Abstract
The alveolar epithelium is composed of two cell types: type I cells comprise 95% of the gas exchange surface area, whereas type II cells secrete surfactant, while retaining the ability to convert into type I cells to induce alveolar repair. Using lineage-tracing analyses in the mouse model of Pseudomonas aeruginosa–induced lung injury, we identified a population of stem cell antigen (Sca)-1–expressing type II cells with progenitor cell properties that mediate alveolar repair. These cells were shown to be distinct from previously reported Sca-1–expressing bronchioalveolar stem cells. Microarray and Wnt reporter studies showed that surfactant protein (Sp)-C+Sca-1+ cells expressed Wnt signaling pathway genes, and inhibiting Wnt/β-catenin signaling prevented the regenerative function of Sp-C+Sca-1+ cells in vitro. Thus, P. aeruginosa–mediated lung injury induces the generation of a Sca-1+ subset of type II cells. The progenitor phenotype of the Sp-C+Sca-1+ cells that mediates alveolar epithelial repair might involve Wnt signaling.
Keywords: lung, progenitor cell, repair, stem cell antigen-1, type II cell
Clinical Relevance
We identified a distinct population of type II cells expressing the mouse antigen stem cell antigen-1 that appeared after Pseudomonas aeruginosa–induced pneumonia and exhibited progenitor cell properties. Our results add one more example showing that different subsets of alveolar progenitor cells are preferentially activated for repair depending on the context surrounding the damage to the alveolar epithelium. Therefore, it will be necessary to develop different pharmacological strategies to use the regenerative potential of distinct alveolar progenitor cell subsets in different contexts of lung injury.
The exposure of lung alveoli to pathogens renders them especially susceptible to inflammation and injury; hence, they have developed intricate repair mechanisms to protect the gas exchange function (1–3). During unchecked inflammation, such as in adult respiratory distress syndrome, inflammatory cytokines, proteases, and oxidants released by inflammatory cells induce injury and death in the alveolar epithelium (1–3). The repair of the epithelial barrier is likely induced in the inflammatory milieu itself (1, 3, 4), but the underlying mechanisms are not clear.
The alveolar epithelium is composed of type I and type II cells (5). Type I cells, which comprise 95% of the gas exchange surface, have an attenuated structure ideal for gas exchange (6, 7). The cuboidal type II cells, which reside in alveoli corners, produce surfactant (8) and have a key role in mediating epithelial repair. Type II cells are prone to rapid induction of proliferation, and can differentiate into type I cells (8–10). Studies using the oxidant, NO2, to injure lung epithelia showed that a 3H label was first incorporated in proliferating type II cells and later in type I cells (11, 12). Lineage-tracing experiments using Cre-LoxP in mouse lungs also showed that type II cells were the source of type I cells after bleomycin-induced alveolar injury (9, 10, 13). In the resting state, type II cells are quiescent, with a normal turnover time of 2–3 weeks (8, 13). After injury, however, some type II cells enter the cell cycle to proliferate and later differentiate into type I cells (9, 10, 13, 14). However, which fraction of type II cells gives rise to type I cells remains unresolved. This question has been particularly difficult to address, because multiple studies have shown that type II cells, defined as being positive for surfactant protein (Sp)-C, comprise a heterogeneous population (15–17). For example, some cells express higher levels of E-cadherin (15), whereas others express CC10 (also known as Scgb1a1), a marker of airway Club cells (16, 17). Given the uncertainty surrounding the particular cells that mediate alveolar epithelial repair, we performed a lineage-tracing analysis to identify those type II cells mediating alveolar epithelial repair. We chose the clinically relevant Pseudomonas aeruginosa (PA) pneumonia model that we described previously, as this model can be calibrated and is associated with severe disruption of the alveolar epithelial barrier (18). We identified a distinct population of Sp-C+ cells expressing the stem cell antigen (Sca)-1, which appeared at the onset of repair after PA challenge. These cells were highly proliferative compared with their Sca-1− counterparts. The Sca-1+ cells ultimately lost Sp-C expression and appeared to differentiate into type I epithelial cells through a process involving the Wnt signaling pathway.
Materials and Methods
Mouse Strains and PA Injury Model
All animal experiments were approved by the Institutional Animal Care Committee and Biosafety Committees of the University of Illinois College of Medicine (Chicago, IL). SPC-rtTA/TetO-Cre/ROSA26-YFP mice were fed doxycycline (Dox) as described previously (18). For SpC-CreER/ROSA-GFP/tomato and Scgb1a1-CreER/ROSA-GFP/tomato mice, tamoxifen (Sigma, St. Louis, MO) was administered four times for a total dosage of 1 mg/g mouse weight, as described previously (17). PA (strain 103) was prepared, as described previously (18, 19). Mice were challenged with PA103 via intratracheal instillation (18).
Isolation and Culture of Type II Cells
Type II cells were isolated as previously described (18, 20). We used epithelial cell adhesion molecule (EpCAM) as a selection marker to ensure that the cells we analyzed were over 95% purity for type II cells. For those experiments without the EpCAM marker, type II cell purity was assessed using a modified Papanicolaou (PAP) staining protocol (20), and only preparations with over 90% purity were used. Cells were cultured to assess proliferation and differentiation.
Real-Time RT-PCR
RNA extraction and RT-PCR were performed using primers and analysis methods as described previously (18). Cyclophilin was used as an internal control for calculating relative gene expression (21). Expression was quantified using the comparative threshold cycle (Ct) method. Relative expression versus the control sample was calculated using the equation 2(−ΔΔCt).
Immunofluorescence
Freshly isolated type II cells were immobilized on slides by cytospin centrifugation and fixed. For in situ proliferation experiments, bromodeoxyuridine (BrdU) was injected intraperitoneally in mice as described previously (22). Images were captured on a Zeiss LSM 510 confocal microscope (Zeiss, Oberkochen, Germany). See the online supplement for details and antibodies used.
Flow Cytometry
Freshly isolated type II cells were stained with phycoerythrin- or allophycocyanin (APC)-labeled rat anti-mouse Sca-1 monoclonal antibody (e-Bioscience, San Diego, CA), as well as with rat IgG2ak isotype control (e-Bioscience), and sorted on a Dako-Cytomation MoFlo high-speed cell sorter (Dako-Cytomation, Carpinteria, CA) or analyzed using a CyAn ADP flow cytometer (Beckman Coulter, Brea, CA) located in the institutional Flow Cytometry Core at the University of Illinois.
Microarray Profiling
Type II cells were isolated from non-PA and post–PA-treated SPC-rtTA/TetO-Cre/ROSA26-YFP mice and separated into yellow fluorescent protein (YFP)+Sca-1+ and YFP+Sca-1− populations using MoFlo cell sorting. Total RNA was isolated from four groups of cells (non-PA Sca-1−, non-PA Sca-1+, 72-h post-PA Sca-1−, and 72-h post-PA Sca-1+) using the RNeasy mini kit (Qiagen, Valencia, CA). Each group contained six mice, and cells from two mice of the same group were pooled as one sample. RNA concentration and purity were determined before gene expression profiling using the Illumina mouse WG-6 version 2.0 system (Illumina, San Diego, CA). Microarray labeling, hybridization, and processing were performed according to the manufacturer’s protocol. Quantile-normalized data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (accession no. GSE47600). See the online supplement for details on data analysis.
Statistical Analysis
Microsoft Excel (Microsoft Corp., Redmond, WA) was used to calculate SE. Differences between groups were compared for statistical significance using Student’s t test. P values less than 0.05 were considered significant.
