Abstract
New neurons are continuously produced from neural stem cells in specific regions of the adult brain of animals and humans. In the hippocampus, a region crucial for cognitive function, neurogenesis responds to a multitude of extrinsic stimuli; emerging evidence indicates that it may be important for behavior, pathophysiology, brain repair, and response to drugs. We have developed an approach to identify and quantify the cellular targets of pro- and anti-neurogenic stimuli, based on reporter transgenic mouse lines in which neural stem and progenitor cells or their progeny are marked by fluorescent proteins. Here, we demonstrate the feasibility of using MIMS for studying adult neurogenesis.
Keywords: MIMS, Multi-isotope Imaging Mass Spectrometry, Stem cell division, Hippocampus
INTRODUCTION
Aging impacts maintenance and regeneration of organs and tissues. Neural tissue is particularly sensitive to the effects of aging; this is highly pronounced in the hippocampus. It seems plausible, therefore, that diminished production of new neurons contributes to the decreased cognitive function of the aging brain. The underlying mechanism of the age-related decrease in neurogenesis is not clear and is a matter of intense debate. We seek to clarify the age-related changes in hippocampal neurogenesis and to introduce quantitative methods to the studies of stem cell maintenance and lineage.
A wide range of antidepressant drugs and treatments can increase neurogenesis in the hippocampus2,3 neurogenesis may also be required for the behavioral effect of SSRI antidepressants3, 4. We have already demonstrated that antidepressant treatments or injury modify the division/differentiation cascade in the hippocampus6. Therefore, by revealing the antidepressant- and injury-induced changes in the cascade at different ages, we will clarify the mechanisms of action of antidepressant protocols, particularly in the elderly patients.
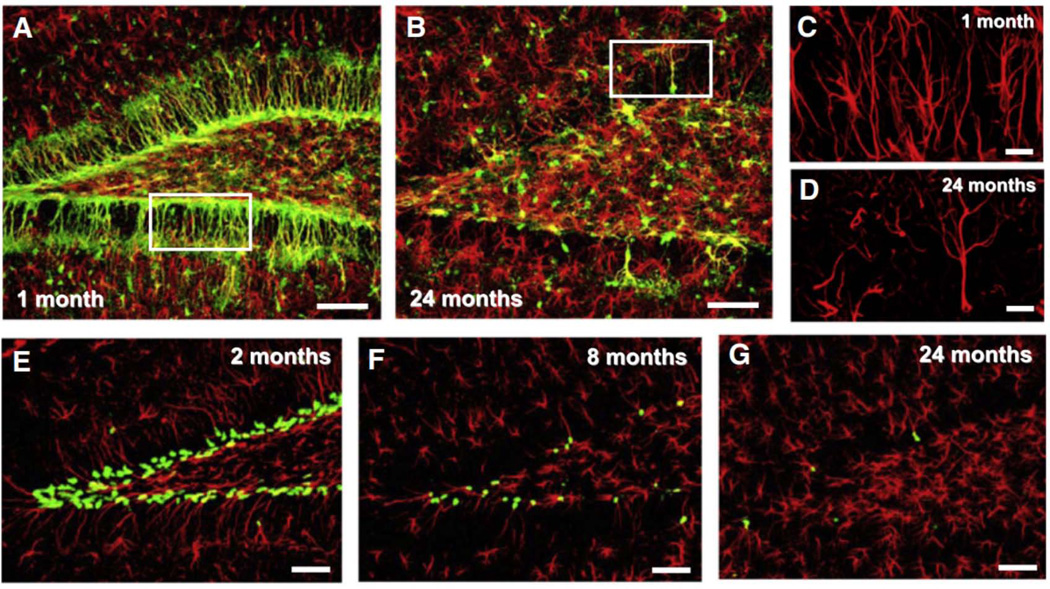
Adult tissues undergo continuous cell turnover in response to stress, damage, or physiological demand. New differentiated cells are generated from dedicated or facultative stem cells or from self-renewing differentiated cells. We generated a series of reporter transgenic mouse lines in which stem and progenitor cells are marked by the expression of fluorescent proteins (including GFP, CFPnuc, H2B-GFP, DsRedTimer and mCherry), as described in Mignone et al. 2004 and Encinas et al (Figure 1). In these lines expression the fluorescent markers in stem cells is directed by the regulatory elements of the rat nestin gene (including its promoter and the crucial enhancer region in the second intron of the gene). Importantly, the same reporter transgene marks stem and progenitor cells in a range of other organs and tissues, including non-neuronal tissues [spinal cord, ciliary margin of the retina, hair follicles, liver, pancreas, skeletal muscle, testis, anterior pituitary, and bone marrow7–13]. Therefore, by using animals carrying multi-type reporter alleles or their combinations, our project will facilitate and standardize the study of tissue-specific stem cells, their lineage and age-dependent changes.
