Abstract
Context
An association between tobacco smoking and prostate cancer (PCa) incidence and mortality was suggested in an earlier meta-analysis of 24 prospective studies in which dose–response associations and risks per unit of tobacco use were not examined.
Objective
We investigated the association between several measures of tobacco use and PCa mortality (primary outcome) and incidence (secondary outcome) including dose–response association.
Evidence acquisition
Relevant articles from prospective studies were identified by searching the PubMed and Web of Science databases (through January 21, 2014) and reference lists of relevant articles. Combined relative risks (RRs) and 95% confidence intervals (CIs) were calculated using random effects methods. We also calculated population attributable risk (PAR) for smoking and PCa mortality.
Evidence synthesis
We included 51 articles in this meta-analysis (11 823 PCa deaths, 50 349 incident cases, and 4 082 606 cohort participants). Current cigarette smoking was associated with an increased risk of PCa death (RR: 1.24; 95% CI, 1.18–1.31), with little evidence for heterogeneity and publication bias. The number of cigarettes smoked per day had a dose–response association with PCa mortality ( p = 0.02; RR for 20 cigarettes per day: 1.20). The PAR for cigarette smoking and PCa deaths in the United States and Europe were 6.7% and 9.5%, respectively, corresponding to >10 000 deaths/ year in these two regions. Current cigarette smoking was inversely associated with incident PCa (RR: 0.90; 95% CI, 0.85–0.96), with high heterogeneity in the results. However, in studies completed in 1995 or earlier (considered as completed before the prostate-specific antigen screening era), ever smoking showed a positive association with incident PCa (RR: 1.06; 95% CI, 1.00–1.12) with little heterogeneity.
Conclusions
Combined evidence from observational studies shows a modest but statistically significant association between cigarette smoking and fatal PCa. Smoking appears to be a modifiable risk factor for PCa death.
Patient summary
Smoking increases the chance of prostate cancer death. Not smoking prevents this harm and many other tobacco-related diseases.
Keywords: Cigarettes, Meta-analysis, Prostate cancer, Prospective, Smoking
1. Introduction
Prostate cancer (PCa) is the second most common cancer and the fifth most common cause of cancer death worldwide [1]. Although PCa is common, few established preventable risk factors have been identified. There is little evidence for any association between alcohol and PCa [2]. The association between diet and PCa has been investigated in a number of studies with little conclusive evidence on the association of nutrients or food items with PCa [3]. Obesity, measured as body mass index (BMI), has shown a modest association with PCa mortality [4,5], but the results with regard to PCa incidence are mixed [5,6]. Several studies reported an inverse association between diabetes mellitus and PCa risk [7,8].
Tobacco use is a known risk factor of several cancers [9]. A meta-analysis of 24 prospective studies published in 2010 found no significant association between current smoking and PCa incidence, but it showed a statistically significant 11–22% increased risk, depending on the exposure measurement method (daily amount of use, cumulative use), in analyses comparing the highest versus lowest categories of use [10]. However, the latter estimates were based on only a few studies (maximum of five studies), and the results for duration of use and cumulative use were combined. A significant 14% increased risk of PCa death associated with current smoking was also reported in that meta-analysis; the highest categories of smoking were associated with a 24–30% increased risk [10]. Although that meta-analysis was published in 2010, it included articles published up to February 2007. Since then, results of several prospective studies have been published [11–32]. Also, no dose–response analysis of the association between tobacco use and PCa is available. One approach to examine dose–response associations, which we sought to do, is via meta-regression. Meta-regression can examine dose–response associations and estimate the risk per exposure unit using actual quantities of exposure and risk estimates for all reported categories of exposure, so it can be more informative than comparing the highest versus the lowest categories of use (only two categories of exposure) that takes into account neither intermediate exposure levels nor any variation in the cut-off point of (and subsequently the quantity of exposure in) the highest categories of use, although it may vary substantially across studies.
An autopsy series of individuals not screened for PCa and who died for various reasons identified asymptomatic undiagnosed PCa in 30–70% of men >60 yr of age [33,34]. A considerable proportion of incident PCa today may be indolent cancers identified through prostate-specific antigen (PSA) screening. Even without treatment, these cancers are unlikely to progress [35,36]. Therefore, although identifying modifiable risk factors for any PCa is important, it is arguably more important for fatal PCa. We aimed to quantify the dose–response associations between tobacco use and PCa mortality and as a secondary aim to examine PCa incidence, separately, using meta-regression models. We also investigated the association between current, former, and ever tobacco use and PCa incidence and mortality.
2. Evidence acquisition
A more detailed version of our methods is available in the Supplement.
2.1. Search methods
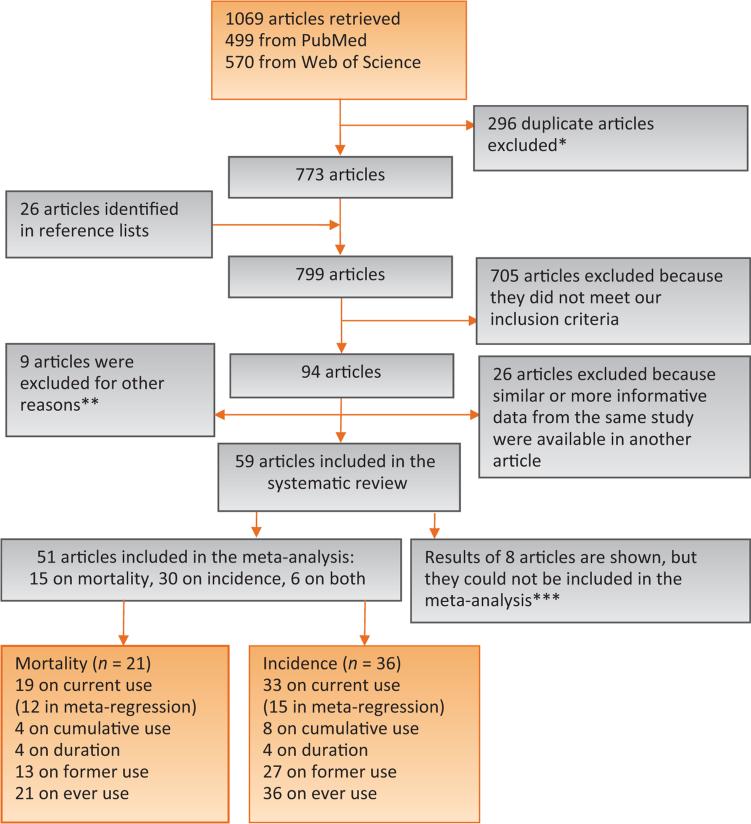
We searched the PubMed and Web of Science databases to identify articles from prospective cohort studies on tobacco use and PCa incidence and mortality, following the Meta-analysis of Observational Studies in Epidemiology guidelines [37]. Only studies published in English were considered. All results were updated on January 21, 2014. Using this approach, we identified a total of 1069 articles, 499 from PubMed and 570 from Web of Science. From this list, 296 articles were excluded because they were retrieved twice (from both databases), and we kept only one of them, leaving 773 articles in the list.