Results
Sca-1+ Type II Cells Appearing during the Alveolar Epithelial Repair Phase Showed Increased Proliferation
We used the mouse model of intratracheal PA injection (18) to study alveolar epithelial progenitor cell populations appearing after PA injury. We previously reported in this model that alveolar type II cells enter the repair phase at 72 hours after PA infection through proliferation and differentiation into type I cells (18). Here, we focused on the function of the Sca-1+ type II cell population, which we showed appearing during the repair phase of PA-induced lung alveolar epithelial injury (18). We tested the hypothesis that these Sca-1+ type II cells represented a population of reparative cells. Sca-1 is a mouse glycosyl phosphatidylinositol–anchored cell surface protein of the Ly6 gene family that is expressed in tissue-resident stem and progenitor cells (23).
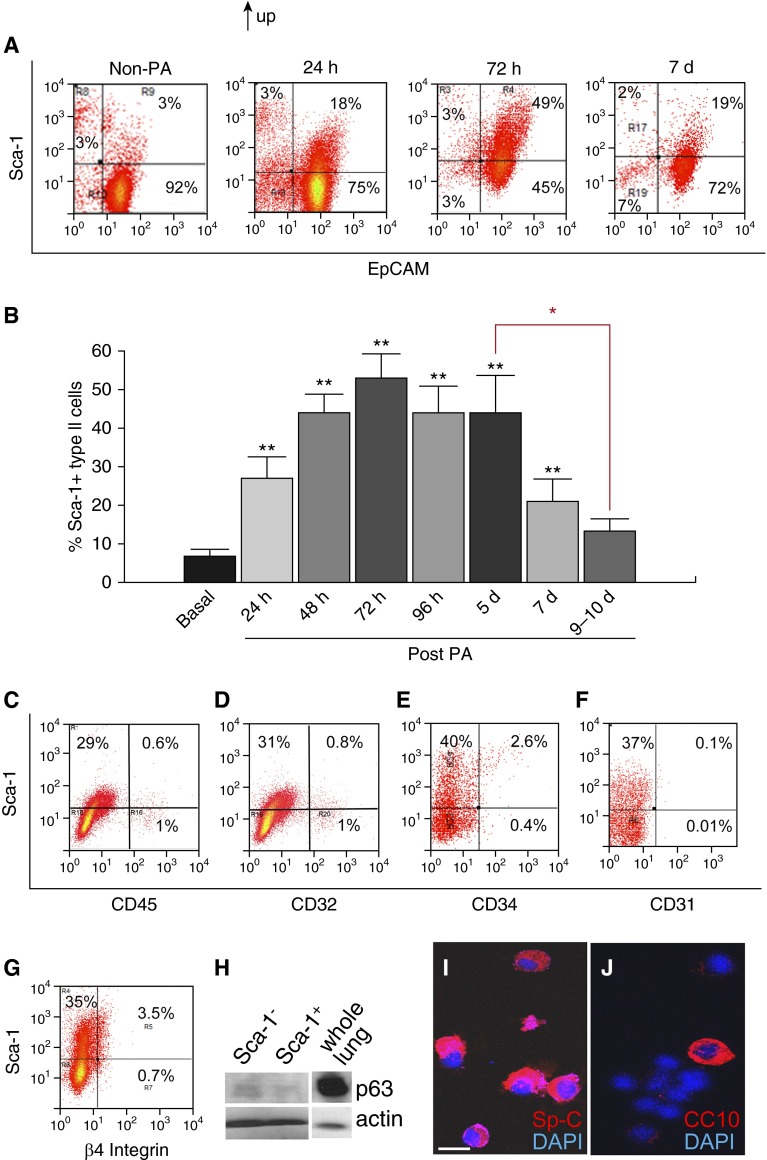
Isolated type II cells from post-PA and control lungs were double stained with antibodies against Sca-1 and the epithelial marker, EpCAM (24). EpCAM was used to minimize inaccuracies arising from impurities in the type II cell isolation. In the method used, the type II cell preparation was over 90% pure, as assessed via PAP staining (see Figures E1A and E1B in the online supplement) (20); the contaminating cells comprised small numbers of endothelial cells, fibroblasts, blood cells, et cetera, and less than 1% T1α+ cells (reflecting type I cells) (25) (Figures 1A and 1C–1E; Figure E1C). The percentage of Sca-1+ type II cells was calculated by dividing the number of Sca-1+ cells in the gated EpCAM+ fraction by the total number of gated EpCAM+ cells (Figures 1A and 1B; Figure E1D). The percentage of Sca-1+ type II cells increased as early as 24 hours after PA, continued to increase up to 48 hours, and peaked at 72 hours, after which it decreased during the repair phase (Figures 1A and 1B). This trend is consistent with our previous Quantitative RT-PCR (qRT-PCR) results showing increased Sca-1 transcript levels in the type II cells isolated 72 hours after PA (18). By 9–10 days after PA, at which time the alveolar epithelial injury was resolved (18), the percentage of Sca-1+ type II cells returned to baseline levels. Importantly, the time at which the Sca-1+ type II cells appeared paralleled the beginning of the alveolar epithelial repair phase, whereas the disappearance of Sca-1+ cells correlated with the termination of repair.
Figure 1.
Stem cell antigen (Sca)-1+ type II cells appear after Pseudomonas aeruginosa (PA) pneumonia in mice. (A and B) Type II cells were isolated from control, non–PA-treated mice, as well as from mice at 24, 48, 72, and 96 hours, and 5, 7, and 9–10 days after PA treatment, and stained with antibodies against Sca-1 and the epithelial cell marker, epithelial cell adhesion molecule (EpCAM). Cells were studied via FACS analysis after gating on forward and side scatter to exclude debris and cell clusters. (A) Representative FACS analysis results. Time points at which the cells were isolated are indicated. Numbers in squares represent the percentage of cells in the indicated fraction out of the total gated cells. (B) The percentage of Sca-1+ type II cells was calculated by dividing the number of Sca-1+ cells in the gated EpCAM+ fraction by the total number of gated EpCAM+ cells and then plotted (n = 3–5 mice per group from at least three independent experiments). **P < 0.01 versus basal (non-PA). Data are plotted as the mean ± SE. Red asterisk indicates P < 0.05 between samples at 5 days after PA and 9–10 days after PA. (C–G) Type II cells were isolated at 3 days after PA, stained with antibodies against Sca-1 and CD45 (C), CD32 (D), CD34 (E), CD31 and EpCAM (F), and β4 integrin and EpCAM (G), and subjected to FACS analysis. Cells were gated on forward and side scatter to exclude debris and cell clusters. (F and G) Cells were also gated for the EpCAM+ fraction. Numbers indicate the percentage of cells in the indicated fraction out of the total gated cells. Data are representative of at least two independent experiments. (H) Type II cells were isolated at 72 hours after PA, separated into Sca-1+ and Sca-1− populations, and processed for Western blot analysis to detect p63 expression. β-actin was used as the protein loading control, and whole-lung lysates run on the same gel were used as a positive control for p63. (I and J) Sca-1+ EpCAM+ cells isolated from 72-hour post-PA mice were fixed on slides by cytospin centrifugation and stained with antibodies against surfactant protein (Sp)-C (I) and CC10 (Scgb1a1) (J). 4′,6-diamidino-2-phenylindole (DAPI) was used to visualize nuclei. Data are representative of more than three independent experiments. Scale bar, 10 μm.
Next, type II cells were isolated at 72 hours after PA, a time point early in the repair phase (18) and characterized by the highest fraction of Sca-1+ cells (Figure 1B), and these cells were further analyzed using FACS. We found that Sca-1+ type II cells were negative for the endothelial and hematopoietic markers, CD45, CD32, CD34, and CD31 (16, 26) (Figures 1C–1F). The majority of Sca-1+ type II cells was also negative for β4 integrin (Figure 1G), indicating that this population was distinct from the integrin α6+β4+ alveoli-regenerating cells reported by Chapman and colleagues (27).
