Abstract
G protein–coupled receptors (GPCRs) are important regulators of cell functions in asthma. We recently reported that regulator of G-protein signaling (RGS) 2, a selective modulator of Gq-coupled GPCRs, is a key regulator of airway hyper-responsiveness (AHR), the pathophysiologic hallmark of asthma. Because RGS2 protein levels in airway cells were significantly lower in patients with asthma compared with patients without asthma, we further investigated the potential pathological importance of RGS2 repression in asthma. The human RGS2 gene maps to chromosome 1q31. We first screened patients with asthma for RGS2 gene promoter single-nucleotide polymorphisms (SNPs) and found significant differences in the distribution of two RGS2 SNPs (A638G, rs2746071 and C395G, rs2746072) between patients with asthma and nonasthmatic subjects. These two SNPs are always associated with each other and have the same higher prevalence in patients with asthma (65%) as compared with nonasthmatic subjects (35%). Point mutations corresponding to these SNPs decrease RGS2 promoter activity by 44%. The importance of RGS2 down-regulation was then determined in an acute IL-13 mouse model of asthma. Intranasal administration of IL-13 in mice also decreased RGS2 expression in lungs by ∼50% and caused AHR. Although naive RGS2 knockout (KO) mice exhibit spontaneous AHR, acute IL-13 exposure further increased AHR in RGS2 KO mice. Loss of RGS2 also significantly enhanced IL-13–induced mouse airway remodeling, including peribronchial smooth muscle thickening and fibrosis, without effects on goblet cell hyperplasia or airway inflammation in mice. Thus, genetic variations and increased inflammatory cytokines can lead to RGS2 repression, which exacerbates AHR and airway remodeling in asthma.
Keywords: asthma, airway hyper-responsiveness, airway remodeling, RGS2, polymorphisms
Clinical Relevance
This study shows for the first time an association between regulator of G-protein signaling (RGS) 2 gene polymorphisms and asthma. It also suggests that both genetic variations and increased inflammatory cytokines can contribute to pulmonary RGS2 repression, which may increase the risk of developing airway hyper-responsiveness and remodeling, key pathologic features of severe asthma. Thus, increased RGS2 expression could provide a new therapy for treatment of severe asthma.
Airway hyper-responsiveness (AHR) is exaggerated constriction of the airways in response to various bronchoconstrictor stimuli, including G protein–coupled receptor (GPCR) agonists such as acetylcholine and histamine (1). It is a key diagnostic criterion of asthma, and decreased AHR is associated with improved asthma control (2). AHR is due in part to hyper-reactivity of airway smooth muscle (ASM), but other factors, including airway remodeling, contribute significantly (2–5). Airway remodeling typically includes thickening of the subepithelial extracellular matrix due to increased deposition of collagens and other extracellular matrix proteins, increased airway smooth muscle mass, and excessive mucus production from goblet cells (6–8). These pathologic features lead to the airway wall thickening and luminal narrowing that characterize the chronic irreversible AHR of severe asthma (7, 8). Currently, antiinflammatory glucocorticoids and bronchodilator β2-adrenoceptor agonists are the most important therapies for treating patients with asthma (9). Although glucocorticoids can potentially slow the progression of airway remodeling in asthma, there are no treatments that can reverse airway remodeling. Thus, elucidating the mechanisms underlying airway remodeling may reveal new therapeutic targets.
GPCRs are important regulators of multiple cell types involved in asthma (10). Numerous agonists for bronchoconstrictor Gq-coupled GPCRs are present or up-regulated in the airway during allergic inflammation (11, 12). Regulator of G-protein signaling (RGS) proteins act just downstream from the point of GPCR signaling convergence, such that one type of RGS protein can potentially inhibit responses from multiple types of GPCRs acting through the same signaling cascade (13). Druey and Panettieri have shown that deletion of the RGS5 gene led to enhanced constriction of mouse lung slices (14) and promoted AHR in mice (15). Panettieri also reported that increased RGS4 in ASM correlates with disease severity in asthma and that RGS4 stimulates ASM cell proliferation but inhibits bronchoconstriction (16). We and others recently reported that RGS2, a selective inhibitor of Gq-coupled GPCR signaling (17), is a key regulator of AHR and that RGS2 knockout (KO) mice exhibit spontaneous AHR (18, 19). RGS2 protein levels in airway cells were significantly lower in subjects with asthma compared with subjects without asthma (18) and could be synergistically increased by corticosteroids plus β2-adrenoceptor agonists (19). However, how RGS2 is down-regulated and whether RGS2 repression contributes to the development of AHR and airway remodeling in asthma remains unknown.
In the present study, we investigated the mechanisms and potential pathological importance of RGS2 repression in asthma. RGS2 gene polymorphisms have been identified and may reduce RGS2 expression in hypertensive patients (20, 21). We found a significant increase in the distribution of two single-nucleotide polymorphisms (SNPs) in the promoter region of the RGS2 gene in patients with asthma as compared with nonasthmatic subjects. Point mutations corresponding to these SNPs decreased RGS2 promoter activity by 44%, suggesting that RGS2 may be genetically repressed in some patients with asthma. We also examined whether the T helper type (Th) 2 inflammatory cytokine IL-13, which is necessary and sufficient to induce many pathologic features of asthma (22–24), could affect RGS2 expression. We found that intranasal administration of IL-13 in mice decreased RGS2 expression in lungs by ∼50%. Thus, our results suggest that both genetic variations and inflammatory cytokines can lead to RGS2 repression. The loss of RGS2 expression increases susceptibility of mice to develop AHR and airway remodeling associated with chronic severe asthma. Further investigation of the molecular mechanisms of RGS2 repression in asthma could identify new approaches to target RGS2 expression in airways to ameliorate AHR and airway remodeling in human asthma.