Figure 1.
MIMS has the potential to revolutionize the study of stem cells by introducing highly quantitative sensitive multiplex measurements of cell and tissue turnover after labeling with non-radioactive, non-toxic isotopes15–17. 15N-thymidine is exactly equivalent to natural thymidine while BrdU is a synthetic nucleoside that is an analog of thymidine. Both are incorporated into newly synthesized DNA. However BrdU may not be used for prolonged periods due to its toxicity18. Our approach, highly sensitive and quantitative, is likely to reveal features of stem cell turnover that are finer than the current level of resolution.
METHOD
C57B6 mice were pulsed with 15N-thymidine from post-natal day 4 for 8 weeks. During weeks 1–4, the animals were dosed subcutaneously twice per day at 50 µg/g. During weeks 5–8, 20 µg/h were delivered via osmotic mini pump. The animals were then maintained without 15N-thymidine dosing for 4 weeks. After the chase, BrdU was administered for 24 hours. A 1 mg bolus was given, followed by an infusion at 20 µg/h. The animals were then euthanized and perfused with 4% PFA to fix the tissues.
The brains were removed and either sectioned with a vibratome at 40 µm thickness (10 µm after vacuum drying) or embedded in TAAB Epon and sectioned at 0.5 µm thickness.
RESULTS
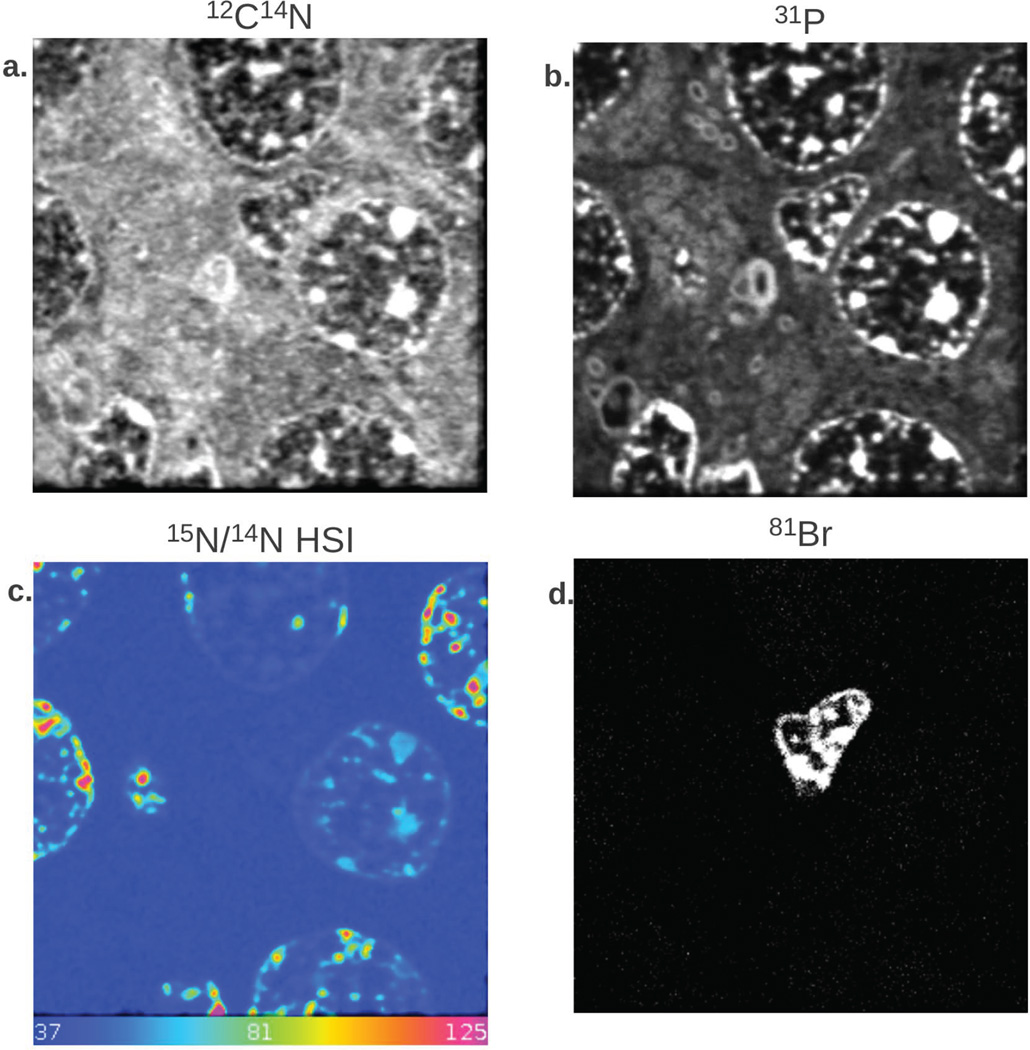
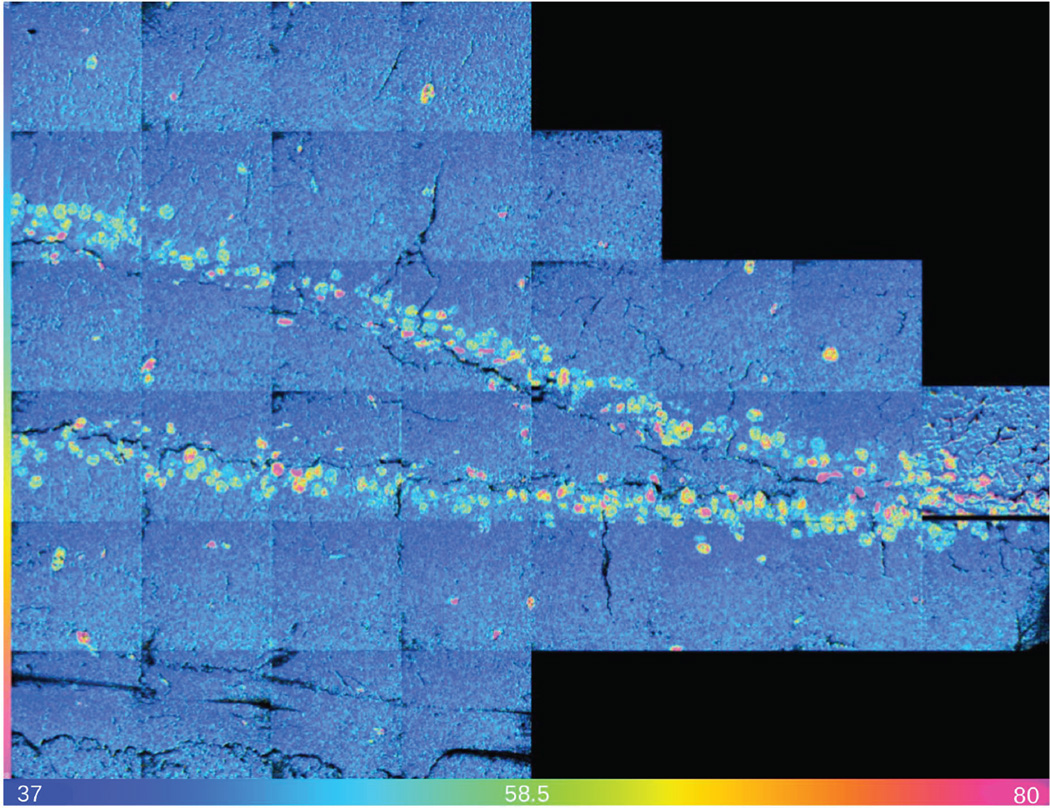
