Abstract
The urinary tract exits to a body surface area that is densely populated by a wide range of microbes. Yet, under most normal circumstances, it is typically considered sterile, i.e., devoid of microbes, a stark contrast to the gastrointestinal and upper respiratory tracts where many commensal and pathogenic microbes call home. Not surprisingly, infection of the urinary tract over a healthy person’s lifetime is relatively infrequent, occurring once or twice or not at all for most people. For those who do experience an initial infection, the great majority (70% to 80%) thankfully do not go on to suffer from multiple episodes. This is a far cry from the upper respiratory tract infections, which can afflict an otherwise healthy individual countless times. The fact that urinary tract infections are hard to elicit in experimental animals except with inoculum 3–5 orders of magnitude greater than the colony counts that define an acute urinary infection in humans (105 cfu/ml), also speaks to the robustness of the urinary tract defense. How can the urinary tract be so effective in fending off harmful microbes despite its orifice in a close vicinity to that of the microbe-laden gastrointestinal tract? While a complete picture is still evolving, the general consensus is that the anatomical and physiological integrity of the urinary tract is of paramount importance in maintaining a healthy urinary tract. When this integrity is breached, however, the urinary tract can be at a heightened risk or even recurrent episodes of microbial infections. In fact, recurrent urinary tract infections are a significant cause of morbidity and time lost from work and a major challenge to manage clinically. Additionally, infections of the upper urinary tract often require hospitalization and prolonged antibiotic therapy. In this chapter, we provide an overview of the basic anatomy and physiology of the urinary tract with an emphasis on their specific roles in host defense. We also highlight the important structural and functional abnormalities that predispose the urinary tract to microbial infections.
NORMAL ANATOMY AND PHYSIOLOGY OF THE URINARY TRACT
The mammalian urinary tract is a contiguous hollow-organ system whose primary function is to collect, transport, store, and expel urine periodically and in a highly coordinated fashion (1, 2). In so doing, the urinary tract ensures the elimination of metabolic products and toxic wastes generated in the kidneys. The process of constant urine flow in the upper urinary tract and intermittent elimination from the lower urinary tract also plays a crucially important part in cleansing the urinary tract, ridding it of microbes that might have already gained access (3). When not eliminating urine, the urinary tract acts effectively as a closed system, inaccessible to the microbes. Comprised, from proximal to distal, of renal papillae, renal pelvis, ureters, bladder, and urethra, each component of the urinary tract has distinct anatomic features and performs critical functions.
The Upper Urinary-Collecting System
The renal papilla, into which each renal tubule-rich pyramid drains, is considered the first gross structure of the upper collecting system. In humans and other higher mammals, renal papillae are individually cupped by a minor calyx, which in turn narrows into an infundibulum. Infundibuli vary in number, length, and diameter but consistently combine to form either 2 or 3 major calyces. These branches are termed upper, middle, and lower-pole calyces depending upon which pole of the kidney they drain. The renal pelvis represents the confluence of these major calyceal branches and itself can vary greatly in size and location (intra-renal vs extra-renal) (Fig. 1). It should be noted that, in rodents, there is only one renal papilla with a corresponding calyx.
FIGURE 1.
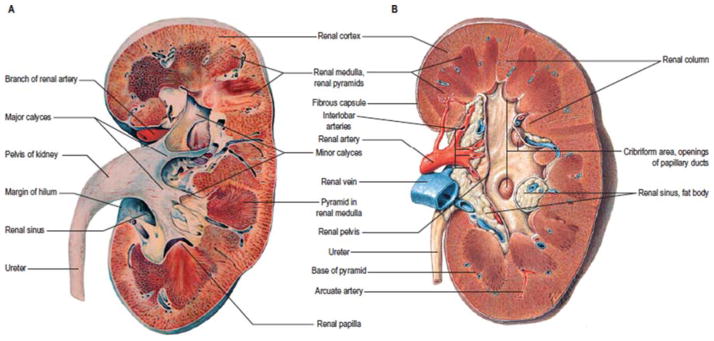
Normal anatomy of the kidney and upper urinary tract. (Reprinted from reference 163, Fig. 74.8, with permission of the publisher.) doi:10.1128/microbiolspec.UTI-0016-2012.f1
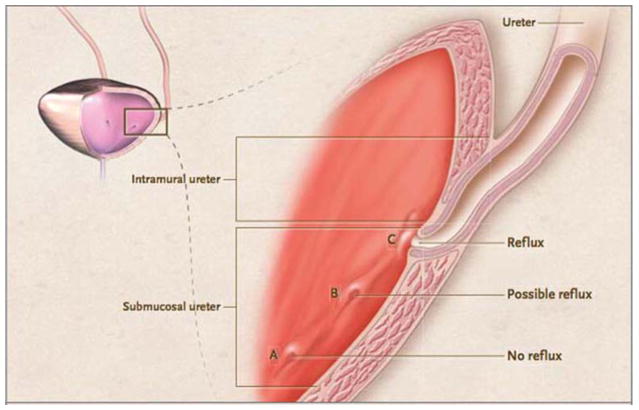
The ureters are bilateral fibromuscular tubes that drain urine from the renal pelvis to the bladder. They are generally 22–30 cm in length and course through the retroperitoneum. They originate at the ureteropelvic junction (UPJ) behind the renal artery and vein and then progress inferiorly along the anterior portion of the psoas muscle. As the ureters enter the pelvic cavity they turn medially and cross in front of the common iliac bifurcation. The ureters pierce the bladder wall obliquely (termed the ureterovesical junction or UVJ and travel in this orientation for 1.5 to 2.0 cm within the bladder wall to terminate in the bladder lumen as ureteral orifices (4). The intramural ureter is compressed by the bladder wall passively during storage and dynamically during emptying. This, in effect, prevents vesicoureteric reflux during steady state and micturition (Fig. 2). Along the length of the ureter there are three segments that physiologically narrow: the ureteropelvic junction, the ureterovesical junction, and where the ureters cross the common iliac vessels. These areas are clinically relevant as they represent the most common locations where ureteral calculi become trapped, causing obstruction.
FIGURE 2.
The ureterovesical junction. In this figure, A represents an orthotopic ureteral orifice. There is adequate length of ureteral tunnel in the bladder and therefore no reflux. Lateral and/or superior insertion of the ureteral orifice (B & C) can lead to inadequate submucosal ureter length and, potentially, reflux. (Reprinted from reference 162 with permission of the publisher.) doi:10.1128/microbiolspec.UTI-0016-2012.f2
Bladder and Urethra
The bladder is a hollow, distensible pelvic viscus that is tetrahedral when empty and ovoid when filled. It is composed primarily of smooth muscle and collagen and, to a much lesser degree, elastin (5). Its superior portion is defined by the urachus, a fibrous remnant of the allantois. The urachus attaches the bladder apex to the anterior abdominal wall. In males the bladder lies between the rectum and pubic symphysis and in females, between the rectum and uterus/vagina. Anterioinferiorly and laterally, the bladder is surrounded by retropubic and perivesical fat and connective tissue. This area is termed the space of Retzius. The trigone of the bladder is a triangular region of smooth muscle between the two ureteral orifices and the internal-urethral meatus. Grossly, thickened muscle between the ureteral orifices (interureteric crest) and between each ureteral orifice and the internal-urethral meatus (Bell’s muscle) distinguishes the trigone from the rest of the bladder. The classic view of bladder and trigone development proposes that the trigone originates from the mesoderm-derived Wolffian ducts and the remainder of the bladder is formed from the endoderm-derived urogenital sinus (6). Recent molecular developmental studies challenge this concept. Thus, the Wolffian ducts have been shown to undergo apoptosis during ureteral transposition and therefore do not contribute to trigone formation (7). Instead, a number of recent mouse models and tissue-transposition studies (7, 8), as well as in vitro studies of urothelial cells (9), suggest that the trigone is endodermal in origin. In males, the bladder base rests on the endopelvic fascia and the pelvic floor musculature, and the bladder neck is 3 to 4 cm behind the symphysis pubis and is fixed by the endopelvic fasciae and the prostate. Here, there is a layer of smooth muscle that surrounds the bladder neck and forms what is known as the involuntary internal-urethral sphincter. In females, the base of the bladder and urethra rest on the anterior wall of the vagina. The internal-urethral sphincter is not as well developed in females (10).
The urethra is contiguous with the bladder neck and begins at the distal end of the internal-urethral sphincter. In males the urethra is typically between 13 and 20 cm in length and is divided into prostatic, membranous, and penile portions. The prostatic urethra is 3–4 cm in length and runs vertically through the length of the prostate. The membranous urethra spans 2 to 2.5 cm from the apex of prostate to the perineal membrane. This portion of the urethra is completely surrounded by striated muscle known as the external-urethral sphincter. The penile portion of the urethra is contained within the corpus spongiosum. It is on average 15-cm long, it dilates slightly in the glans penis (fossa navicularis) and terminates at the external-urethral meatus. The female urethra, 3.8 to 5.1 cm long, is considerably shorter than the male one, and passes obliquely from the bladder neck to external-urethral meatus along the anterior vaginal wall. The distal two-thirds of the female urethra are invested by a slow-twitch striated muscle termed the external-urethral sphincter (10, 11).
Vagina
Although not part of the urinary tract, the vagina plays an integral role in UTI pathogenesis. It is a fibromuscular tube lined by epithelial cells. It extends from the opening of the labia minora (vestibule) to the uterus with its anterior wall approximately 7.5-cm long and its posterior wall approximately 9-cm long. The anterior wall is related to the bladder base superiorly and urethra inferiorly. Posteriorly, the vaginal wall is separated from the rectum by the recto-uterine pouch superiorly and Denonvillier’s fascia and the perineal body inferiorly. The inner vagina is covered by a non-keratinized, stratified, squamous epithelium. With the onset of puberty the vaginal epithelium thickens and its superficial cells accumulate glycogen. There are no mucous glands, but transudate from the underlying lamina propria and mucus from the cervical glands lubricate the vagina. The muscular layers are composed of smooth muscle found in both longitudinal and circular orientation (12). Normal vagina in reproductive women is populated by the lactobacilli which produces lactic acid, producing a low pH condition highly unfavorable for the growth and colonization by uropathogenic microbes (13). This constitutes one of the major host defenses, as alterations in vaginal flora are considered a key predisposing factor to UTIs.