Materials and Methods
Selection of Subjects and Sequence Analysis of the RGS2 Gene
The study was approved by the Creighton University Institutional Review Board as previously described (18). We enrolled 16 normal subjects without history of asthma as controls and 16 treatment-naive subjects with mild-to-moderate asthma and significant AHR. Genomic DNA was extracted from monocytes using DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). Genomic DNA samples of 24 subjects with asthma and 24 nonasthmatic subjects were purchased from BioServe Biotechnologies (Beltsville, MD). The two RGS2 gene SNPs (SNP1, A638G, rs2746071 and SNP2, G395C, rs2746072) in these DNA samples were genotyped by sequence analysis.
Luciferase Reporter Constructs and Assays
The human RGS2 promoter fragment (−757/−1) was amplified and cloned into pGL3-Basic luciferase reporter vector from human genomic DNAs as described (25). Point mutations corresponding to SNP1 and SNP2 were generated using a QuikChange mutagenesis kit (Stratagene, La Jolla, CA). RGS2 promoter activity was assayed in human ASM cells using the dual luciferase assay kit (Promega, Fitchburg, WI) (25).
Acute IL-13 Murine Model of Asthma
All studies were approved by the Institutional Animal Care and Use Committee at Creighton University and were in accordance with the GlaxoSmithKline Policy on the Care, Welfare and Treatment of Laboratory Animals. Anesthetized wild-type (WT) and RGS2 KO C57BL/6J mice (18) were administered intranasal saline or IL-13 (5 μg/d) for 3 days as described previously (26).
Measurement of AHR
Mice were ventilated at a breathing rate of 140 breaths/min, and the lung resistance (RL) and dynamic compliance (Cdyn) responses to increasing concentrations of nebulized methacholine (0–50 mg/ml) were recorded using a FinePointe system (Buxco, St. Paul, MN) (18, 26).
Inflammatory Cell Numbers and Cytokine Levels in Bronchoalveolar Lavage Fluid
Bronchoalveolar lavage (BAL) fluid was collected by lavaging mouse lungs with 1 ml saline twice via a tracheal catheter (27). Leukocytes in the BAL fluid were stained and counted under a microscope (27). Cytokines/chemokines in the BAL fluid were measured using a Mouse Cytokine/Chemokine Magnetic Bead Panel Immunoassay Kit (Millipore, Billerica, MA). Active and total TGF-β1 levels were measured using TGF-β1 Single Plex Kit (Millipore) before and after acidification of the BAL fluid.
Staining and Histopathology of Lung
Parasagittal sections of paraffin-embedded mouse lung were immunostained for α–smooth muscle actin (18), trichrome stained for collagen, hematoxylin and eosin stained for inflammatory infiltrate, and periodic acid–Schiff (PAS) stained for mucus-secreting goblet cells (28).
Measurement of RGS2 Protein Expression and Collagen Deposition in Mouse Lung
Mouse lung was minced in liquid nitrogen. RGS2 protein levels in minced lung tissue were analyzed by Western blot as described (18). The amount of collagen in minced lung tissue was measured using a Sirius red total collagen detection kit (Chondrex, Redmond, WA) according to the manufacturer’s instructions. A collagen standard was used for calibration.
Data Analysis
Data are expressed as means ± SE. Groups were compared using Student’s t test for unpaired observations or two-way ANOVA with the Bonferroni correction where there were multiple comparisons. A probability level of P < 0.05 was statistically significant.
Results
RGS2 Gene Polymorphisms Repress Its Promoter Activity
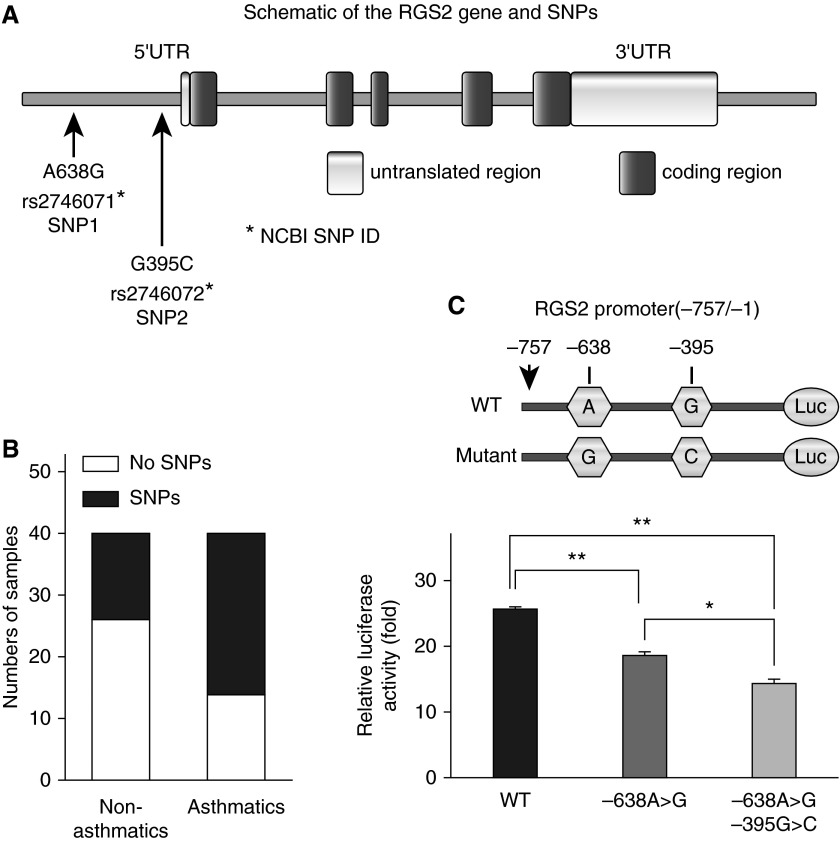
We screened patients with asthma for sequence variations in the RGS2 gene promoter regions and found significant differences in distribution of two SNPs (SNP1, A638G, rs2746071 and SNP2, G395C, rs2746072) between patients with asthma and nonasthmatic subjects (Figures 1A and 1B). These two SNPs are associated with each other. In 40 nonasthmatic subjects, only 14 samples (35%) had both SNP1 and SNP2, versus 26 samples (65%) in 40 patients with asthma (P = 0.0073). All SNPs found in nonasthmatic subjects are heterozygous (A/G in SNP1 and G/C in SNP2) and carry the WT allele. In contrast, three patients with asthma were found to carry homozygous mutants (G/G in SNP1 and C/C in SNP2). The single point mutation corresponding to SNP1 decreased the RGS2 promoter activity by ∼27%, whereas double mutations corresponding to SNP1 and SNP2 decreased the RGS2 promoter activity by 44% (P < 0.01) (Figure 1C).