We examined article abstracts and retrieved and reviewed full texts of potentially eligible articles. We included publications that met the following four criteria: reporting original research, human studies, prospective cohorts, and providing information about tobacco use and PCa. We included in our meta-analysis studies reporting relative risk (RR) estimates for the association between tobacco use and PCa incidence or mortality or information to calculate these estimates (eg, the number of cases and person-years of follow-up or number of noncases). We included in our systematic review, but we were not able to include in our meta-analysis, articles that explicitly reported no significant associations between tobacco use and PCa but did not present risk estimates or information to calculate them, or articles in which some information about the association was provided but not enough to be included in the meta-analysis. We also searched bibliographies of relevant articles to identify other publications not retrieved in our electronic search; we found 26 additional publications potentially eligible, resulting in 799 potentially eligible articles. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered.
We excluded 705 articles that did not meet the inclusion criteria. Among this group, there were 162 articles on risk factors of PCa incidence or mortality with no information on smoking and PCa, as follows: 22 articles from studies in which smoking data had not been collected (mainly census-based studies and retrospective occupational cohorts); 77 articles from studies whose results were included in our meta-analysis using another article; and 63 articles from 52 other studies with 459 PCa deaths and 16 288 incident PCa cases, of which only 776 cases were from studies completed in 1995 or earlier (Supplement). Of remaining articles with information on smoking and PCa (n = 94), 26 articles were excluded because similar or more complete data from the same cohort were available in another included publication. An additional nine articles were excluded for other reasons (Fig. 1). Of the remaining 59 articles [11–32,38–74], 51 articles were included in the meta-analysis. We report results of the other eight articles without including them in the meta-analysis [16,40,41,51,52,58,73,74] because they did not provide enough information to do so. Two authors (F.I. and D.M.M.) independently performed the search, evaluated the articles, and abstracted the data. Any inconsistency was resolved by consensus.
Fig. 1.
Flowchart of selection of studies.
* When articles were indexed in both databases, only one was considered for further review.
** Two pooled studies because results from individual studies were included, three articles from studies on atomic bomb survivors (results might not generalize to the population), and four articles from two studies because there were few nonsmokers among study participants.
*** Some results were provided, but the information was not sufficient to be included in the meta-analysis.
2.2. Data abstraction
Ever tobacco use was defined as follows. Some articles reported on ever tobacco use. Some reported on both former and current tobacco use, so results on ever use could be calculated by combining the results for former and current users. Also, some studies only reported on current tobacco use (ie, tobacco use at baseline). In this case, current users were considered as ever users. The RRs and 95% confidence intervals (CIs) were abstracted separately by tobacco product type. We indicated in the figures and tables the articles reporting on smoking of all tobacco products combined only or without specifying the tobacco product, but in the meta-analysis, those results were included in the analyses of cigarette smoking, assuming all or most of the tobacco users were cigarette smokers. We used the maximally adjusted results when several risk estimates with various adjustments were reported.
2.3. Statistical analysis
We calculated the summary risk estimates and 95% CIs and plotted forest plots using random effects models (DerSimonian-Laird method) [75] for the association between current, former, and ever tobacco use and PCa incidence and mortality separately. We reported results for cigarette smoking and for other tobacco products (when available). Heterogeneity among articles was estimated using the I2 statistic and p values associated with Q statistics. The 2 statistic indicates the percentage of total variability explained by heterogeneity [76]. We plotted funnel plots and used the Egger weighted regression method and the Begg and Mazumdar adjusted rank correlation test to examine publication bias.
We also applied random effects meta-regression models when information on the quantitative use of tobacco and PCa was reported, and presented a linear prediction of the fitted values. We did not apply meta-regression analysis to former cigarette smoking because few articles reported quantitative measures for former smokers. Furthermore, the association between former tobacco use and PCa risk, if any, could be influenced by the duration of time since quitting smoking. Also, because little quantitative data were available on tobacco products other than cigarettes, we only include cigarette smoking in our meta-regression analysis. The midpoint of each exposure category was considered as the dose associated to the RR (95% CI) for that category. For the open-ended upper category of use, we multiplied its lower bound by 1.5 to estimate the exposure level [77].
Subgroup analyses were conducted for results controlling (either by standardization or statistical adjustments) for age, race/ethnicity, socioeconomic status (any of income, education level, occupation, or insurance status), BMI, and history of diabetes mellitus as the main potential confounding factors. We performed analyses stratified by geographic area and by the time of study completion (last follow-up before/during vs after 1995). The latter was done to investigate the associations in the era before PSA screening for PCa became widespread, which first started around the mid-1990s, mainly in the United States [78–80]. The widespread use of PSA screening after the mid-1990s may not be homogeneous across countries because the starting time and extent of use were not the same in various regions. Nevertheless, although no cut-off is perfect, a cut-off of 1995 is reasonable to identify pre–PSA screening era studies that were the main focus of this subgroup analysis.
We also calculated population attributable risk (PAR) for smoking and PCa death in the United States and Europe because most studies were from these two regions, using the following formula [81]:
in which pr was the smoking prevalence in the population [82,83]. Using these PARs and the number of PCa deaths in those region [79,84], the number of PCa deaths attributable to smoking were calculated. All statistical analyses were performed using Stata v.11 software (StataCorp, College Station, TX, USA). Throughout the article, associations with 95% CIs that do not include unity or two-sided p values <0.05 were considered statistically significant.
3. Evidence synthesis
Supplementary Table 1a summarizes the characteristics and results of 51 articles included in this meta-analysis. Overall, 15 articles reported on mortality, 30 on incidence, and 6 on both, totaling 11 823 PCa deaths, 50 349 incident cases, and 4 082 606 participants. Articles were published between 1958 and 2013 and were from studies conducted in the following geographic regions: 26 from the United States, 17 from Europe, and 8 from Asia (mainly East Asia) or Australia/New Zealand. Eight additional articles provided some results but not enough information to be included in the meta-analysis (Supplementary Table 1b). Results of these articles are shown separately at the end of this section.
3.1. Prostate cancer mortality
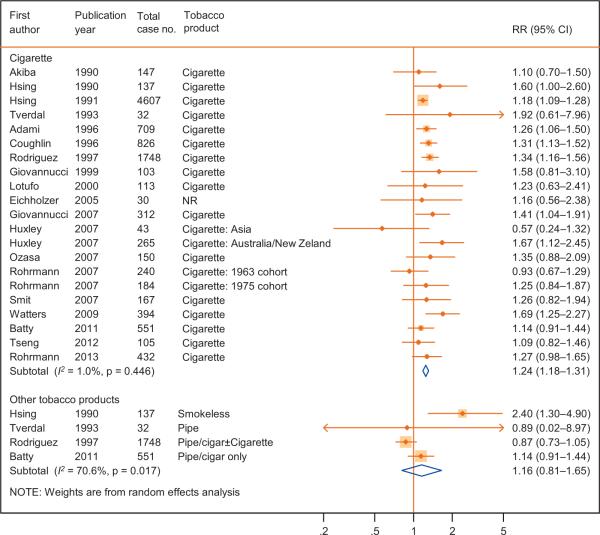
Current cigarette smoking at baseline was associated with an increased risk of death from PCa (RR: 1.24; 95% CI, 1.18–1.31) based on 19 articles (Fig. 2; Table 1), with little heterogeneity in results (I2 = 1%; p = 0.45) and little evidence for publication bias ( p = 0.48; Supplementary Fig. 1). Data on tobacco products other than cigarette were available from four studies only with significant heterogeneity in the results (Fig. 2).