Microscopic Anatomy and Physiology of the Urinary Tract
The luminal surface of the urinary tract, from minor calyx to prostatic urethra, is lined by a specialized epithelium known as urothelium. Although the term urothelium has been used to describe the epithelia covering the mucosal surfaces of the bulk of the urinary tract, which share the expression of a group of integral membrane proteins called uroplakins, recent data indicate that the urothelia of the ureters, bladder, and possibly other areas are distinguishable with respect to the detailed morphological and biochemical features, their in vitro proliferative behaviors when placed under identical tissue-culture conditions, and their embryological origin. For example, it has been shown that ureteral urothelium contains lower amounts of uroplakins and fewer cytoplasmic fusiform vesicles than bladder urothelium (9, 14, 15). It is now clear that these phenotypic differences are due to intrinsic divergence instead of extrinsic modulation (9). These findings are consistent with the fact that the renal pelvic and ureteral urothelia are derived from the mesoderm, whereas bladder and urethral urothelia are derived from the endoderm. It is therefore misleading to describe urothelial cells derived from various regions of the urinary tract as if they were all equal, or to study a particular type of urothelial cells and generalize that the results must be applicable to the urothelial cells from other zones of the urinary tract. To avoid confusion, it is always necessary to be explicit about the tissue origin of the urothelial cells, e.g., bladder-urothelial cells or ureteral-urothelial cells (16).
The most extensively studied urothelium is that of the bladder. This epithelium performs important biological functions, including the formation of a physically stable apical surface and a highly effective permeability barrier, even as the surface area of the urinary tract undergoes dramatic changes during different phases of the micturition cycle. These attributes are thought to be a function of slow urothelial-cell turnover (~200 days) (16, 17) and the elaboration of the uroplakin-containing urothelial plaques and highly efficient tight junctions. The superficial urothelium consists of a single layer of large, multinucleated, and highly differentiated ‘umbrella’ cells. Umbrella cells accumulate a large amount of uroplakin proteins that form urothelial plaques. These plaques cover approximately 90% of the apical/luminal surface and are also present in high concentrations in association with the cytoplasmic fusiform vesicles (18, 19). Urothelial plaques are essentially two-dimensional crystals of hexagonally packed 16-nm uroplakin (UP) proteins (18, 20–23). There are four major UPs: Ia, Ib, II, and IIIa and one minor UP: IIIb (24–27). UPIa/II and UPIb/IIIa (or IIIb) heterodimer formation is necessary before these proteins can exit the endoplasmic reticulum and eventually form 16-nm particles and then plaques (19, 24, 28, 29). UP-knockout studies reveal that uroplakins are critical to the formation of urothelial plaques, formation of a normal UVJ, and a normal permeability barrier function (30–32). The cytoplasmic fusiform vesicles rich in urothelial plaques are thought to play a major role in delivering the uroplakin plaques to the apical surface (33, 34) (Fig. 3).
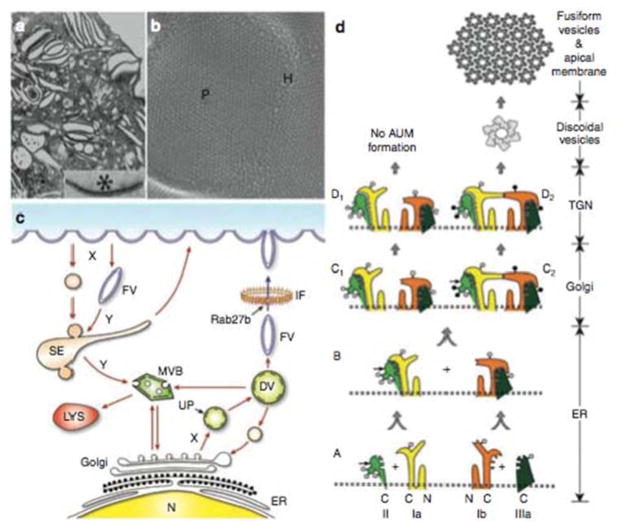
FIGURE 3.
Assembly, intracelluar trafficking and structure of uroplakins. (a) Luminal portion of a superficial umbrella cell of mouse urothelium visualized by transmission electron microscopy (inset: an urothelial plaque exhibiting asymmetric unit membrane or AUM). (b) Quick-freeze deep-etch showing 16-nm uroplakin particles arranged in hexagonal arrays comprising the urothelial plaques (P) interconnected by particle-free hinges (H). (c) Vesicular trafficking in umbrella cells. Uroplakin heterodimer formation takes place in the endoplasmic reticulum (ER) and undergoes modification in the Golgi apparatus. Assembled uroplakins then amass in small vesicles and bud off the trans-Golgi network (TGN), forming discoidal vesicles (DVs). The next- stage, fusiform vesicles (FVs) pass through an intermediate-filament (IF) network and ultimately fuse with the apical membrane, a process mediated by Rab27b. Apical plaque-associated UPs are internalized via endocytic pathways and/or modified FVs that form sorting endosomes (SE) and multivesicular bodies (MVB), which merge with lysosomes (LYS) for degradation. (d) A hypothetical model of uroplakin assembly into 2-D crystals. Stages A and B: The four major uroplakins (UPIa, Ib, II, and IIIa) are modified with high-mannose glycans in the ER and hetero- dimerize forming UPIa/II and UPIb/IIIa and undergo major conformational changes. Symbols: the small, horizontal arrows on UPII denote the furin cleavage site at the end of the prosequence; the open and closed circles denote high-mannose and complex glycans, respectively. With in vivo urothelium (pathway on the right), the glycans on two of the three N-glycosylation sites on the prosequence of UPII become complex glycans in the TGN (stage C2), and the cleavage of the prosequence by furin in the TGN (stage D2) then triggers oligomerization to form a 16-nm particle. In cultured urothelial cells (pathway on the left), the differentiation-dependent glycosylation of pro-UPII is defective, preventing the formation of the uroplakin heterotetramer and the 16-nm particle, thus the lack of asymmetric-unit membrane. (Reprinted and adapted from reference 16 with permission of the publisher.) doi:10.1128/microbiolspec.UTI-0016-2012.f3
Uroplakins appear to play a major role during the pathogenesis of urinary tract infections.
UPIa presents a high level of terminally exposed, unmodified mannose residues and has been identified as the sole urothelial receptor to interact with the FimH lectin of the type 1-fimbriated uropathogenic E. coli (UPEC) (35–37). In addition to the bladder, UPIa has been found on the mucosal surfaces of the ureters, renal pelvis, and major and minor calyces (9, 14). It has been proposed that interaction of the FimH adhesin of type 1-fimbriated UPEC with UPIa at these locations help bacteria resist the flow of urine and, coupled with bacterial flagella formation, may facilitate the ascent of bacteria from the bladder into the upper urinary tract (16, 37).
Although both FimH and flagella are known to exhibit phase variation (38, 39), the temporal expression of these virulence factors in relation to the ascent of UPEC along the urinary tract has not been established. UPEC that cause pyelonephritis typically express P fimbriae in addition to type-1 fimbriae (40). Once bacteria reach the kidney, the P fimbriae interact with glycolipids in the renal tubular cells removing the need for type-1 fimbriae/UPIa interaction. UPIIIa has also been shown to be important in UTI pathogenesis. Thumbikat et al. demonstrated that the phosphorylation of UPIIIa’s cytoplasmic tail is a critical step in urothelial signaling associated with bacterial invasion and host-cell apoptosis (41).
The intermediate and basal layers of the urothelium contain smaller, less well- differentiated epithelial cells. It is within the basal-cell layer that urothelial stem cells are believed to reside (16, 42, 43). The intermediate and basal layers may service as a reservoir for rapid umbrella-cell regeneration.
Different urothelial layers not only differ in morphology, proliferative potential, and degree of differentiation, they also seem have divergent abilities to support intracellular bacterial growth and propagation. For instance, intracellular bacterial communities (IBCs) of the UPEC strains are found almost exclusively in the urothelial umbrella-cell layer, but not in intermediate and basal layers (44). The cells in the latter two layers, however, can harbor so-called quiescent intracellular reservoirs (QIRs), a possible source for recurrent UTIs. Whether differences in the intracellular architecture, in particular vesicular trafficking, e.g., endocytic and exocytic machineries, between various urothelial-cell layers (45–48) are responsible for the observed differences in bacterial growth remains to be seen.
NORMAL URINE TRANSPORT AND MICTURITION
Urine production is a function of both renal-glomerular filtration and tubular reabsorption and is tightly regulated by systemic hydration state and electrolyte balance. Urinary filtrate is passed through the nephron as it winds through the cortex and medulla and is concentrated via a counter-current mechanism. Urine exits the kidney at the renal papillae and is transported through the upper collecting system. The smooth muscle surrounding the calyces, renal pelvis, and ureters is of the syncytial type without discrete neuromuscular junctions. Instead, smooth-muscle excitation is spread from one muscle cell to the next. In humans, atypical smooth-muscle cells, located near the pelvicalyceal border, are thought to act as the pacemakers of urinary-tract peristalsis (49, 50). These cells initiate unidirectional peristaltic contractions which, in turn, promote the forward flow of urine. Recently, Hurato et al. demonstrated that disruption of the pelvicalyceal region from the more distal urinary-tract segments prevented downstream peristalsis. Furthermore, hyperpolarization-activated cation-3 (HCN3), an isoform of a channel family known for initiating electrical activity in the brain and heart, was isolated in the same spatial distribution as the atypical smooth-muscle cells of the pelvicalyceal junction. Inhibition of this channel protein caused a loss of electrical activity in the pelvicalyceal junction and led to randomized electrical activity and loss of coordinated peristalsis (51). Whether HCN3-positive cells are the same as the atypical smooth muscle remains to be seen. Normal ureteral contractions occur two to six times per minute and it is the advancing contraction wave that forces the urine bolus down the length of the ureters and then into the bladder (52). Some uropathogenic bacteria appear to have evolved a way to overcome the normally protective forward flow of urine that results from the peristaltic ureteral contractions. Recent studies demonstrated that most UPEC have the ability to impair ureteric contractility via a calcium-dependent mechanism and that mechanism is dependent upon FimH-urothelial interaction (53, 54).
The micturition cycle is best thought of as two distinct phases: urine storage/bladder filling and voiding/bladder emptying (55). The viscoelastic properties of the bladder allow for increases in bladder volume with little change in detrusor or intravesical pressures. Additionally, during bladder filling, spinal sympathetic reflexes (T12–L2) are activated that, through modulation of parasympathetic-ganglionic transmission, inhibit bladder contractions and increase bladder-outlet resistance via smooth-muscle activation (56). Bladder-outlet resistance also increases during filling secondary to increased external urethral-sphincter activity via a spinalsomatic reflex (guarding reflex) (57). As the bladder reaches its capacity, afferent activity from tension, volume, and nociceptive receptors are conveyed via Aδ and C fibers through the pelvic and pudenal nerves to the sacral spinal cord (56). Afferent signals ascend in the spinal cord to the pontine micturition center in the rostral brainstem. Here signals are processed under the strong influence of the cerebral cortex and other areas of the brain. If voiding is deemed appropriate, the voiding/bladder-emptying reflex is initiated. The pattern of efferent activity that follows is completely reversed, producing sacral parasympathetic outflow and inhibition of sympathetic and somatic pathways. First the external urethral-sphincter relaxes and shortly thereafter a coordinated contraction of the bladder causes the expulsion of urine (56, 58) (Fig. 4).
