Background: SUMO conjugation to the proteome critically controls cell growth, but mechanisms for regulating SUMO ligases are poorly defined.
Results: Desumoylation of the major fission yeast SUMO ligase by a nuclear pore-associated protease protects it from proteolysis.
Conclusion: Desumoylation of a SUMO ligase antagonizes autoinhibition of the SUMO pathway.
Significance: These data demonstrate cooperation between STUbL and Cdc48(p97) in proteasome-mediated degradation of SUMO conjugates.
Keywords: nuclear pore, proteasome, protein degradation, small ubiquitin-like modifier (SUMO), sumoylation, Cdc48-Ufd1-Npl4, Pli1, STUbL, Ulp1
Abstract
Covalent modification of the proteome by SUMO is critical for genetic stability and cell growth. Equally crucial to these processes is the removal of SUMO from its targets by the Ulp1 (HuSENP1/2) family of SUMO proteases. Ulp1 activity is normally spatially restricted, because it is localized to the nuclear periphery via interactions with the nuclear pore. Delocalization of Ulp1 causes DNA damage and cell cycle defects, phenotypes thought to be caused by inappropriate desumoylation of nucleoplasmic targets that are normally spatially protected from Ulp1. Here, we define a novel consequence of Ulp1 deregulation, with a major impact on SUMO pathway function. In fission yeast lacking Nup132 (Sc/HuNUP133), Ulp1 is delocalized and can no longer antagonize sumoylation of the PIAS family SUMO E3 ligase, Pli1. Consequently, SUMO chain-modified Pli1 is targeted for proteasomal degradation by the concerted action of a SUMO-targeted ubiquitin ligase (STUbL) and Cdc48-Ufd1-Npl4. Pli1 degradation causes the profound SUMO pathway defects and associated centromere dysfunction in cells lacking Nup132. Thus, perhaps counterintuitively, Ulp1-mediated desumoylation can promote SUMO modification by stabilizing a SUMO E3 ligase.
Introduction
Covalent modification of the proteome by the small ubiquitin-like modifier SUMO2 regulates most aspects of cell growth, including cell cycle transitions and genome stability (1–4). The addition of SUMO and its removal from target proteins must be tightly regulated to avoid the pathological consequences of defects in pathway homeostasis (5–7).
SUMO is attached to lysine residues in target proteins via an enzymatic cascade of E1 activating, E2 conjugating, and E3 ligase factors (4). E3 ligases provide target specificity and enhance SUMO conjugation. A major family of SUMO E3 ligases is the PIAS (protein inhibitor of activated STAT) family, which has several members in mammalian cells e.g. PIAS1–4, two in budding yeast SIZ1/2, and one called Pli1 in fission yeast (8, 9). Pli1 catalyzes the majority of sumoylation (>90%) including SUMO chain formation, and its deletion causes meiotic defects, centromeric heterochromatin dysfunction, and telomere elongation (8, 10).
SUMO is removed by one of a small family of Ubl-specific proteases (11, 12). In yeasts there are two Ubl-specific proteases, Ulp1 and Ulp2, of which Ulp1 processes SUMO into a mature form by removing a C-terminal peptide to reveal a diglycine motif. Both Ulp1 and Ulp2 desumoylate a subset of SUMO conjugates, with specificity likely driven in large part by their spatial separation (12). Ulp1 localizes to the nuclear rim by associating with nuclear pores, whereas Ulp2 is nucleoplasmic (12–18). In higher eukaryotes, SENP1/2 localize to nuclear pores like Ulp1 (19–21), and SENP6/7, like Ulp2, are nucleoplasmic (22, 23).
Sumoylated proteins can also be ubiquitinated by a SUMO-targeted ubiquitin ligase (STUbL) to promote their degradation at the proteasome (24–26). Correlative evidence suggests that SUMO chains act as targeting signals for STUbLs. Consistent with this, high molecular weight SUMO chains accumulate in STUbL mutant cells (24–26), a phenotype also caused by Ulp2 inactivation (10, 27). Moreover, in fission yeast, the growth and genome stability defects caused by both STUbL and Ulp2 inactivation are suppressed by blocking SUMO chain formation (10, 28). In contrast, preventing SUMO chain formation in budding yeast is lethal to STUbL mutants but suppresses some ulp2Δ defects (27, 29, 30). These findings highlight the importance of SUMO pathway homeostasis and that the “wiring” of the STUbL-SUMO interface differs notably between these yeasts (see above and Refs. 10 and 27–30).
The nuclear pore has emerged as a broadly conserved hub of SUMO-mediated signaling, impacting key processes such as transcription, chromosome segregation, and DNA repair (31, 32). However, despite functional overlap, mutants of orthologous nuclear pore components cause different phenotypes in fission and budding yeast, the latter of which has been used in most studies (33, 34). Therefore, to robustly model how the nuclear pore could impact SUMO pathway homeostasis in higher eukaryotes, it is important to determine how the nuclear pore impacts sumoylation in fission yeast.
Herein we reveal that sumoylation is globally reduced in fission yeast deleted for Nup132, which contrasts with the relatively mild and selective sumoylation defects of analogous budding yeast mutants (16, 35, 36). Nevertheless, we show that as in budding yeast (36), Ulp1 is both delocalized and less abundant in nup132Δ (Sc nup133Δ) cells. What then causes the profound SUMO conjugation defect in nup132Δ cells? Intriguingly, we found that Ulp1 dysfunction in nup132Δ cells allows an accumulation of SUMO chains on the major SUMO E3 ligase Pli1, which promote its degradation in a STUbL-, Cdc48-Ufd1-Npl4-, and proteasome-dependent manner. Moreover, this novel phenomenon allowed us to execute a detailed mechanistic dissection of SUMO pathway and cofactor requirements in the turnover of a specific STUbL substrate. Overall, our data define how the activity of a PIAS family SUMO ligase, and thus its physiological impact, e.g. centromere function, hang in the balance between its autosumoylation and desumoylation by a nuclear pore localized SUMO protease.
Experimental Procedures
Yeast Strains and Growth Conditions
Standard yeast methods were performed as described previously (37). The strains used in this study are listed in Table 1.
TABLE 1.
