Abstract
Inflorescences of the tribe Triticeae, which includes wheat (Triticum sp. L.) and barley (Hordeum vulgare L.) are characterized by sessile spikelets directly borne on the main axis, thus forming a branchless spike. ‘Compositum-Barley’ and tetraploid ‘Miracle-Wheat’ (T. turgidum convar. compositum (L.f.) Filat.) display noncanonical spike-branching in which spikelets are replaced by lateral branch-like structures resembling small-sized secondary spikes. As a result of this branch formation ‘Miracle-Wheat’ produces significantly more grains per spike, leading to higher spike yield. In this study, we first isolated the gene underlying spike-branching in ‘Compositum-Barley,’ i.e., compositum 2 (com2). Moreover, we found that COM2 is orthologous to the branched headt (bht) locus regulating spike branching in tetraploid ‘Miracle-Wheat.’ Both genes possess orthologs with similar functions in maize BRANCHED SILKLESS 1 (BD1) and rice FRIZZY PANICLE/BRANCHED FLORETLESS 1 (FZP/BFL1) encoding AP2/ERF transcription factors. Sequence analysis of the bht locus in a collection of mutant and wild-type tetraploid wheat accessions revealed that a single amino acid substitution in the DNA-binding domain gave rise to the domestication of ‘Miracle-Wheat.’ mRNA in situ hybridization, microarray experiments, and independent qRT-PCR validation analyses revealed that the branch repression pathway in barley is governed through the spike architecture gene Six-rowed spike 4 regulating COM2 expression, while HvIDS1 (barley ortholog of maize INDETERMINATE SPIKELET 1) is a putative downstream target of COM2. These findings presented here provide new insights into the genetic basis of spike architecture in Triticeae, and have disclosed new targets for genetic manipulations aiming at boosting wheat’s yield potential.
Keywords: ‘Miracle-Wheat’, ‘Wunder-Weizen’, ‘Compositum-Barley’, inflorescence branching, yield potential
INFLORESCENCES of the tribe Triticeae, containing wheat (Triticum sp. L.), barley (Hordeum vulgare L.), and rye (Secale cereale L.), display a raceme-like branchless shape and are therefore called a spike. Each spike is normally composed of spikelets arranged in two opposite rows along the main axis (rachis). Individual spikelets contain one or several florets, each producing one grain. Conventionally, in wheat, single spikelets arise from single rachis nodes (Figure 1A). Noncanonical spike forms showing ramified or branched wheat spikes (Figure 1, B–D), have been described as ‘Miracle-Wheat’ [Triticum turgidum convar. compositum (L.f.) Filat.] also recognized as ‘Wunder-Weizen,’ ‘Blé de Miracle,’ or ‘Blé d’Osiris.’ The branching-appearance of the ‘Miracle-Wheat’ inflorescence is evidently due to a naturally occurring mutation that has been known since ancient times (L'Obel 1591; Tschermak 1914; Sharman 1944). Spike-branching is of particular importance for enhancing sink capacity and boosting the yield potential of the crop, because in the case of wheat cultivars, current performance is generally thought to be sink restricted (Miralles and Slafer 2007; Lawlor and Paul 2014). The spike branching has been observed in diploid wheat (2n = 2x = 14, bhm locus; Amagai et al. 2014), tetraploid wheat (2n = 4x = 28, bht locus; Klindworth et al. 1997), as well as barley [2n = 2x = 14; compositum 2 (com2) locus], and rye [2n = 2x = 14; monstrosum ear 1 (mo1) locus; Devries and Sybenga 1984]. The loci maintaining the branchless inflorescence form of the tribe Triticeae are all located in syntenic chromosome positions. This suggests that in Triticeae, the spike form (i.e., branch repression) is controlled by a major orthologous gene. Defects in this gene result in lateral branch formation that, in its completely developed form, resembles a small-sized indeterminate spike (Figure 1, B–D). These lateral branches are distinct from the supernumerary spikelets (SS) phenotype, which comprises only additional spikelets per rachis node (Pennell and Halloran 1983). The underlying genetic factors for the SS phenotype can be diverse as it has been exemplified for the multi-rowed spike (mrs) locus (Dobrovolskaya et al. 2015) or paired spikelets phenotype (Boden et al. 2015). Moreover, a recent genome-wide QTL analysis in common wheat (T. aestivum L.) identified seven QTL regulating SS formation located on five chromosomes (2D, 5B, 6A, 6B, and 7B) (Echeverry-Solarte et al. 2014). Despite the long scientific scrutiny, “true spike-branching” in tetraploid wheat or barley, which represents the formation of laterally formed branch-like structures within the spike, has always remained elusive.