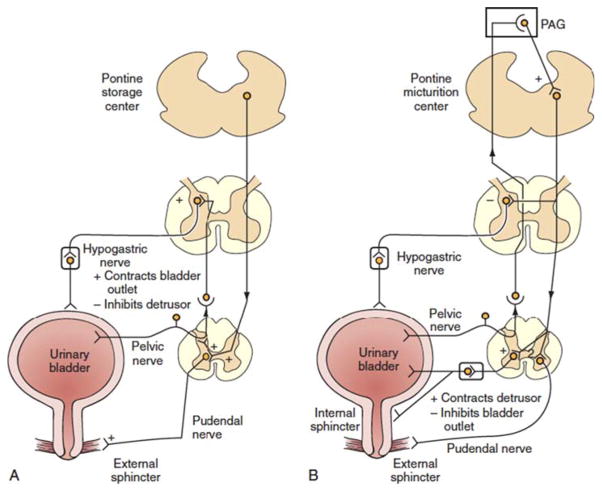
FIGURE 4.
Mechanism of storage and voiding. A. Storage of urine. Low-level bladder afferent firing, secondary to bladder distension, increases sympathetic outflow to the bladder outlet and external urethral sphincter (‘guarding reflex’). Sympathetic signaling also acts to inhibit detrusor-muscle contractions. B. Voiding. At bladder capacity, high-level bladder afferent activity activates the pontine-micturition center. This, in turn, inhibits the guarding reflex. The activated pontine-micturition center, under appropriate conditions, will lead to parasympathetic outflow to the bladder and internal-sphincter smooth muscle. Urinary sphincter relaxation is soon followed by a large, coordinated detrusor contraction leading to expulsion of urine from the bladder. (Reprinted and adapted from reference 58 with permission of the publisher.) doi:10.1128/microbiolspec.UTI-0016-2012.f4
The forward flow of urine is imperative to the maintenance of a healthy urinary tract. Any structural or functional process that impedes the flow of urine has the potential to promote urine stasis, hence UTI pathogenesis. In the next few sections, we will elaborate upon those anatomic and physiologic abnormalities that can affect either storage or emptying of urine and, in turn, promote UTI pathogenesis.
ANATOMIC ABNORMALITIES
Medullary Sponge Kidney
Medullary sponge kidney (MSK) is a renal disorder that is characterized by distal collecting-duct dilatation and multiple cysts and diverticula within the renal medullary pyramids. It is associated with a higher risk of nephrocalcinosis, urolithiasis, renal failure, and UTI (59–61). The prevalence of this disorder in the general population is unknown. However, a large series of intravenous pyelograms (IVPs), performed for any reason, revealed radiologic signs of MSK in 0.5% to 1% (62). The incidence of MSK in individuals who are known to form urolithiasis is higher, ranging from 2.6% to 12% (61, 63, 64). Clinical presentations of MSK include renal colic (51.8%), UTI (7.1%), and/or gross hematuria (16.1%) (64). MSK is diagnosed radiographically and has traditionally been accomplished via IVP. Pathognomic features on IVP include elongated ectatic papillary tubules, papillary contrast blush, and persistent medullary opacification which, taken together, give a ‘bouquet of flowers’ appearance. Today, IVPs have been replaced in favor of ultrasonography, computed tomography, and magnetic-resonance imaging. MSK can be diagnosed with these imaging modalities but with much less sensitivity (65–67). MSK was once thought to be an isolated congenital abnormality. However, there is increasing data that links MSK with other malformative disorders such as hemihypertrophy, Beckwith-Wiedemann syndrome, congenital dilatation of intrahepatic bile ducts, and hepatic fibrosis, and autosomal-dominant polycystic-kidney disease (68–70). This has led some to suggest that MSK is a developmental disorder of renal embryogenesis. Gambaro et al. have hypothesized that MSK may be a consequence of disruption of the ureteral-bud/metanephric-blastema interface, which is critical to normal renal and ureteric development (71).
The increased risk of UTI in MSK patients has not been systematically studied. One might hypothesize that the increased risk of UTI could be due to urinary stasis within the ectatic collecting ducts, renal dysfunction, formation of urolithiasis, or any combination of the above. A better understanding of UTI pathogenesis in these patients is needed.
Calyceal Diverticula
A calyceal diverticulum is a congenital, urothelium-lined cavity within the renal parenchyma. They are relatively uncommon, occurring in 0.21% to 0.45% of people undergoing renal imaging (72). Diverticula development is believed to be due to failure of small ureteral bud regression (73). The majority of diverticula are unilateral, less than 1 cm in diameter, and within the posterior aspect of the upper collecting system (74). Diverticula are distributed in the upper (70%), lower (18%), and mid (12%) calyx. Urine moves passively in a retrograde manner through narrow infundibulum to fill the diverticulum. This pooling of urine within the diverticulum predisposes to calculi (9.5% to 39%) and recurrent urinary tract infections (25%) (73, 75). Additionally, obstruction at the diverticular neck can lead to rupture and hemorrhage, abscess formation, and potentially life-threatening sepsis. If symptomatic, a percutaneous approach to diverticulum ablation (+/− stone removal) is preferred.
Ureteral Obstruction
There are a number of intrinsic and extrinsic causes of ureteral obstruction (Table 1). Obstruction of the ureters can cause urinary stasis and, in severe cases, renal dysfunction; both of which are risk factors for UTI. Urinary stasis is believed to prolong the time for which bacteria can adhere to and invade the urothelium, whereas renal dysfunction prevents adequate concentration of antibiotics in the urine (76). Hematogenous infection of the kidney, a rare entity under normal conditions, is increased with ureteral obstruction (77). Transient ureteral obstruction, followed by E.coli infection of the lower urinary tract, has also been shown to predispose to ascending pyelonephritis in rats (78). However, the mechanisms underlying ureteral obstruction and increased ascending infection are not well understood. It has been posed that the release of ureteral obstruction alters urodynamics (i.e., secondary VUR) and may delay antegrade emptying, which acts to promote ascending infection (78). It has also been suggested that obstruction causes papillary necrosis and that sloughed papilla may act as a nidus for infection (76). Other intrinsic causes (Table 1) for ureteral obstruction may also act as a nidus for recurrent UTI. For example, urinary calculi can provide a surface for bacteria to adhere and proliferate upon.
TABLE 1.
Anatomic causes of ureteral obstruction
| Intrinsic | Extrinsic |
|---|---|
| Calculi | Retroperitoneal fibrosis |
| Sloughed renal papillae trauma | Neoplasm |
Congenital
|
Pregnancy Pelvic lipomatosis Aortic aneurysm Abscess Lymphocele Urinoma |
Neoplasm
|
|
Inflammatory
|
Ureters may become obstructed secondary to ectopic insertion. In females, ectopic insertion can occur anywhere from the bladder neck to the perineum including the vagina, uterus, and rectum. This typically leads to incontinence. In males, the ectopic ureter may insert anywhere in the urogenital system above the external sphincter. Insertion can occur in the vas deferens, seminal vesicles, or ejaculatory ducts. Because insertion is above the external sphincter it does not cause incontinence, but it can be associated with infection. In a duplicated urinary tract the upper-renal moiety is associated with an ectopic ureter and this is thought to occur secondary to late ureteral budding from the mesonephric duct. The ureter subsequently inserts medial and inferior to its normal orthotopic position in the trigone. As a consequence of this abnormal insertion, the ureter must travel obliquely for greater length through the bladder and therefore can become obstructed (79).
Primary Vesicoureteric Reflux
Primary VUR is defined by the retrograde flow of urine, from bladder to the upper urinary tract, in the absence of obvious pathogenic cause. It occurs in approximately 1% of the general population and is responsible for 12% of antenatally detected hydronephrosis (80, 81). Children with normal perinatal ultrasonography who develop UTI have VUR 37.4% of the time (82). VUR is associated with recurrent UTIs, renal malformations, hypertension, and renal scarring and impairment known as reflux nephropathy (83). Primary VUR represents a congenital defect in which the structure, and therefore function, of the UVJ is compromised. The length of intravesical ureter has been shown to be critical for the normal anti-reflux mechanism of UVJ (84, 85). Children with VUR were found to have a tunnel length-to-diameter ratio of 1.4:1 compared to 5:1 ratio in non-VUR children (85). VUR appears to be hereditary as VUR is present in 32% of siblings and 66% of offspring of known VUR patients (86, 87). Mouse models have confirmed that mutations in some of the genes expressed by the developing kidney and urinary tract can cause VUR (88, 89). Additionally, UP II and UP IIIa knockout mice been shown to have severe VUR (30, 32). Linkages between human VUR and genes involved in renal/urinary tract development have also been shown. Specifically, ACE, AGTR2, and RET polymorphisms have been positively associated with VUR (90–93). However, newer data do not support some of these findings (94, 95). Case-control studies of UPII and UPIIIa have failed to show significant association between single-nucleotide polymorphisms and VUR (96, 97). Jiang et al. genotyped all four UP genes in a population of 76 VUR patients. Of the 18 single-nucleotide polymorphisms identified, only 2 had a weak association with VUR. These data suggest that missense changes of UP genes cannot play a dominant role in causing VUR in humans (98). It has been speculated that major UP mutations are not compatible with human life (16). A large clinical and DNA database has recently been established from families containing sibling pairs with documented VUR (99). This will be an important resource for researchers and will hopefully bring light to the genetic components predisposing to VUR and reflux nephropathy.
Bladder-Outlet Obstruction
Bladder-outlet obstruction (BOO) in men can develop for a number of reasons including bladder calculi, medications, prostate cancer, urethral scarring, and benign prostatic hyperplasia (BPH). While most causes for BOO are relatively uncommon, BPH will develop in nearly all men by the age of 80 (100). Histologically, BPH represents a variable proliferative process of the stromal and epithelial elements of the periurethral and transition zones of the prostate (101). The pathophysiology of BOO in men with BPH is thought to be both static, due to physical blockage of the urethra and bladder outlet, and dynamically related to smooth-muscle tension. Interestingly, prostate volume does not seem to be an important determinant of BPH symptom severity (102, 103). Instead, the proportion of prostatic smooth muscle in the inner-transition zone of prostate appears to be an important determinant of clinical BPH (104). The molecular pathogenesis of BPH is not well understood. A number of growth factors have been associated with BPH, including fibroblast growth factor 2 and insulin growth factor. A number of cytokines and inflammatory mediators are also upregulated in BPH including interleukin (IL)-1α, −2, −8, −15, and −17 and nuclear-factor κB. Madigan et al. compared molecular differences between symptomatic and asymptomatic BPH, and found 4 genes involved in the innate antiviral immune response to be upregulated in symptomatic BPH: CFI, OAS2, APOBEC3G, and IFIT1 (105). A causal relationship between BPH/BOO and UTI risk has been difficult to establish as there is little data pertaining to this area. However, it is generally thought that urinary retention secondary to BOO increases UTI risk. Chronic urinary retention is defined as a non-painful bladder that remains palpable or percussable after urination and implies a significant residual volume of urine greater than 300 ml (106). Chronic urinary retention is a relatively uncommon finding in the general population, but incidence increases dramatically with age, lower urinary tract symptom severity, and prostate volume (107).