To compare the Sca-1+ cells with two other reported alveolar progenitor cells, Sca-1+ type II cells were isolated using MoFlo cell sorting at 72 hours after PA and subjected to Western blotting and antibody staining. We found that Sca-1+ type II cells only expressed trace levels of p63 (28) (Figure 1H), were positive for the type II cell marker, Sp-C (29) (Figure 1I), and were mostly negative for Club cell marker CC10 (17, 29) (Figure 1J). Therefore, the identified Sca-1+ cells were distinct from both the p63+ cells reported by Kumar and colleagues (28) and the bronchioalveolar stem cells (BASCs) reported by Kim and colleagues (16).
Next, to determine the difference between Sca-1+ type II cells and their Sca-1− counterparts, we isolated Sca-1+ and Sca-1− type II cells by FACS at 24 and 72 hours after PA and compared their expression profiles for the key proliferative factors, CDC25C and Cyclin B1 (18). By 24 hours, Sca-1+ type II cells expressed similar amounts of CDC25C and Cyclin B1 messenger RNA (mRNA) as their Sca-1− counterparts, and these levels were similar to those observed in Sca-1− cells derived from non–PA-challenged lungs (basal) (Figures 2A and 2B). However, Sca-1+ type II cells obtained at 72 hours after PA, the early phase of repair (18), had significantly greater CDC25C and Cyclin B1 mRNA levels than Sca-1− cells (Figures 2A and 2B).
Figure 2.
Proliferation of Sca-1+ type II cells in a P. aeruginosa model of pneumonia. (A and B) Type II cells were isolated at 24 and 72 hours after PA and separated into Sca-1+ and Sca-1− populations. RNA was isolated from these cells and processed for real-time RT-PCR analysis. Relative CDC25C (A) and CLNB1 (B) expression was compared between Sca-1+ and Sca-1− cells. Non–PA-treated Sca-1− type II cells were used as basal control. Data are plotted as mean ± SE. *P < 0.05. (C) Bromodeoxyuridine (BrdU) was injected into non–PA-treated (Basal) or 24- and 72-hour post–PA-treated mice intraperitoneally. Type II cells were isolated at 5 hours after BrdU injection and stained with fluorescence-labeled antibodies against EpCAM, Sca-1, and BrdU. Cells were studied by FACS analysis after gating on forward and side scatter to exclude debris and clusters, and cells were also gated for EpCAM+ fraction. For each time point after PA, the percentages of cells in the indicated fraction out of total gated cells are shown. Data are representative of at least two independent experiments.
To assess the proliferative potential of these cells, BrdU was injected intraperitoneally in non–PA-treated mice, as well as in 24- and 72-hour post-PA mice. Type II cells were isolated at 5 hours after BrdU injection and stained using antibodies against EpCAM, Sca-1, and BrdU. As shown in Figure 2C, there were few BrdU+ cells in type II cells isolated from non-PA or 24-hour post-PA lungs. In contrast, the percentage of BrdU+ type II cells increased significantly at 72 hours after PA, and almost all BrdU+ type II cells were also Sca-1+ at this time (Figure 2C). Thus, Sca-1+ type II cells developed a hyperproliferative phenotype at approximately 72 hours after PA, which corresponds to the beginning of the repair phase.
Sp-C+ Cells Give Rise to Sca-1+ Cells
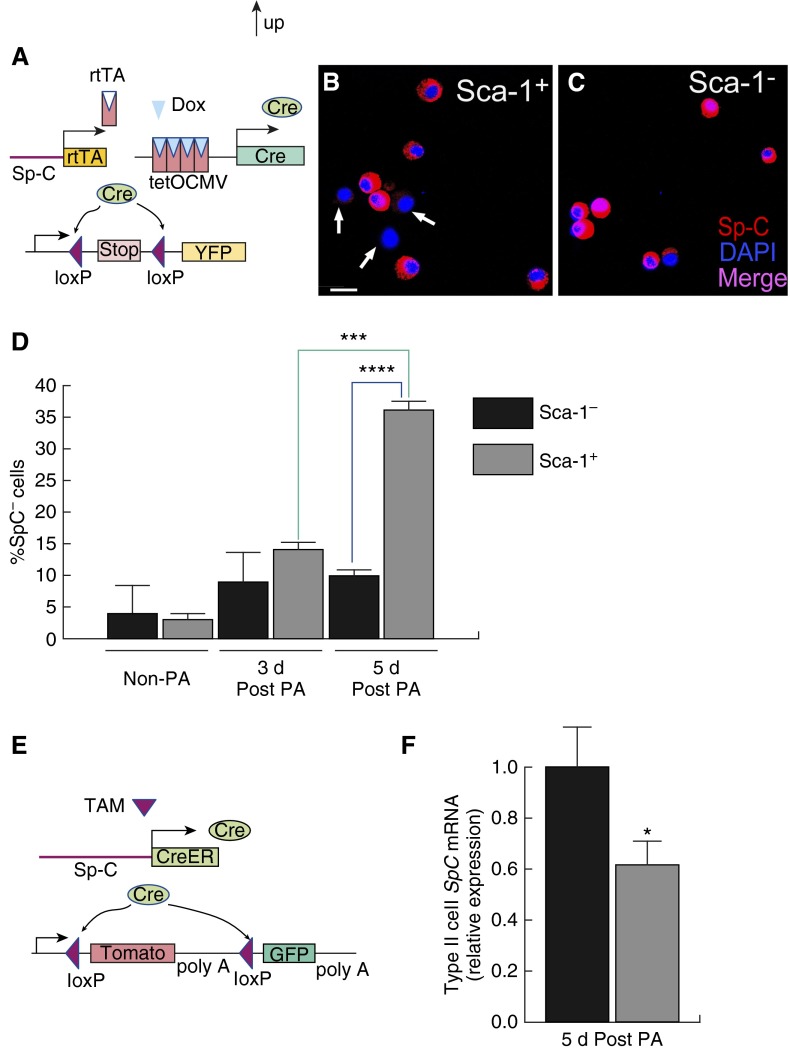
We next performed a lineage-tracing analysis to address the origin of Sca-1+ type II cells. Specifically, we examined whether the Sca-1+ cells were derived from type II cells expressing the marker, Sp-C. Here we used the SPC-rtTA/TetO-Cre/ROSA-YFP mouse line (17, 18, 30). Dox was administered in adult mice before PA injury. Reverse tetracycline-controlled transactivator (rtTA) expressed by the type II cell–specific Sp-C promoter interacts with Dox to produce Cre recombinase and induce the expression of YFP in Sp-C+ cells and in their progeny (Figure 3A; Figures E2A–E2D). Type II cells were isolated at 3 days after PA and stained with antibodies against Sca-1 and EpCAM. We observed that ∼70% of EpCAM+ cells were labeled with YFP (Figure 3B). This is consistent with our previous results showing that approximately 70% of the type II cells had Cre-mediated recombination, and thus YFP expression (18). Among both YFP+EpCAM+ and YFP−EpCAM+ cells, ∼45% of the cells were Sca-1+ (Figure 3B), indicating that most Sca-1+ cells were derived from Sp-C+ cells.
Figure 3.