Figure 1.
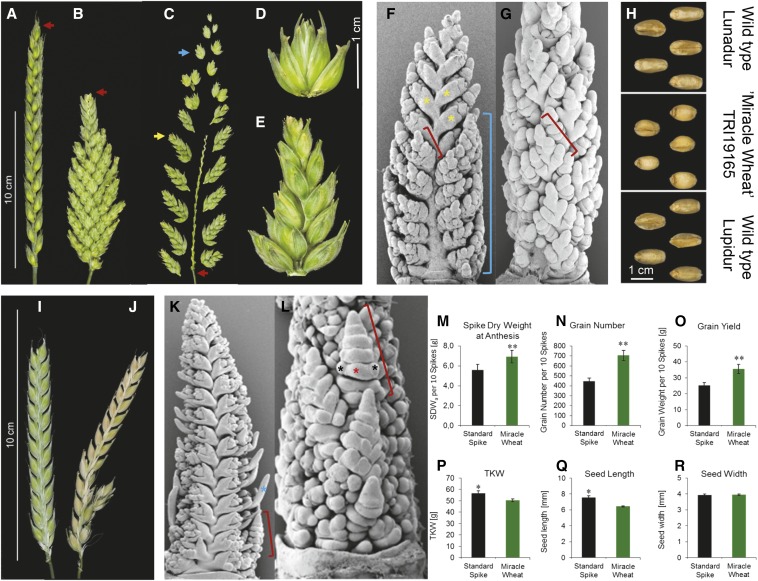
Spike morphology of wild-type and branched (mutant) genotypes in wheat and barley. (A) Hexaploid awnless wheat cv. ‘Kanzler’ with terminal spikelet (red arrowhead) and determinate spike. (B) ‘Wunder-Weizen’ accession TRI 1781 carrying the bht allele displays loss of terminal spikelet (red arrowhead) and indeterminate spike development; awns are removed for clarity. (C) Detached spike’s main axis (red arrowhead), associated single spikelet (blue arrowhead), and multispikelet branch (yellow arrowhead indicates small-sized secondary spike; see also E). (D) Single spikelet containing multiple florets. (E) A multispikelet branch bearing fertile spikelets. (F) Lateral and (G) dorsal view on immature spike from ‘Miracle-Wheat’ TRI 27966 at the terminal spikelet stage showing ectopic branch-like structures emerging from glume primordia (GP, yellow asterisks); blue brackets enclose the branched region along the spike and red brackets delimits early small-sized secondary spike. (H) Seed shape differences (length and width) between two elite tetraploid durum wheat cultivars (top, ‘Lunadur’; bottom, ‘Lupidur’) and ‘Miracle-Wheat’ accession TRI19165. (I) Wild-type barley spike cv. Bowman; awns clipped off for clarity. (J) A branched spike of com2-type barley in BW-NIL(com2.g). (K) Lateral and (L) dorsal view of immature BW-NIL(com2.g) mutant spike at the AP stage (AP, blue asterisk), red bracket delimits early developmental stage of small-sized secondary spike, and red and black asterisks in L represent central and lateral SMs, respectively, of secondary spike. (M–R) Comparison of yield components between elite durum wheat cultivars and ‘Miracle-Wheat’ accessions in the field. Data are based on averages of 200 spikes per phenotypic class. Asterisks indicate significant difference between pairwise comparisons as calculated by Student’s t-test at 95% (*) and 99% (**) confidence intervals.