List of yeast strains used in this study
| Strain | Genotypea | Source |
|---|---|---|
| NBY752 | imr1R (dg-glu)NcoI::ura4 oriI ade6-210/ade6-216 | Ref. 51 |
| NBY5450 | nup132::ura4+ | Ref. 33 |
| NBY780 | h+ | |
| NBY781 | h− | |
| NBY1493 | pli1::kanMx6 | |
| NBY1820 | pli1-TAP:kanMx6 | |
| NBY1967 | pli1::hphMx6 imr1R (dg-glu)NcoI::ura4 oriI ade6-210/ade6-216 | |
| NBY2471 | slx8-29:kanMx6 | |
| NBY2754 | pREP2:ura4+ integrated at ars1 | |
| NBY2756 | slx8-29:kanMx6 pREP2:ura4+ integrated at ars1 | |
| NBY2927 | ulp2::kanMx6 | |
| NBY3063 | ulp1-GFP:kanMx6 | |
| NBY3172 | ulp2::hphMx6 | |
| NBY5159 | ulp1-myc13:kanMx6 | Ref. 14 |
| NBY5428 | nup132::ura4+ slx8-29:kanMx6 | |
| NBY5467 | nup132::ura4+ ulp2::kanMx6 | |
| NBY5468 | nup132::ura4+ ulp1-GFP:kanMx6 | |
| NBY5471 | pREP41-SUMOGG:LEU2 pREP2:ura4+ integrated at ars1 | |
| NBY5472 | pREP41-SUMOFL:LEU2 pREP2:ura4+ integrated at ars1 | |
| NBY5473 | slx8-29:kanMx6 pREP41-SUMOGG:LEU2 pREP2:ura4+ integrated at ars1 | |
| NBY5474 | slx8-29:kanMx6 pREP41-SUMOFL:LEU2 pREP2:ura4+ integrated at ars1 | |
| NBY5475 | nup132::ura4+ pREP41-SUMOGG:LEU2 | |
| NBY5476 | nup132::ura4+ pREP41-SUMOFL:LEU2 | |
| NBY5477 | nup132::ura4+ slx8-29:kanMx6 pREP41-SUMOGG:LEU2 | |
| NBY5478 | nup132::ura4+ slx8-29:kanMx6 pREP41-SUMOFL:LEU2 | |
| NBY5506 | pli1-TAP:kanMx6 nup132::ura4+ | |
| NBY5528 | ulp1-1:kanMx6 | |
| NBY5544 | pli1-TAP:kanMx6 nup132::ura4+ pREP41-ulp1:LEU2 | |
| NBY5546 | pli1-TAP:kanMx6 nup132::ura4+ slx8-29:hphMx6 | |
| NBY5547 | pli1-TAP:kanMx6 slx8-29:hphMx6 | |
| NBY5548 | pli1-TAP:kanMx6 nup132::ura4+ ufd1-1:natMx6 | |
| NBY5549 | pli1-TAP:kanMx6 ufd1-1:natMx6 | |
| NBY5550 | pli1-TAP:kanMx6 nup132::ura4+ mts3-1 | |
| NBY5551 | pli1-TAP:kanMx6 mts3-1 | |
| NBY5569 | pli1-TAP:kanMx6 nup132::ura4+ slx8-29:hphMx6 pREP41-6HIS-SUMO:LEU2 | |
| NBY5573 | pli1-TAP:kanMx6 ulp1-1:kanMx6 | |
| NBY5577 | pli1::kanMx6 pREP41-pli1:LEU2 | |
| NBY5579 | nup132::ura4+ pREP41-pli1:LEU2 | |
| NBY5582 | pli1-TAP:kanMx6 nup132::ura4+ SUMOK14,K30R | |
| NBY5593 | nup132::ura4+ slx8-29:kanMx6 pREP41-pli1:LEU2 | |
| NBY5620 | nup132::ura4+ imr1R (dg-glu)NcoI::ura4 oriI ade6-210/ade6-216 | |
| NBY5621 | pli1-TAP:kanMx6 nup132::ura4+ SUMOD81R | |
| NBY5633 | pli1::kanMx6 ulp2::hphMx6 | |
| NBY5665 | ulp1-myc13:kanMx6 nup132:hphMx6 |
a All strains are of ura4-D18 leu1-32 background genotype, unless otherwise stated. Double colons represent knockouts; single colons represent tagging. A reference is given for strains not generated in this study.
Spot Assays
The cells were grown at 25 °C to logarithmic phase (A600 of 0.6–0.8), spotted on agar plates in 5-fold dilutions from a starting A600 of 0.5 and then incubated at 25–35 °C for 3–5 days.
Fluorescence Microscopy
GFP fusion proteins and DAPI staining were imaged in live cells using an Eclipse E800 microscope (Nikon Metrology) with a 100× Plan Differential Interference Contrast H oil immersion objective. The images were captured through an INFINITY 3 charge-coupled device camera using the INFINITY ANALYZE software (Lumenera Corporation).
Western Blotting
Exponentially growing cells (∼15 A600 units) were washed with STOP buffer (10 mm EDTA, 50 mm NaF, 150 mm NaCl, 1 mm NaN3). The pellets were flash-frozen in liquid nitrogen, and the cells were lysed by beating twice at 5.0 m/s for 20 s in a FastPrep-24 instrument (MP Biochemicals) in 200 μl of 20% TCA supplied with 100 ml silica-zirconia beads (BioSpec Products). After bead beating, 400 μl of 5% TCA was added, and the total cell lysate was centrifuged at 16,000 × g for 5 min at 4 °C. The pellet was washed twice with 0.1% TCA. The precipitated proteins were resuspended in 8 m urea, 50 mm Tris, pH 8.5, 150 mm NaCl. Protein was quantitated by measuring absorbance at A280, and 60 μg of proteins were resolved on a gradient SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was blocked in 1% (w/v) nonfat milk in phosphate-buffered saline solution with 0.1% (v/v) Tween 20, probed with peroxidase anti-peroxidase and then detected using an ECL Dura system (Pierce) or probed with antibodies against α-tubulin, Pmt3 (SUMO) and then an IRDye-conjugated secondary antibody and imaged on an ODYSSEY scanner (Li-Cor).
Nickel-Nitrilotriacetic Acid Pulldown
Proteins from cell pellets were extracted by TCA precipitation as described above, and 20 mg of proteins were incubated with 50 μl of nickel-nitrilotriacetic acid beads (Qiagen) in the presence of 15 mm imidazole at room temperature for 1 h. The beads were then washed three times with 8 m urea, 50 mm Tris, pH 8, 150 mm NaCl, and 20 mm imidazole and eluted with 2× LDS sample loading buffer (Invitrogen) at 70 °C for 10 min. Input, flow through, and one-third of the nickel pulldown were resolved by SDS-PAGE and detected by peroxidase anti-peroxidase Western blotting.
RNA Extraction and RT-Quantitative PCR
Total RNA was extracted from ∼5 A600 units of exponentially growing cells. The cells were lysed by bead beating in 0.8 ml of TRIzol (Invitrogen), using the equipment and settings described above. RNA extraction with TRIzol was performed by following the manufacturer's instructions. The extracted RNA was treated with DNase I (New England Biolabs) and cleaned up with the RNeasy Plus minikit (Qiagen). A total of 1 μg of DNase-treated RNA was reverse transcribed using the ProtoScript® II first strand cDNA synthesis kit (New England Biolabs). The completed reactions were diluted 10-fold, and 5 μl of the dilutions was used as the template in 20-μl quantitative PCR mixtures using the SensiFAST SYBR No-ROX kit (Bioline), and the quantitative PCRs were carried out using the Chromo4 system (Bio-Rad).
Plasmid Construction
Unless otherwise stated, cDNAs were cloned by PCR amplification using specific primers and PrimeStar DNA polymerase (Clontech) followed by ligation into the pREP series of yeast shuttle vectors (38). Details regarding plasmid construction are available on request.
Results
Nuclear Pore Mutant Suppresses Phenotypes of STUbL Dysfunction
To assess roles of fission yeast Nup132 in SUMO pathway homeostasis and genome stability, we analyzed genetic interactions between its deletion (nup132Δ) and alleles of STUbL (slx8-29) and Ulp2 (ulp2Δ). As in previous reports, nup132Δ cells grew similarly to wild type and exhibited little to no sensitivity to genotoxins or elevated temperatures (Fig. 1A) (33, 34). These subtle phenotypes are consistent with the fact that protein import/export and RNA export pathways are intact in nup132Δ cells and that Nup132 has an expressed paralog called Nup131 (33, 34).
FIGURE 1.