Abnormal Pelvic Anatomy
Although not extensively studied, differences in pelvic anatomy may predispose UTI. A single case-control study of 213 young women assessed perineal measurements and urethral length in those with and without a history of recurrent UTI (rUTI). In women not using spermicidals, and after controlling for sexual intercourse frequency, the urethra-to-anus distance and posterior fourchette-to-anus distance were found to be significantly shorter in those with a history of rUTI (4.8 vs 5.0 cm, P = .03 and 2.6 vs 2.8 cm, P = .04, respectively). This difference did not exist between groups using spermicidal products. Urethral length was measured with the aid of a urethral catheter and was not associated with a statistically significant increased risk of rUTI (3.6 vs 3.5 cm, P = .41). The authors concluded that perineal and urethral anatomy are likely important in the absence of other risk factors for rUTI (108).
Aging Female
The prevalence of UTI has been shown to increase with age (109). Anatomic and functional theories to explain this phenomenon include bladder dysfunction, pelvicorgan prolapse, urinary and fecal incontinence, and changes in estrogen status.
When healthy postmenopausal women with a history of rUTI were compared to non- recurrent controls, three strong risk factors emerged: incontinence (41% cases vs 9% controls; P < .001), pelvic-organ prolapse (cystocele) (19% vs 0%; P < .001) and postvoid residual (28% vs 2%; P < .001). Urinary incontinence was most strongly associated with rUTI in multivariate analysis (odds ratio [OR] 5.79; 95% confidence interval [CI] 2.05–16.42) (110).
There are two main theories that strive to explain normal female urinary continence. In the integral theory, proposed by Petros and Ulmsten, the urethra is closed from behind via the pelvic-floor muscles and their ability to stretch the vaginal hammock against the pubourethral ligaments (111). With this theory, weakness in the pubourethral ligaments predisposes to stress urinary incontinence (112). Delancey has suggested an alternate theory for female urinary continence called the hammock theory. This theory proposes that both urethral support and constriction are important and depend upon support from the anterior vaginal wall, the endopelvic fascia between the arcus tendineus facia pelvis, and the pelvic-floor muscles. Weakening or attenuation of these supports can therefore predispose to incontinence (113). It is unclear exactly how incontinence predisposes to rUTI. Anti-incontinence procedures may cause obstruction and urinary retention, increasing the risk of UTI. It is also conceivable that a continuously damp perineum and introitus may facilitate uropathogen colonization and ascent into the urinary tract leading to rUTI.
Anterior vaginal-wall prolapse (cystocele) is defined as pathologic descent of the anterior vaginal wall and overlying bladder base. The etiology of anterior vaginal prolapse is likely multifactorial and the result of damage or impairment of the pelvic muscles and/or connective tissues that normally provide support. Nitti et al. have shown that cystocele is an important cause of bladder-outlet obstruction and incomplete bladder emptying in females (114).
With the decline of circulating estrogen that accompanies menopause, physiologic and structural changes can occur to the vulvovaginal epithelium. In the low-estrogen state, the normally predominant lactobacilli diminish due to decreased vaginal-epithelial glycogen. Lactobacilli, via anaerobic metabolism of glycogen, normally produce lactic acid and hydrogen peroxide. These are both essential in maintaining an acidic and hostile vaginal environment to E. coli and other potentially uropathogenic organisms (115).
PHYSIOLOGIC ABNORMALITIES
Diabetes Mellitus
Individuals with diabetes mellitus are at a high risk for UTI and rUTIs. Epidemiologic studies show a 1.2 to 2.2-fold increase in the relative risk of UTI in diabetics when compared with non-diabetics (116–118). However, the underlying mechanisms that increase UTI susceptibility in diabetes are not completely understood. Hyperglycosuria has long been held as a causative factor in diabetic UTI pathogenesis with the theory being that higher glucose levels promote bacterial growth. However, there are no studies to date that clearly demonstrate a direct relationship between elevated urinary glucose and increased UTI risk. Clinical studies have also demonstrated that there is no dose-response relationship between serum HbA1c levels and UTI risk (119–121). One ex vivo study found that type-1 fimbriated E. coli adhered more to urothelial cells of diabetic women than non-diabetic controls; it was hypothesized that increased glycosylation of UPs might explain this finding(122). Abnormal glycosylation might also affect soluble glycoproteins in the urine, making them less effective competitive inhibitors for the type-1 fimbriae (123), which could in turn increase the adherence of UPEC to the urothelial surface. Diabetic cystopathy (DC) is a condition marked by the insidious onset of impaired bladder sensation, decreased detrusor contractility, increased post-void residual volume and, in severe cases, detrusor areflexia. Voiding dysfunction and urinary retention can lead to decreased clearance of bacteria via micturition and therefore predispose diabetic patients to UTI. Traditionally, autonomic neuropathy was felt to be the pathophysiological cause of DC (124). A more contemporary view of DC centers on a multifactorial etiology including alterations in detrusor muscle, urethra, autonomic nerves, and urothelium secondary to oxidative stress and hyperglycemia-induced polyuria (125, 126).
Neutrophil and lymphocyte dysfunction generally found in diabetics may also impair the ability to clear bacteria from the urinary tract in the later stages of infection. Using a streptozocin-induced diabetic-mouse model, Rosen et al. provided evidence to support this theory. These authors were able to show that E. coli titers of diabetic C3H/HeJ mouse kidneys and bladders were 10,000-fold higher than non-diabetic C3H/HeJ controls (127).
Pregnancy
The prevalence of bacteruria in pregnancy is similar to non-gravid females and ranges from 2% to 10%. However, 25% to 35% of pregnant woman with bacteruria will progress to pyelonephritis (128, 129). Screening and treatment of bacteruria in this population is recommended to prevent pyelonephritis and its associated adverse perinatal outcomes such as prematurity and/or low birth weight (128, 129). Physiologic and anatomic changes in pregnancy occur that lead to urinary stasis, secondary vesicoureteric reflux, and increased risk of UTI.
Hydronephrosis develops in the majority of pregnant women and is present in 15% during the first trimester, 20% in the second trimester, and 50% in the third trimester (130). The finding of hydronephrosis in the first trimester, before the gravid uterus reaches the pelvic brim to cause obstruction, supports a hormonal etiology. Progesterone has been hypothesized to promote ureteral dilation and therefore development of hydronephrosis (131). Hydronephrosis of pregnancy may also be mechanical. The gravid uterus after the 20th week of gestation may extrinsically compress the ureters. This idea is supported by both the increased incidence and degree of hydronephrosis after 20-weeks’ gestation. Further support comes from the finding that the right collecting system is dilated two to three times more commonly then the left and that the gravid uterus is typically dextrorotated (130).
Ureteropelvic-Junction Obstruction
Ureteropelvic-junction obstruction (UPJO) is the most common cause of obstructive nephropathy in children with an estimated incidence of 1 in 1,000–1,500 (132). The widespread use of prenatal screening ultrasonography has led to the early detection of hydronephrosis and, subsequently, the majority of UPJO are diagnosed in the neonatal and infant period. However, UPJO can present at any age with symptoms including flank pain, episodic upper abdominal pain (Dietl’s crisis), renal failure, hematuria, and recurrent UTI. UPJO is generally considered a functional abnormality. Accumulating evidence suggests that abnormal smooth-muscle development and differentiation in the renal pelvis and proximal ureter is responsible for creating an aperistaltic segment at the level of the UPJ. Analyses of human UPJ tissues obtained at the time of surgical correction for UPJO (pyeloplasty) have demonstrated decreased neuronal innervation, significant fibrosis, and abnormal smooth-muscle cell arrangement (133, 134).
Murine models of UPJO have also provided valuable insight into UPJO pathogenesis. Mouse lines harboring conditional knockouts of sonic hedgehog, ADAMTS-1, and calcineurin B have smooth-muscle deficiency in the renal pelvis and ureters and display phenotypes similar to human UPJO (132, 135, 136). Further studies of the genetic and cellular defects underlying UPJO are needed. In some cases of UPJO, an aberrant renal vessel is observed crossing the UPJ. Whether or not crossing vessels alone can cause extrinsic compression sufficient for UPJO remains unclear (137). Recent clinical studies demonstrate that the rate of UTI in children with high-grade UPJO is low (138, 139). Therefore, most experts do not recommend antibiotic prophylaxis in this population. However, rUTIs in the context of UPJO and urinary stasis are generally considered an indication for either antibiotic prophylaxis and/or surgical correction.
Dysfunctional Voiding
Dysfunctional voiding (DV) is characterized by an intermittent and/or fluctuating flow rate due to involuntary intermittent contractions of the peri-urethral striated or levator muscles during voiding in neurologically normal individuals (140). The prevalence of adult DV in the general population is unknown. In patients referred for urodynamic evaluation, Jorgensen et al. found DV prevalence rate of 0.5% (141). Groutz et al. retrospectively reviewed the videourodynamics of 1,015 consecutive adults and found that 2% met their criteria for DV (142). These figures likely underestimate the true prevalence of this condition. Of the 21 patients who met videourodynamic criteria for DV in the Groutz study, 14 were asked about childhood voiding. All 14 denied any form of dysfunctional voiding as a child (142). This clearly challenges the previously held notion that all adult DV stems from behavior learned and carried over from childhood. In fact, there are data that now suggest that DV can be learned in adulthood. Using urodynamic criteria of obstruction (maximum flow rate ≤12 ml/sec and detrusor pressure at maximum flow ≥25 cm H20), Cameron et al. demonstrated bladder-outlet obstruction in 48.1% of women with interstitial cystitis/bladder-pain syndrome. Although electromyograph (EMG) data was not reported, the authors postulated that painful voiding leads to pelvic-floor spasticity and subsequent DV (143). DV has also been shown to have an increased incidence in sexual-abuse victims and has been linked to the exposure of psychological stressors (144, 145). The goal of DV treatment is to facilitate a patient’s return back to normal micturition. To achieve this any combination of behavioral, cognitive, and pharmacologic therapies can be utilized. Recurrent UTIs may occur in up to 42% of women with DV (146). Minardi et al. have recently demonstrated that in women with both DV and recurrent UTI, pelvic-floor physiotherapy can significantly reduce the rate of UTI recurrence (147).