In the present report, we investigated the genetic and molecular basis of true spike-branching in ‘Compositum-Barley’ and tetraploid ‘Miracle-Wheat.’ Here we positionally cloned the gene com2 underlying spike-branching in barley and found that it is orthologous to bht, which regulates spike branching in ‘Miracle-Wheat.’ Both genes possess orthologs with similar functions in maize BRANCHED SILKLESS 1 (BD1) (Chuck et al. 2002), rice FRIZZY PANICLE/BRANCHED FLORETLESS 1 (FZP/BFL1) (Komatsu et al. 2003; Zhu et al. 2003), and Brachypodium distachyon MORE SPIKELETS 1 (MOS1) (Derbyshire and Byrne 2013). Moreover, bht is orthologous to mrs identified in hexaploid wheat (Dobrovolskaya et al. 2015). Sequence analysis of the bht locus in a collection of mutant and wild-type tetraploid wheat accessions revealed that a single mutation gave rise to the domestication of ‘Miracle-Wheat.’ As a result of branch formation, this mutant allele produces significantly more grains per spike, leading to higher spike yield.
Materials and Methods
Plant material
The ‘Compositum-Barley’ mutants were obtained from the Nordic Genetic Resource center, the National Small Grains Collection (US Department of Agriculture), and the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) gene bank (Supporting Information, Table S1). For haplotype analysis, barley accessions from a previous report were used (Castiglioni et al. 1998) (Table S3). Mutant allele com2.g, its two-rowed progenitor Ackermann’s Donaria, and Bowman near isogenic line (BW-NIL=BW 192) carrying com2.g were used for phenotypic descriptions and SEM analysis. Plant material used to generate mapping populations is reported in the corresponding section for genetic mapping. In the case of wheat, for allelism tests and genetic mapping in wheat, branched head wheat mutants were received from the National Small Grains Collection (US Department of Agriculture), and the IPK gene bank (Table S4 and Table S5).
Marker development
Barley chromosome 2H genome zipper (GZ) (Mayer et al. 2011) was utilized for initial marker development. Barley sequence information, the homologs of the rice genes ordered along the 2H-GZ was used for primer design (File S2). Publicly available wheat SSR markers (Röder et al. 1998) were used for genetic mapping. The barley and wheat orthologs of the rice FZP/BFL1 gene sequence (Os07g0669500) were used for candidate gene marker development (File S2).
Genetic mapping
The barley F2 mapping population was developed by crossing Bowman introgression line BW-NIL(com2.g) and barley cv. Haruna Nijo. For initial mapping, 286 individuals were analyzed (File S2). Two different wheat F2 mapping populations (Tamaroi45 × TRI 27966; 279 F2 individuals; (Tamaroi42 × TRI 19165; 159 F2 individuals) were created. In both species, segregation between mutant and WT F2 plants fitted a 3:1 ratio typical for a monogenic recessive gene. Linkage analysis of segregation data were carried out using the maximum likelihood algorithm of Joinmap 4.0. Kosambi mapping function was used to convert recombination fractions into map distances. High-resolution mapping was performed only in barley (File S2).
Targeting induced local lesions in genomes analysis
For identifying further mutant alleles of COM2 in barley, two different targeting induced local lesions in genomes (TILLING) populations, including ethyl methanesulfonate (EMS)-treated population of cv. Barke and sodium azide-induced TILLMore population of cv. Morex, were screened (File S2). To identify the TtBH-1 mutants in tetraploid wheat, an EMS-treated TILLING population of cv. Kronos was screened. In all cases, the open reading frame (ORF) region of the corresponding gene was targeted for detection of causal SNPs (File S2).
Haplotype analysis
Genomic DNA from a diverse set of barley accessions (Table S3) was PCR amplified using specific primers to amplify full coding sequence of the barley COM2 gene (File S2).
Microarray hybridization and data analysis
Total RNA was isolated from spike meristems collected at glume, stamen, and awn primordium stages from mutants BW-NIL(com2.g) and respective wild type cv. Bowman using the RNA-queous MircroKit (Invitrogen). A detailed description of the genes present on the array and the experimental procedure are described in Koppolu et al. (2013). Microarray hybridizations were performed in three biological replications per stage.