Lineage-tracing analysis demonstrating derivation of Sca-1+ type II progenitor cells from Sp-C+ cells. (A and B) Lineage-tracing using the SPC-rtTA/TetO-Cre/ROSA-YFP line. (A) Strategy for using yellow fluorescent protein (YFP) as a marker to lineage trace the Sp-C+ cells and their progenies. Doxycycline (Dox) was administered in adult mice before injury. Reverse tetracycline-controlled transactivator (rtTA) protein, which is expressed by type II cell–specific Sp-C promoter, interacts with Dox to drive tetO-CMV promoter to produce Cre recombinase. Cre deletes the stop codon and allows expression of YFP in the Sp-C+ cells and in their progenies. (B) Type II cells from Dox-treated SPC-rtTA/TetO-Cre/ROSA-YFP mice were isolated at 3 days after PA, stained with phycoerythrin–EpCAM and allophycocyanin (APC)–Sca-1 antibodies, and subjected to FACS analysis. Cells were gated on forward and side scatter to exclude debris and cell clusters, and also gated for EpCAM+ cells. Numbers indicate the percentage of cells in the indicated fraction out of the total gated cells. Data are representative of two independent experiments. (C–D) Lineage-tracing analysis using the SpC-CreER/ROSA-GFP/tomato line. (C) Strategy for the use of green fluorescent protein (GFP) as a marker to lineage trace the Sp-C+ cells and their progenies. This line harbors a tamoxifen (TAM)-inducible Cre gene through Sp-C promoter. TAM was injected into adult mice before injury to induce the expression of Cre from the Sp-C promoter. Cre deletes the Tomato gene and enables expression of GFP in cells expressing Sp-C before injury, and the GFP expression continues in their progenies. (D) Type II cells were isolated at 72 hours after PA from TAM-injected SpC-CreER/ROSA-GFP/tomato mice, stained with APC–Sca-1 antibody, and subjected to FACS analysis. Cells were gated on forward and side scatter to exclude debris and cell clusters. Numbers indicate the percentage of cells in the indicated fraction out of total gated cells. Data are representative of two independent experiments. (E–G) Lineage-tracing studies using the Scgb1a1-CreER/ROSA-GFP/tomato line. (E) Strategy for the use of GFP as a marker to lineage trace the Scgb1a1+ cells and their progenies. TAM was injected into adult mice before injury to induce the expression of Cre from the Scgb1a1 promoter. Cre deletes the Tomato gene and enables expression of GFP in cells expressing Scgb1a1 and in their progenies. (F and G) Type II cells were isolated from TAM-injected Scgb1a1-CreER/ROSA-GFP/tomato mice without PA (F) or at 3 days after PA (G), stained with APC–Sca-1 antibodies, and subjected to FACS analysis. Cells were gated on forward and side scatter to exclude debris and cell clusters. Numbers indicate the percentage of cells in the indicated fraction out of total gated cells. Data are representative of two independent experiments. (H–J) Lungs were isolated from TAM-injected Scgb1a1-CreER/ROSA-GFP/tomato mice at 72 hours after PA, 5 hours after BrdU injection, and subjected to paraffin sectioning and immunofluorescent antibody staining. (H–J) The same section showing costaining of Sp-C (red) and Scgb1a1 (CC10)-GFP (green) (H), BrdU (blue) and Scgb1a1(CC10)-GFP (green) (I), and all three stainings (J). In these pictures, there were cells double labeled by Sp-C and Scgb1a1 (CC10)-GFP (yellow in H), and cells double labeled by BrdU and Sp-C (purple in J, arrows), but few cells were double labeled by BrdU and Scgb1a1(CC10)-GFP (I). Scale bar, 20 μm. Data are representative of three independent experiments.
To address the possible concern that the SPC-rtTA/TetO-Cre/ROSA-YFP line also labeled a small number of bronchiole cells (Figures E2A–E2D), we confirmed the above findings using another lineage-tracing line, SpC-CreER/ROSA-GFP/tomato (9, 27). When tamoxifen was injected into these mice before injury, Cre was expressed in Sp-C–expressing cells to activate green fluorescent protein (GFP) expression, leading these cells and their progeny to be permanently labeled with GFP (Figure 3C; Figures E2E–E2H). This line was generated by knocking in CreERT into the endogenous Sp-C locus (9, 27) and was more specific in labeling Sp-C+ type II cells compared with the SPC-rtTA/TetO-Cre/ROSA-YFP line. This was evident by the complete absence of GFP expression in bronchioles (Figure E2E) and by the colocalization of Sp-C and GFP (Figure E2F–E2H). By counting roughly 200 Sp-C+ cells in lung sections prepared from this line, we estimated that over 70% of Sp-C+ cells were labeled with GFP, indicating that the labeling efficiency of this line was greater than 70%. Type II cells from tamoxifen-treated mice were isolated at 3 and 5 days after PA for FACS analysis. The results from 72-hour post-PA samples are shown in Figure 3D. We observed that 80% of the type II cells were GFP+. The cells were not stained for EpCAM in this experiment; thus, a small fraction of the cells were GFP− non–type II cells. The other GFP− cells were likely type II cells that were not efficiently labeled with SpC-CreER–dependent GFP expression. Most of the Sca-1+ cells were also GFP+ (Figure 3D). Cells isolated at 5 days after PA showed similar results (Figure E3). Thus, both lineage-tracing experiments described above demonstrated that most of the Sca-1+ cells were derived from Sp-C–expressing type II cells.
Most Sca-1+ Cells Appearing during the Early Repair Phase Were Derived from CC10− Cells
We next used the Scgb1a1-CreER/ROSA-GFP/tomato mouse line (17, 27) to determine whether Sca-1+ cells could also be derived from Scgb1a1-expressing cells (Figure 3E). Scgb1a1, also known as CC10, is considered a marker of Club cells (17), and it is expressed in the BASCs present in bronchioalveolar ductal junction (16). Scgb1a1-CreER/ROSA-GFP/tomato mice were injected with tamoxifen before injury, and cells expressing CC10, as well as their progeny, were thus permanently labeled with GFP (Figure 3E; Figures E2I–E2L). The specificity and efficiency of labeling in this line were confirmed using immunohistochemistry (Figure E2I–E2L). By counting over 200 CC10+ cells in lung sections prepared from this line, we determined that over 90% of the CC10+ cells were labeled with GFP. In this lineage-tracing experiment, type II cells were isolated from non–PA-treated and 3-day post–PA-treated mice for FACS analysis. In non–PA-treated mice, less than 10% of the type II cells were Sca-1+, and almost half of the Sca-1+ cells were GFP+, suggesting that these cells were BASCs (Figure 3F) (16). In contrast, at 3 days after PA, the percentage of Sca-1+ cells increased significantly, and, importantly, most of the Sca-1+ cells were GFP−, whereas less than 10% of Sca-1+ cells were GFP+ (Figure 3G). Therefore, most of the Sca-1+ type II cells appearing during the repair phase of PA-induced injury were derived from CC10− cells. Our results indicate that a population of Sca-1+ cells derived from Sp-C+CC10− cells appears during the early repair phase after PA. Consistent with this observation, Sca-1+ type II cells were isolated at 72 hours after PA, and immunofluorescence staining showed that most of these cells did not express CC10, whereas they did express Sp-C (Figures 1I and 1J).