Primary Bladder-Neck Obstruction
Primary bladder-neck obstruction (PBNO) is a condition in which the flow of urine is obstructed due to incomplete bladder-neck opening. Diagnostic features of PBNO include high-voiding pressures and low urine flow (maximum detrusor pressure >20 cm H20 and maximum urine flow <12 mL/s), fluoroscopic absence of bladder-neck opening/funneling and minimal EMG activity during volitional voiding. Recently, Brucker et al. evaluated urodynamic differences between DV and PBNO. Patients with DV had a higher mean Qmax (12 ml/s vs 7 ml/s; P = 1.27) and a lower mean post-voiding residual (PVR) (125 ml vs 400 ml; P = .012) when compared to PBNO. In their analysis, the authors found that EMG alone would have led to misdiagnosis in 20% of DV and 14.3% of PBNO patients. This data supports the use of fluoroscopy in diagnosing and differentiating DV and PBNO (148). It has been theorized that the etiology of PBNO is either morphologic (smooth-muscle hypertrophy or fibrosis) or neurogenic in nature (149). Between 8.7% and 16% of women presenting with bladder-outlet obstruction will have PBNO as their underlying cause (114, 150). Treatment options for PBNO include observation, pharmacotherapy, and surgical intervention. Obstruction of urine flow at the bladder neck can lead to elevated PVR and presumably increase the risk of UTI. However, UTI incidence in patients with PBNO has not been systematically studied.
Neurologic Patients
Individuals with suprasacral and subpontine spinal-cord injuries develop bladder overactivity and involuntary contractions of the external urinary sphincter. The resulting loss of coordinated micturition, termed detrusor-external sphincter dyssynergia (DESD), can lead to dangerously high storage pressures and the development of serious urologic and nephrologic complications including secondary VUR, incomplete bladder emptying, bacteriuria, and recurrent UTI. Ideally, a combination of clean intermittent catheterization (CIC) and anticholinergic medications are used to reduce intravesical storage pressures and allow for bladder emptying (151). Alternative treatments may be needed for those who cannot tolerate anticholinergic agents or cannot reliably catheterize (e.g., quadriplegic or non-compliant patients).
External sphincterotomy has historically been considered the next best alternative to CIC/anticholinergic therapy for DESD. However, it is now recognized that this technique is associated with a high complication rate (151, 152). Urethral stenting, sphincter-injected botulinum toxin A and, in refractory cases, urinary diversion can also be considered for the treatment of DESD (153, 154).
Other Physiological Defects
It has long been speculated that genetic modifiers may play a role in influencing host susceptibility to recurrent UTIs. For instance, the female relatives of women with recurrent UTIs are more prone to UTIs than the general population (155). The severity and disease course in experimental animals, particularly mice, can also be dependent on different strains and genetic backgrounds under study (156). The availability of knockout mice deficient for functionally divergent genes with convergent UTI phenotype is suggestive of a polygenic nature of genetic predisposition to UTIs. Thus far, knockout-mouse strains lacking/deficient for toll-like receptors (e.g., TLR2, 4, and 11), anti-microbial peptides (e.g., defensins and cathelicidin), anti-bacterial adherence factor (e.g., Tamm-Horsfall protein), and growth factors (e.g., transforming growth factor [TGF]-β1 and vascular endothelial growth factor [VEGF]) have been found to have increased spontaneous or experimentally induced UTIs (155,157–162). Interestingly, single-nucleotide polymorphisms (SNPs) of some these genes have also been identified in humans. As reviewed by Zaffanello and colleagues, most of these gene SNPs are actually associated with developmental anomalies of the urinary tract, including urinary-tract malformation and VUR (155). Perhaps not surprisingly, there is evidence that other genes involved in the innate and adaptive immunity can also alter host defense and hence its susceptibility to UTIs. The effects of these genes in the global host responses to microbial infections in multi-organ systems should be distinguished from those only affecting the urinary tract. Such a distinction is not only important for investigative purposes, but also for clinical decision making on whether management strategies should be global or local.
SUMMARY AND PERSPECTIVE
The ability of the urinary tract to defend against microbial infections relies on its normal anatomic architecture as well as a functional physiological state. Defects in either or both aspects, whether they be congenital or acquired, can increase the access, retention, and spread, and the decreased clearance of microbes from the urinary tract, leading to microbial infections and even recurrent episodes. A thorough understanding of anatomy and physiology of the urinary tract is therefore necessary for those who investigate and those who diagnose and treat urinary tract infections. An appreciation of the normal architecture and functions of the upper and lower collecting systems and external genitalia is also required before one can hope to identify and/or systematically study abnormalities that may predispose to UTIs. While the pathogenic processes behind anatomic abnormality-associated UTIs, such as obstruction and reflux, are well understood, those behind the physiological risk factors that predispose to uncomplicated and recurrent UTIs in anatomically normal individuals are much less clear, and this is an area that clearly requires much greater research efforts. Fundamental biological and biochemical studies, genetic analyses, gene targeting with genetically engineered animals, and gene-wide association studies in humans could be instrumental in further advancing our knowledge regarding the physiological functions of the urinary tract and its disease pathogenesis. Successful translation of such knowledge to the bedside should in turn help more effectively treat UTI and/or reduce its recurrence.
Footnotes
Conflicts of interest: We declare no conflicts.
References
- 1.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elbadawi A. Functional anatomy of the organs of micturition. Urol Clin North Am. 1996;23:177–210. doi: 10.1016/s0094-0143(05)70304-9. [DOI] [PubMed] [Google Scholar]
- 3.O’Grady F, Cattell WR. Kinetics of urinary tract infection. II. The bladder. Br J Urol. 1966;38:156–162. doi: 10.1111/j.1464-410x.1966.tb09694.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JK, Cadeddu JA. Surgical anatomy of the retroperitoneum, adrenals, kidneys, and ureters. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10. Saunders Elsevier; Philadelphia: 2012. pp. 3–6. [Google Scholar]
- 5.Macarak EJ, Howard PS. The role of collagen in bladder filling. Adv Exp Med Biol. 1999;462:215–223. doi: 10.1007/978-1-4615-4737-2_17. [DOI] [PubMed] [Google Scholar]
- 6.Tanagho ER, Pugh RC. The anatomy and function of the ureterovesical junction. Br J Urol. 1963;35:151–165. doi: 10.1111/j.1464-410x.1963.tb02610.x. [DOI] [PubMed] [Google Scholar]
- 7.Viana R, Batourina E, Huang H, Dressler GR, Kobayashi A, Behringer RR, Shapiro E, Hensle T, Lambert S, Mendelsohn C. The development of the bladder trigone, the center of the anti-reflux mechanism. Development. 2007;134:3763–3769. doi: 10.1242/dev.011270. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka ST, Ishii K, Demarco RT, Pope JC, IV, Brock JW, III, Hayward SW. Endodermal origin of bladder trigone inferred from mesenchymal-epithelial interaction. J Urol. 2010;183:386–391. doi: 10.1016/j.juro.2009.08.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng F-M, Wu XT, Shapiro E, Sun TT. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol. 2005;171:835–844. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung BI, Sommer GDBJ. Anatomy of the lower urinary tract and male genitalia. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10. Saunders Elsevier; Philadelphia: 2012. pp. 59–60. [Google Scholar]
- 11.Standring S. Gray’s Anatomy. 40. Churchill Livingstone Elsevier; Philadelphia: 2008. The anatomical basis of clinical practice. [Google Scholar]
- 12.Standring S. Gray’s Anatomy. 40. Churchill Livingstone Elsevier; Philadelphia: 2008. Female reproductive system; pp. 1279–1304. [Google Scholar]
- 13.Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Tiedler T, Cox M, Stamm WE. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212–1217. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedel I, Liang FX, Deng F-M, Tu L, Kreibich G, Wu XR, Sun TT, Hergt M, Moll R. Urothelial umbrella cells of human ureter are heterogeneous with respect to their uroplakin composition: different degrees of urothelial maturity in ureter and bladder? Eur J Cell Biol. 2005;84:393–405. doi: 10.1016/j.ejcb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Romih R, Korošec P, de Mello W, Jr, Jezernik K. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res. 2005;320:259–268. doi: 10.1007/s00441-004-1005-4. [DOI] [PubMed] [Google Scholar]
- 16.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun T-T. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009;75:1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker B. Renewal of cell populations in the female mouse. Am J Anat. 1960;107:95–105. doi: 10.1002/aja.1001070202. [DOI] [PubMed] [Google Scholar]
- 18.Kachar B, Liang F, Lins U, Ding M, Wu XR, Stoffler D, Aebi U, Sun TT. Three-dimensional analysis of the 16 nm urothelial plaque particle: luminal surface exposure, preferential head-to-head interaction, and hinge formation. J Mol Biol. 1999;285:595–608. doi: 10.1006/jmbi.1998.2304. [DOI] [PubMed] [Google Scholar]
- 19.Hu CC, Liang FX, Zhou G, Tu L, Tang CH, Zhou J, Kreibich G, Sun TT. Assembly of urothelial plaques: tetraspanin function in membrane protein trafficking. Mol Biol Cell. 2005;16:3937–3950. doi: 10.1091/mbc.E05-02-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergara J, Longley W, Robertson JD. A hexagonal arrangement of subunits in membrane ofmouse urinary bladder. J Mol Biol. 1969;46:593–596. doi: 10.1016/0022-2836(69)90200-9. [DOI] [PubMed] [Google Scholar]