Figure 1.
Polymorphisms and promoter activity of the regulator of G-protein signaling (RGS) 2 gene in patients with asthma. (A) Diagram of the RGS2 gene and single-nucleotide polymorphisms (SNPs). (B) Association of RGS2 polymorphisms with asthma. Two common SNPs, SNP1 (A638G) and SNP2 (G395C), were genotyped in 40 nonasthmatic subjects and 40 patients with asthma. Data were analyzed using a χ2 test, which gave a P value of 0.0073. (C) RGS2 gene polymorphisms repress promoter activity. Human airway smooth muscle (ASM) cells were transfected with pGL3-luciferase (Luc) plasmids driven by SV-40 or the RGS2 wild-type (WT) or mutant promoter corresponding to SNP1 and SNP2. Results are presented as fold increase compared with pGL3 containing a SV-40 promoter. Data are means ± SE of three independent experiments in triplicate done in two primary ASM cell lines. *P < 0.05 and **P < 0.01 between groups. NCBI, National Center for Biotechnology Information; UTR, untranslated region.
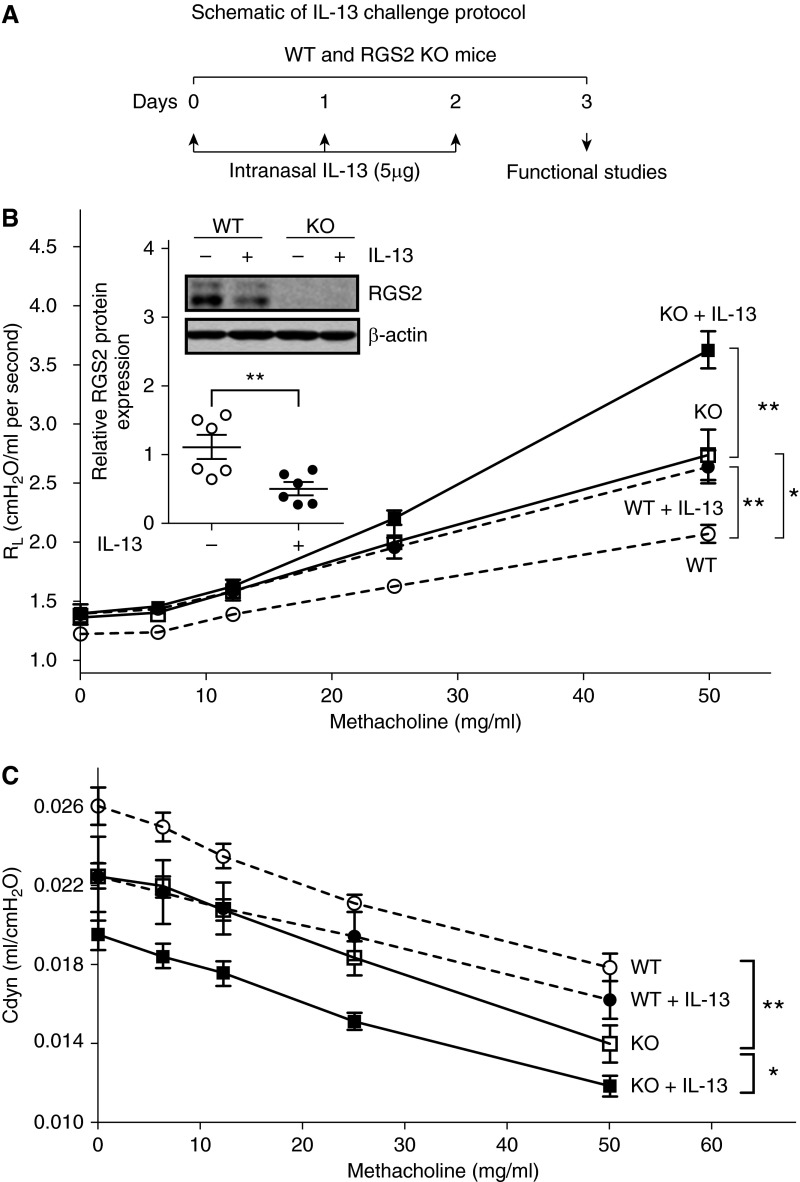
Acute IL-13 Exposure Down-Regulated Lung RGS2 Expression and Caused AHR in WT Mice
Increased IL-13 plays a central role in the development of AHR in allergic asthma. Intranasal administration of murine IL-13 into WT mice (Figure 2A) decreased RGS2 protein expression in mouse lung tissue by ∼50% (Figure 2B, inset) and caused AHR, as reflected by higher RL (Figure 2B) and lower Cdyn (Figure 2C).
Figure 2.
Loss of RGS2 increases susceptibility of mice to acute IL-13–induced airway hyper-responsiveness (AHR). (A) Diagram of IL-13 challenge protocol. AHR was determined by direct measurement of lung resistance (RL) and dynamic compliance (Cdyn) using a Buxco FinePointe apparatus. Changes in RL (B) and Cdyn (C) of WT (circle) and RGS2 knockout (KO) (square) mice without (open circle or open square) or with IL-13 challenge (solid circle or solid square) in response to increasing concentrations of nebulized methacholine. Data are mean ± SE (n = 8–11 mice per group). *P < 0.05 and **P < 0.01 between groups. Inset: Representative Western blot of RGS2 protein expression in lung homogenates of WT and RGS2 KO mice without or with IL-13 challenge, analyzed by measuring band density and normalized by β-actin. Data are means ± SE (n = 6 mice per group). **P < 0.01 between WT mice without and with IL-13 challenge.