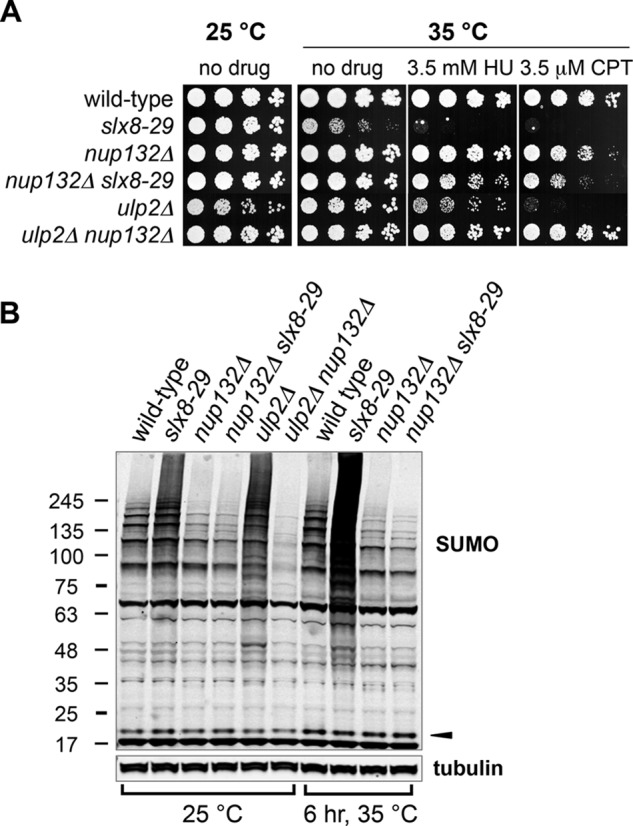
Nup132 deletion suppresses lethality and accumulation of SUMO conjugates in slx8-29 and ulp2Δ mutants. A, serial dilutions of the indicated strains were spotted onto media with indicated drugs, grown at the indicated temperatures. B, Western blots of sumoylated proteins or tubulin of indicated strains grown at 25 °C to log phase or to mid log phase then shifted to 35 °C for 6 h. The black arrowhead indicates the position of free mature SUMO.
Intriguingly, deletion of Nup132 strongly suppressed the growth defects and genotoxin sensitivity of slx8-29 (Fig. 1A). This was surprising, because combined deletions of the analogous factors in budding yeast are synthetically sick (36). As observed for slx8-29, we found that ulp2Δ phenotypes were also suppressed by deleting Nup132 (Fig. 1A). Because an accumulation of SUMO conjugates, particularly chains, drives slx8-29 and ulp2Δ phenotypes (10, 28, 39), we analyzed global sumoylation in Nup132 deleted cells.
At permissive temperature, hypomorphic slx8-29 cells have increased levels of high molecular weight (HMW) SUMO conjugates as compared with wild type (Fig. 1B) (28). In contrast, both nup132Δ and slx8-29 nup132Δ cells had less HMW SUMO conjugates than wild type (Fig. 1B). Moreover, at restrictive temperature, the strong accumulation of HMW SUMO conjugates in slx8-29 cells was absent in slx8-29 nup132Δ cells (Fig. 1B). There was also a lower level of HMW SUMO conjugates in ulp2Δ nup132Δ double mutant versus ulp2Δ single mutant cells (Fig. 1B). Therefore, for as yet undefined reasons, nup132Δ cells exhibit reduced global sumoylation that correlates with the rescue of STUbL and Ulp2 SUMO protease dysfunction.
Altered Ulp1 Activity in nup132Δ Cells
Ulp1 endogenously tagged with GFP (Ulp1-GFP) gives a weak fluorescence signal but shows that fission yeast Ulp1 exhibits a perinuclear localization in live as well as fixed cells (Fig. 2A) (14). Based on analyses of Ulp1 orthologs in other species, this likely reflects its nuclear pore association (32). Budding yeast Ulp1 is anchored at the nuclear pore via a complex set of interactions between the Ulp1 N terminus and several nuclear pore-associated factors, and both its anchoring and protein stability are disrupted in cells lacking Nup133 (fission yeast Nup132; reviewed in Ref. 32). Similarly, we found that both the perinuclear signal of Ulp1 and the level of full-length protein were reduced in nup132Δ cells (Fig. 2, A and B). Thus, the anchoring mechanism of Ulp1 at the nuclear pore is broadly conserved, but the consequences of Ulp1 delocalization and destabilization for SUMO pathway homeostasis are different.
FIGURE 2.
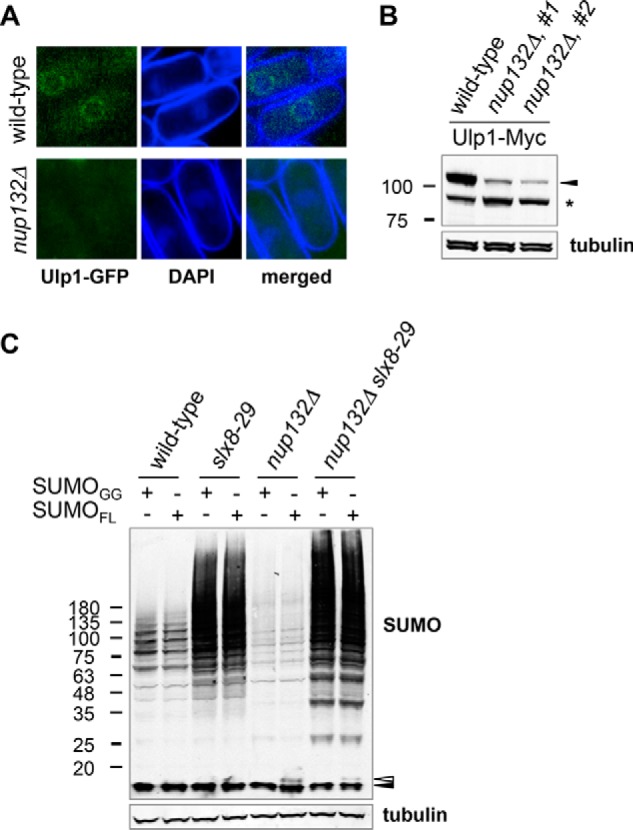
Cells lacking Nup132 have altered Ulp1 activity. A, live cell imaging of GFP fluorescence of Ulp1-GFP and DAPI staining in wild type (top panels) and nup132Δ cells (bottom panels). In addition to defining the nucleus, live cell staining of fission yeast with DAPI also delineates the cell wall and septum. B, Western blots of Ulp1 endogenously tagged with Myc13 or tubulin of indicated strains. The black arrowhead points to the position of full-length Ulp1-Myc13. The asterisk marks the position of a truncated form of Ulp1-Myc13. C, Western blots of sumoylated proteins or tubulin of indicated strains. Ectopic expression of SUMOGG or SUMOFL under the nmt41 promoter was induced by growing cells in the absence of thiamine (B1) for 22 h at 25 °C and then an additional 6 h at 35 °C. The black arrowhead indicates the position of free mature SUMO, and the white arrowhead indicates the position of immature SUMO.
Because Ulp1 functions in SUMO maturation, we next tested whether reduced sumoylation in nup132Δ cells was due to inefficient SUMO maturation. We ectopically expressed either full-length (SUMOFL) or the mature form (SUMOGG) of SUMO, which both ran at the size of mature SUMO (Fig. 2C, black arrowhead) in wild type cells, indicating that normal Ulp1 activity can fully process overexpressed full-length SUMO. Ulp1 maturation activity is slightly compromised in nup132Δ cells, because they show a small amount of residual immature full-length SUMO (Fig. 2C, white arrowhead). However, because SUMO conjugation in nup132Δ cells is low and unchanged whether they express full-length or mature SUMO (Fig. 2C), we conclude that reduced sumoylation in nup132Δ cells is not caused by a SUMO maturation defect.