Quantitative RT-PCR
Purelink RNA mini kit (Invitrogen) was applied to extract total RNA from immature spike tissues (double ridge, triple mound, glume primordium, lemma primordium, stamen primordium, and awn primordium stages) followed by removal of genomic DNA contamination using RNAse-free DNAse (Invitrogen). RNA integrity and quantities were analyzed via Agilent bioanalyzer and nanodrop (peq lab), respectively. QuantiTect reverse transcription kit (Qiagen) was utilized for cDNA synthesis using 1 μg of total RNA. Real-time PCR was performed using QuantiTect SYBR green PCR kit (Qiagen) and the ABI prism 7900HT sequence detection system (Applied Biosystems). qRT-PCR results were analyzed using SDS2.2 tool (Applied Biosystems). The reference housekeeping gene used in all cases was HvActin.
mRNA in situ hybridization
A portion of COM2 gene segment (444 bp in length; starting from CDS nucleotide position 888 toward 3′ UTR) was amplified using cDNA isolated from immature spikes of cv. Bonus with specific primers (Table S6). The PCR product was cloned into pBluescript II KS (+) vector (Stratagene, La Jolla, CA). Linearized clones by HindIII or NotI were used as templates to generate antisense (HindIII) and sense (NotI) probes using T3 or T7 RNA polymerase. In situ hybridization was conducted as described previously (Komatsuda et al. 2007).
Scanning electron microscopy
Scanning electron microscopy (SEM) was performed on immature spike tissues at five stages including triple mound, glume, lemma, stamen, and awn primordium from greenhouse-grown plants. SEM was conducted as described elsewhere (Lolas et al. 2010).
DNA preparation
DNA was extracted from leaf samples at the three-leaf stage. Plants for which the DNA was prepared included all genotypes of F2 plants of wheat and barley, fine mapping population of barley, diverse wheat and barley genotypes used for haplotype analysis, and the wheat and barley TILLING lines.
Sequence information and analysis
Unpublished sequence information for the two BAC contigs (44575 and 47813; spanning the interval between M1 and M2) was made available from the international barley sequencing consortium (through Nils Stein). This sequence information was analyzed for gene annotation (File S2).
Data availability
Mutant plants from wheat and barley TILLING analysis are available upon request. File S3 contains the reference DNA sequence information of the TtBH and COM2 of some wheat and barley cultivars, respectively.
Results
Inflorescence form in ‘Miracle-Wheat’ and ‘Compositum-Barley’
‘Miracle-Wheat’ and ‘Compositum-Barley’ display altered, branched inflorescence architecture (Figure 1, A–E, I, and J). Branch formation is more pronounced at the basal part of the spike. In ‘Miracle-Wheat,’ spikes show an indeterminate pattern of growth due to the loss of terminal spikelet formation (Figure 1, compare A to B). In wheat and barley, the inflorescence meristem (IM) progressively initiates lateral meristems acropetally, which give rise to the spikelet meristems (SMs). The SMs develop florets along the rachilla (spikelet axis). Wheat and barley mutants show a normal inflorescence development until the glume primordium (GP) stage at which the SM begins to differentiate. At this stage, predominantly in the basal part of the spike, the SMs revert to branch- or IM-like meristems (Figure 1, F, G, K, and L). This is demonstrated by the failure to initiate florets, and instead, indeterminately produce further spikelets in a distichous manner (Figure 1, compare D to E). It thus seems that in ‘Miracle-Wheat’ and ‘Compositum-Barley’ SMs have acquired an IM-like identity that potentially is able to produce a small-sized indeterminate spike in the form of a branch-like structure (Figure 1, F, G, K, and L). Similar branched-spike phenotypes are also found in the bd1 (Chuck et al. 2002) and fzp/bfl1 mutants of maize and rice (Komatsu et al. 2003; Zhu et al. 2003), respectively. ‘Miracle-Wheat’ and ‘Compositum-Barley’ may thus undergo identical developmental defects while acquiring SM determinacy with ‘Miracle-Wheat’ also losing the spike determinacy as it fails to produce a terminal spikelet (Figure 1, A and B). Two years of field experiments with 12 tetraploid ‘Miracle-Wheat’ landrace accessions showed a significant increase in spike dry weight at anthesis, grain number, and grain yield per spike as compared to the canonical spike forms of tetraploid elite durum wheat cultivars (Figure 1, M–O). Though seed width remained almost unaltered between both groups of wheats, there was a slight decrease in thousand kernel weight (TKW) and seed length in ‘Miracle-Wheats’ (Figure 1, P–R). In contrast, com2 mutants of diploid barley usually show similar or slightly lower spikelet and floret fertility (Figure S1). For instance, barley plants carrying the more severe com2 mutant allele irregular spike 25 display drastically reduced fertility and seed set (Figure S1).