Loss of RGS2 Increased Susceptibility of Mice to Develop AHR
RGS2 KO mice exhibited spontaneous AHR as compared with WT mice, consistent with our previous report (18). Loss of RGS2 also exacerbated IL-13–induced AHR, reflected by further augmented RL and reduced Cdyn in RGS2 KO mice as compared with WT mice (Figures 2B and 2C).
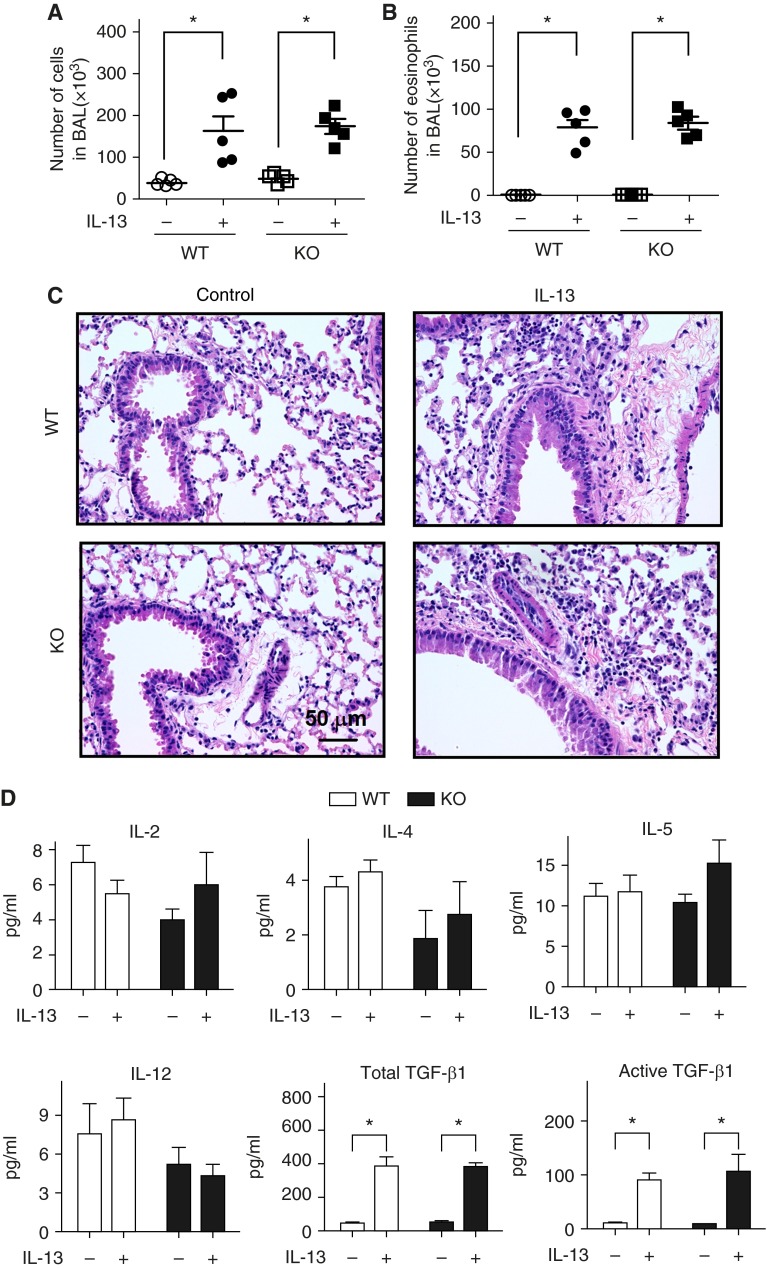
Loss of RGS2 Did Not Alter IL-13–Induced Airway Inflammation in Mice
An increase in total cells and eosinophils was observed in WT and RGS2 KO mice after IL-13 treatment (P < 0.01) (Figures 3A and 3B). However, no differences were observed in total cells or eosinophils in BAL fluid between WT and RGS2 KO mice. Hematoxylin and eosin staining of lung sections showed similar peribronchial and perivascular inflammatory infiltrates in both groups of mice after IL-13 treatment (Figure 3C). In addition, WT and RGS2 KO mice showed significant and comparable elevations in total and active TGF-β1 in the BAL fluid after IL-13 treatment, whereas no changes were observed in levels of IL-2, IL-4, IL-5, or IL-12 (Figure 3D). The total TGF-β1 levels in mouse lung homogenates were also measured. Again, IL-13 caused equal increase in lung TGF-β1 levels in WT and RGS2 KO mice (see Figure 1 in the online supplement).
Figure 3.
Loss of RGS2 does not alter IL-13–induced airway inflammation in mice. Total inflammatory cell numbers (A) and eosinophils (B) in bronchoalveolar lavage (BAL) of WT and RGS2 KO mice without or with IL-13 challenge were counted (n = 5 mice per group). (C) Hematoxylin and eosin–stained lung sections from control and IL-13–treated WT and RGS2 KO mice. (D) Levels of cytokine IL-2, IL-4, IL-5, IL-12, and total and active transforming growth factor (TGF)-β1 in the BAL of WT and RGS2 KO mice without or with IL-13 challenge. Data are means ± SE (n = 5 mice per group). *P < 0.01 between groups.