MIMS atomic mass images of CN− ions in biological samples are highly contrasted, even though they are obtained without any staining (Figure 2a). The 31P− image (Figure 2b) enables identification of other cells with unreplicated DNA. An Hue Saturation Intensity (HSI) transformation of the 12C15N−/12C14N− ratio image is shown in Figure 2c. The colors indicate the fractional excess 15N derived from the measured 12C15N−/12C14N− isotope ratios and reveal the 15N-tymidine labeled DNA of actively proliferating cells. A post-chase replicating nucleus labeled with BrdU is seen in the 81Br− mass image (Figure 2d). This nucleus has a markedly different morphology compared to the other nuclei in the field, which implies that it is a different cell type. This underscores the need for direct cellular recognition. MIMS analysis identified 15N-tymidine labeled DNA of actively proliferating cells across all regions of the detatate gyrus (Figure 3). The majority of labeled cells lie within the granular cell layer of the dentate gyrus (Figure 2).
Figure 2.
Figure 3.
CONCLUSION
MIMS is exceptionally well suited for measuring the changes in the amount of label as a function of divisions because the method is: (a) highly sensitive, with a level of detection that is orders of magnitude lower than that of conventional autoradiography or immunocytochemistry; (b) highly quantitative over a scale that spans several orders of magnitude; (c) allows multiple labels to be resolved simultaneously15; (d) allows for fluorescence signal detection for cell type or protein identification, or even better direct identification using MIMS-compatible gold nanoparticle immuno-labels19; (e) allows 3D reconstruction of the imaged cell, thus enabling analysis of organelles or their subregions16.