Positional cloning of the barley spike-branching allele com2.g
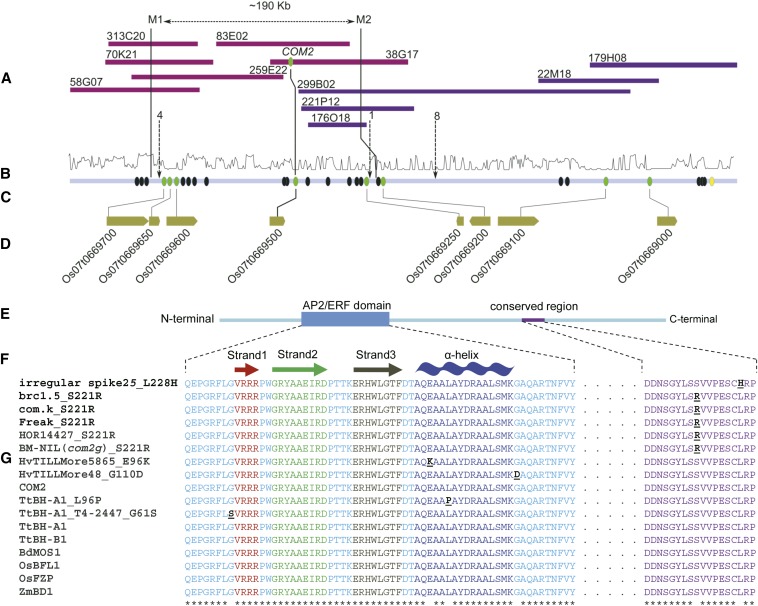
Low-resolution genetic mapping was performed in both tetraploid wheat and barley (File S2). Linkage maps localized the bht and com2.g phenotypes genetically to an interval on the short arm of wheat chromosome 2A (2AS) and barley chromosome 2H (2HS), respectively, at a similar region (File S2 and Figure S2, A and B). The genetic maps were established by including a genetic marker derived from a candidate gene; the wheat and barley orthologs of maize BD1 and rice FZP/BFL1 known to be located in this syntenic chromosome region (Rossini et al. 2006) (for marker details see File S2). The phenotype cosegregated with the candidate gene-based markers, confirming previous findings of bht and com2.g genetic positions (Klindworth et al. 1997; Rossini et al. 2006). Considering the lower complexity of the barley genome, fine mapping was performed in barley by screening 1750 F2 plants for recombination events. A total of 52 F2 recombinant plants and their corresponding F3 families were analyzed whereby six families initially showed a discrepant phenotype compared to the corresponding genotypic score. (See File S2 for more details on deviant plants). com2.g was ultimately mapped into an interval on 2HS, flanked by M1 and M2 CAPS markers (Figure 2, A–D and Table S6). Overlapping BAC clones (∼190 kb) between markers M1 and M2 were sequenced. After annotation, 11 gene fragments and five putative complete gene models were identified, including the barley ortholog of rice FZP/BFL1 (Os07g0669500) (Figure 2C). This candidate gene represents a putative transcription factor consisting of a single exon, encoding a protein of 307 amino acids containing an ethylene-responsive element DNA binding factor (i.e., AP2/ERF) (Figure 2, E–G). Sequence analysis of the barley mutant parental allele [Bowman Near Isogenic Line of com2.g =BW192; i.e., BW-NIL(com2.g)] (Druka et al. 2010) revealed a single amino acid substitution of serine to arginine at position 221 (S221R) in a highly conserved region of the ORF (Figure 2G, File S2, and Figure S3). Nonfunctional forms of this gene in rice and maize result in the conversion of determinate SMs to indeterminate branch meristems reminiscent of the com2.g phenotype (Chuck et al. 2002; Komatsu et al. 2003). The barley ortholog of the BD1/FZP/BFL1 gene, COM2, was thus identified as an eligible candidate for the com2.g allele.
Figure 2.