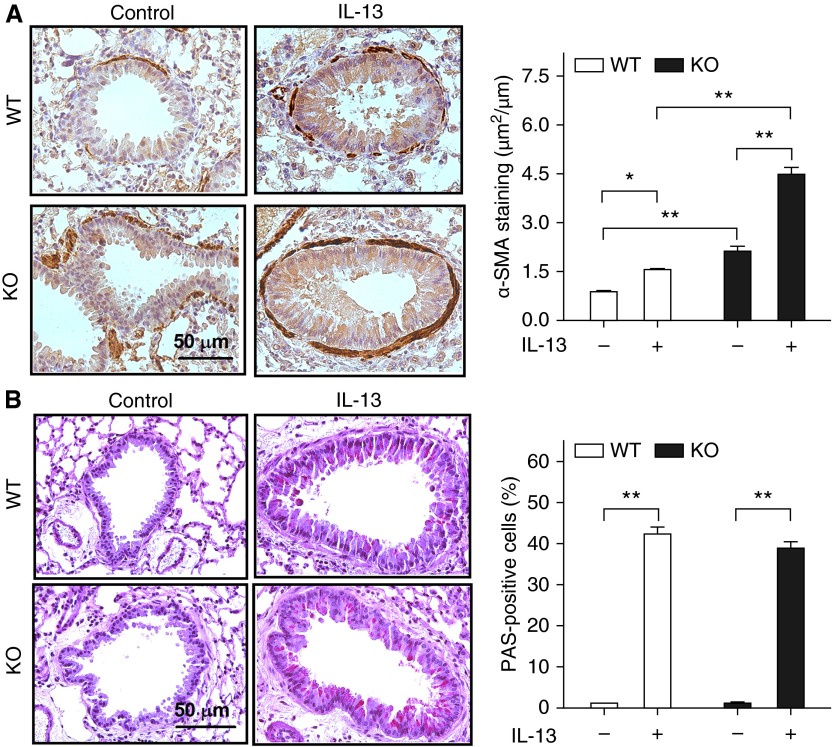
Loss of RGS2 Exacerbated IL-13–Increased Peribronchial Smooth Muscle Thickness but Not Goblet Cell Numbers in Mice
The area of peribronchial α–smooth muscle actin immunostaining in RGS2 KO mice was significantly greater than in WT mice (2.1 ± 0.1 versus 0.9 ± 0.1 μm2/μm circumference of bronchiole; P < 0.01) (Figure 4A). IL-13 treatment increased the thickness of the peribronchial smooth muscle layer in WT mice (0.9 ± 0.1 versus 1.6 ± 0.1; P < 0.05) and in RGS2 KO mice (2.1 ± 0.1 versus 4.5 ± 0.2; P < 0.01) (Figure 4A). Lung sections of WT and RGS2 KO mice exposed to IL-13 showed an increased number of PAS-positive epithelial cells (goblet cells) compared with that in control animals (Figure 4B). However, the percentage of PAS-positive epithelial cells observed in lung sections of IL-13–treated RGS2 KO mice (38.9 ± 1.6%) was similar to that of IL-13–treated WT mice (41.4 ± 2.6%).
Figure 4.
Loss of RGS2 exacerbates IL-13–increased peribronchial smooth muscle thickness but not goblet cell numbers in mice. (A) Representative α-smooth muscle actin (α-SMA) staining images of ASM cells in WT and RGS2 KO mice without (Control) or with IL-13 challenge (left). The area of α-SMA staining was expressed per μm length of basement membrane of bronchioles (right). (B) Representative periodic acid–Schiff (PAS) staining images for airway goblet cells (left). Results are expressed as percentage of PAS-positive cells per bronchiole (right), calculated from the number of PAS-positive cells divided by the number of epithelial cells. Data are means ± SE (15 bronchioles from five mice). *P < 0.05 and **P < 0.01 between groups.
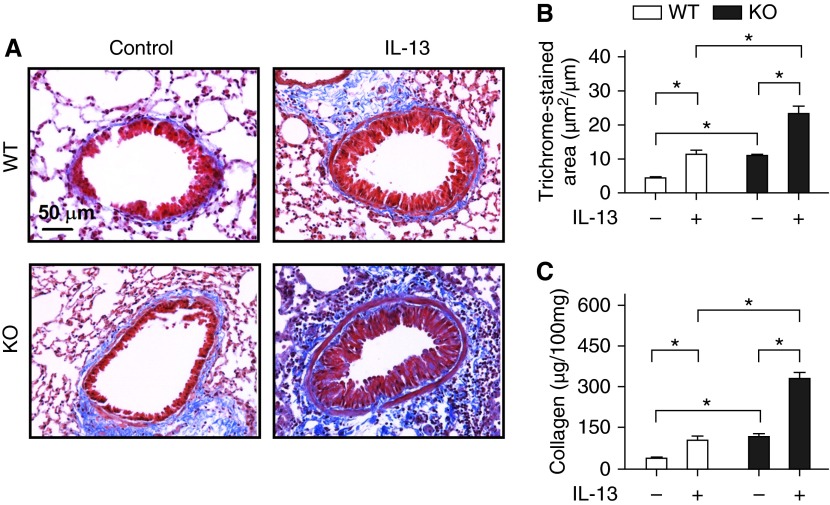
Increased Subepithelial Fibrosis and Collagen Deposition in RGS2 KO Mice
The area of peribronchial trichrome staining for collagen was significantly larger in RGS2 KO mice compared with WT mice (11.0 ± 0.8 versus 4.5 ± 0.4 μm2/μm circumference of bronchiole; P < 0.01) (Figures 5A and 5B). IL-13 increased the area of peribronchial trichrome staining in both WT and RGS2 KO mice, but the area of trichrome staining in IL-13–treated RGS2 KO mice was still significantly greater than in IL-13–treated WT mice (23.3 ± 2.1 versus 11.4 ± 1.1; P < 0.01). Total lung collagen assays showed increased lung collagen in RGS2 KO mice compared with WT mice (115.4 ± 12.7 versus 38.3 ± 6.5 μg per 100 mg of lung tissue; P < 0.01) (Figure 5C). This elevated collagen was further increased to 329.6 ± 23.6 μg per 100 mg of lung tissue after IL-13 treatment. In contrast, lung collagen in IL-13–treated WT mice was only 105.8 ± 12.1 μg per 100 mg of lung tissue.
Figure 5.
Loss of RGS2 increases IL-13–induced peribronchial fibrosis in mice. (A) Representative trichrome staining images for collagen in parasagittal sections of lung from WT and RGS2 KO mice without (Control) or with IL-13 challenge. (B) The area of trichrome staining was expressed per micrometer length of basement membrane of bronchioles. Data are means ± SE (35 bronchioles from five mice). (C) Total lung collagen content was measured by the Sirius red collagen assay. Results are expressed as micrograms of collagen per 100 mg of lung tissue. Data are means ± SE (five lungs from five mice). *P < 0.01 between groups.