As shown in Figure 2C, most of the proliferating (BrdU+) type II cells that appeared at 72 hours after PA were Sca-1+. To determine whether these proliferative Sca-1+ cells were derived from Sp-C+CC10+ cells or Sp-C+CC10− cells, PA was injected into tamoxifen-treated Scgb1a1-CreER/ROSA-GFP/tomato mice. BrdU was then injected intraperitoneally in these mice at 72 hours after PA, and lung sections were prepared at 5 hours after BrdU injection and subjected to immunofluorescent antibody staining. As shown in Figures 3H–3J, few Scgb1a1 lineage–labeled GFP+ cells showed BrdU staining. In contrast, there were many Scgb1a1-GFP−Sp-C+BrdU+ cells. In our PA model, the injury, inflammation, and repair responses are not evenly distributed in all areas of the lung: some areas showed morphology similar to control lungs, whereas other areas were more cellular, indicating a strong response to pneumonia. To take this regional difference into account, we randomly selected several areas with relatively high cellularity from peripheral lungs of three mice, and within these areas we randomly selected 14 areas to score the BrdU+ cells. Of the 136 Scgb1a1-GFP+Sp-C+ cells in these areas, only 14% were BrdU+. In contrast, out of approximately 300 Scgb1a1-GFP−Sp-C+ cells, 48% were BrdU+. Therefore, our results in Figures 2C and 3H–3J collectively show that most of the hyperproliferative Sca-1+ alveoli epithelial cells appearing during the regenerative phase after PA were derived from Sp-C+CC10− cells.
Loss of Sp-C in Sca-1+ Type II Cells Undergoing Differentiation into Type I Cells
As most of the Sca-1+ type II cells isolated at 72 hours after PA expressed Sp-C (Figure 1I), we next assessed whether the hyperproliferative Sca-1+ type II cells observed after PA were associated with the time-dependent loss of the type II cell marker, Sp-C, during the repair phase. For these experiments, we first used the SPC-rtTA/TetO-Cre/ROSA-YFP mouse line described previously here, in which YFP expression was induced before injury in the cells expressing Sp-C (Figures 3A and 4A). Type II cells were isolated from control (non–PA-treated) lungs and lungs at 3 and 5 days after PA. The cells were then separated using flow cytometry into YFP+Sca-1+ and YFP+Sca-1− cells and stained with an antibody against Sp-C. At 5 days after PA, some Sca-1+ YFP+ type II cells were Sp-C− (Figure 4B), whereas most Sca-1−YFP+ type II cells were Sp-C+ (Figure 4C). By counting the numbers of YFP+Sp-C+ and YFP+Sp-C− cell, we observed that, at 5 days after PA, greater than 30% of the YFP+Sca-1+ cells were Sp-C− (Figure 4D), whereas only 10% of the YFP+Sca-1− cells were Sp-C− (Figure 4D). Furthermore, in cells isolated from non-PA and 3-day post-PA lungs, only 10% of the YFP+ cells were Sp-C−, irrespective of whether they were Sca-1+ or Sca-1− (Figure 4D).
Figure 4.
Lineage-tracing analysis demonstrating loss of Sp-C expression in reparative Sca-1+ cells. (A) The SPC-rtTA/TetO-Cre/ROSA-YFP line was used to examine expression of Sp-C in post–PA-treated type II cells. In these mice, reverse tetracycline-controlled transactivator (rtTA) is expressed from Sp-C promoter. Dox was introduced before injury to enable rtTA binding to the TetO-CMV promoter to induce Cre expression. Cre cleaves the stop codon and enables expression of YFP. The cells that expressed Sp-C before injury and their progenies were thus permanently labeled with YFP. (B and C) YFP+ type II cells were isolated at 5 days after PA, separated into Sca-1+ and Sca-1− populations, and stained with type II cell marker Sp-C. Some Sca-1+ YFP+ type II cells were Sp-C− (arrows; scale bar, 10 μm) (B), whereas almost all Sca-1−YFP+ type II cells were Sp-C+ (C). (D) YFP+ Sca-1− and YFP+ Sca-1+ type II cells were isolated from non–PA-treated mice as well as mice at 3 and 5 days after PA and stained with Sp-C. Percentage of YFP+SP-C− cells versus total number of YFP+ cells were compared between Sca-1− and Sca-1+ populations at different time points. Over 100 cells were scored in each cell group from each mouse (n = 3–5 mice from each time point from at least three independent experiments). Data are plotted as mean ± SE. ***P = 0.0006, comparing 72-hour Sca-1+ and 5-day Sca-1+ samples; ****P = 2.25E-05, comparing 5-day Sca-1+ and 5-day Sca-1− samples. (E) SpC-CreER/ROSA-GFP/tomato line was used to examine Sp-C expression in 5-day post-PA type II cells. Cre expression from the Sp-C promoter was induced by TAM injection in adult mice before injury. Cre enables expression of GFP in cells expressing Sp-C before injury, and these cells and their progenies are permanently labeled with GFP. (F) GFP+Sca-1− and GFP+Sca-1+ type II cells were isolated at 5 days after PA from TAM-injected SpC-CreER/ROSA-GFP/tomato mice, and the expression of Sp-C in these two groups of cells were compared by real-time RT-PCR analysis. *P < 0.05 versus control (Sca-1− cells); n = 5 mice from two independent experiments. Data are plotted as mean ± SE.
We next used the SpC-CreER/ROSA-GFP/tomato line described previously here, in which tamoxifen is introduced in adult mice before PA to induce the expression of GFP in Sp-C+ cells (Figures 3C and 4E). GFP+ type II cells were isolated 5 days after PA and separated into Sca-1+ and Sca-1− populations. RT-PCR analysis showed that the GFP+Sca-1+ cells expressed significantly lower amounts of Sp-C mRNA than their GFP+Sca-1− counterparts (Figure 4F). Therefore, data from both mouse lines showed that Sca-1+ cells lost the type II marker, Sp-C, by 5 days after PA, which is consistent with their conversion into type I cells.
Sca-1+ Type II Epithelial Cells Differentiate into Type I Cells
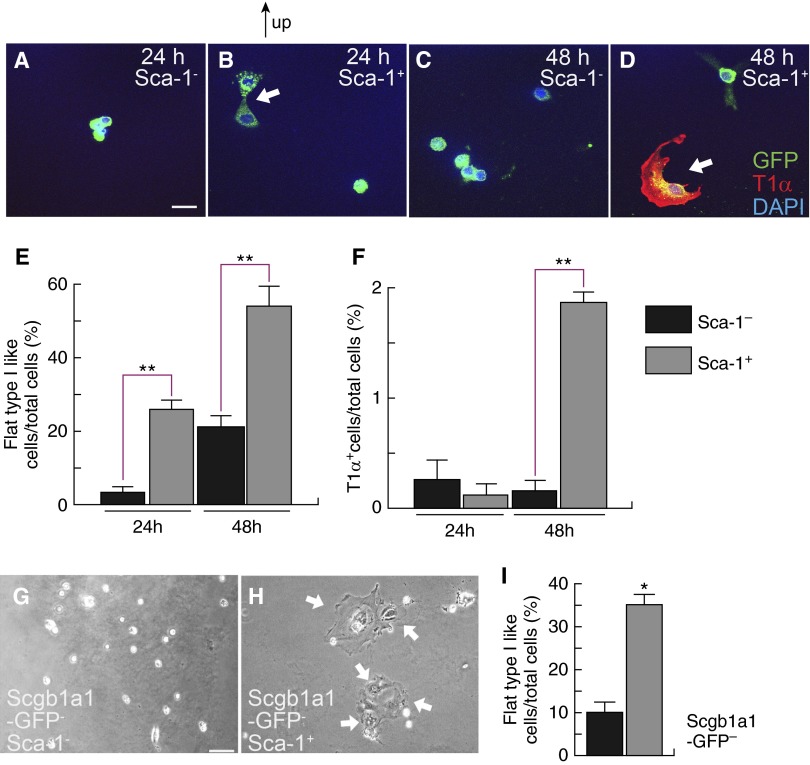
To compare the potential for Sca-1+ and Sca-1− type II cells to differentiate into type I cells, we isolated cells at 72 hours after PA. All cells appeared cuboidal before culturing (Figure E1A). At 24 hours in culture, flat type I–like cells were commonly observed in the Sca-1+ population, but were rare in the Sca-1− cells (Figures 5A, 5B, and 5E). However, these cells did not, as yet, express the type I cell marker, T1α (Figures 5B and 5F). At 48 hours in culture, some Sca-1+ cells began expressing T1α (Figures 5D and 5F). By contrast, most Sca-1− cells failed to flatten or express T1α (Figures 5A, 5C, 5E, and 5F). Some type I–like cells generated by in vitro differentiation from Sca-1+ type II cells also expressed another type I cell marker, HOP homeobox (Figure E4A) (10). qRT-PCR analysis showed that 5-day–cultured Sca-1+ type II cells expressed higher amounts of Aqp5, another type I cell marker (25), than Sca-1− cells (Figure E4B).