Interestingly, nup132Δ slx8-29 cells exhibited a major increase in SUMO conjugates, to a level exceeding that of slx8-29 single mutant cells (Fig. 2C). Therefore, the inactivation of STUbL, when combined with higher levels of SUMO, restores global sumoylation in nup132Δ cells. Enhanced global sumoylation in nup132Δ slx8-29 as compared with slx8-29 cells is consistent with reduced Ulp1-mediated desumoylation in nup132Δ mutants.
The PIAS Family Ligase Pli1 Is Destabilized in Cells Lacking Nup132
In fission yeast, the sole PIAS family ligase called Pli1 is responsible for generating the majority (>90%) of SUMO conjugates detectable by Western blotting (8, 10, 28, 40). Although Pli1 deletion does not overtly affect cell growth, it strongly suppresses STUbL mutants, disrupts centromere function, and causes telomere elongation (8, 10, 28, 40, 41). Because nup132Δ cells share the pli1Δ phenotypes of global hyposumoylation, elongated telomeres, and suppression of STUbL dysfunction (Fig. 1, A and B) (28, 42, 43), we analyzed Pli1 levels in nup132Δ cells.
The stability of endogenous Pli1 was monitored over time in cells treated with cycloheximide, an inhibitor of protein synthesis. Intriguingly, we found that Pli1 was both less abundant and more rapidly degraded in nup132Δ versus wild type cells (Fig. 3A). Given that STUbL inactivation can restore global sumoylation in nup132Δ cells (Fig. 2C), we tested whether the slx8-29 mutation affected Pli1 stability. Strikingly, whereas Pli1 was only weakly detectable in nup132Δ cells, Pli1 abundance and a series of lower mobility species were dramatically increased in slx8-29 nup132Δ cells (Fig. 3B). Neither Pli1 levels nor its gel migration were affected in slx8-29 single mutant cells (Fig. 3B).
FIGURE 3.
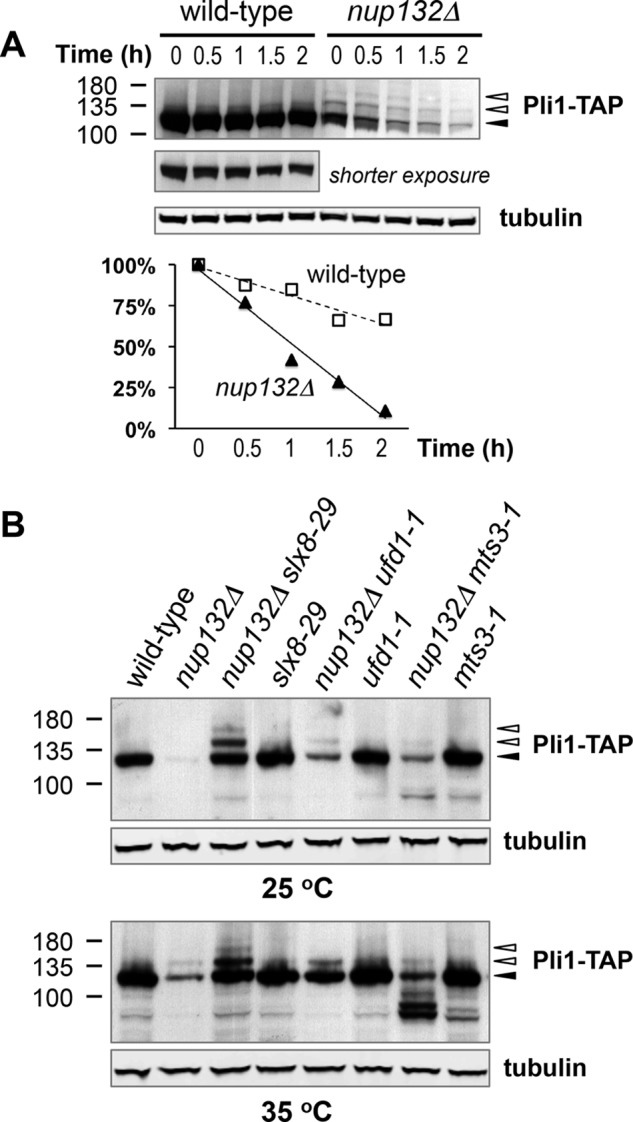
Pli1 SUMO ligase is destabilized in nup132Δ cells. A, wild type or nup132Δ cells grown at 25 °C to mid log phase and then shifted to 35 °C for 4 h. Cycloheximide was added to cell cultures at 200 μg/ml, and cells were harvested at 0, 15, 30, 60, or 120 min after cycloheximide addition and analyzed by Western blotting. The levels of Pli1-TAP proteins (unmodified and all SUMO modified species) were normalized to the levels of tubulin in each sample and quantified with National Institutes of Health ImageJ software. The graph is representative of results from three experiments. B, Western blots of Pli1-TAP or tubulin of indicated strains grown at 25 °C to log phase (top panels) or to mid log phase then shifted to 35 °C for 6 h (bottom panels). Black and white arrowheads mark the positions of unmodified and SUMO modified Pli1-TAP bands, respectively.
We and others recently proposed a cooperative role for STUbL and Cdc48 (p97) AAA+ ATPase in the proteasomal degradation of SUMO conjugates, at a global level (44, 45). Consistent with this proposal, mutations in the Cdc48 cofactor Ufd1 (ufd1-1) or in the 19S proteasome regulatory subunit Mts3 (mts3-1) also stabilized Pli1 in nup132Δ cells (Fig. 3B).
Direct Evidence that SUMO Chains Are Required for STUbL Targeting
The role of STUbL in targeting SUMO-conjugated proteins is now well established, and notably, slower migrating forms of Pli1 accumulate in slx8-29 nup132Δ cells (Fig. 3B). To confirm that these species are SUMO-conjugated Pli1, we purified SUMO conjugates under denaturing conditions from slx8-29 nup132Δ cells and probed for Pli1 (Fig. 4A). This approach enriched only the slower migrating Pli1 species and revealed higher order polysumoylation of Pli1 (Fig. 4A, white arrowheads).
FIGURE 4.
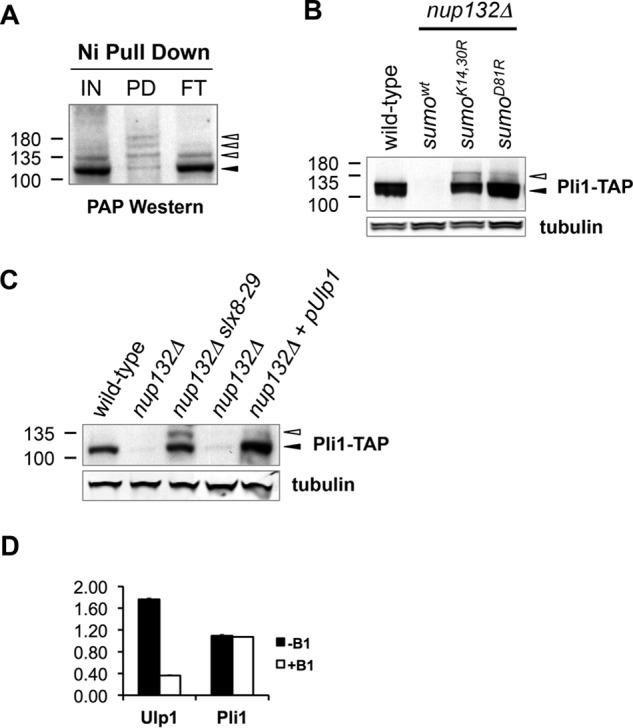
Pli1 SUMO ligase is stabilized by Ulp1-mediated desumoylation. A, His6-SUMO was expressed from the nmt41 promoter in slx8-29 nup132Δ cells. Cells were grown in the absence of B1 for 24 h, and proteins were extracted by TCA and pulled down on nickel-nitrilotriacetic acid (Ni) beads. Input (IN), pulldown (PD), and flow through (FT) were analyzed for the presence of Pli1-TAP by peroxidase anti-peroxidase (PAP) Western blotting. B, Western blots of Pli1-TAP or tubulin of indicated strains grown at 25 °C to log phase. C, cells were grown in the absence of B1 for 24 h to induce the ectopic expression of nmt41-driven Ulp1. In the A–C, black and white arrowheads mark the position of unmodified and modified Pli1-TAP bands. D, the transcript levels of ulp1 and pli1 of cells grown in the absence of B1 for 24 h to induce the ectopic expression of nmt41-driven Ulp1 or under repressed condition (+ B1) were analyzed by RT-quantitative PCR.