Fig. 2.
The association between tobacco smoking at baseline and prostate cancer mortality in cohort studies.
CI = confidence interval; NR = not reported; RR = relative risk. Huxley [12] and Rohrmann [72] had two subpopulations.
Table 1.
Association between cigarette smoking (current smokers at baseline) and prostate cancer incidence and mortality
| Cigarette use | No. of articles | RR (95% CI) | p for trenda | I2 statistics | p for heterogeneity |
|---|---|---|---|---|---|
| Mortality | |||||
| Overall | |||||
| Current use | 19 | 1.24 (1.18-1.31) | - | 1 | 0.45 |
| Former use | 13 | 1.06 (1.00-1.13) | - | 0 | 0.62 |
| Ever use | 21 | 1.18 (1.11-1.24) | - | 36 | 0.04 |
| Cigarettes per day b | 12 | 1.006 (1.001-1.011) | 0.02 | 0 | - |
| 1995 or earlier * | |||||
| Current use | 10 | 1.24 (1.17-1.31) | - | 0 | 0.79 |
| Former use | 7 | 1.10 (0.99-1.21) | - | 27 | 0.23 |
| Ever use | 12 | 1.23 (1.13-1.33) | - | 47 | 0.04 |
| Cigarettes per day b | 8 | 1.006 (1.001-1.011) | 0.03 | 0 | - |
| After 1995 * | |||||
| Current use | 8 | 1.24 (1.11-1.39) | - | 14 | 0.32 |
| Former use | 6 | 1.00 (0.90-1.11) | - | 0 | 0.99 |
| Ever use | 8 | 1.09 (1.01-1.18) | - | 0 | 0.78 |
| Cigarettes per day b | 4 | 1.009 (0.988-1.030) | 0.38 | 10 | - |
| Incidence | |||||
| Overall | |||||
| Current use | 33 | 0.90 (0.85-0.96) | - | 68 | <0.001 |
| Former use | 27 | 1.00 (0.95-1.06) | - | 61 | <0.001 |
| Ever use | 36 | 0.94 (0.90-0.98) | - | 68 | <0.001 |
| Cigarettes per day b | 15 | 0.995 (0.990-1.001) | 0.09 | 55 | - |
| 1995 or earlier * | |||||
| Current use | 15 | 1.06 (0.98-1.15) | - | 25 | 0.18 |
| Former use | 12 | 1.08 (1.01-1.16) | - | 0 | 0.63 |
| Ever use | 16 | 1.06 (1.00-1.12) | - | 19 | 0.23 |
| Cigarettes per day b | 8 | 1.004 (0.995-1.012) | 0.38 | 15 | - |
| After 1995 * | |||||
| Current use | 18 | 0.84 (0.79-0.89) | - | 58 | 0.001 |
| Former use | 15 | 0.97 (0.91-1.03) | - | 68 | <0.001 |
| Ever use | 20 | 0.90 (0.86-0.93) | - | 62 | <0.001 |
| Cigarettes per day b | 7 | 0.991 (0.985-0.998) | 0.009 | 54 | - |
CI = confidence interval; RR = relative risk.
The p value for the association in the meta-regression analysis.
Quantitative measure for number of cigarettes smoked (cigarettes per day). The RRs (95% CIs) are calculated using meta-regression models and show the risk associated with increments of smoking one cigarette per day.
Last follow-up in 1995 or earlier versus after 1995. In the results for prostate cancer mortality, the last follow-up could not be abstracted from one of the articles [12], so the numbers of articles in the two time periods do not add up to the total number of articles.
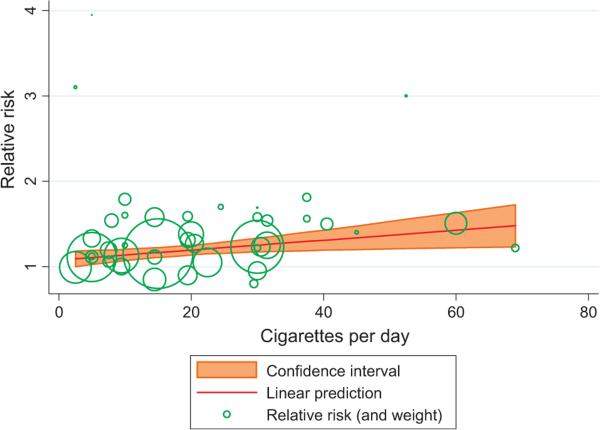
In meta-regression models, the amount of cigarette smoking at baseline (cigarettes per day) showed a dose– response association with PCa death ( p = 0.02; RR for 20 cigarettes per day = 1.20) based on 12 studies [13,18,31,45–48,53,54,57,64,72] (Fig. 3; Table 1). Three point estimates related to one of the categories of tobacco use in three different studies appeared to be markedly different from other point estimates (RRs ≥3 in Fig. 3), but those estimates were based on small numbers of cases and consequently had small weights. Exclusion of those point estimates did not substantially change the results (data not shown). Few studies reported on cumulative or duration of tobacco use and PCa mortality (Supplementary Table 1a), so we did not apply meta-regression to these measures. Of the four articles that reported on cumulative cigarette smoking use and PCa mortality, one with 103 deaths showed a significant positive association [60], and one with 150 deaths showed a nonsignificant positive association [13]; two others (with 98 and 37 deaths, respectively) found a null association [40,64]. All four articles that reported on smoking duration showed an increased risk [13,31,53,57] but with little evidence for dose–response associations in two studies [53,57]. These four articles also reported on amount of use, and they were included in the meta-regression analysis of that measure.
Fig. 3.
The association between amount of cigarette smoking at baseline and prostate cancer mortality using the meta-regression method. The relative risk (RR) (95% confidence interval) calculated using this model for smoking one cigarette per day was 1.006 (1.001– 1.010) ( p = 0.02). The RR for other selected amount of cigarette smoking per day was as follows: 20 cigarettes, 1.20; 30 cigarettes, 1.25; and 40 cigarettes, 1.31 (RR = 0.0057994 × number of cigarettes smoked per day + 1.079222).
The RR (95% CI) for the association between previous cigarette smoking at baseline and PCa mortality was 1.06 (1.00–1.13) based on 13 articles (Supplementary Fig. 2), with little heterogeneity (I2 = 0%; p = 0.62). The RR (95% CI) for the association between ever cigarette smoking and PCa mortality was 1.18 (1.11–1.24) based on 21 articles (Supplementary Fig. 3) but with moderate, statistically significant heterogeneity (I2 = 36%; p = 0.04).