Figure 5.
Differentiation of Sca-1+ type II epithelial cells into type I cells. Sca-1+ and Sca-1− type II cells were isolated at 72 hours after PA and cultured on gelatin-coated plates. Within 24 hours of culture, some Sca-1+ type II cells changed into flat type I–like cells (B). By 48 hours, more Sca-1+ type II cells changed into a flat shape, and some expressed the type I cell marker T1α (D). In contrast, most Sca-1− type II cells showed cuboidal shape at 24 and 48 hours after initiation of culture (A and C). (E) Numbers of type I–like cells versus total cells were counted at different time points after initiation of culture for both Sca-1+ and Sca-1− cells, and their percentages were compared. (F) T1α+ cells versus total cells were compared between Sca-1− and Sca-1+ cells at 24 and 48 hours after initiation of culture. (E and F) Over 100 cells were counted in each cell type at each time point from each mouse; n = 3–5 mice at each time point from three independent experiments. Data are plotted as mean ± SE. **P < 0.01 versus control. (G–I) Type II cells were isolated from TAM-injected Scgb1a1-CreER/ROSA-GFP/tomato mice at 72 hours after PA, and EpCAM+GFP−Sca-1− (G) as well as EpCAM+GFP−Sca-1+ (H) cells were further isolated by MoFlo cell sorting and cultured. Arrows show flat type I–like cells. Scale bar, 20 μm. Data are representative of three independent experiments. (I) Numbers of type I–like cells versus total cells were counted at 4 days after initiation of culture for both EpCAM+GFP−Sca-1+ and EpCAM+GFP−Sca-1− cells, and their percentages were compared; n = 3 mice from three independent experiments; more than 100 cells were counted in each cell group from each mouse. Data are plotted as mean ± SE. *P < 0.05 versus control. Arrows show flat type I–like cells. Scale bar, 20 μm. Scgb1a1, secretoglobin 1A member 1.
Because some of the Sca-1+ cells in our preparation might have been CC10+ BASCs, we further isolated EpCAM+GFP− cells from tamoxifen-treated Scgb1a1-CreER/ROSA-GFP/tomato mice, separated these cells into Sca-1+ and Sca-1− fractions, and cultured them under the conditions described previously here. As shown in Figures 5G and 5H, GFP−Sca-1+ cells frequently gave rise to flat, type I–like cells, whereas almost all GFP−Sca-1− cells remained cuboidal. At 4 days after culture initiation, the percentage of flat, type I–like cells in the GFP−Sca-1+ culture was three times higher than that for the GFP−Sca-1− cells (Figure 5I). Thus, Sca-1+ cells from the Sp-C+CC10− lineage appearing after PA exhibited higher potential for type II–to–type I cell differentiation than their Sca-1− counterparts.
Distinct Gene Expression Profile of Sca-1+ Type II Cells
To determine whether Sca-1+ type II cells display the expression of progenitor cell–specific genes, we compared the expression profiles of isolated Sca-1+ and Sca-1− type II cells. To increase the purity of type II cells, we used SPC-rtTA/TetO-Cre/ROSA-YFP mice and only analyzed the YFP+ cells. Type II cells were isolated from mice before PA (basal state), as well as at 72 hours after PA, and were separated into YFP+Sca-1+ and YFP+Sca-1− populations. Gene expression profiles were then determined for the following four groups of cells: non-PA Sca-1−, non-PA Sca-1+, 72-hour post-PA Sca-1−, and 72-hour post-PA Sca-1+ (Figure 6A). We identified 2,200 differentially expressed genes between 72-hour post-PA Sca-1− and Sca-1+ cells (Figure 6B). As a positive control, we observed that both Sca-1 and FoxM1, a transcription factor that regulates the differentiation of type II cells into type I cells (18), showed significantly increased expression in 72-hour post-PA Sca-1+ cells, and the increased expression of these two transcripts was confirmed using qRT-PCR (data not shown). As shown in Figure 6B, all four groups of cells had distinct gene expression patterns. The expression of genes involved in cell cycle progression was particularly enriched in the Sca-1+ cells from 72-hour post-PA lungs (Figure 6C), which is consistent with the progenitor function of Sca-1+ cells appearing after PA.
Figure 6.
Gene expression profile comparison of Sca-1+ and Sca-1− type II cells. (A) Type II cells were isolated from non-PA and 72-hour post–PA-challenged lungs, and separated into Sca-1− and Sca-1+ populations. RNA was isolated from four group of cells: non-PA Sca-1− (0 h neg), non-PA Sca-1+ (0 h pos), 72-hour post-PA Sca-1− (72 h neg), and 72-hour post-PA Sca-1+ (72 h pos), and gene expression profiles of these four groups were compared using the Ill mouse WG-6 version 2.0 chips. Quantile-normalized data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (accession no. GSE47600). (B) A total of 2,200 differentially expressed genes were analyzed by heatmap to show the different patterns of gene expression profile among the four cell groups. (C) The expression levels of 52 genes, the products of which are involved in the cell cycle, are compared among the four groups of cells using heatmap analysis. (D) Heatmap analysis of wingless type MMTV integration site family (Wnt) pathway genes using data generated by the aforementioned microarray study. Expression levels of 11 genes, the products of which are involved in canonical wingless type MMTV integration site family (Wnt) signaling pathway, were compared between Sca-1− and Sca-1+ cells isolated at 72 hours after PA. Red letters, positive regulators in canonical Wnt pathway; blue letters, negative regulator in this pathway. (E–G) Real-time RT-PCR analysis of expression level of APC (E), glycogen synthase kinase 3 β (Gsk3β) (F), and Wnt4 (G) using Sca-1− and Sca-1+ type II cells isolated at 72 hours after PA (n = 3–5 mice from each cell group from three independent experiments). Data are plotted as mean ± SE. *P < 0.05 versus control.
Sca-1+ Type II Cells Showed Up-Regulation of Wnt Pathway Genes
The microarray analysis also revealed that genes involved in the Wnt/β-catenin signaling pathways were differentially expressed between Sca-1+ and Sca-1− cells at 72 hours after PA (Figure 6D). Several genes encoding negative regulators of Wnt (31–33) were highly expressed in the Sca-1− cells, whereas several positive regulators (31, 34) were highly expressed in Sca-1+ cells (Figure 6D). RT-PCR confirmed that the negative regulators, APC (31) (Figure 6E; Figure E5A) and Gsk3β (31) (Figure 6F; Figure E5A), were expressed at higher levels in Sca-1− cells, whereas the positive regulator, Wnt4 (31) (Figure 6G; Figure E5A), was expressed at a higher level in Sca-1+ cells. qRT-PCR using EpCAM+GFP− type II cells isolated from tamoxifen-treated Scgb1a1-CreER/ROSA-GFP/tomato mice also showed that non–Scgb1a1 lineage–labeled Sca-1+ cells expressed lower levels of APC and Gsk3β and higher levels of Wnt4 than Sca-1− cells at 72 hours after PA (Figure E6). β-catenin expression did not differ significantly between Sca-1+ and Sca-1− cells isolated 72 hours after PA (Figure E5B). However, after these cells were cultured for 5 days, Sca-1+ cells displayed higher β-catenin transcript levels than Sca-1− cells (Figures E5C and E5D).