STUbLs are thought to target proteins that are modified by SUMO chains, and strong correlative data have been published (see Refs. 25, 26, and 46–48). To test the role of SUMO chains more directly, we used two SUMO mutants that we previously showed block SUMO chain formation in different ways (10).
SUMO chains form through Lys14 and Lys30 in fission yeast SUMO and are therefore absent in SUMOK14,30R mutant cells (10, 49). Strikingly, Pli1 was stabilized in nup132Δ SUMOK14,30R cells (Fig. 4B), to an extent similar to that seen in nup132Δ slx8-29 cells (Fig. 3B). Moreover, the SUMOD81R mutation, which abolishes the noncovalent SUMO:Ubc9 complex required for SUMO chain formation (10), also stabilized Pli1 in nup132Δ cells (Fig. 4B).
In light of the above data, it is interesting to note that the absence of SUMO chains in SUMOK14,30R, SUMOD81R, or pli1Δ cells suppresses all tested STUbL mutant phenotypes (10, 28). Explaining this epistatic relationship, as demonstrated for Pli1, STUbL is only engaged when SUMO chains are present. Overall, these data provide direct mechanistic insight into the role of SUMO chains in STUbL targeting.
Ulp1 Protects Pli1 from Sumoylation and STUbL-mediated Degradation
Given that the Ulp1 SUMO protease is deregulated in nup132Δ cells, we tested whether increased Ulp1 dosage would impact the SUMO conjugation state and stability of Pli1. Strikingly, overexpression of Ulp1 in nup132Δ cells restored normal Pli1 levels (Fig. 4C). Moreover, Pli1 was restored in a hyposumoylated form, consistent with Ulp1 desumoylating and protecting it against STUbL-dependent degradation (Fig. 4C). Because budding yeast Ulp1 modulates transcription of the GAL1 gene (50), we monitored transcript levels of Pli1 upon Ulp1 overexpression. Whereas Ulp1 overexpression yields the anticipated increase in Ulp1 transcript levels, no difference in Pli1 transcription was detected (Fig. 4D). These results suggest that Ulp1 is normally optimally positioned to desumoylate and stabilize Pli1 and that increased Ulp1 dosage can compensate for this loss of spatial regulation.
Pli1 Overexpression Reverses SUMO-related Phenotypes of nup132Δ Cells
Pli1 acts at the end of the SUMO conjugation pathway to attach activated SUMO to its substrates. Therefore, if mature SUMO or E1/E2 factors were limiting, this would render the SUMO pathway unresponsive to the presence of Pli1. Therefore, to test whether Pli1 is the major or sole SUMO pathway factor deregulated by deleting Nup132, we overexpressed Pli1 in pli1Δ, nup132Δ, and nup132Δ slx8-29 cells. As anticipated, expression of Pli1 in pli1Δ control cells restored global SUMO conjugates to a level higher than that seen in wild type (Fig. 5A). Increased Pli1 dosage also restored SUMO conjugates in nup132Δ cells but to levels that were lower than those in pli1Δ cells (Fig. 5A). This is expected based on the constitutive degradation of Pli1 in nup132Δ but not pli1Δ cells. In slx8-29 nup132Δ double mutant cells, SUMO conjugates were higher than those in the nup132Δ single mutant upon Pli1 overexpression (Fig. 5A). Again, this is consistent with the role of STUbL in degrading Pli1 and antagonizing HMW SUMO conjugates. Overall, these data indicate that the SUMO pathway is largely intact upstream of Pli1 in nup132Δ cells.
FIGURE 5.
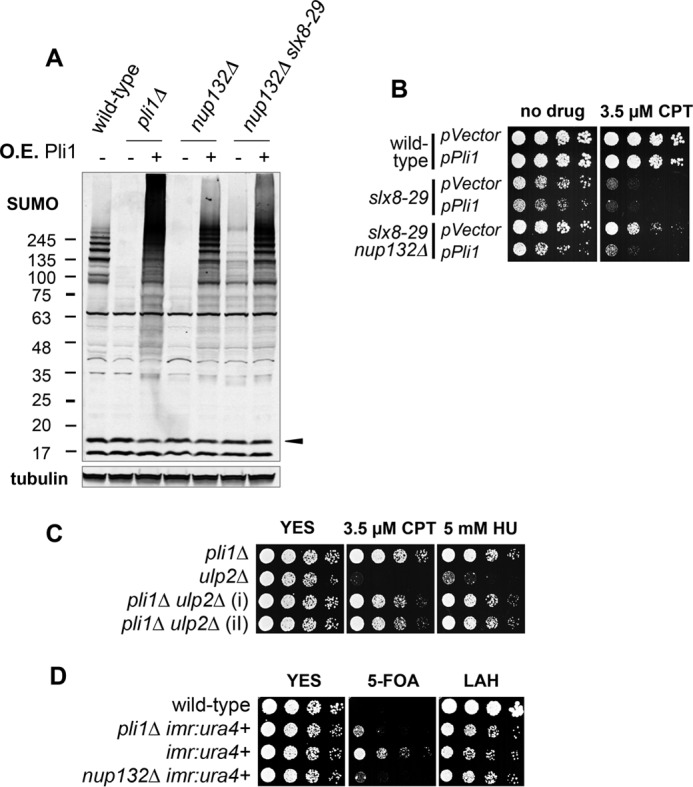
Pli1-related phenotypes in nup132Δ reverted by Pli1 expression. A, Western blots of SUMO conjugates or tubulin in the indicated strains in which Pli1 expression was induced (+), or not (−), from the nmt41 promoter for 24 h. The black arrowhead indicates the position of free mature SUMO. B, serial dilutions of the indicated strains transformed with empty control vector or pREP41-Pli1 that expresses Pli1 were spotted onto media without or with CPT grown at 35 °C. C and D, serial dilutions of the indicated strains were spotted onto rich (YES) or minimum selection (LAH) media at 32 °C, without or with the indicated drugs. YES, yeast extract, dextrose, amino acid supplement mixture; HU, hydroxyurea; CPT, camptothecin.