When we included only results adjusted for potential confounders, the combined risk estimates did not change substantially (Supplementary Table 2). The associations between cigarette smoking and PCa death in studies completed in 1995 or earlier (RR: 1.24; 95% CI, 1.17–1.31) or afterward (RR: 1.24; 95% CI, 1.11–1.39) were similar and comparable with overall associations (Supplementary Fig. 4; Table 1). Current smoking was similarly associated with PCa mortality in studies from the United States and Europe, but not in studies from Asia; however, the latter was only based on four studies (Supplementary Table 3).
Only a few of the studies included in our analysis provided information about PCa screening in their study populations (Supplementary Table 4), probably because this information was not available from most cancer registers that were the main source of outcome measurements. This limited information suggests that the association between smoking and PCa death is slightly stronger in those with no screening compared with those with PCa screening [18].
3.2. Prostate cancer incidence
Cigarette smoking at baseline was inversely associated with incident PCa (RR: 0.90; 95% CI, 0.85–0.96) based on 33 studies (Supplementary Fig. 5; Table 1). However, results showed high heterogeneity (2 = 68%; p < 0.001). In meta-regression analysis, based on 15 articles [14,17,18,22,26,31,49, 50,53,56,59,62,64,65,72], number of cigarettes smoked per day was not significantly associated with PCa risk ( p = 0.09; Supplementary Fig. 6; Table 1). Eight articles reported on cumulative use [26,29,32,50,56,64–66] with no significant association with incident PCa risk in meta-regression analysis (Supplementary Fig. 7). Only four articles reported on smoking duration and PCa risk [17,26,31,53], and none showed a clear pattern of association (Supplementary Table 1a).
Previous smoking showed no association (RR: 1.00; 95% CI, 0.95–1.06) based on 27 articles (Supplementary Fig. 8), but ever smoking showed an inverse association (RR: 0.94; 95% CI, 0.90–0.98) based on 36 articles (Supplementary Fig. 9) with incident PCa; however, heterogeneity in results for both groups was high (2 = 61%; p < 0.001, and 2 = 68%; p < 0.001, respectively).
Exclusive analysis of the results adjusted for potential confounders did not substantially change the results (Supplementary Table 2). The patterns of the associations between smoking and PCa risk in studies completed in 1995 or earlier were different from those in studies completed afterward (Table 1; Supplementary Fig. 10). For current smoking, the RR (95% CI) was 1.06 (0.98–1.15) for studies completed in 1995 or earlier and 0.84 (0.79–0.89) for studies completed afterward. In studies completed in 1995 or earlier, former (RR: 1.08; 95% CI, 1.01–1.16) and ever (RR: 1.06; 95% CI, 1.00–1.12) cigarette smoking showed positive associations with PCa incidence (Table 1). The results of meta-regression analyses followed the patterns just described. This subgroup analysis substantially reduced the heterogeneity for the studies completed in 1995 or earlier: The p values for heterogeneity for current, former, and ever cigarette smoking changed from <0.001 to ≥0.18 (Table 1). In overall analysis, incident PCa was not associated with current smoking in studies from the United States, but studies from other regions showed inverse associations. The proportion of articles from the United States to those from Europe was higher in the PSA prescreening era (10 to 6) than afterward (5 to 8). In each time period, the associations in the United States and Europe were similar (Supplementary Table 3).
3.3. Population attributable risk
The PARs for cigarette smoking and PCa deaths in the United States and Europe were 6.7% and 9.5%, respectively (Table 2). The total number of PCa deaths attributable to cigarette smoking in these two regions combined was approximately 10 400 deaths per year.
Table 2.
Population attributable risk and the number of prostate cancer deaths attributable to cigarette smoking in the United States and Europe*
| PAR and no. of prostate cancer deaths | United States | Europe |
|---|---|---|
| No. of annual prostate cancer deaths | 28 170 [84] | 89 600 [79] |
| % ever smoker men | 40% [82] | 58% [83] |
| PAR for smoking | 6.7% | 9.5% |
| Total no. of prostate cancer deaths attributable to smoking | 1887 | 8512 |
PAR = population attributable risk.
Assuming an 18% increase in prostate cancer mortality associated with ever cigarette smoking. Mortality data for the United States are from 2012 and for Europe from 2008. Smoking data for the United States are from 2010 to 2011 and for Europe from 2012.
3.4. Studies included in the systematic review but not in the meta-analysis
Of these eight articles (Supplementary Table 1b), three reported on morality [40,52,73], four reported on incidence [16,51,58,74], and one reported on both [41], with an additional 1000 PCa deaths, 1344 incident cancers, and 225 451 cohort participants. One study reporting on current cigarette smoking (37 PCa deaths) [40], and another study reporting on ever smoking (12 deaths) [41] found no significant associations between smoking and PCa mortality. Another study reported a RR (95% CI) of 1.02 (0.93–1.12) for the association between increments of smoking five cigarettes per day and PCa death [52]. Because these studies were small (including only a total of 122 PCa deaths), inclusion of those studies, if they had provided enough information, was unlikely to substantially change our results on PCa mortality. Results of another study with 878 PCa deaths were suggestive of a nonsignificant increased risk with smoking ≥15 cigarettes per day (RR: 1.27 for smoking ≥25 cigarettes per day), but RR was <1 for those smoking <15 cigarettes per day [73].
Two articles (with 16 cases [41] and 524 cases [58]) reported no significant associations between smoking and PCa risk. Another study reported a RR (95% CI) of 1.08 (0.90–1.30) for the association between increments of 10 cigarettes smoked per day and PCa risk (based on 211 cases) [51], and two other studies reported an inverse association between smoking and PCa (363 and 230 cases; both completed after 1995) [16,74].
4. Discussion
Smoking at baseline was associated with a 24% increased risk of PCa death. Consistent with this, the meta-regression analysis showed a positive association between the number of cigarettes smoked per day and PCa death. The association between cigarette smoking and incident PCa was mixed. The overall analyses showed no or inverse associations, whereas studies completed in 1995 or earlier pointed to a positive association. Associations between tobacco products other than cigarettes and PCa were reported in a few studies, generally with high heterogeneity in the results.
4.1. Prostate cancer mortality
The association between cigarette smoking and PCa death was robust. It was observed in analyses of current, former, and ever use and in meta-regression models, which indicates a dose–response association, and persisted in subgroup analyses including when stratified by geography or study completion time. We cannot exclude residual confounding (due to imperfect measurement of other risk factors) or confounding effects of unknown risk factors, but the association was not strongly related to the confounding effects of known potential confounding factors such as age, race, diabetes, BMI, or socioeconomic status. The association did not also seem to be related to publication bias. Overall, the current epidemiological evidence suggests an association between smoking and PCa death.