To determine whether Wnt signaling is activated in Sca-1+ type II cells after PA injury, we used Wnt reporter mice Tg(TCF/Lef1-HIST1H2BB/EGFP)61H (35). This line expresses GFP under the control of the Wnt-dependent transcription factor (TCF/Lef1), thereby reflecting Wnt/β-catenin activity at the single-cell level (35). By isolating type II cells from non-PA and 72-hour post–PA-treated Tg(TCF/Lef1-HIST1H2BB/EGFP)61H mice, we showed that there were more GFP+ (i.e., Wnt-active) type II cells in the PA-treated lungs, and that most of the GFP+ cells were in the Sca-1+ fraction (Figures 7A and 7B). Thus, our results were consistent with the microarray data (Figures 6D–6G), and showed that Wnt/β-catenin signaling is activated in Sca-1+ type II cells at the early repair phase after PA injury.
Figure 7.
Wnt signaling is involved in differentiation of Sca-1+ type II into type I cells in culture. (A and B) Type II cells were isolated from Tg(TCF/Lef1-HIST1H2BB/EGFP)61H mice without PA (A) or at 72 hours after PA (B), and subjected to FACS analysis. Cells were gated on forward and side scatter to exclude debris and clusters and were also gated for EpCAM+ fraction. The percentages of cells in the indicated fraction out of the total gated cells are shown. (C–G) Sca-1− and Sca-1+ cells were isolated at 72 hours after PA and cultured with 1 ml media for 5 days. Sca-1− cells were cultured with control media (C), and Sca-1+ cells were cultured with either control media (D) or with Wnt inhibitors, secreted frizzled-related protein (sFRP) 2 (E), Dickkopf (DKK) 2 (F), and XAV939 (G). Sca-1+ cells cultured in control media changed into a flat shape after culture (D). In contrast, almost all Sca-1+ cells cultured with Wnt inhibitors showed a cuboidal shape after culture (E–G), similar to Sca-1− type II cells cultured in control media (C). Scale bar, 100 μm. Data are representative of more than three independent experiments. (H) A model describing the three phases of alveolar epithelial repair: (1) type II cells are activated into Sca-1+ cells; (2) these Sca-1+ cells exhibit higher proliferation rates; and (3) in the final phase, Sca-1+ cells lose the type II marker, Sp-C, and differentiate into type I epithelial cells in a Wnt-mediated manner.
Next, to determine whether Wnt/β-catenin signaling was involved in the differentiation of Sca-1+ cells to into type I cells, Sca-1− and Sca-1+ type II cells were isolated at 72 hours after PA and cultured with the Wnt inhibitors, secreted frizzled-related protein 2 (36), Dickkopf 2 (37), and XAV939 (38, 39), or in control media. Sca-1+ cells cultured with control media differentiated into flat, type I–like cells, whereas Sca-1− cells remained cuboidal (Figures 7C and 7D), consistent with the results shown in Figure 5. In contrast, Sca-1+ cells cultured with all three Wnt inhibitors remained cuboidal (Figures 7E–7G). RT-PCR showed that Sca-1+ cells cultured with control media expressed significantly higher levels of the type I cell marker, Aqp5 (18), and Cyclin B1 (18) than those cultured with XAV939 (Figures E7A and E7C). XAV939-treated cells expressed similar levels of type II cell marker, Sp-C, as control (Figure E7B). Furthermore, terminal deoxynucleotidyl transferase 2′-deoxyuridine 5′-triphosphate (dUTP) nick end labeling assay (TUNEL assay) was performed to access cell death using Wnt inhibitor–treated cell cultures. None of the three inhibitor treatments significantly increased the rate of cell death compared with control; most of the cuboidal cells in inhibitor-treated cultures were terminal deoxynucleotidyl transferase dUTP nick end labeling negative (Figures E7D–E7H). Thus, our data suggest a critical role for Wnt/β-catenin signaling in the mechanism of converting Sca-1+ type II cells into type I cells.
Discussion
By performing lineage tracing, we identified a distinct population of type II cells expressing the mouse antigen, Sca-1, that appeared within 24 hours after PA pneumonia and exhibited increased proliferation along with the ability to differentiate into type I alveolar epithelial cells. The Sca-1+ type II cell number peaked at the alveolar epithelial repair phase and returned to resting levels at the time of full recovery. Through the use of microarray analyses, Wnt reporter mice, and inhibitor experiments, we demonstrated that the Sca-1+ type II cells showed up-regulation of Wnt pathway genes, suggesting a role of Wnt signaling in the type II–to–type I transition.
Type II cells are presumed to function as progenitor cells that repair the injured alveolar epithelium (8, 9, 13). The present results are consistent with this role of type II cells, but, importantly, we showed that this Sca-1+ type II cell subpopulation has a high potential for regenerative activity. Several lines of evidence support this conclusion. First, the Sca-1+ type II cells appeared in parallel with the beginning of the repair phase in the PA lung injury model. Sca-1+ type II cells also exhibited significantly greater proliferation rates compared with their control counterparts, the Sca-1− cells. The time-dependent loss of the type II marker, Sp-C, and the acquisition of the type I phenotype were also well correlated. Gene profiling studies showed that these cells, in contrast to Sca-1− cells, carried the signatures of regenerative cells, specifically, genes involved in cell cycle regulation, the Wnt signaling pathway, and the transcription factor, FoxM1, which we previously showed to be essential for the type II–to–type I cells transition and for alveolar epithelial barrier repair (18). From the comprehensive lineage-tracing studies, we demonstrated that most of the Sca-1+ progenitor cells that emerge after PA-induced pneumonia were derived from not only Sp-C+, but also CC10− type II cells.
It is not entirely clear whether the Sca1+ cells arose through the expansion of a pre-existing subset of progenitor cells with higher regenerative potential or whether they represent type II cells residing within the severely injured areas of the lung, which were activated to express Sca-1 after injury. However, we found that Sca-1+ type II cells isolated at 24 hours after PA expressed basal levels of CDC25C and CyclinB1 and had little BrdU incorporation, whereas, by 72 hours after PA, Sca-1+ type II cells expressed high levels of CDC25C and CyclinB1 and showed high rates of BrdU incorporation compared with controls. Thus, the initial appearance of Sca-1+ type II cells did not elicit cell proliferation, suggesting that most of them were not simply derived from the expansion of a pre-existing Sca-1+ stem cell pool. It is likely that quiescent Sca-1− type II cells were activated by as yet unknown mechanisms in the inflammatory milieu. Recent studies have suggested that the inflammatory milieu itself can create alveolar regeneration signals (4, 40).
In the present study, we demonstrated the up-regulation of canonical Wnt pathway genes in Sca-1+ progenitor cells during their repairing process after PA, based on gene arrays, Wnt reporter mice and Wnt inhibitor studies. Therefore, we postulate that alveolar repair after PA-induced injury followed a sequence: a subset of type II cells are activated and express Sca-1+ at the onset of the regeneration phase and exhibit a higher proliferation rate than Sca-1− cells; these Sca-1+ cells subsequently lose Sp-C and undergo a transition into type I epithelial cells, which is likely to be mediated by Wnt signaling (Figure 7H).