Thus far, our data indicate that the limited Pli1 activity present in nup132Δ cells rescues the lethality of slx8-29 cells. To directly test this, we assayed the effect of restoring Pli1 expression on the growth of slx8-29 nup132Δ cells. The growth of wild type or slx8-29 cells was similar whether they carried an empty vector control or Pli1 plasmid (Fig. 5B). As expected for the vector control (i.e. nup132Δ suppresses slx8-29), slx8-29 nup132Δ double mutant cells grew well compared with the slx8-29 single mutant (Fig. 5B). Notably, however, expression of Pli1 in slx8-29 nup132Δ cells caused poor growth and drug sensitivity that was similar to that of slx8-29 cells (Fig. 5B). Therefore, consistent with our biochemical analyses, restoring Pli1 expression is sufficient to nullify the rescue of slx8-29 phenotypes by nup132Δ.
We also tested whether, as was the case for cells lacking STUbL activity (28), deletion of Pli1 could suppress the growth and genome instability phenotypes of ulp2Δ cells. Indeed, the pli1Δ ulp2Δ double mutant phenocopies pli1Δ cells, indicating that pli1Δ is epistatic to ulp2Δ (Fig. 5C). We previously demonstrated that Pli1-dependent SUMO chain formation is toxic to both slx8-29 and ulp2Δ cells (10). Therefore, in keeping with our biochemical analysis, Pli1 degradation and reduction of HMW SUMO conjugates can explain the suppression of both slx8-29 and ulp2Δ phenotypes.
Compromised Centromere Function in Cells Lacking Nup132
Centromere function is disrupted in pli1Δ cells, whereby a ura4+ marker inserted at the heterochromatic inner repeats (imr) is constitutively expressed, versus the variegating phenotype in wild type cells (40). We therefore assessed chromatin function at imr in nup132Δ cells. As expected, wild type cells carrying the ura4+ insertion at imr (imr:ura4+) exhibited variegated expression, being able to grow both in the absence of uracil (leucine adenine histidine) and in the presence of 5-fluoroorotic acid (51). However, both pli1Δ imr:ura4+ and nup132Δ imr:ura4+ cells grew poorly in the presence of FOA but robustly in the absence of uracil (Fig. 5D). This result is consistent with the constitutive degradation of Pli1 in nup132Δ cells, causing hyposumoylation of key epigenetic regulators.
Discussion
Herein, we reveal a key novel consequence of nuclear pore dysfunction and subsequent spatial deregulation of Ulp1, which has a major impact on global sumoylation and centromere function. In budding yeast that lack Nup133 (SpNup132), Nup60, or Nup120, Ulp1 is destabilized and mislocalized in the nucleoplasm, causing altered global SUMO conjugate patterns and associated defects in genetic stability (16, 31, 35, 36, 52). A gain of Ulp1 function that promotes the desumoylation of normally spatially protected nucleoplasmic SUMO conjugates is proposed to cause these phenotypes. Such promiscuous desumoylation of nucleoplasmic SUMO conjugates by delocalized Ulp1 in nup132Δ fission yeast is also likely.
Interestingly, however, our results demonstrate that the residual Ulp1 activity in nup132Δ cells is unable to counteract Pli1 sumoylation. This leads to Pli1 degradation and associated hyposumoylation phenotypes. It will be interesting to determine whether this novel mechanism contributes to the SUMO conjugation defects reported for certain budding yeast nucleoporin mutants (16, 31, 35, 36, 52). In this regard, STUbL degrades the nuclear pool of a budding yeast PIAS family ligase, Siz1, particularly when its nuclear export is inhibited (53). Therefore, although untested, it is possible that nuclear pore-associated Ulp1 desumoylates Siz1 and protects it from degradation. If so, given the evolutionary divergence of fission and budding yeast, conservation of this mechanism in human cells seems likely.
How Ulp1, which is normally tethered at the nuclear periphery, accesses nucleoplasmic Pli1 is an interesting question for the future. We determined that increasing Ulp1 dosage in nup132Δ cells stabilized Pli1. This is consistent with increased Ulp1 activity compensating for the loss of a normally more coordinated interaction with Pli1. For example, fission yeast centromeres cluster at the nuclear periphery and are subject to functionally critical Pli1-dependent sumoylation (40, 54). Therefore, Pli1 acting at centromeres is also spatially organized at the nuclear periphery, more proximal to Ulp1.
In addition, budding yeast Ulp1 was recently shown to desumoylate transcription factors on chromatin to facilitate transcriptional activation, providing an example of recruitment of Ulp1 targets to the nuclear pore (50). Interestingly, a subcomplex of the human nuclear pore including Nup133 transiently localizes to centromeres and contributes to their mitotic function (55). Therefore, it will be interesting to determine whether Nup133-associated SENP2 (56) also modulates centromere/kinetochore sumoylation to support its function. Relocalization of the human pore complex and SUMO protease, rather than the target, may be a result of the open mitosis in human cells versus the closed mitosis of yeast.
Based on high throughput analysis, nup132Δ cells share another phenotype of pli1Δ cells, that is, highly elongated telomeres (40, 43). Notably, like centromeres, telomeres are also clustered at the nuclear periphery and are subject to Pli1-dependent regulation (40, 41). Pli1 sumoylates the telomere protein Tpz1 to support a mechanism for inhibiting excessive telomerase activity at chromosome ends (57). Therefore, based on the synonymous centromere silencing roles of Pli1 and Nup132, we envisage that Tpz1 will be hyposumoylated in cells lacking Nup132, leading to telomere elongation.
Identification of Pli1 as a STUbL substrate has enabled a comprehensive analysis of SUMO pathway and ancillary factor requirements. The role of SUMO chains in STUbL targeting has been largely inferred from (i) the accumulation of SUMO chain species when STUbL activity is compromised, (ii) the multivalent SUMO-interacting motif arrangement in STUbLs, and (iii) SUMO chain-induced autoubiquitination/degradation of RNF4 (25, 47, 58). We now demonstrate directly that the STUbL pathway is dependent on the chain forming lysine residues of SUMO, as well as the noncovalent SUMO-Ubc9 interface that promotes SUMO chain formation. These data fit well with the epistatic relationship of SUMO chain and STUbL mutants in fission yeast (10).
In addition, Cdc48-Ufd1-Npl4 was recently identified as a cofactor for STUbL in managing global SUMO conjugate levels (44, 45). However, evidence for a cooperative role in degrading a specific substrate was lacking. Herein, we show that both STUbL and Ufd1 are indeed involved in the proteasome-dependent turnover of Pli1 in nup132Δ cells.
As in fission yeast (44, 45), budding yeast Cdc48 also acts as a SUMO-targeted segregase that removes sumoylated DNA repair factors from chromatin (59). Given this degree of functional conservation, it seems likely that the human STUbL RNF4 and p97 cooperate in the remodeling of chromatin e.g. at DNA repair sites, as reported independently for each factor (60–63).
Author Contributions
M. N. and M. N. B. were both involved in study design, experimental execution, and writing of the manuscript.
Acknowledgments
We thank Dr. Takegawa (Kyushu University) for the nup132::ura4+ strain and Dr. Felicity Watts (University of Sussex) for the ulp1-myc:kanMx6 strain. We thank Emily Arner for technical assistance. We also thank members of the Cell Cycle Groups at the Scripps Research Institute for support.
This work was supported, in whole or in part, by National Institutes of Health Grants GM068608 and GM081840 (to M. N. B.). The authors declare that they have no conflicts of interest with the contents of this article.
- SUMO
- small ubiquitin-like modifier
- HMW
- high molecular weight
- STUbL
- SUMO-targeted ubiquitin ligase.