Adherence to PSA testing may also be negatively associated with tobacco smoking for various reasons including lower socioeconomic status [85–88]. The limited evidence shown in Supplementary Table 4 indicates that PSA screening is slightly more common in nonsmokers than smokers. However, because PSA screening reduces PCa death by approximately 21% [89], adherence to screening must be extremely high in nonsmokers and extremely low in smokers if differences in screening adherence alone are to explain the 24% increased mortality of PCa in smokers. The patterns of association between smoking and PCa death before and after PSA screening era were almost similar, refuting any major influence of PSA screening on this association. Also, the association between smoking and PCa mortality persisted in the few studies that adjusted their results for indicators of socioeconomic status. Although for the reasons just cited socioeconomic status and screening/ detection biases are unlikely to explain all increased risk of PCa death in smokers, the slightly lower screening coverage in smokers in recent years (after the common use of PSA screening) may lead to a slightly stronger association between smoking and fatal PCa in the future if this trend continues, given that screening does reduce PCa mortality, albeit modestly.
Several studies suggested smokers may have more advanced disease at surgery and consequently a higher risk of recurrence, metastasis, and death [90–92]. This may be related to a delayed diagnosis in smokers versus nonsmokers or a stronger association of smoking with progression than PCa initiation. Smokers may also have a suboptimal response to treatment (radical prostatectomy or radiotherapy) [92–94]. Some studies have shown possible mechanistic pathways linking smoking and PCa progression; however, the evidence is sparse, and these results are sometimes only based on experimental or in vitro models. For example, increased heme oxygenase 1 (HO-1) messenger RNA expression and upregulated HO-1 protein levels are present in PCa cell lines [95]. HO-1 may have a role in tumor angiogenesis [96]. An effect of smoking on PCa progression through CpG hypermethylation of several genes has also been suggested [97]. Although this has not been studied in the prostate, nicotine can induce angiogenesis in some tissues that may lead to faster cancer progression [98]. Because aspirin use may reduce PCa mortality and recurrence [99–101], inflammation may also have a role in PCa progression or even initiation [102]. Smoking induces inflammation in various tissues [103], and smokers have more inflammation within the prostate than nonsmokers [104].
4.2. Prostate cancer incidence
Our results suggest a possible association between smoking and increased PCa incidence in studies completed in 1995 or earlier, but studies completed afterward showed a null or even inverse association. It is unlikely the difference in pattern of association over time is solely related to differences in the quality of studies. Some earlier studies were large well-conducted studies. Also, if a difference in study quality was the reason for this pattern, we would expect similar patterns in the association between smoking and PCa mortality, whereas the latter association barely changed over time. The reason for this pattern is unclear. One possible explanation is that smoking may reduce the risk of indolent nonaggressive cancers that have predominated in more recent years while promoting more aggressive cancers.
The difference in the patterns of association between smoking and incident PCa in the United States and Europe in the overall analysis seems to be related to a higher proportion of studies from the United States in the pre-PSA screening era (many showing positive associations) than afterward (many showing inverse associations). Results for the United States and Europe in the analysis stratified by study completion time were not different.
4.3. Public health implications
Even if the association between smoking and PCa death is established as causal, the magnitude of association is smaller versus those reported for other smoking-related cancers including cancers of the lung and upper aero-digestive tract [105]. Therefore, the proportion of the PCa deaths attributed to smoking will be modest. However, because PCa is a common cause of cancer death, this association may have a considerable impact on cancer mortality at the population level. With a PAR of about 10%, the number of PCa deaths in Europe in 2008 attributable to smoking was approximately 8500 deaths. This further emphasizes the importance of widespread efforts to reduce cigarette smoking initiation and smoking rates, to increase smoking cessation, and perhaps to reduce the use of other tobacco products that are being aggressively marketed. Any beneficial effect of efficient tobacco control programs can also include reduced PCa mortality, beyond other protective effects against other smoking-related cancers and cardiovascular disease. Because most PCa deaths are attributable to factors other than smoking, further studies to identify these factors are required.
4.4. Strength and limitations
Strengths of this analysis include independent search and data abstraction by two researchers experienced in conducting systematic reviews; inclusion of a relatively large number of studies including several earlier articles that were missing and 22 articles published after the earlier meta-analysis [10]; using meta-regression to examine dose–response relationships; conducting several subgroup analyses; and calculating PAR for smoking and PCa deaths in the United States and Europe. Also, because all included studies were prospective cohort studies, little information bias was expected.
Similar to any other meta-analysis of observational studies, combining the results of studies with various qualities of design and conduct may sometimes be misleading. For example, although we found little association between smoking and PCa death in Asian countries, this was based on few studies and thus no firm conclusions can be drawn. However, we performed several subgroup analyses to examine the associations in various settings. Also, the association between smoking and PCa death showed little heterogeneity, indicating that although some differences in study design and conduct across studies were inevitable, these differences had little impact on the overall associations. In other words, the results reported in various studies were significantly consistent. Similarly, there was little heterogeneity in the association between smoking and PCa incidence in studies completed in 1995 or earlier. The high heterogeneity in results when all studies or studies completed after 1995 were considered is therefore probably because these results were from studies in populations with various screening statuses.
Although there was no indication of publication bias in our analysis, there still might be some unpublished studies with null results. Because the results of large cohort studies (even null results) are more likely to be published, any unpublished study is likely to be relatively small and consequently with no major effect on our pooled estimates that were based on 11 823 PCa deaths and 50 349 incident cases. Consistent with this, in the 63 articles on PCa risk factors that did not report any information on tobacco use and PCa (Supplement), there were only 459 PCa deaths in total and 776 incident cases in studies completed in 1995 or earlier, which is unlikely to change the corresponding results even if the associations in those studies were different from ours. There might also be errors in the attribution of PCa as the cause of death. However, because smoking history was obtained prospectively and the error is unlikely to be differential with regard to smoking status (ie, it is unlikely that a higher proportions of smokers’ deaths are attributed erroneously to PCa than nonsmokers’ deaths), this error is unlikely to cause the observed associations. Similarly, although we could not completely exclude the role of errors in exposure (smoking) measurement, it is unlikely that these errors at baseline were differential for eventual noncases and PCa cases. Errors in attribution of PCa stage at diagnosis would not affect the association between smoking and PCa incidence or death because our outcome occurred after the assessment of smoking and included all incident cases or deaths irrespective of the cancer stage. Another potential limitation is that only studies published in English language were considered. However, this may not have a major impact on our pooled estimates because we expected few large cohort studies on smoking and PCa that were not published in English. Finally, the association between smoking and PCa incidence and/or mortality can be underestimated in this analysis as a result of competing risks because most of the other smoking-related deaths (eg, cardiovascular and lung cancer) are more likely to happen at earlier ages compared with PCa deaths, so those smokers could no longer be in the group of smokers at risk of PCa.
5. Conclusions
We found a modest but statistically significant association between cigarette smoking and PCa death, with a dose– response relationship. However, the association between cigarette smoking and PCa incidence was mixed. Although our results may suggest a modest association between cigarette smoking and PCa incidence before the screening era, such an association was not observed in the studies published afterward. This may suggest that smoking is not associated with indolent PCa as identified with PSA screening. The positive association in earlier years and the association with mortality collectively provide evidence that smoking is related to aggressive PCa.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: The work of Stephen J. Freedland was supported by a National Institutes of Health grant (K24CA160653). There was no specific funding for this project.
Footnotes
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Islami, Moreira, Boffetta, Freedland.
Acquisition of data: Islami, Moreira.