Discussion
We previously reported that expression of RGS2 was reduced in airway cells of human asthma patients and that naive RGS2 KO mice spontaneously display AHR, supporting RGS2 as a key regulator of AHR (18). RGS2 gene polymorphisms have been identified in hypertensive patients and might reduce RGS2 expression (20, 21). The human RGS2 gene maps to chromosome 1q31 (29) and SNPs at this region were strongly associated with asthma in children (30). Thus, we first screened patients with asthma for RGS2 gene promoter SNPs and found significant differences in the distribution of two RGS2 SNPs (A638G, rs2746071 and C395G, rs2746072) between patients with asthma and nonasthmatic subjects. These two SNPs are always associated with each other, and both have a 1.8-fold higher prevalence in patients with asthma (65%) as compared with nonasthmatic subjects (35%). Although an association of these two RGS2 SNPs with suicide in the Japanese population has been reported (31), to our knowledge, our study is the first report of an association between RGS2 gene polymorphisms and asthma. The point mutation corresponding to SNP1 decreased RGS2 promoter activity, and additional mutation corresponding to SNP2 further decreased RGS2 promoter activity. Thus, both of these SNPs might contribute to repression of the RGS2 gene expression seen in patients with asthma. Furthermore, because heterozygous RGS2 KO mice exhibit spontaneous AHR (19), partial repression of RGS2 due to its genetic variations in patients should be sufficient to trigger AHR in the absence of inflammation, which could contribute to non–Th2-dependent asthma development (32). A limitation of these findings is that the sample size used for the genetic association study was relatively small. We are currently collecting more patient samples to further investigate the regulation of RGS2 gene expression by polymorphisms.
The Th2 inflammatory cytokine IL-13 is necessary and sufficient to induce many pathologic features of asthma (22–24). We previously showed that targeting downstream effectors of GPCRs attenuated IL-13–induced AHR in mice (26). We found that intranasal administration of IL-13 decreased RGS2 expression in mouse lungs by ∼50% and caused AHR in mice in vivo. The expression of RGS2 is known to be dynamically regulated and responsive to the cell’s microenvironment (33–35). We previously reported that RGS2 was also selectively down-regulated in lung tissues of an ovalbumin-sensitized/challenged murine model of asthma (18). Because RGS2 functions as a key negative regulator of AHR (18, 19), it is likely that down-regulation of RGS2 is one mechanism underlying the development of AHR in IL-13 and ovalbumin models of asthma. Thus, our study suggests that increased Th2 cytokines such as IL-13 might contribute to the repression of RGS2 gene expression seen in patients with asthma.
The pathological importance of RGS2 down-regulation was determined in an acute IL-13 mouse model of asthma. Consistent with our previous report (18), naive RGS2 KO mice exhibit spontaneous AHR. However, in vivo IL-13 treatment caused additional AHR in RGS2 KO mice, which was much higher than that of IL-13–treated WT mice. Because there were no differences in inflammatory cell numbers or key Th1 and Th2 cytokines in the BAL and lung tissue of RGS2 KO mice compared with WT mice, augmented airway inflammation is unlikely to be a major contributor to increased AHR in RGS2 KO mice. This caused us to speculate that the loss of RGS2 may augment other AHR-promoting factors.
Airway wall remodeling, including goblet cell hyperplasia in airway epithelium, increased peribronchial ASM mass, and airway fibrosis, has been recognized as an important factor contributing to AHR and asthma severity. We found that IL-13 markedly increased airway mucus-producing goblet cells in mice, as reported by others (36). However, no differences were observed between WT and RGS2 KO mice, suggesting that goblet cell hyperplasia is unlikely to be a major contributor to augmented AHR in IL-13–treated RGS2 KO mice. We previously showed that loss of RGS2 enhanced mitogenic stimulation of ASM growth in culture (18). Consistent with these in vitro data, the thickness of the peribronchial smooth muscle layer in RGS2 KO mice was much greater than in WT mice. IL-13 treatment of WT mice caused an increase in thickness of the peribronchiolar smooth muscle layer to a level comparable to that in untreated RGS2 KO mice. Moreover, IL-13 treatment of RGS2 KO mice caused an approximate doubling in size of the smooth muscle layer from its already elevated baseline. Thus, loss of RGS2 further increases IL-13–induced airway smooth muscle remodeling, which may contribute to augmented AHR observed in IL-13–treated RGS2 KO mice.
Subepithelial fibrosis, the result of deposition of extracellular matrix, primarily collagens, also contributes to airway remodeling and AHR (36, 37). Indeed, treatment of mice with IL-13 increased the subepithelial collagen deposition in WT and RGS2 KO mice to the same extent (3-fold) above the control deposition. However, with or without IL-13 treatment, the amount of collagen deposition in lungs of RGS2 KO mice was much greater than that of WT mice, suggesting that loss of RGS2 increases subepithelial fibrosis in lungs of mice. In addition, loss of RGS2 reduced basal airway compliance and augmented IL-13–induced reduction of airway compliance, an indication of reduced airway distensibility, presumably due to increased collagens. This result is consistent with the clinical observation that decreased airway compliance correlates inversely with increases in subepithelial fibrosis in patients with asthma (38–40). The increase in airway fibrosis observed in IL-13–treated RGS2 KO mice is most likely attributable to increased fibroblast activation and differentiation. A previous study suggested that RGS2 functions as a negative regulator of angiotensin II–induced fibroblast responses in the myocardium (41). By analogy, it seems likely that loss of RGS2 could augment pulmonary fibroblast responses, leading to exacerbated airway fibrosis and AHR in mice, but this has yet to be proved.