- 21.Hicks RM, Ketterer B. Hexagonal lattice of subunits in the thick luminal membrane of the rat urinary bladder. Nature. 1969;224:1304–1305. doi: 10.1038/2241304a0. [DOI] [PubMed] [Google Scholar]
- 22.Taylor KA, Robertson JD. Analysis of the three-dimensional structure of the urinary bladder epithelial cell membranes. J Ultrastruct Res. 1984;87:23–30. doi: 10.1016/s0022-5320(84)90113-8. [DOI] [PubMed] [Google Scholar]
- 23.Walz T, Häner M, Wu XR, Henn C, Engel A, Sun TT, Aebi U. Towards the molecular architecture of the asymmetric unit membrane of the mammalian urinary bladder epithelium: a closed “twisted ribbon” structure. J Mol Biol. 1995;248:887–900. doi: 10.1006/jmbi.1995.0269. [DOI] [PubMed] [Google Scholar]
- 24.Wu XR, Sun TT. Molecular cloning of a 47 kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J Cell Sci. 1993;106:31–43. doi: 10.1242/jcs.106.1.31. [DOI] [PubMed] [Google Scholar]
- 25.Lin JH, Wu XR, Kreibich G, Sun TT. Precursor sequence, processing, and urothelium-specific expression of a major 15-kDa protein subunit of asymmetric unit membrane. J Biol Chem. 1994;269:1775–1784. [PubMed] [Google Scholar]
- 26.Yu J, Lin JH, Wu XR, Sun TT. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J Cell Biol. 1994;125:171–182. doi: 10.1083/jcb.125.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng FM, Liang FX, Tu L, Resing KA, Hu P, Supino M, Hu CC, Zhou G, Ding M, Kreibich G, Sun TT. Uroplakin IIIb, a urothelial differentiation marker, dimerizes with uroplakin Ib as an early step of urothelial plaque assembly. 2002. J Cell Biol. 2002;59:685–694. doi: 10.1083/jcb.200204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu XR, Medina JJ, Sun TT. Selective interactions of UPIa and UPIb, two members of the transmembrane 4 superfamily, with distinct single transmembrane-domained proteins in differentiated urothelial cells. J Biol Chem. 1995;270:29752–29759. doi: 10.1074/jbc.270.50.29752. [DOI] [PubMed] [Google Scholar]
- 29.Tu L, Sun TT, Kreibich G. Specific heterodimer formation is a prerequisite for uroplakins to exit from the endoplasmic reticulum. Mol Biol Cell. 2002;13:4221–4230. doi: 10.1091/mbc.E02-04-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu P, Deng FM, Liang FX, Hu CM, Auerbach AB, Shapiro E, Wu XR, Kachar B, Sun TT. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol. 2000;151:961–972. doi: 10.1083/jcb.151.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol. 2002;283:F1200–1207. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- 32.Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, Geieser N, Nelson PK, Robbins ES, Shapiro E, Kachar B, Sun TT. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol. 2004;167:1195–1204. doi: 10.1083/jcb.200406025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minsky BD, Chlapowski FJ. Morphometric analysis of the translocation of lumenal membrane between cytoplasm and cell surface of transitional epithelial cells during the expansion-contraction cycles of mammalian urinary bladder. J Cell Biol. 1978;77:685–697. doi: 10.1083/jcb.77.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis SA, de Moura JL. Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature. 1982;297:685–688. doi: 10.1038/297685a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R, Wu XR, Sun TT, Kong XP. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001;114:4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 36.Xie B, Zhou G, Chan SY, Shapiro E, Kong XP, Wu XR, Sun TT, Costello CE. Distinct glycan structures of uroplakins Ia and Ib: structural basis for the selective binding of FimH adhesin to uroplakin Ia. J Biol Chem. 2006;281:14644–14653. doi: 10.1074/jbc.M600877200. [DOI] [PubMed] [Google Scholar]
- 37.Wu XR, Sun TT, Medina JJ. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc Natl Acad Sci U S A. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen SL, Hung CS, Pinkner JS, Walker JN, Cusumano CK, Li Z, Bouckaert J, Gordon JI, Hultgren SJ. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc Natl Acad Sci U S A. 2009;106:22439–22444. doi: 10.1073/pnas.0902179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B, Hu B, Zhou Z, Guo D, Guo X, Ding P, Feng L, Wang L. A novel non-homologous recombination-mediated mechanism for Escherichia coli unilateral flagellar phase variation. Nucleic Acids Res. 2012;40:4530–4538. doi: 10.1093/nar/gks040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane MC, Mobley HL. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007;72:19–25. doi: 10.1038/sj.ki.5002230. [DOI] [PubMed] [Google Scholar]
- 41.Thumbikat P, Berry RE, Zhou G, Billips BK, Yaggie RE, Zaichuk T, Sun TT, Schaeffer AJ, Klumpp DJ. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog. 2009;5:e1000415. doi: 10.1371/journal.ppat.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho PL, Kurtova A, Chan KS. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol. 2012;9:583–594. doi: 10.1038/nrurol.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. Host-pathogen checkpoints and population bottle-necks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev. 2012;36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 46.Guo X, Tu L, Gumper I, Plesken H, Novak EK, Chintala S, Swank RT, Pastores G, Torres P, Izumi T, Sun TT, Sabatini DD, Kreibich G. Involvement of Vps33a in the fusion of uroplakin-degrading multivesicular bodies with lysosomes. Traffic. 2009;10:1350–1361. doi: 10.1111/j.1600-0854.2009.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y. Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc Natl Acad Sci U S A. 2003:g14012–14017. doi: 10.1073/pnas.2436350100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou G, Liang FX, Romih R, Wang Z, Liao Y, Ghiso J, Luque-Garcia JL, Neubert TA, Kreibich G, Alonso MA, Schaeren-Wiemers N, Sun TT. MAL facilitates the incorporation of exocytic uroplakin-delivering vesicles into the apical membrane of urothelial umbrella cells. Mol Biol Cell. 2012;23:1354–1366. doi: 10.1091/mbc.E11-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang RJ, Hashitani H, Tonta MA, Bourke JL, Parkington HC, Suzuki H. Spontaneous electrical and Ca2+ signals in the mouse renal pelvis that drive pyeloureteric peristalsis. Clin Exp Pharmacol Physiol. 2010;37:509–515. doi: 10.1111/j.1440-1681.2009.05226.x. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal J, Tonta MA, Mitsui R, Li Q, Kett M, Li J, Parkington HC, Hashitani H, Lang RJ. Potassium and ANO1/TMEM16A chloride channel profiles distinguish atypical and typical smooth muscle cells from interstitial cells in the mouse renal pelvis. Br J Pharmacol. 2012;165:2389–2408. doi: 10.1111/j.1476-5381.2011.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurtado R, Bub G, Herzlinger D. The pelvis-kidney junction contains HCN3, a hyperpolarization-activated cation channel that triggers ureter peristalsis. Kidney Int. 2010;77:500–508. doi: 10.1038/ki.2009.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss RM. Physiology and pharmacology of the renal pelvis and ureter. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10. Ch 59. Saunders Elsevier; Philadelphia: 2012. [Google Scholar]
- 53.Floyd RV, Winstanley C, Bakran A, Wray S, Burdyga TV. Modulation of ureteric Ca signaling and contractility in humans and rats by uropathogenic E. coli. Am J Physio Renal Physiol. 2010;298:F900–908. doi: 10.1152/ajprenal.00468.2009. [DOI] [PubMed] [Google Scholar]
- 54.Floyd RV, Upton M, Hultgren SJ, Wray S, Burdyga TV, Winstanley C. Escherichia coli-mediated impairment of ureteric contractility is uropathogenic E. coli-specific. J Infect Dis. 2012;206:1589–1596. doi: 10.1093/infdis/jis554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wein AJ. Classification of neurogenic voiding dysfunction. J Urol. 1981;125:605–609. doi: 10.1016/s0022-5347(17)55134-4. [DOI] [PubMed] [Google Scholar]
- 56.de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Ann Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 57.Park JM, Bloom DA, McGuire EJ. The guarding reflex revisited. Br J Urol. 1997;80:940–945. doi: 10.1046/j.1464-410x.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimura N, Chancellor MB. Physiology and pharmacology of the bladder and urethra. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10. Saunders Elsevier; Philadelphia: 2012. pp. 1787–1789. [Google Scholar]
- 59.Miller NL, Humphreys MR, Coe FL, Evan AP, Bledsoe SB, Handa SE, Lingeman JE. Nephrocalcinosis: re-defined in the era of endourology. Urol Res. 2010;38:421–427. doi: 10.1007/s00240-010-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginalski JM, Portmann L, Jaeger P. Does medullary sponge kidney cause nephrolithiasis? Am J Roentgenol. 1990;155:299–302. doi: 10.2214/ajr.155.2.2115256. [DOI] [PubMed] [Google Scholar]
- 61.Laube M, Hess B, Terrier F, Vock P, Jaeger P. Prevalence of medullary sponge kidney in patients with and without nephrolithiasis. Praxis (Bern 1994) 1995;84:1224–1230. [PubMed] [Google Scholar]
- 62.Palubinskas AJ. Renal pyramidal structure opacification in excretory urography and its relation to medullary sponge kidney. Radiology. 1963;81:963–970. doi: 10.1148/81.6.963. [DOI] [PubMed] [Google Scholar]
- 63.Forster JA, Taylor J, Browning AJ, Biyani CS. A review of the natural progression of medullary sponge kidney and a novel grading system based on intravenous urography findings. Urol Int. 2007;78:264–269. doi: 10.1159/000099350. [DOI] [PubMed] [Google Scholar]
- 64.McPhail EF, Gettman MT, Patterson DE, Rangel LJ, Krambeck AE. Nephrolithiasis in medullary sponge kidney: evaluation of clinical and metabolic features. Urology. 2012;79:277–281. doi: 10.1016/j.urology.2011.07.1414. [DOI] [PubMed] [Google Scholar]
- 65.Toyoda K, Miyamoto Y, Ida M, Tada S, Utsunomiya M. Hyperechoic medulla of the kidneys. Radiology. 1989;173:431–434. doi: 10.1148/radiology.173.2.2678257. [DOI] [PubMed] [Google Scholar]
- 66.Ginalski JM, Schnyder P, Portmann L, Jaeger P. Medullary sponge kidney on axial computed-tomography: comparison with excretory urography. Eur J Radiol. 1991;2:104–107. doi: 10.1016/0720-048x(91)90107-7. [DOI] [PubMed] [Google Scholar]
- 67.Hida T, Nishie A, Asayama Y, Ishigami K, Fujita N, Inokuchi J, Naito S, Ando S, Honda H. MR imaging of focal medullary sponge kidney: case report. Magn Reson Med Sci. 2012;11:65–69. doi: 10.2463/mrms.11.65. [DOI] [PubMed] [Google Scholar]