Analysis and interpretation of data: Islami, Moreira, Boffetta, Freedland.
Drafting of the manuscript: Islami, Freedland.
Critical revision of the manuscript for important intellectual content: Islami, Moreira, Boffetta, Freedland.
Statistical analysis: Islami.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Freedland, Boffetta.
Other (specify): None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2014.08.059.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Rota M, Scotti L, Turati F, et al. Alcohol consumption and prostate cancer risk: a meta-analysis of the dose-risk relation. Eur J Cancer Prev. 2012;21:350–9. doi: 10.1097/CEJ.0b013e32834dbc11. [DOI] [PubMed] [Google Scholar]
- 3.Masko EM, Allott EH, Freedland SJ. The relationship between nutrition and prostate cancer: is more always better? Eur Urol. 2013;63:810–20. doi: 10.1016/j.eururo.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer–a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 6.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Jiang HW, Ding GX, et al. Diabetes mellitus and prostate cancer risk of different grade or stage: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2013;99:241–9. doi: 10.1016/j.diabres.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013;16:151–8. S1. doi: 10.1038/pcan.2012.40. [DOI] [PubMed] [Google Scholar]
- 9.Secretan B, Straif K, Baan R, et al. A review of human carcinogens–part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–4. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 10.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez A, Peters U, Lampe JW, White E. Boron intake and prostate cancer risk. Cancer Causes Control. 2007;18:1131–40. doi: 10.1007/s10552-007-9052-2. [DOI] [PubMed] [Google Scholar]
- 12.Huxley R. The impact of modifiable risk factors on mortality from prostate cancer in populations of the Asia-Pacific region. Asian Pac J Cancer Prev. 2007;8:199–205. [PubMed] [Google Scholar]
- 13.Ozasa K. Japan Collaborative Cohort Study for Evaluation of Cancer. Smoking and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev. 2007;8(Suppl):89–96. [PubMed] [Google Scholar]
- 14.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2007;121:1339–45. doi: 10.1002/ijc.22805. [DOI] [PubMed] [Google Scholar]
- 15.Smit E, Garcia-Palmieri MR, Figueroa NR, et al. Protein and legume intake and prostate cancer mortality in Puerto Rican men. Nutr Cancer. 2007;58:146–52. doi: 10.1080/01635580701328206. [DOI] [PubMed] [Google Scholar]
- 16.Chamie K, DeVere White RW, Lee D, Ok JH, Ellison LM. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008;113:2464–70. doi: 10.1002/cncr.23695. [DOI] [PubMed] [Google Scholar]
- 17.Butler LM, Wang R, Wong AS, Koh WP, Yu MC. Cigarette smoking and risk of prostate cancer among Singapore Chinese. Cancer Causes Control. 2009;20:1967–74. doi: 10.1007/s10552-009-9391-2. [DOI] [PubMed] [Google Scholar]
- 18.Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2427–35. doi: 10.1158/1055-9965.EPI-09-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batty GD, Kivimaki M, Clarke R, Davey SG, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011;22:311–8. doi: 10.1007/s10552-010-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundmark B, Zethelius B, Garmo H, Holmberg L. Serum levels of selenium and smoking habits at age 50 influence long term prostate cancer risk; a 34 year ULSAM follow-up. BMC Cancer. 2011;11:431. doi: 10.1186/1471-2407-11-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geybels MS, Verhage BA, van Schooten FJ, van den Brandt PA. Measures of combined antioxidant and pro-oxidant exposures and risk of overall and advanced stage prostate cancer. Ann Epidemiol. 2012;22:814–20. doi: 10.1016/j.annepidem.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Karlsen RV, Bidstrup PE, Christensen J, et al. Men with cancer change their health behaviour: a prospective study from the Danish diet, cancer and health study. Br J Cancer. 2012;107:201–6. doi: 10.1038/bjc.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karppi J, Kurl S, Laukkanen JA, Kauhanen J. Serum beta-carotene in relation to risk of prostate cancer: the Kuopio Ischaemic Heart Disease Risk Factor study. Nutr Cancer. 2012;64:361–7. doi: 10.1080/01635581.2012.658949. [DOI] [PubMed] [Google Scholar]
- 24.Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Coffee consumption and prostate cancer risk: further evidence for inverse relationship. Nutr J. 2012;11:42. doi: 10.1186/1475-2891-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng CH. Insulin use is not significantly predictive for prostate cancer mortality in diabetic patients: a 12-year follow-up study. BJU Int. 2012;110:668–73. doi: 10.1111/j.1464-410X.2011.10924.x. [DOI] [PubMed] [Google Scholar]
- 26.Bae JM, Li ZM, Shin MH, Kim DH, Lee MS, Ahn YO. Cigarette smoking and prostate cancer risk: negative results of the Seoul Male Cancer Cohort Study. Asian Pac J Cancer Prev. 2013;14:4667–9. doi: 10.7314/apjcp.2013.14.8.4667. [DOI] [PubMed] [Google Scholar]
- 27.Heikkila K, Nyberg ST, Theorell T, et al. Work stress and risk of cancer: meta-analysis of 5700 incident cancer events in 116,000 European men and women. BMJ. 2013;346:f165. doi: 10.1136/bmj.f165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutros S, Meyer TE, Fox SD, et al. Prospective evaluation of serum sarcosine and risk of prostate cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Carcinogenesis. 2013;34:2281–5. doi: 10.1093/carcin/bgt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemogne C, Consoli SM, Melchior M, et al. Depression and the risk of cancer: a 15-year follow-up study of the GAZEL cohort. Am J Epidemiol. 2013;178:1712–20. doi: 10.1093/aje/kwt217. [DOI] [PubMed] [Google Scholar]
- 30.Onitilo AA, Berg RL, Engel JM, et al. Prostate cancer risk in pre-diabetic men: a matched cohort study. Clin Med Res. 2013;11:201–9. doi: 10.3121/cmr.2013.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohrmann S, Linseisen J, Allen N, et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2013;108:708–14. doi: 10.1038/bjc.2012.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawada N, Inoue M, Iwasaki M, et al. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: the Japan Public Health Center-based prospective study. Int J Cancer. 2014;134:971–8. doi: 10.1002/ijc.28423. [DOI] [PubMed] [Google Scholar]
- 33.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 34.Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst. 2013;105:1050–8. doi: 10.1093/jnci/djt151. [DOI] [PubMed] [Google Scholar]
- 35.Ploussard G, Epstein JI, Montironi R, et al. The contemporary concept of significant versus insignificant prostate cancer. Eur Urol. 2011;60:291–303. doi: 10.1016/j.eururo.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Roobol MJ, Carlsson SV. Risk stratification in prostate cancer screening. Nat Rev Urol. 2013;10:38–48. doi: 10.1038/nrurol.2012.225. [DOI] [PubMed] [Google Scholar]
