer Ther. 2009;8:321–328. doi: 10.1177/1534735409352027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundar IK, Ahmad T, Yao H, Hwang JW, Gerloff J, Lawrence BP, Sellix MT, Rahman I. Influenza A virus–dependent remodeling of pulmonary clock function in a mouse model of COPD. Sci Rep. 2015;4:9927. doi: 10.1038/srep09927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haspel JA, Chettimada S, Shaik RS, Chu JH, Raby BA, Cernadas M, Carey V, Process V, Hunninghake GM, Ifedigbo E, et al. Circadian rhythm reprogramming during lung inflammation. Nat Commun. 2014;5:4753. doi: 10.1038/ncomms5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20:919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallmann R, Brown SA, Gachon F. Chronopharmacology: new insights and therapeutic implications. Annu Rev Pharmacol Toxicol. 2014;54:339–361. doi: 10.1146/annurev-pharmtox-011613-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of ccl2 expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, et al. The nuclear receptor rev-erbalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang G, Wright CJ, Hinson MD, Fernando AP, Sengupta S, Biswas C, La P, Dennery PA. Oxidative stress and inflammation modulate rev-erbalpha signaling in the neonatal lung and affect circadian rhythmicity. Antioxid Redox Signal. 2014;21:17–32. doi: 10.1089/ars.2013.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanimura N, Kusunose N, Matsunaga N, Koyanagi S, Ohdo S. Aryl hydrocarbon receptor–mediated cyp1a1 expression is modulated in a clock-dependent circadian manner. Toxicology. 2011;290:203–207. doi: 10.1016/j.tox.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in bmal1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene bmal1 regulates diurnal oscillations of ly6c(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burioka N, Fukuoka Y, Takata M, Endo M, Miyata M, Chikumi H, Tomita K, Kodani M, Touge H, Takeda K, et al. Circadian rhythms in the CNS and peripheral clock disorders: function of clock genes: influence of medication for bronchial asthma on circadian gene. J Pharmacol Sci. 2007;103:144–149. doi: 10.1254/jphs.fmj06003x4. [DOI] [PubMed] [Google Scholar]
- 24.Yao H, Sundar IK, Huang Y, Gerloff J, Sellix MT, Sime PJ, Rahman I.Disruption of sirt1-mediated regulation of circadian molecular clock and inflammation in copd Am J Respir Cell Mol BiolIn press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M, Davidson AJ. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondratov RV, Antoch MP. Circadian proteins in the regulation of cell cycle and genotoxic stress responses. Trends Cell Biol. 2007;17:311–317. doi: 10.1016/j.tcb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F. The circadian clock component bmal1 is a critical regulator of p21waf1/cip1 expression and hepatocyte proliferation. J Biol Chem. 2008;283:4535–4542. doi: 10.1074/jbc.M705576200. [DOI] [PubMed] [Google Scholar]
- 28.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Ronty M, et al. Sirt1 protects against emphysema via foxo3-mediated reduction of premature senescence in mice. J Clin Invest. 2012;122:2032–2045. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burioka N, Takata M, Okano Y, Ohdo S, Fukuoka Y, Miyata M, Takane H, Endo M, Suyama H, Shimizu E. Dexamethasone influences human clock gene expression in bronchial epithelium and peripheral blood mononuclear cells in vitro. Chronobiol Int. 2005;22:585–590. doi: 10.1081/CBI-200062416. [DOI] [PubMed] [Google Scholar]
- 30.Takata M, Burioka N, Ohdo S, Fukuoka Y, Miyata M, Endo M, Suyama H, Shimizu E. Beta2-adrenoceptor agonists induce the mammalian clock gene, hper1, mRNA in cultured human bronchial epithelium cells in vitro. Chronobiol Int. 2005;22:777–783. doi: 10.1080/07420520500179167. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cell Mol Life Sci. 2013;70:2985–2998. doi: 10.1007/s00018-012-1207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojetin DJ, Burris TP. Rev-erb and ror nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB.A circadian gene expression atlas in mammals: implications for biology and medicine Proc Natl Acad Sci USA 201411116219–16224 [DOI] [PMC free article] [PubMed] [Google Scholar]