- 68.Chesney RW, Kaufman R, Stapleton FB, Rivas ML. Association of medullary sponge kidney and medullary dysplasia in Beckwith-Wiedemann syndrome. J Pediatr. 1989;115:761–764. doi: 10.1016/s0022-3476(89)80659-6. [DOI] [PubMed] [Google Scholar]
- 69.Kerr DN, Warrick CK, Hart-Mercer J. A lesion resembling medullary sponge kidney in patients with congenital hepatic fibrosis. Clin Radiol. 1962;13:85–91. doi: 10.1016/s0009-9260(62)80024-5. [DOI] [PubMed] [Google Scholar]
- 70.Torres VE, Erickson SB, Smith LH, Wilson DM, Hattery RR, Segura JW. The association of nephrolithiasis and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1988;11:318–325. doi: 10.1016/s0272-6386(88)80137-9. [DOI] [PubMed] [Google Scholar]
- 71.Gambaro G, Fabris A, Citron L, Tosetto E, Anglani F, Bellan F, Conte M, Bonfante L, Lupo A, D’Angelo A. An unusual association of contralateral congenital small kidney, reduced renal function and hyperparathyroidism in sponge kidney patients: on the track of the molecular basis. Nephrol Dial Transplant. 2005;20:1042–1047. doi: 10.1093/ndt/gfh798. [DOI] [PubMed] [Google Scholar]
- 72.Hulbert JC, Reddy PK, Hunter DW, Castaneda-Zuniga W, Amplatz K, Lange PH. Percutaneous techniques for the management of caliceal diverticula containing calculi. J Urol. 1986;135:225–227. doi: 10.1016/s0022-5347(17)45590-x. [DOI] [PubMed] [Google Scholar]
- 73.Monga M, Smith R, Ferral H, Thomas R. Percutaneous ablation of caliceal diverticulum: long-term followup. J Urol. 2000;163:28–32. [PubMed] [Google Scholar]
- 74.Auge BK, Munver R, Kourambas J, Newman GE, Wu NZ, Preminger GM. Neoinfundibulotomy for the management of symptomatic caliceal diverticula. J Urol. 2002;167:1616–1620. [PubMed] [Google Scholar]
- 75.Bellman GC, Silverstein JI, Blickensderfer S, Smith AD. Technique and follow-up of percutaneous management of caliceal diverticula. Urology. 1993;42:21–25. doi: 10.1016/0090-4295(93)90327-7. [DOI] [PubMed] [Google Scholar]
- 76.Heyns CF. Urinary tract infection associated with conditions causing urinary tract obstruction and stasis, excluding urolithiasis and neuropathic bladder. World J Urol. 2012;30:77–83. doi: 10.1007/s00345-011-0725-9. [DOI] [PubMed] [Google Scholar]
- 77.Beeson PB, Guze LB. Experimental pyelonephritis. I. Effect of ureteral ligation on the course of bacterial infection in the kidney of the rat. J Exp Med. 1956;104:803–815. doi: 10.1084/jem.104.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bitz H, Darmon D, Goldfarb M, Shina A, Block C, Rosen S, Brezis M, Heyman SN. Transient urethral obstruction predisposes to ascending pyelonephritis and tubulo- interstitial disease: studies in rats. Urol Res. 2001:67–73. doi: 10.1007/s002400000153. [DOI] [PubMed] [Google Scholar]
- 79.Fernbach SK, Feinstein KA, Spencer K, Lindstrom CA. Ureteral duplication and its complications. Radiographics. 1997;17:109–127. doi: 10.1148/radiographics.17.1.9017803. [DOI] [PubMed] [Google Scholar]
- 80.Chapman CJ, Bailey RR, Janus ED, Abbott GD, Lynn KL. Vesicoureteric reflux: segregation analysis. Am J Med Genet. 1985;20:577–584. doi: 10.1002/ajmg.1320200403. [DOI] [PubMed] [Google Scholar]
- 81.Farhat W, McLorie G, Geary D, Capolicchio G, Bägli D, Merguerian P, Khoury A. The natural history of neonatal vesicoureteral reflux associated with antenatal hydronephrosis. J Urol. 2000;164:1057–1060. doi: 10.1097/00005392-200009020-00033. [DOI] [PubMed] [Google Scholar]
- 82.Hannula A, Venhola M, Renko M, Pokka T, Huttunen N-P, Uhari M. Vesicoureteral reflux in children with suspected and proven urinary tract infection. Pediatr Nephrol. 2010;25:1463–1469. doi: 10.1007/s00467-010-1542-x. [DOI] [PubMed] [Google Scholar]
- 83.Risdon RA, Yeung CK, Ransley PG. Reflux nephropathy in children submitted to unilateral nephrectomy: a clinicopathological study. Clin Nephrol. 1993;40:308–314. [PubMed] [Google Scholar]
- 84.Vermillion CD, Heale WF. Position and configuration of the ureteral orifice and its relationship to renal scarring in adults. J Urol. 1973;109:579–584. doi: 10.1016/s0022-5347(17)60484-1. [DOI] [PubMed] [Google Scholar]
- 85.Paquin AJ., Jr Ureterovesical anastomosis: the description and evaluation of a technique. J Urol. 1959;82:573–583. doi: 10.1016/S0022-5347(17)65934-2. [DOI] [PubMed] [Google Scholar]
- 86.Hollowell JG, Greenfield SP. Screening siblings for vesicoureteral reflux. J Urol. 2002;168:2138–2141. doi: 10.1016/S0022-5347(05)64337-6. [DOI] [PubMed] [Google Scholar]
- 87.Noe HN, Wyatt RJ, Peeden JN, Jr, Rivas ML. The transmission of vesicoureteral reflux from parent to child. J Urol. 1992;148:1869–1871. doi: 10.1016/s0022-5347(17)37053-2. [DOI] [PubMed] [Google Scholar]
- 88.Murawski IJ, Myburgh DB, Favor J, Gupta IR. Vesico-ureteric reflux and urinary tract development in the Pax21Neu+/ mouse. Am J Physiol Renal Physiol. 2007;293:F1736–1745. doi: 10.1152/ajprenal.00221.2007. [DOI] [PubMed] [Google Scholar]
- 89.Yu OH, Murawski IJ, Myburgh DB, Gupta IR. Overexpression of RET leads to vesicoureteric reflux in mice. Am J Physiol Renal Physiol. 2004;287:F1123–1130. doi: 10.1152/ajprenal.00444.2003. [DOI] [PubMed] [Google Scholar]
- 90.Ohtomo Y, Nagaoka R, Kaneko K, Fukuda Y, Miyano T, Yamashiro Y. Angiotensin converting enzyme gene polymorphism in primary vesicoureteral reflux. Pediatr Nephrol. 2001;16:648–652. doi: 10.1007/s004670100634. [DOI] [PubMed] [Google Scholar]
- 91.Rigoli L, Chimenz R, di Bella C, Cavallaro E, Caruso R, Briuglia S, Fede C, Salpietro CD. Angiotensin-converting enzyme and angiotensin type 2 receptor gene genotype distributions in Italian children with congenital uropathies. Pediatr Res. 2004;56:988–993. doi: 10.1203/01.PDR.0000145252.89427.9E. [DOI] [PubMed] [Google Scholar]
- 92.Loré F, Talidis F, Di Cairano G, Renieri A. Multiple endocrine neoplasia type 2 syndromes may be associated with renal malformations. J Intern Med. 2001;250:37–42. doi: 10.1046/j.1365-2796.2001.00846.x. [DOI] [PubMed] [Google Scholar]
- 93.Yang Y, Houle AM, Letendre J, Richter A. RET Gly691Ser mutation is associated with primary vesicoureteral reflux in the French-Canadian population from Quebec. Hum Mutat. 2008;29:695–702. doi: 10.1002/humu.20705. [DOI] [PubMed] [Google Scholar]
- 94.Yoneda A, Cascio S, Green A, Barton D, Puri P. Angiotensin II type 2 receptor gene is not responsible for familial vesicoureteral reflux. J Urol. 2002;168:1138–1141. doi: 10.1016/S0022-5347(05)64611-3. [DOI] [PubMed] [Google Scholar]
- 95.Shefelbine SE, Khorana S, Schultz PN, Huang E, Thobe N, Hu ZJ, Fox GM, Jing S, Cote GJ, Gagel RF. Mutational analysis of the GDNF/RET-GDNFR alpha signaling complex in a kindred with vesicoureteral reflux. Hum Genet. 1998;102:474–478. doi: 10.1007/s004390050724. [DOI] [PubMed] [Google Scholar]
- 96.Giltay JC, van de Meerakker J, van Amstel HK, de Jong TP. No pathogenic mutations in the uroplakin III gene of 25 patients with primary vesicoureteral reflux. J Urol. 2004;171:931–932. doi: 10.1097/01.ju.0000094802.50650.3d. [DOI] [PubMed] [Google Scholar]
- 97.Jenkins D, Bitner-Glindzicz M, Malcolm S, Allison J, de Bruyn R, Flanagan S, Thomas DF, Belk RA, Feather SA, Bingham C, Southgate J, Woolf AS. Mutation analyses of Uroplakin II in children with renal tract malformations. Nephrol Dial Transplant. 2006;21:3415–3421. doi: 10.1093/ndt/gfl465. [DOI] [PubMed] [Google Scholar]
- 98.Jiang S, Gitlin J, Deng FM, Liang FX, Lee A, Atala A, Bauer SB, Ehrlich GD, Feather SA, Goldberg JD, Goodship JA, Goodship TH, Hermanns M, Hu FZ, Jones KE, Malcolm S, Mendelsohn C, Preston RA, Retik AB, Schneck FX, Wright V, Ye XY, Woolf AS, Wu XR, Ostrer H, Shapiro E, Yu J, Sun TT. Lack of major involvement of human uroplakin genes in vesicoureteral reflux: implications for disease heterogeneity. Kidney Int. 2004;66:10–19. doi: 10.1111/j.1523-1755.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 99.Lambert HJ, Stewart A, Gullett AM, Cordell HJ, Malcolm S, Feather SA, Goodship JA, Goodship TH, Woolf AS UK VUR Study Group. Primary, nonsyndromic vesicoureteric reflux and nephropathy in sibling pairs: a United Kingdom cohort for a DNA bank. Clin J Am Soc Nephrol. 2011;6:760–766. doi: 10.2215/CJN.04580510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 101.Lepor H. Pathophysiology of benign prostatic hyperplasia in the aging male population. Rev Urol. 2005;7(Suppl 4):S3–S12. [PMC free article] [PubMed] [Google Scholar]
- 102.Nitti VW, Kim Y, Combs AJ. Correlation of the AUA symptom index with urodynamics in patients with suspected benign prostatic hyperplasia. Neurourol Urodyn. 1994;13:521–527. doi: 10.1002/nau.1930130504. [DOI] [PubMed] [Google Scholar]
- 103.Barry MJ, Cockett AT, Holtgrewe HL, McConnell JD, Sihelnik SA, Winfield HN. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993;150:351–358. doi: 10.1016/s0022-5347(17)35482-4. [DOI] [PubMed] [Google Scholar]
- 104.Shapiro E, Becich MJ, Hartanto V, Lepor H. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J Urol. 1992;147:1293–1297. doi: 10.1016/s0022-5347(17)37546-8. [DOI] [PubMed] [Google Scholar]
- 105.Madigan AA, Sobek KM, Cummings JL, Green WR, Bacich DJ, O’Keefe DS. Activation of innate anti-viral immune response genes in symptomatic benign prostatic hyperplasia. Genes Immun. 2012;13:566–572. doi: 10.1038/gene.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Kerrebroeck PV, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 107.Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, Lieber MM. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 108.Hooton TM, Stapleton AE, Roberts PL, Winter C, Scholes D, Bavendam T, Stamm WE. Perineal anatomy and urine-voiding characteristics of young women with and without recurrent urinary tract infections. Clin Infect Dis. 1999;29:1600–1601. doi: 10.1086/313528. [DOI] [PubMed] [Google Scholar]
- 109.Nicolle LE. Asymptomatic bacteriuria: when to screen and when to treat. Infect Dis Clin North Am. 2003;17:367–394. doi: 10.1016/s0891-5520(03)00008-4. [DOI] [PubMed] [Google Scholar]
- 110.Raz R, Gennesin Y, Wasser J, Stoler Z, Rosenfeld S, Rottensterich E, Stamm WE. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152–156. doi: 10.1086/313596. [DOI] [PubMed] [Google Scholar]
- 111.Petros PE, Ulmsten UI. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7–31. doi: 10.1111/j.1600-0412.1990.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 112.Petros PP, Ulmsten U. An anatomical classification–a new paradigm for management of female lower urinary tract dysfunction. Eur J Obstet Gynecol Reprod Biol. 1998;80:87–94. doi: 10.1016/s0301-2115(98)00092-x. [DOI] [PubMed] [Google Scholar]