High-resolution genetic linkage map of COM2 region on chromosome 2HS of barley and protein domain structure conservation among grasses. (A) Overlapped BAC clones (clones of the minimal tiling path) originated from two barley physical map contigs 44575 (purple) and 47813 (blue). The two contigs were merged as they showed significant edge sequence homology. (B) Depicts k-mer method-based repeat frequency (log-scaled; 0, 10). (C) Respective and predicted consensus sequence derived from BAC sequences in which circles represent either the Augustus gene model without sequence homology to Brachypodium, rice, sorghum, or barley genes (black), or with sequence homology to Brachypodium, rice, sorghum, or barley gene (green) as well as those with only sequence homology to predicted barley genes (yellow). The one-directional arrows connect the number of recombination to the corresponding position. (D) Rice genes syntenic to detected barley genes. (For corresponding low-resolution genetic mapping, see Figure S2 and File S2). (E) Protein sequence of COM2 with AP2/ERF DNA-binding domain and a highly conserved terminal region. (F) Position and structure of the AP2/ERF subdomains; including β-sheet (consists of strands 1, 2, and 3) and α-helix. (G) Alignment of AP2/ERF domain and conserved terminal region of COM2/ BHt-A1 with other grass orthologs and mutant alleles in barley and wheat. (F and G) Functional amino acid substitutions are underlined and shown in black. Asterisks indicate no amino acid changes at the corresponding position. For phenotype of respective mutant wheat and barley, see Figure S4.
More com2 mutants and natural sequence diversity in barley
Resequencing a set of available barley spike-branching mutants revealed that four of them shared the same mutation (S221R) as found in the BW-NIL(com2.g) mutant, i.e., brc1.5, com.k, Freak, HOR14427 (a double mutant of com2/hooded spike; see Table S1), while one showed a different amino acid substitution (L228H; irregular spike 25) (Figure 2G, File S2, and Figure S3). These com2 mutants were the result of both induced or natural mutations and are affected in highly conserved nucleotide and protein regions outside of the AP2/ERF domain (Figure 2G). This high level of nucleotide similarity among diverse grass genera might suggest a post-transcriptional regulation of COM2 transcripts. Such regulation seems in line with our observations that the com2 phenotype can vary between different genetic backgrounds (e.g., S221R substitution in several accessions; see Table S1) and/or due to environmental conditions.
We also screened two different barley TILLING populations from cv. Barke (two rowed) and cv. Morex (six rowed). Of these populations, 16 M2 plants (11 homozygous and 5 heterozygous) revealed amino acid substitutions in the ORF region (Table S2). Neither homozygous nor heterozygous lines with mutations outside of the AP2/ERF domain in 12 M2 plants, and their corresponding M3 families, transmitted a branched spike. In contrast to this, two of the remaining four M2 plants (TILLMore48 and TILLMore5865) carrying mutations inside the AP2/ERF domain did transmit a branched spike as was revealed by the phenotypes of the corresponding M3 plants (Figure 2G, Table S2, and Figure S4). This observation further confirmed that the COM2 gene is underlying com2.g.
To evaluate natural variation at the ORF of COM2, the respective region was sequenced and analyzed in a set of 85 diverse barley accessions (Castiglioni et al. 1998) (Table S3). Sequence analysis revealed a low level of COM2 natural sequence variation. Nevertheless, we identified 10 different SNPs that resulted in seven different haplotypes, including two major and five minor groups. None of the groups could be assigned toward a particular geographical region. Among the 10 nucleotide changes, 4 caused amino acid substitutions and the remaining 6 resulted in silent mutations. Of the four amino acid substitutions, two were in conserved regions and two were in nonconserved regions. Of all the identified SNPs, amino acid substitutions, and haplotypes, only S221R (haplotype II) was associated with spike branching and exclusively present in com2.g and brc1.5 mutant stocks (Table S3). The natural variation for COM2 in barley further supports the uniqueness of all causal mutations detected for the com2 locus. However, no allelism test among barley spike-branching mutants was performed.