- 37.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 38.Hammond EC, Horn D. Smoking and death rates; report on forty-four months of follow-up of 187,783 men. II. Death rates by cause. JAMA. 1958;166:1294–308. doi: 10.1001/jama.1958.02990110030007. [DOI] [PubMed] [Google Scholar]
- 39.Hammond EC. Smoking in relation to the death rates of one million men and women. Natl Cancer Inst Monogr. 1966;19:127–204. [PubMed] [Google Scholar]
- 40.Weir JM, Dunn JE., Jr Smoking and mortality: a prospective study. Cancer. 1970;25:105–12. doi: 10.1002/1097-0142(197001)25:1<105::aid-cncr2820250115>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Whittemore AS, Paffenbarger RS, Jr, Anderson K, Lee JE. Early precursors of urogenital cancers in former college men. J Urol. 1984;132:1256–61. doi: 10.1016/s0022-5347(17)50118-4. [DOI] [PubMed] [Google Scholar]
- 42.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49:1857–60. [PubMed] [Google Scholar]
- 43.Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64:598–604. doi: 10.1002/1097-0142(19890801)64:3<598::aid-cncr2820640306>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Thompson MM, Garland C, Barrett-Connor E, Khaw KT, Friedlander NJ, Wingard DL. Heart disease risk factors, diabetes, and prostatic cancer in an adult community. Am J Epidemiol. 1989;129:511–7. doi: 10.1093/oxfordjournals.aje.a115162. [DOI] [PubMed] [Google Scholar]
- 45.Akiba S, Hirayama T. Cigarette smoking and cancer mortality risk in Japanese men and women–results from reanalysis of the sixprefecture cohort study data. Environ Health Perspect. 1990;87:19–26. doi: 10.1289/ehp.908719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsing AW, McLaughlin JK, Schuman LM, et al. Diet, tobacco use, and fatal prostate cancer: results from the Lutheran Brotherhood Cohort Study. Cancer Res. 1990;50:6836–40. [PubMed] [Google Scholar]
- 47.Hsing AW, McLaughlin JK, Hrubec Z, Blot WJ, Fraumeni JF., Jr Tobacco use and prostate cancer: 26-year follow-up of US veterans. Am J Epidemiol. 1991;133:437–41. doi: 10.1093/oxfordjournals.aje.a115910. [DOI] [PubMed] [Google Scholar]
- 48.Tverdal A, Thelle D, Stensvold I, Leren P, Bjartveit K. Mortality in relation to smoking history: 13 years’ follow-up of 68,000 Norwegian men and women 35–49 years. J Clin Epidemiol. 1993;46:475–87. doi: 10.1016/0895-4356(93)90025-v. [DOI] [PubMed] [Google Scholar]
- 49.Hiatt RA, Armstrong MA, Klatsky AL, Sidney S. Alcohol consumption, smoking, and other risk factors and prostate cancer in a large health plan cohort in California (United States). Cancer Causes Control. 1994;5:66–72. doi: 10.1007/BF01830728. [DOI] [PubMed] [Google Scholar]
- 50.Le Marchand L, Kolonel LN, Wilkens LR, Myers BC, Hirohata T. Animal fat consumption and prostate cancer: a prospective study in Hawaii. Epidemiology. 1994;5:276–82. doi: 10.1097/00001648-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Thune I, Lund E. Physical activity and the risk of prostate and testicular cancer: a cohort study of 53,000 Norwegian men. Cancer Causes Control. 1994;5:549–56. doi: 10.1007/BF01831383. [DOI] [PubMed] [Google Scholar]
- 52.Gann PH, Daviglus ML, Dyer AR, Stamler J. Heart rate and prostate cancer mortality: results of a prospective analysis. Cancer Epidemiol Biomarkers Prev. 1995;4:611–6. [PubMed] [Google Scholar]
- 53.Adami HO, Bergstrom R, Engholm G, et al. A prospective study of smoking and risk of prostate cancer. Int J Cancer. 1996;67:764–8. doi: 10.1002/(SICI)1097-0215(19960917)67:6<764::AID-IJC3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 54.Coughlin SS, Neaton JD, Sengupta A. Cigarette smoking as a predictor of death from prostate cancer in 348,874 men screened for the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143:1002–6. doi: 10.1093/oxfordjournals.aje.a008663. [DOI] [PubMed] [Google Scholar]
- 55.Engeland A, Andersen A, Haldorsen T, Tretli S. Smoking habits and risk of cancers other than lung cancer: 28 years’ follow-up of 26,000 Norwegian men and women. Cancer Causes Control. 1996;7:497–506. doi: 10.1007/BF00051881. [DOI] [PubMed] [Google Scholar]
- 56.Cerhan JR, Torner JC, Lynch CF, et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control. 1997;8:229–38. doi: 10.1023/a:1018428531619. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez C, Tatham LM, Thun MJ, Calle EE, Heath CW., Jr Smoking and fatal prostate cancer in a large cohort of adult men. Am J Epidemiol. 1997;145:466–75. doi: 10.1093/oxfordjournals.aje.a009129. [DOI] [PubMed] [Google Scholar]
- 58.Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6:863–73. [PubMed] [Google Scholar]
- 59.Veierod MB, Laake P, Thelle DS. Dietary fat intake and risk of prostate cancer: a prospective study of 25,708 Norwegian men. Int J Cancer. 1997;73:634–8. doi: 10.1002/(sici)1097-0215(19971127)73:5<634::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 60.Giovannucci E, Rimm EB, Ascherio A, et al. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol Biomarkers Prev. 1999;8:277–82. [PubMed] [Google Scholar]
- 61.Heikkila R, Aho K, Heliovaara M, et al. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal study. Cancer. 1999;86:12–5. [PubMed] [Google Scholar]
- 62.Parker AS, Cerhan JR, Putnam SD, Cantor KP, Lynch CF. A cohort study of farming and risk of prostate cancer in Iowa. Epidemiology. 1999;10:452–5. doi: 10.1097/00001648-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Will JC, Vinicor F, Calle EE. Is diabetes mellitus associated with prostate cancer incidence and survival? Epidemiology. 1999;10:313–8. [PubMed] [Google Scholar]
- 64.Lotufo PA, Lee IM, Ajani UA, Hennekens CH, Manson JE. Cigarette smoking and risk of prostate cancer in the physicians’ health study (United States). Int J Cancer. 2000;87:141–4. doi: 10.1002/1097-0215(20000701)87:1<141::aid-ijc21>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 65.Lund Nilsen TI, Johnsen R, Vatten LJ. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br J Cancer. 2000;82:1358–63. doi: 10.1054/bjoc.1999.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nomura AM, Lee J, Stemmermann GN, Combs GF., Jr Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:883–7. [PubMed] [Google Scholar]
- 67.Visvanathan K, Helzlsouer KJ, Boorman DW, et al. Association among an ornithine decarboxylase polymorphism, androgen receptor gene (CAG) repeat length and prostate cancer risk. J Urol. 2004;171:652–5. doi: 10.1097/01.ju.0000108384.74718.73. [DOI] [PubMed] [Google Scholar]
- 68.Eichholzer M, Bernasconi F, Jordan P, Stahelin HB. Body mass index and the risk of male cancer mortality of various sites: 17-year follow-up of the Basel cohort study. Swiss Med Wkly. 2005;135:27–33. doi: 10.4414/smw.2005.10415. [DOI] [PubMed] [Google Scholar]