AHR and airway remodeling are key factors associated with a decline in lung function in patients with severe asthma, but the relationship between AHR and airway remodeling is poorly defined. Our data suggest that a combination of increased smooth muscle mass and fibrosis most likely explains the augmented AHR in RGS2 KO mice exposed to IL-13. Loss of RGS2 therefore increases susceptibility of mice to development of key features of severe asthma, suggesting the pathological importance of RGS2 repression in the development and progression of asthma. Thus, targeting RGS2 expression could be an attractive therapeutic approach to attenuate the development of AHR, airway smooth muscle hyperplasia, and airway fibrosis in patients with severe asthma. Our study also shows that genetic variations can lead to the RGS2 repression seen in patients with asthma. Asthma has been the focus of a sizeable number of genetic studies (42, 43). Our study identified a significant increase of two common SNPs in the RGS2 gene in patients with asthma, which could reduce RGS2 gene expression. Further clinical investigation could lead to development of these two SNPs of RGS2 as a novel biomarker for early identification of patients who have increased risk to develop AHR and asthma. In addition, current therapies for treating patients with asthma, including β2-adrenoceptor agonists and corticosteroids, target the hypercontractile and inflammatory components of asthma. RGS2 regulates ASM contraction and loss of RGS2 leads to hypercontractility of ASM cells (18, 19). Our study is the first to suggest that increases in the Th2 cytokine IL-13 can down-regulate pulmonary RGS2 expression in vivo. Because “Th2-mediated” airway inflammation is a major feature of many human patients with asthma, our studies provide a new and exciting link among airway inflammation, hypercontractility, and remodeling seen in chronic persistent asthma. Furthermore, understanding the mechanisms and specific cell types (i.e., airway smooth muscle, epithelium, or fibroblasts) in which Th2-ctyokines down-regulate RGS2 expression could provide new avenues for the treatment of patients with chronic persistent asthma.
Acknowledgments
Acknowledgments
The authors thank Dr. David Siderovski at West Virginia University School of Medicine for providing the original RGS2 KO breeding mice.
Footnotes
This work was supported in part by American Asthma Foundation Early Excellence Award (Y.T.); by GlaxoSmithKline Research Fund grant ADV115426 (Y.T. and M.L.T.); by National Institutes of Health grants R01 HL116849 (Y.T. and T.B.C.), R01 HL097796 (R.A.P.), and P30 ES013508 (R.A.P.); and by the National Institutes of Health National Center for Research Resources G20-RR024001.
Author Contributions: H.J., Y.X., P.W.A., D.W.W., M.L.T., R.A.P., T.B.C., and Y.T. participated in research design. H.J., Y.X., P.W.A., and Y.T. conducted experiments. T.B.C., M.L.T., and R.A.P. contributed materials and reagents. H.J., Y.X., P.W.A., T.B.C., and Y.T. performed data analysis. Y.X., P.W.A., D.W.W., M.L.T., T.B.C., and Y.T. wrote or contributed to the writing of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0319OC on November 4, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hargreave FE, O’Byrne PM, Ramsdale EH. Mediators, airway responsiveness, and asthma. J Allergy Clin Immunol. 1985;76:272–276. doi: 10.1016/0091-6749(85)90641-4. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138(Suppl):4S–10S. doi: 10.1378/chest.10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahy JV, Corry DB, Boushey HA. Airway inflammation and remodeling in asthma. Curr Opin Pulm Med. 2000;6:15–20. doi: 10.1097/00063198-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Casale TB, Stokes JR. Immunomodulators for allergic respiratory disorders. J Allergy Clin Immunol. 2008;121:288–296, quiz 297–298. doi: 10.1016/j.jaci.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Berend N, Salome CM, King GG. Mechanisms of airway hyperresponsiveness in asthma. Respirology. 2008;13:624–631. doi: 10.1111/j.1440-1843.2008.01330.x. [DOI] [PubMed] [Google Scholar]
- 6.Fish JE, Peters SP. Airway remodeling and persistent airway obstruction in asthma. J Allergy Clin Immunol. 1999;104:509–516. doi: 10.1016/s0091-6749(99)70315-5. [DOI] [PubMed] [Google Scholar]
- 7.Paré PD, Roberts CR, Bai TR, Wiggs BJ. The functional consequences of airway remodeling in asthma. Monaldi Arch Chest Dis. 1997;52:589–596. [PubMed] [Google Scholar]
- 8.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116:477–486, quiz 487. doi: 10.1016/j.jaci.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Biochemical basis of asthma therapy. J Biol Chem. 2011;286:32899–32905. doi: 10.1074/jbc.R110.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards SW, Tan CM, Limbird LE. Localization of G-protein-coupled receptors in health and disease. Trends Pharmacol Sci. 2000;21:304–308. doi: 10.1016/s0165-6147(00)01513-3. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal. 2006;18:2105–2120. doi: 10.1016/j.cellsig.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Saxena H, Deshpande DA, Tiegs BC, Yan H, Battafarano RJ, Burrows WM, Damera G, Panettieri RA, DuBose TD, Jr, An SS, et al. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol. 2012;166:981–990. doi: 10.1111/j.1476-5381.2011.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Cooper PR, Damera G, Mukhopadhyay I, Cho H, Kehrl JH, Panettieri RA, Jr, Druey KM. Beta-agonist-associated reduction in RGS5 expression promotes airway smooth muscle hyper-responsiveness. J Biol Chem. 2011;286:11444–11455. doi: 10.1074/jbc.M110.212480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balenga NA, Jester W, Jiang M, Panettieri RA, Jr, Druey KM. Loss of regulator of G protein signaling 5 promotes airway hyperresponsiveness in the absence of allergic inflammation. J Allergy Clin Immunol. 2014;134:451–459. doi: 10.1016/j.jaci.