References
- 1. Cubeñas-Potts C., Matunis M. J. (2013) SUMO: a multifaceted modifier of chromatin structure and function. Dev. Cell 24, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Girdwood D. W., Tatham M. H., Hay R. T. (2004) SUMO and transcriptional regulation. Semin. Cell Dev. Biol. 15, 201–210 [DOI] [PubMed] [Google Scholar]
- 3. Jackson S. P., Durocher D. (2013) Regulation of DNA damage responses by ubiquitin and SUMO. Mol. Cell 49, 795–807 [DOI] [PubMed] [Google Scholar]
- 4. Kerscher O., Felberbaum R., Hochstrasser M. (2006) Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22, 159–180 [DOI] [PubMed] [Google Scholar]
- 5. Felberbaum R., Hochstrasser M. (2008) Ulp2 and the DNA damage response: desumoylation enables safe passage through mitosis. Cell Cycle 7, 52–56 [DOI] [PubMed] [Google Scholar]
- 6. Flotho A., Melchior F. (2013) Sumoylation: a regulatory protein modification in health and disease. Annu. Rev. Biochem. 82, 357–385 [DOI] [PubMed] [Google Scholar]
- 7. Bawa-Khalfe T., Yeh E. T. (2010) SUMO losing balance: SUMO proteases disrupt SUMO homeostasis to facilitate cancer development and progression. Genes Cancer 1, 748–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watts F. Z., Skilton A., Ho J. C., Boyd L. K., Trickey M. A., Gardner L., Ogi F. X., Outwin E. A. (2007) The role of Schizosaccharomyces pombe SUMO ligases in genome stability. Biochem. Soc. Trans. 35, 1379–1384 [DOI] [PubMed] [Google Scholar]
- 9. Rytinki M. M., Kaikkonen S., Pehkonen P., Jääskeläinen T., Palvimo J. J. (2009) PIAS proteins: pleiotropic interactors associated with SUMO. Cell. Mol. Life Sci. 66, 3029–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prudden J., Perry J. J., Nie M., Vashisht A. A., Arvai A. S., Hitomi C., Guenther G., Wohlschlegel J. A., Tainer J. A., Boddy M. N. (2011) DNA repair and global sumoylation are regulated by distinct Ubc9 noncovalent complexes. Mol. Cell. Biol. 31, 2299–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mukhopadhyay D., Dasso M. (2007) Modification in reverse: the SUMO proteases. Trends Biochem. Sci. 32, 286–295 [DOI] [PubMed] [Google Scholar]
- 12. Hickey C. M., Wilson N. R., Hochstrasser M. (2012) Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 13, 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jongjitwimol J., Feng M., Zhou L., Wilkinson O., Small L., Baldock R., Taylor D. L., Smith D., Bowler L. D., Morley S. J., Watts F. Z. (2014) The S. pombe translation initiation factor eIF4G is Sumoylated and associates with the SUMO protease Ulp2. PLoS One 9, e94182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor D. L., Ho J. C., Oliver A., Watts F. Z. (2002) Cell-cycle-dependent localisation of Ulp1, a Schizosaccharomyces pombe Pmt3 (SUMO)-specific protease. J. Cell Sci. 115, 1113–1122 [DOI] [PubMed] [Google Scholar]
- 15. Li S. J., Hochstrasser M. (2000) The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol. Cell. Biol. 20, 2367–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao X., Wu C. Y., Blobel G. (2004) Mlp-dependent anchorage and stabilization of a desumoylating enzyme is required to prevent clonal lethality. J. Cell Biol. 167, 605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panse V. G., Küster B., Gerstberger T., Hurt E. (2003) Unconventional tethering of Ulp1 to the transport channel of the nuclear pore complex by karyopherins. Nat. Cell Biol. 5, 21–27 [DOI] [PubMed] [Google Scholar]
- 18. Li S. J., Hochstrasser M. (2003) The Ulp1 SUMO isopeptidase: distinct domains required for viability, nuclear envelope localization, and substrate specificity. J. Cell Biol. 160, 1069–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hang J., Dasso M. (2002) Association of the human SUMO-1 protease SENP2 with the nuclear pore. J. Biol. Chem. 277, 19961–19966 [DOI] [PubMed] [Google Scholar]
- 20. Zhang H., Saitoh H., Matunis M. J. (2002) Enzymes of the SUMO modification pathway localize to filaments of the nuclear pore complex. Mol. Cell. Biol. 22, 6498–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bailey D., O'Hare P. (2004) Characterization of the localization and proteolytic activity of the SUMO-specific protease, SENP1. J. Biol. Chem. 279, 692–703 [DOI] [PubMed] [Google Scholar]
- 22. Mukhopadhyay D., Ayaydin F., Kolli N., Tan S. H., Anan T., Kametaka A., Azuma Y., Wilkinson K. D., Dasso M. (2006) SUSP1 antagonizes formation of highly SUMO2/3-conjugated species. J. Cell Biol. 174, 939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maison C., Romeo K., Bailly D., Dubarry M., Quivy J. P., Almouzni G. (2012) The SUMO protease SENP7 is a critical component to ensure HP1 enrichment at pericentric heterochromatin. Nat. Struct. Mol. Biol. 19, 458–460 [DOI] [PubMed] [Google Scholar]
- 24. Sriramachandran A. M., Dohmen R. J. (2014) SUMO-targeted ubiquitin ligases. Biochim. Biophys. Acta 1843, 75–85 [DOI] [PubMed] [Google Scholar]
- 25. Geoffroy M. C., Hay R. T. (2009) An additional role for SUMO in ubiquitin-mediated proteolysis. Nat. Rev. Mol. Cell Biol. 10, 564–568 [DOI] [PubMed] [Google Scholar]
- 26. Perry J. J., Tainer J. A., Boddy M. N. (2008) A SIM-ultaneous role for SUMO and ubiquitin. Trends Biochem. Sci. 33, 201–208 [DOI] [PubMed] [Google Scholar]
- 27. Bylebyl G. R., Belichenko I., Johnson E. S. (2003) The SUMO isopeptidase Ulp2 prevents accumulation of SUMO chains in yeast. J. Biol. Chem. 278, 44113–44120 [DOI] [PubMed] [Google Scholar]
- 28. Prudden J., Pebernard S., Raffa G., Slavin D. A., Perry J. J., Tainer J. A., McGowan C. H., Boddy M. N. (2007) SUMO-targeted ubiquitin ligases in genome stability. EMBO J. 26, 4089–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mullen J. R., Das M., Brill S. J. (2011) Genetic evidence that polysumoylation bypasses the need for a SUMO-targeted Ub ligase. Genetics 187, 73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uzunova K., Göttsche K., Miteva M., Weisshaar S. R., Glanemann C., Schnellhardt M., Niessen M., Scheel H., Hofmann K., Johnson E. S., Praefcke G. J., Dohmen R. J. (2007) Ubiquitin-dependent proteolytic control of SUMO conjugates. J. Biol. Chem. 282, 34167–34175 [DOI] [PubMed] [Google Scholar]
- 31. Bukata L., Parker S. L., D'Angelo M. A. (2013) Nuclear pore complexes in the maintenance of genome integrity. Curr. Opin. Cell Biol. 25, 378–386 [DOI] [PubMed] [Google Scholar]
- 32. Palancade B., Doye V. (2008) Sumoylating and desumoylating enzymes at nuclear pores: underpinning their unexpected duties? Trends Cell Biol. 18, 174–183 [DOI] [PubMed] [Google Scholar]