- 69.Hultdin J, Van Guelpen B, Bergh A, Hallmans G, Stattin P. Plasma folate, vitamin B12, and homocysteine and prostate cancer risk: a prospective study. Int J Cancer. 2005;113:819–24. doi: 10.1002/ijc.20646. [DOI] [PubMed] [Google Scholar]
- 70.Baglietto L, Severi G, English DR, Hopper JL, Giles GG. Alcohol consumption and prostate cancer risk: results from the Melbourne collaborative cohort study. Int J Cancer. 2006;119:1501–4. doi: 10.1002/ijc.21983. [DOI] [PubMed] [Google Scholar]
- 71.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohrmann S, Genkinger JM, Burke A, et al. Smoking and risk of fatal prostate cancer in a prospective U.S. study. Urology. 2007;69:721–5. doi: 10.1016/j.urology.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer. 2005;92:426–9. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q, Kuriyama S, Kakizaki M, et al. History of cholelithiasis and the risk of prostate cancer: the Ohsaki Cohort Study. Int J Cancer. 2011;128:185–91. doi: 10.1002/ijc.25303. [DOI] [PubMed] [Google Scholar]
- 75.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 76.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38:1497–511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- 78.von Eschenbach A, Ho R, Murphy GP, Cunningham M, Lins N. American Cancer Society guidelines for the early detection of prostate cancer: update, June 10, 1997. Cancer. 1997;80:1805–7. doi: 10.1002/(sici)1097-0142(19971101)80:9<1805::aid-cncr17>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 79.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 80.Kvale R, Auvinen A, Adami HO, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst. 2007;99:1881–7. doi: 10.1093/jnci/djm249. [DOI] [PubMed] [Google Scholar]
- 81.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18:571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 82.The tobacco use supplement to the current population survey 2011–2012. National Cancer Institute; [January 20, 2014]. Web site. http://applied research.cancer.gov/tus-cps/2013. [Google Scholar]
- 83.Attitudes of Europeans towards tobacco. European Commission; Special Eurobarometer 385/Wave EB77.1 – TNA Opinion and Social. Web site. http://ec.europa.eu/health/tobacco/docs/eurobaro_attitudes_towards_tobacco_2012_en.pdf. [Google Scholar]
- 84.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 85.Ross LE, Coates RJ, Breen N, Uhler RJ, Potosky AL, Blackman D. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med. 2004;38:732–44. doi: 10.1016/j.ypmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Hoffman RM, Stone SN, Espey D, Potosky AL. Differences between men with screening-detected versus clinically diagnosed prostate cancers in the USA. BMC Cancer. 2005;5:27. doi: 10.1186/1471-2407-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan RM, Steele RJ, Nabi G, McCowan C. Socioeconomic variation and prostate specific antigen testing in the community: a United Kingdom based population study. J Urol. 2013;190:1207–12. doi: 10.1016/j.juro.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 88.Rundle A, Neckerman KM, Sheehan D, et al. A prospective study of socioeconomic status, prostate cancer screening and incidence among men at high risk for prostate cancer. Cancer Causes Control. 2013;24:297–303. doi: 10.1007/s10552-012-0108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts WW, Platz EA, Walsh PC. Association of cigarette smoking with extraprostatic prostate cancer in young men. J Urol. 2003;169:512–6. doi: 10.1097/01.ju.0000046160.80804.7f. [DOI] [PubMed] [Google Scholar]
- 91.Joshu CE, Mondul AM, Meinhold CL, et al. Cigarette smoking and prostate cancer recurrence after prostatectomy. J Natl Cancer Inst. 2011;103:835–8. doi: 10.1093/jnci/djr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moreira DM, Aronson WJ, Terris MK, et al. Cigarette smoking is associated with an increased risk of biochemical disease recurrence, metastasis, castration-resistant prostate cancer, and mortality after radical prostatectomy: results from the SEARCH database. Cancer. 2014;120:197–204. doi: 10.1002/cncr.28423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pickles T, Liu M, Berthelet E, Kim-Sing C, Kwan W, Tyldesley S. The effect of smoking on outcome following external radiation for localized prostate cancer. J Urol. 2004;171:1543–6. doi: 10.1097/01.ju.0000118292.25214.a4. [DOI] [PubMed] [Google Scholar]
- 94.Pantarotto J, Malone S, Dahrouge S, Gallant V, Eapen L. Smoking is associated with worse outcomes in patients with prostate cancer treated by radical radiotherapy. BJU Int. 2007;99:564–9. doi: 10.1111/j.1464-410X.2006.06656.x. [DOI] [PubMed] [Google Scholar]
- 95.Birrane G, Li H, Yang S, Tachado SD, Seng S. Cigarette smoke induces nuclear translocation of heme oxygenase 1 (HO-1) in prostate cancer cells: nuclear HO-1 promotes vascular endothelial growth factor secretion. Int J Oncol. 2013;42:1919–28. doi: 10.3892/ijo.2013.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyake M, Fujimoto K, Anai S, et al. Heme oxygenase-1 promotes angiogenesis in urothelial carcinoma of the urinary bladder. Oncol Rep. 2011;25:653–60. doi: 10.3892/or.2010.1125. [DOI] [PubMed] [Google Scholar]
- 97.Enokida H, Shiina H, Urakami S, et al. Smoking influences aberrant CpG hypermethylation of multiple genes in human prostate carcinoma. Cancer. 2006;106:79–86. doi: 10.1002/cncr.21577. [DOI] [PubMed] [Google Scholar]
- 98.Lee J, Cooke JP. Nicotine and pathological angiogenesis. Life Sci. 2012;91:1058–64. doi: 10.1016/j.lfs.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.La Vecchia C, Bosetti C. Urological cancer: aspirin and the risk of prostate cancer mortality. Nat Rev Clin Oncol. 2012;9:616–7. doi: 10.1038/nrclinonc.2012.182. [DOI] [PubMed] [Google Scholar]
- 100.Algotar AM, Thompson PA, Ranger-Moore J, et al. Differences in characteristics of men with localised prostate cancer who demonstrate low, intermediate or high prostate-specific antigen velocity. Intern Med J. 2012;42:374–80. doi: 10.1111/j.1445-5994.2011.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flahavan EM, Bennett K, Sharp L, Barron TI. A cohort study investigating aspirin use and survival in men with prostate cancer. Ann Oncol. 2014;25:154–9. doi: 10.1093/annonc/mdt428. [DOI] [PubMed] [Google Scholar]
- 102.Mahmud SM, Franco EL, Aprikian AG. Use of nonsteroidal anti-inflammatory drugs and prostate cancer risk: a meta-analysis. Int J Cancer. 2010;127:1680–91. doi: 10.1002/ijc.25186. [DOI] [PubMed] [Google Scholar]
- 103.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 104.Moreira D, Nickel JC, Gerber L, et al. Smoking is associated with acute prostatic inflammation in men with a negative prostate biopsy: results from the REDUCE study. J Urol. 2013;189:e480. [Google Scholar]
- 105.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3–9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.