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damera G, Druey KM, Cooper PR, Krymskaya VP, Soberman RJ, Amrani Y, Hoshi T, Brightling CE, Panettieri RA., Jr An RGS4-mediated phenotypic switch of bronchial smooth muscle cells promotes fixed airway obstruction in asthma. PLoS One. 2012;7:e28504. doi: 10.1371/journal.pone.0028504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci USA. 1997;94:14389–14393. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Jiang H, Nguyen H, Jia S, Berro A, Panettieri RA, Jr, Wolff DW, Abel PW, Casale TB, Tu Y. Regulator of G protein signaling 2 is a key modulator of airway hyperresponsiveness. J Allergy Clin Immunol. 2012;130:968–976, e3. doi: 10.1016/j.jaci.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Holden NS, Bell MJ, Rider CF, King EM, Gaunt DD, Leigh R, Johnson M, Siderovski DP, Heximer SP, Giembycz MA, et al. β2-Adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proc Natl Acad Sci USA. 2011;108:19713–19718. doi: 10.1073/pnas.1110226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Kamide K, Kokubo Y, Takiuchi S, Tanaka C, Banno M, Miwa Y, Yoshii M, Horio T, Okayama A, et al. Genetic variations of regulator of G-protein signaling 2 in hypertensive patients and in the general population. J Hypertens. 2005;23:1497–1505. doi: 10.1097/01.hjh.0000174606.41651.ae. [DOI] [PubMed] [Google Scholar]
- 21.Semplicini A, Lenzini L, Sartori M, Papparella I, Calò LA, Pagnin E, Strapazzon G, Benna C, Costa R, Avogaro A, et al. Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. J Hypertens. 2006;24:1115–1124. doi: 10.1097/01.hjh.0000226202.80689.8f. [DOI] [PubMed] [Google Scholar]
- 22.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 23.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 25.Wolff DW, Xie Y, Deng C, Gatalica Z, Yang M, Wang B, Wang J, Lin MF, Abel PW, Tu Y. Epigenetic repression of regulator of G-protein signaling 2 promotes androgen-independent prostate cancer cell growth. Int J Cancer. 2012;130:1521–1531. doi: 10.1002/ijc.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H, Xie Y, Abel PW, Toews ML, Townley RG, Casale TB, Tu Y. Targeting phosphoinositide 3-kinase γ in airway smooth muscle cells to suppress interleukin-13-induced mouse airway hyperresponsiveness. J Pharmacol Exp Ther. 2012;342:305–311. doi: 10.1124/jpet.111.189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guedes AG, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1286–L1293. doi: 10.1152/ajplung.00187.2006. [DOI] [PubMed] [Google Scholar]
- 28.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HK, Heng HH, Shi XM, Forsdyke DR, Tsui LC, Mak TW, Minden MD, Siderovski DP. Differential expression of a basic helix-loop-helix phosphoprotein gene, G0S8, in acute leukemia and localization to human chromosome 1q31. Leukemia. 1995;9:1291–1298. [PubMed] [Google Scholar]
- 30.Sleiman PM, Flory J, Imielinski M, Bradfield JP, Annaiah K, Willis-Owen SA, Wang K, Rafaels NM, Michel S, Bonnelykke K, et al. Variants of DENND1B associated with asthma in children. N Engl J Med. 2010;362:36–44. doi: 10.1056/NEJMoa0901867. [DOI] [PubMed] [Google Scholar]
- 31.Cui H, Nishiguchi N, Ivleva E, Yanagi M, Fukutake M, Nushida H, Ueno Y, Kitamura N, Maeda K, Shirakawa O. Association of RGS2 gene polymorphisms with suicide and increased RGS2 immunoreactivity in the postmortem brain of suicide victims. Neuropsychopharmacology. 2008;33:1537–1544. doi: 10.1038/sj.npp.1301557. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 33.Ingi T, Krumins AM, Chidiac P, Brothers GM, Chung S, Snow BE, Barnes CA, Lanahan AA, Siderovski DP, Ross EM, et al. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J Neurosci. 1998;18:7178–7188. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zmijewski JW, Song L, Harkins L, Cobbs CS, Jope RS. Oxidative stress and heat shock stimulate RGS2 expression in 1321N1 astrocytoma cells. Arch Biochem Biophys. 2001;392:192–196. doi: 10.1006/abbi.2001.2430. [DOI] [PubMed] [Google Scholar]
- 35.Endale M, Kim SD, Lee WM, Kim S, Suk K, Cho JY, Park HJ, Wagley Y, Kim S, Oh JW, et al. Ischemia induces regulator of G protein signaling 2 (RGS2) protein upregulation and enhances apoptosis in astrocytes. Am J Physiol Cell Physiol. 2010;298:C611–C623. doi: 10.1152/ajpcell.00517.2008. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai TR, Knight DA. Structural changes in the airways in asthma: observations and consequences. Clin Sci (Lond) 2005;108:463–477. doi: 10.1042/CS20040342. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JW, Li X, Pain MC. The lack of distensibility of asthmatic airways. Am Rev Respir Dis. 1993;148:806–809. doi: 10.1164/ajrccm/148.3.806. [DOI] [PubMed] [Google Scholar]
- 39.Brackel HJL, Pedersen OF, Mulder PGH, Overbeek SE, Kerrebijn KF, Bogaard JM. Central airways behave more stiffly during forced expiration in patients with asthma. Am J Respir Crit Care Med. 2000;162:896–904. doi: 10.1164/ajrccm.162.3.9905034. [DOI] [PubMed] [Google Scholar]
- 40.Ward C, Johns DP, Bish R, Pais M, Reid DW, Ingram C, Feltis B, Walters EH. Reduced airway distensibility, fixed airflow limitation, and airway wall remodeling in asthma. Am J Respir Crit Care Med. 2001;164:1718–1721. doi: 10.1164/ajrccm.164.9.2102039. [DOI] [PubMed] [Google Scholar]
- 41.Zhang P, Su J, King ME, Maldonado AE, Park C, Mende U. Regulator of G protein signaling 2 is a functionally important negative regulator of angiotensin II-induced cardiac fibroblast responses. Am J Physiol Heart Circ Physiol. 2011;301:H147–H156. doi: 10.1152/ajpheart.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torgerson DG, Capurso D, Mathias RA, Graves PE, Hernandez RD, Beaty TH, Bleecker ER, Raby BA, Meyers DA, Barnes KC, et al. Resequencing candidate genes implicates rare variants in asthma susceptibility. Am J Hum Genet. 2012;90:273–281. doi: 10.1016/j.ajhg.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.March ME, Sleiman PM, Hakonarson H. Genetic polymorphisms and associated susceptibility to asthma. Int J Gen Med. 2013;6:253–265. doi: 10.2147/IJGM.S28156. [DOI] [PMC free article] [PubMed] [Google Scholar]