- 113.DeLancey JO. The pathophysiology of stress urinary incontinence in women and its implications for surgical treatment. World J Urol. 1997;15:268–274. doi: 10.1007/BF02202011. [DOI] [PubMed] [Google Scholar]
- 114.Nitti VW, TU LM, Gitlin J. Diagnosing bladder outlet obstruction in women. J Urol. 1999;161:1535–1540. [PubMed] [Google Scholar]
- 115.Stika CS. Atrophic vaginitis. Dermatol Ther. 2010;23:514–522. doi: 10.1111/j.1529-8019.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 116.Ronald A, Ludwig E. Urinary tract infections in adults with diabetes. Inter J Antimicrob Agents. 2001;17:287–292. doi: 10.1016/s0924-8579(00)00356-3. [DOI] [PubMed] [Google Scholar]
- 117.Chen SL, Jackson SL, Boyko EJ. Diabetes mellitus and urinary tract infection: epidemiology, pathogenesis and proposed studies in animal models. J Urol. 2009;182(6 Suppl):S51–56. doi: 10.1016/j.juro.2009.07.090. [DOI] [PubMed] [Google Scholar]
- 118.Hirji I, Guo Z, Andersson SW, Hammar N, Gomez-Caminero A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD) J Diabetes Complications. 2012;26:513–516. doi: 10.1016/j.jdiacomp.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 119.Boyko EJ, Fihn SD, Scholes D, Chen CL, Normand EH, Yarbro P. Diabetes and the risk of acute urinary tract infection among post-menopausal women. Diabetes Care. 2002;25:1778–1783. doi: 10.2337/diacare.25.10.1778. [DOI] [PubMed] [Google Scholar]
- 120.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol. 2005;161:557–564. doi: 10.1093/aje/kwi078. [DOI] [PubMed] [Google Scholar]
- 121.Geerlings SE, Stolk RP, Camps MJ, Netten PM, Collet TJ, Hoepelman AI Diabetes Women Asymptomatic Bacteriuria Utrecht Study Group. Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care. 2000;23:1737–1741. doi: 10.2337/diacare.23.12.1737. [DOI] [PubMed] [Google Scholar]
- 122.Geerlings SE, Meiland R, van Lith EC, Brouwer EC, Gaastra W, Hoepelman AIM. Adherence of type 1-fimbriated Escherichia coli to uroepithelial cells: more in diabetic women than in control subjects. Diabetes Care. 2002;25:1405–1409. doi: 10.2337/diacare.25.8.1405. [DOI] [PubMed] [Google Scholar]
- 123.Taganna J, de Boer AR, Wuhrer M, Bouckaert J. Glycosylation changes as important factors for the susceptibility to urinary tract infection. Biochem Soc Trans. 2011;39:349–354. doi: 10.1042/BST0390349. [DOI] [PubMed] [Google Scholar]
- 124.Frimodt-Møller C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med. 1980;92:318–321. doi: 10.7326/0003-4819-92-2-318. [DOI] [PubMed] [Google Scholar]
- 125.Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005;95:733–738. doi: 10.1111/j.1464-410X.2005.05392.x. [DOI] [PubMed] [Google Scholar]
- 126.Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009;182(6 Suppl):S18–26. doi: 10.1016/j.juro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosen DA, Hung CS, Kline KA, Hultgren SJ. Streptozocin-induced diabetic mouse model of urinary tract infection. Infect Immun. 2008;76:4290–4298. doi: 10.1128/IAI.00255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta- analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73:576–582. [PubMed] [Google Scholar]
- 129.Bolton M, Horvath DJ, Li B, Cortado H, Newsom D, White P, Paritida-Sanchez S, Justice SS. Intrauterine growth restriction is a direct consequence of localized maternal uropathogenic Escherichia coli cystitis. PLoS One. 2012;7:e33897. doi: 10.1371/journal.pone.0033897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Faúndes A, Brícola-Filho M, Pinto e Silva JL. Dilatation of the urinary tract during pregnancy: proposal of a curve of maximal caliceal diameter by gestational age. Am J Obstet Gynecol. 1998;178:1082–1086. doi: 10.1016/s0002-9378(98)70552-6. [DOI] [PubMed] [Google Scholar]
- 131.Marchant DJ. Effects of pregnancy and progestational agents on the urinary tract. Am J Obstet Gynecol. 1972;112:487–501. doi: 10.1016/0002-9378(72)90309-2. [DOI] [PubMed] [Google Scholar]
- 132.Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113:1051–1058. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang PL, Peters CA, Rosen S. Ureteropelvic junction obstruction: morphological and clinical studies. Pediatr Nephrol. 2000;14:820–826. doi: 10.1007/s004679900240. [DOI] [PubMed] [Google Scholar]
- 134.Murakumo M, Nonomura K, Yamashita T, Ushiki T, Abe K, Koyanagi T. Structural changes of collagen components and diminution of nerves in congenital ureteropelvic junction obstruction. J Urol. 1997;157:1963–1968. [PubMed] [Google Scholar]
- 135.Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 136.Kuwayama F, Miyazaki Y, Ichikawa I. Embryogenesis of the congenital anomalies of the kidney and the urinary tract. Nephrol Dial Transplant. 2002;17:45–47. doi: 10.1093/ndt/17.suppl_9.45. [DOI] [PubMed] [Google Scholar]
- 137.Yiee JH, Johnson-Welch S, Baker LA, Wilcox DT. Histologic differences between extrinsic and intrinsic ureteropelvic junction obstruction. Urology. 2010;76:181–184. doi: 10.1016/j.urology.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 138.Roth CC, Hubanks JM, Bright BC, Heinlen JE, Donovan BO, Kropp BP, Frimberger D. Occurrence of urinary tract infection in children with significant upper urinary tract obstruction. Urology. 2009;73:74–78. doi: 10.1016/j.urology.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 139.Islek A, Güven AG, Koyun M, Akman S, Alimoglu E. Probability of urinary tract infection in infants with ureteropelvic junction obstruction: is antibacterial prophylaxis really needed? Pediatr Nephrol. 2011;26:1837–1841. doi: 10.1007/s00467-011-1889-7. [DOI] [PubMed] [Google Scholar]
- 140.Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand P, Schaer GN. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 141.Jørgensen TM, Djurhuus JC, Schrøder HD. Idiopathic detrusor sphincter dyssynergia in neurologically normal patients with voiding abnormalities. Eur Urol. 1982;8:107–110. doi: 10.1159/000473490. [DOI] [PubMed] [Google Scholar]
- 142.Groutz A, Blaivas JG, Pies C, Sassone AM. Learned voiding dysfunction (non- neurogenic, neurogenic bladder) among adults. Neurourol Urodyn. 2001;20:259–268. doi: 10.1002/nau.1003. [DOI] [PubMed] [Google Scholar]
- 143.Cameron AP, Gajewski JB. Bladder outlet obstruction in painful bladder syndrome/interstitial cystitis. Neurourol Urodyn. 2009;28:944–948. doi: 10.1002/nau.20729. [DOI] [PubMed] [Google Scholar]
- 144.Pannek J, Einig EM, Einig W. Clinical management of bladder dysfunction caused by sexual abuse. Urol Int. 2009;82:420–425. doi: 10.1159/000218531. [DOI] [PubMed] [Google Scholar]
- 145.Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1671–1678. doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carlson KV, Rome S, Nitti VW. Dysfucntional voiding in women. J Urol. 2001;165:143–148. doi: 10.1097/00005392-200101000-00035. [DOI] [PubMed] [Google Scholar]
- 147.Minardi D, d’Anzeo G, Parri G, Polito M, Jr, Piergallina M, El Asmar Z, Marchetti M, Muzzonigro G. The role of uroflowmetry biofeedback and biofeedback training of the pelvic floor muscles in the treatment of recurrent urinary tract infections in women with dysfunctional voiding: a randomized controlled prospective study. Urology. 2010;75:1299–1304. doi: 10.1016/j.urology.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 148.Brucker BM, Fong E, Shah S, Kelly C, Rosenblum N, Nitti VW. Urodynamic differences between dysfunctional voiding and primary bladder neck obstruction in women. Urology. 2012;80:55–60. doi: 10.1016/j.urology.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 149.Leadbetter GW, Jr, Leadbetter WF. Diagnosis and treatment of congenital bladder- neck obstruction in children. N Engl J Med. 1959;260:633–637. doi: 10.1056/NEJM195903262601304. [DOI] [PubMed] [Google Scholar]
- 150.Kuo HC. Videourodynamic characteristics and lower urinary tract symptoms of female bladder outlet obstruction. Urology. 2005;66:1005–1009. doi: 10.1016/j.urology.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 151.Ahmed HU, Shergill IS, Arya M, Shah PJ. Management of detrusor–external sphincter dyssynergia. Nat Clin Pract Urol. 2006;3:368–380. doi: 10.1038/ncpuro0521. [DOI] [PubMed] [Google Scholar]
- 152.Kim YH, Kattan MW, Boone TB. Bladder leak point pressure: the measure for sphincterotomy success in spinal cord injured patients with external detrusor-sphincter dyssynergia. J Urol. 1998;159:493–497. doi: 10.1016/s0022-5347(01)63957-0. [DOI] [PubMed] [Google Scholar]
- 153.Abdul-Rahman A, Ismail S, Hamid R, Shah J. A 20-year follow-up of the mesh wallstent in the treatment of detrusor external sphincter dyssynergia in patients with spinal cord injury. BJU Int. 2010;106:1510–1513. doi: 10.1111/j.1464-410X.2010.09379.x. [DOI] [PubMed] [Google Scholar]
- 154.Chen SL, Bih LI, Huang YH, Tsai SJ, Lin TB, Kao YL. Effect of single botulinum toxin A injection to the external urethral sphincter for treating detrusor external sphincter dyssynergia in spinal cord injury. J Rehabil Med. 2008;40:744–748. doi: 10.2340/16501977-0255. [DOI] [PubMed] [Google Scholar]
- 155.Zaffanello M, Malerba G, Cataldi L, Antoniazzi F, Franchini M, Monti E, Fanos V. Genetic risk for recurrent urinary tract infections in humans: A systematic review. J Biomed Biotechnol. 2010;2010:321082. doi: 10.1155/2010/321082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 158.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004;286:F795–802. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 159.Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, Hökfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1681/01.asn.0000926856.92699.53. [DOI] [PubMed] [Google Scholar]
- 160.Morrison G, Kilanowski F, Davidson D, Dorin J. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect Immun. 2002;70:3053–3060. doi: 10.1128/IAI.70.6.3053-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 162.Diamond DA, Mattoo TK. Endoscopic treatment of primary vesicoureteral reflux. N Engl J Med. 2012;366:1218–1226. doi: 10.1056/NEJMct1108922. [DOI] [PubMed] [Google Scholar]
- 163.Standring S. Gray’s Anatomy. 40. Churchill Livingstone Elsevier; Philadelphia: 2008. Kidneys and ureter; pp. 1230–1231. [Google Scholar]