In recent studies, several cell types with progenitor cell potential localized in alveolar and lower airway compartments have been postulated to meditate alveolar repair. Chapman and colleagues (27) identified a group of α6β4 integrin-positive alveolar epithelial cells that appeared after bleomycin-induced injury and showed regenerative potential. However, the Sca-1+ cells identified in the present study were negative for β4 integrin. In addition, most of the cells identified by Chapman and colleagues were Sp-C− and were derived from Sp-C− progenitor cells of unknown origin (H. A. Chapman, personal communication). In another study, p63+ cells derived from keratin 14+ bronchiolar epithelial cells migrated into alveoli after H1N1 influenza infection and induced alveolar regeneration (28). The Sca-1+ type II cells identified in the present study are different from these cells, as the Sca-1+ cells were negative for p63 expression. Another group showed that Sca-1–expressing Sp-C+CC10+ cells, termed BASCs, have the potential to mediate alveolar repair after bleomycin-induced injury, but not after hyperoxia-induced injury (10, 13, 16, 17). Even though some of our type II cell preparations may contain a small number of BASCs and possibility have some overlap with other previously identified putative progenitor populations, our lineage-tracing studies demonstrated that most (∼90%) Sca-1+ progenitor cells that emerge after PA-induced pneumonia were derived from Sp-C+CC10− type II cells, and were CC10−. In addition, our lineage-tracing studies showed that cells derived from CC10+ cells (including BASCs) did not exhibit the same high proliferation rates at the 72-hour time point after PA as the Sp-C+Sca-1+ type II cells. Analyses of the cells derived from CC10− cells showed that the non-BASC Sca-1+ type II cells had a higher potential for type I cell differentiation than their Sca-1− counterparts. Therefore, Sca-1 not only labels BASCs and some airway cells (16, 41), but, based on our results, also labels activated type II cells that engage in alveolar repair.
We found that Sca-1+ type II cells also appeared after lung injury caused by bleomycin (Figure E8), suggesting the generality of our findings, whereas Sca-1+ type II cells were not observed in hyperoxia-induced lung injury (Figure E8). Thus, it may be that the magnitude, site, and type of injury, as well as the specific aspects of the inflammatory milieu, such as the differential release of cytokines, determine the cell types activated to repair alveoli (42). In the context of previous studies, such as those on the appearance of p63+ cells after H1N1 influenza infection (28) and α6β4 cells after bleomycin-induced injury (27), our results add one more example showing that different subsets of alveolar progenitor cells are preferentially activated for repair depending on the context surrounding the damage to the alveolar epithelium. Therefore, it will be necessary to develop different pharmacological strategies to use the regenerative potential of distinct alveolar progenitor cell subsets in different contexts of lung injury.
Acknowledgments
Acknowledgments
The authors thank Dr. Brigid Hogan for discussion and insights, the Flow Cytometry Core at the University of Illinois at Chicago for assistance in MoFlo sorting and FACS analysis, and Dr. Brad Merrill for help with the experiments on Wnt signaling.
Footnotes
This work was supported by National Institutes of Health grants HL105947-01 (Y.L.), HL07829-16 (A.B.M.), HL090152 (A.B.M.), and GM094220 (J.R.).
Author Contributions: Y.L.—study concept and design, collection and analysis of data, manuscript writing; V.S.K.—collection of data; W.Z.—data analysis and interpretation; J.R. and A.B.M.—study concept and design, manuscript writing.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0497OC on December 4, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Shimabukuro DW, Sawa T, Gropper MA. Injury and repair in lung and airways. Crit Care Med. 2003;31(8 suppl):S524–S531. doi: 10.1097/01.CCM.0000081437.06466.B3. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley S, Shi W, Carraro G, Sedrakyan S, Da Sacco S, Driscoll BA, Perin L, De Filippo RE, Warburton D. The milieu of damaged alveolar epithelial type 2 cells stimulates alveolar wound repair by endogenous and exogenous progenitors. Am J Respir Cell Mol Biol. 2011;45:1212–1221. doi: 10.1165/rcmb.2010-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzog EL, Brody AR, Colby TV, Mason R, Williams MC. Knowns and unknowns of the alveolus. Proc Am Thorac Soc. 2008;5:778–782. doi: 10.1513/pats.200803-028HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- 7.Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem. 2010;25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- 8.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 9.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkauskas CE, Michael J, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 12.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973;70:175–198. [PMC free article] [PubMed] [Google Scholar]
- 13.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 14.Stripp BR. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell? Proc Am Thorac Soc. 2008;5:695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy R, Buckley S, Doerken M, Barsky L, Weinberg K, Anderson KD, Warburton D, Driscoll B. Isolation of a putative progenitor subpopulation of alveolar epithelial type 2 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L658–L667. doi: 10.1152/ajplung.00159.2003. [DOI] [PubMed] [Google Scholar]
- 16.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel SM, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, Natarajan V, Zhao YY, Malik AB. Foxm1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med. 2011;208:1473–1484. doi: 10.1084/jem.20102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen–host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 21.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross JJ, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–2343. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 24.Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-cam: a human epithelial antigen is a homophilic cell–cell adhesion molecule. J Cell Biol. 1994;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElroy MC, Kasper M. The use of alveolar epithelial type I cell–selective markers to investigate lung injury and repair. Eur Respir J. 2004;24:664–673. doi: 10.1183/09031936.04.00096003. [DOI] [PubMed] [Google Scholar]
- 26.Hombach-Klonisch S, Panigrahi S, Rashedi I, Seifert A, Alberti E, Pocar P, Kurpisz M, Schulze-Osthoff K, Mackiewicz A, Los M. Adult stem cells and their trans-differentiation potential—perspectives and therapeutic applications. J Mol Med. 2008;86:1301–1314. doi: 10.1007/s00109-008-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wert SE, Glasser SW, Korfhagen TR, Whitsett JA. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol. 1993;156:426–443. doi: 10.1006/dbio.1993.1090. [DOI] [PubMed] [Google Scholar]
- 30.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Dimitrova YN, Li J, Lee YT, Rios-Esteves J, Friedman DB, Choi HJ, Weis WI, Wang CY, Chazin WJ. Direct ubiquitination of beta-catenin by Siah-1 and regulation by the exchange factor TBL1. J Biol Chem. 2010;285:13507–13516. doi: 10.1074/jbc.M109.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Wang M, Tan X, Li TF, Zhang YE, Chen D. Smad3 prevents beta-catenin degradation and facilitates beta-catenin nuclear translocation in chondrocytes. J Biol Chem. 2010;285:8703–8710. doi: 10.1074/jbc.M109.093526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/β-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Descamps S, Arzouk H, Bacou F, Bernardi H, Fedon Y, Gay S, Reyne Y, Rossano B, Levin J. Inhibition of myoblast differentiation by Sfrp1 and Sfrp2. Cell Tissue Res. 2008;332:299–306. doi: 10.1007/s00441-008-0574-z. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Wang K, Shao Y, Huang J, Li X, Shan J, Wu D, Zheng JJ. Structural insight into the mechanisms of Wnt signaling antagonism by DKK. J Biol Chem. 2008;283:23364–23370. doi: 10.1074/jbc.M802375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang YE, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 39.Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S, Fernandez Y, Herance JR, Gispert JD, Mendizabal L, et al. Beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 40.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol. 2013;182:1286–1296. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, et al. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan AE, Chapman HA. Regenerative activity of the lung after epithelial injury. Biochim Biophys Acta. 2013;1832:922–930. doi: 10.1016/j.bbadis.2012.11.020. [DOI] [PubMed] [Google Scholar]