- 33. Asakawa H., Yang H. J., Yamamoto T. G., Ohtsuki C., Chikashige Y., Sakata-Sogawa K., Tokunaga M., Iwamoto M., Hiraoka Y., Haraguchi T. (2014) Characterization of nuclear pore complex components in fission yeast Schizosaccharomyces pombe. Nucleus 5, 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baï S. W., Rouquette J., Umeda M., Faigle W., Loew D., Sazer S., Doye V. (2004) The fission yeast Nup107–120 complex functionally interacts with the small GTPase Ran/Spi1 and is required for mRNA export, nuclear pore distribution, and proper cell division. Mol. Cell. Biol. 24, 6379–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis A., Felberbaum R., Hochstrasser M. (2007) A nuclear envelope protein linking nuclear pore basket assembly, SUMO protease regulation, and mRNA surveillance. J. Cell Biol. 178, 813–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palancade B., Liu X., Garcia-Rubio M., Aguilera A., Zhao X., Doye V. (2007) Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent sumoylation processes. Mol. Biol. Cell 18, 2912–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moreno S., Klar A., Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 38. Maundrell K. (1993) Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127–130 [DOI] [PubMed] [Google Scholar]
- 39. Kosoy A., Calonge T. M., Outwin E. A., O'Connell M. J. (2007) Fission yeast Rnf4 homologs are required for DNA repair. J. Biol. Chem. 282, 20388–20394 [DOI] [PubMed] [Google Scholar]
- 40. Xhemalce B., Seeler J. S., Thon G., Dejean A., Arcangioli B. (2004) Role of the fission yeast SUMO E3 ligase Pli1p in centromere and telomere maintenance. EMBO J. 23, 3844–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xhemalce B., Riising E. M., Baumann P., Dejean A., Arcangioli B., Seeler J. S. (2007) Role of SUMO in the dynamics of telomere maintenance in fission yeast. Proc. Natl. Acad. Sci. U.S.A. 104, 893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han T. X., Xu X. Y., Zhang M. J., Peng X., Du L. L. (2010) Global fitness profiling of fission yeast deletion strains by barcode sequencing. Genome Biol. 11, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu N. N., Han T. X., Du L. L., Zhou J. Q. (2010) A genome-wide screen for Schizosaccharomyces pombe deletion mutants that affect telomere length. Cell Res. 20, 963–965 [DOI] [PubMed] [Google Scholar]
- 44. Køhler J. B., Jørgensen M. L., Beinoraité G., Thorsen M., Thon G. (2013) Concerted action of the ubiquitin-fusion degradation protein 1 (Ufd1) and Sumo-targeted ubiquitin ligases (STUbLs) in the DNA-damage response. PLoS One 8, e80442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nie M., Aslanian A., Prudden J., Heideker J., Vashisht A. A., Wohlschlegel J. A., Yates J. R. 3rd, Boddy M. N. (2012) Dual Recruitment of Cdc48 (p97)-Ufd1-Npl4 Ubiquitin-selective segregase by small ubiquitin-like modifier protein (SUMO) and ubiquitin in SUMO-targeted ubiquitin ligase-mediated genome stability functions. J. Biol. Chem. 287, 29610–29619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hunter T., Sun H. (2008) Crosstalk between the SUMO and ubiquitin pathways. Ernst Schering Found Symp Proc. 1–16 [DOI] [PubMed] [Google Scholar]
- 47. Rojas-Fernandez A., Plechanovová A., Hattersley N., Jaffray E., Tatham M. H., Hay R. T. (2014) SUMO chain-induced dimerization activates RNF4. Mol. Cell 53, 880–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Praefcke G. J., Hofmann K., Dohmen R. J. (2012) SUMO playing tag with ubiquitin. Trends Biochem. Sci. 37, 23–31 [DOI] [PubMed] [Google Scholar]
- 49. Skilton A., Ho J. C., Mercer B., Outwin E., Watts F. Z. (2009) SUMO chain formation is required for response to replication arrest in S. pombe. PLoS One 4, e6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Texari L., Dieppois G., Vinciguerra P., Contreras M. P., Groner A., Letourneau A., Stutz F. (2013) The nuclear pore regulates GAL1 gene transcription by controlling the localization of the SUMO protease Ulp1. Mol. Cell 51, 807–818 [DOI] [PubMed] [Google Scholar]
- 51. Allshire R. C., Nimmo E. R., Ekwall K., Javerzat J. P., Cranston G. (1995) Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9, 218–233 [DOI] [PubMed] [Google Scholar]
- 52. Bennett C. B., Lewis L. K., Karthikeyan G., Lobachev K. S., Jin Y. H., Sterling J. F., Snipe J. R., Resnick M. A. (2001) Genes required for ionizing radiation resistance in yeast. Nat. Genet. 29, 426–434 [DOI] [PubMed] [Google Scholar]
- 53. Westerbeck J. W., Pasupala N., Guillotte M., Szymanski E., Matson B. C., Esteban C., Kerscher O. (2014) A SUMO-targeted ubiquitin ligase is involved in the degradation of the nuclear pool of the SUMO E3 ligase Siz1. Mol. Biol. Cell 25, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Funabiki H., Hagan I., Uzawa S., Yanagida M. (1993) Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 121, 961–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zuccolo M., Alves A., Galy V., Bolhy S., Formstecher E., Racine V., Sibarita J. B., Fukagawa T., Shiekhattar R., Yen T., Doye V. (2007) The human Nup107–160 nuclear pore subcomplex contributes to proper kinetochore functions. EMBO J. 26, 1853–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goeres J., Chan P. K., Mukhopadhyay D., Zhang H., Raught B., Matunis M. J. (2011) The SUMO-specific isopeptidase SENP2 associates dynamically with nuclear pore complexes through interactions with karyopherins and the Nup107–160 nucleoporin subcomplex. Mol. Biol. Cell 22, 4868–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miyagawa K., Low R. S., Santosa V., Tsuji H., Moser B. A., Fujisawa S., Harland J. L., Raguimova O. N., Go A., Ueno M., Matsuyama A., Yoshida M., Nakamura T. M., Tanaka K. (2014) SUMOylation regulates telomere length by targeting the shelterin subunit Tpz1(Tpp1) to modulate shelterin-Stn1 interaction in fission yeast. Proc. Natl. Acad. Sci. U.S.A. 111, 5950–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heideker J., Perry J. J., Boddy M. N. (2009) Genome stability roles of SUMO-targeted ubiquitin ligases. DNA Repair 8, 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bergink S., Ammon T., Kern M., Schermelleh L., Leonhardt H., Jentsch S. (2013) Role of Cdc48/p97 as a SUMO-targeted segregase curbing Rad51-Rad52 interaction. Nat. Cell Biol. 15, 526–532 [DOI] [PubMed] [Google Scholar]
- 60. Acs K., Luijsterburg M. S., Ackermann L., Salomons F. A., Hoppe T., Dantuma N. P. (2011) The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat. Struct. Mol. Biol. 18, 1345–1350 [DOI] [PubMed] [Google Scholar]
- 61. Meerang M., Ritz D., Paliwal S., Garajova Z., Bosshard M., Mailand N., Janscak P., Hübscher U., Meyer H., Ramadan K. (2011) The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat. Cell Biol. 13, 1376–1382 [DOI] [PubMed] [Google Scholar]
- 62. Yin Y., Seifert A., Chua J. S., Maure J. F., Golebiowski F., Hay R. T. (2012) SUMO-targeted ubiquitin E3 ligase RNF4 is required for the response of human cells to DNA damage. Genes Dev. 26, 1196–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Galanty Y., Belotserkovskaya R., Coates J., Jackson S. P. (2012) RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 26, 1179–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]