For the first time we have directly located and measured stem cell generation in the hippocampus by combining MIMS with reporter lines (Nestin-GFP and Nestin-mCherry) that facilitate the identification of specific progenitor populations based on specific molecular markers. To determine the parameters of the neurogenic division/ differentiation cascade in the adult brain, we will perform quantitative MIMS measurements of stem cell division/amplification/turnover in these reporter mice. The parallel analysis of genetically-driven fluorescent reporters and cell division by MIMS has recently been demonstrated in the heart17. We will move forward and apply a combination of fluorescent reporters with MIMS to determine age-related changes in division and differentiation of neural stem and progenitor cells in the adult brain in response to aging, antidepressant treatments, and injury. Furthermore, we will study similar effects in non-neural stem and progenitor cells highlighted by the expression of the reporters.
ACKNOWLEDGEMENTS
Funded by the NIH (5P41EB001974-13, AG034641, R01AG040019, R21AG034641-01, R01 AG040209), Human Frontier Science Program (RGP0048) and the Ellison Medical Foundation (AG-SS-2215-08), Watkins Cardiovascular Leadership Award, K08 DK090147-02 and Harvard Stem Cell Institute Seed Grant.
REFERENCES
- 1.Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Cell Stem Cell. 2011;8(5):566–579. doi: 10.1016/j.stem.2011.03.010. PMCID: PMC3286186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. PMID: 12907793. [DOI] [PubMed] [Google Scholar]
- 3.Dranovsky A, Hen R. Biol. Psychiatry. 2006;59(12):1136–1143. doi: 10.1016/j.biopsych.2006.03.082. PMID: 16797263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner-Schmidt JL, Duman RS. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. PMID: 16425236. [DOI] [PubMed] [Google Scholar]
- 5.Encinas JM, Vaahtokari A, Enikolopov G. PNAS. 2006;103(21):8233–8238. doi: 10.1073/pnas.0601992103. PMCID: PMC1461404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enikolopov G, Overstreet-Wadiche L. In: Adult Neurogenesis. Gage F, Kempermann G, Song H, editors. New York: CSHL Press; 2008. [Google Scholar]
- 7.Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Dev. Biol. 2007;304(1):246–259. doi: 10.1016/j.ydbio.2006.12.026. PMCID: PMC1888564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleiberman AS, Encinas JM, Mignone JL, Michurina T, Rosenfeld MG, Enikolopov G. Dev. Dyn. 2005;234(2):413–421. doi: 10.1002/dvdy.20536. PMCID: PMC2751636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G. PNAS. 2008;105(17):6332–6337. doi: 10.1073/pnas.0801644105. PMCID: PMC2359820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mignone JL, Roig-Lopez JL, Fedtsova N, Schones DE, Manganas LN, Maletic-Savatic M, Keyes WM, Mills AA, Gleiberman A, Zhang MQ, Enikolopov G. Cell Cycle. 2007;6(17):2161–2170. doi: 10.4161/cc.6.17.4593. PMID: 17873521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Nat. Biotechnol. 2004;22(9):1115–1124. doi: 10.1038/nbt1004. PMID: 15322557. [DOI] [PubMed] [Google Scholar]
- 12.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. PMCID: PMC3146551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D. J. Cell Biol. 2004;167(5):935–944. doi: 10.1083/jcb.200409107. PMCID: PMC2172461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. J. Comp. Neurol. 2004;469(3):311–324. doi: 10.1002/cne.10964. PMID: 14730584. [DOI] [PubMed] [Google Scholar]
- 15.Steinhauser ML, Bailey AP, Senyo SE, Guillermier C, Perlstein TS, Gould AP, Lee RT, Lechene CP. Nature. 2012;481(7382):516–519. doi: 10.1038/nature10734. PMCID: PMC3267887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang DS, Piazza V, Perrin BJ, Rzadzinska AK, Poczatek JC, Wang M, Prosser HM, Ervasti JM, Corey DP, Lechene CP. Nature. 2012;481(7382):520–524. doi: 10.1038/nature10745. PMCID: PMC3267870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Nature. 2013;493(7432):433–436. doi: 10.1038/nature11682. [Pubmed In Progress - PMCID: PMC3548046] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Littlefield JW, Gould EA. Journal of Biological Chemistry. 1960;235:1129–1133. [PubMed] [Google Scholar]
- 19.Thiery-Lavenant G, Guillermier C, Wang M, Lechene CP. Surface and Interface Analysis. 2014 doi: 10.1002/sia.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]