Expression pattern of COM2 during barley spike development
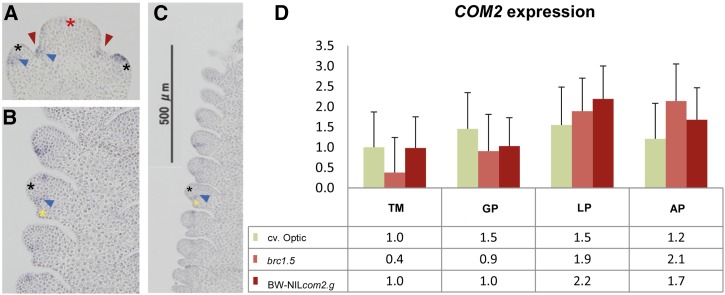
The mRNA in situ hybridization experiments, performed in two-rowed barley (cv. Bonus), revealed that COM2 expression starts early during spikelet development at the triple mound (TM) stage, when spikelet primordia differentiate (Figure 3A). Expression is initially localized at the boundary between central (CS) and lateral spikelets (LS). In the less developed lateral spikelets, expression is first detected in the apical region of the lateral SM (Figure 3A). When GP develop, expression shifts to the area between the SM and the emerging GP (Figure 3B). This resembles the expression pattern of BD1 (maize) and FZP (rice) mRNA (Chuck et al. 2002; Komatsu et al. 2003). Signals for COM2 mRNA expression were consistent along the longitudinal axis of the wild-type spike (Figure 3C, blue triangle). Since COM2 is expressed very early in SM differentiation, it may be involved in mediating SM identity. COM2 expression was also measured between the barley mutants BW-NIL(com2.g), brc1.5, and the wild-type cv. Optic at TM, GP, LP (lemma primordium), and AP (awn primordium) stages. We found no differences in COM2 expression between the mutants and the wild type tested, indicating that branch formation is probably caused by changes at the protein level. In both wild-type and mutant plants, a slight elevation of COM2 transcripts was observed toward lemma primordium stage, while the SM continued to enlarge (Figure 3D). Moreover, in earlier stages (TM and GP) the wild type shows slightly greater expression than the mutants.
Figure 3.
Expression pattern and quantification of COM2 in two-rowed barley. (A–C) mRNA in situ hybridization of COM2 in two-rowed wild-type barley cv. Bonus. (A) Transverse section at triple-mound stage shows COM2 expression at sites of future glume primordia (GP) (red arrowhead). (B) Detail of immature spike with lateral spikelets (LSs) at GP stage reveal accumulation of COM2 transcripts between LS and outer glume. (C) Immature spike with LS in the GP stage showing semicircular COM2 expression between GP and LS primordia along the spike. Expression is clearer in the developmentally advanced basal part of the spike. Asterisks indicate central spikelet (CS) meristem (red), LS meristem (black), and GP (yellow), blue triangles point to the regions of gene expression. (D) COM2 expression in mutants BW-NIL(com2.g) and brc1.5 and wild type cv. Optic. LP and AP stand for lemma primordium and awn primordium stages, respectively. Mean ± SE of three biological replicates. Relative expression values are given at the bottom of the graph. The y-axis value shows the expression relative to HvActin, while genotype differences were tested at a significance level of P > 0.05.
Identification of the gene underlying the bht locus in ‘Miracle-Wheat’
Since phenotypes of com2.g and bht were mapped to the same chromosome group 2 of wheat and barley in syntenic regions (File S2 and Figure S2, A and B), COM2 is likely the orthologous tetraploid wheat gene (TtBH-A1) underlying the bht locus in ‘Miracle-Wheat.’ Sequence analysis of the TtBH-A1 ORF revealed that the two bht mutant parents of the corresponding two mapping populations carried the same recessive allele (Figure S2B). This bht allele contained a single amino acid substitution of leucine to proline at position 96 (L96P) within the AP2/ERF protein domain (Figure 2G). Three different ‘Miracle-Wheat’ landraces with naturally occurring branched phenotype were selected for allelism tests. Crosses among these lines always produced a spike-branching phenotype (Table S4). Resequencing TtBH-A1 ORF from these lines revealed the identical L96P mutation as present in the parents of the mapping populations. Thus, lack of genetic complementation for spike branching in the F1 progenies further indicates that the same L96P mutation at bht may be the casual factor for the branch phenotype. Further resequencing of TtBH-A1 ORF in 30 wild-type accessions as well as 29 ‘Miracle-Wheat’ landraces confirmed that all spike-branching accessions carried the L96P substitution (File S2 and Table S5). This suggests a monophyletic origin of this mutant during the domestication process of tetraploid wheat. To further confirm that TtBH-A1 is the gene underlying spike branching in ‘Miracle-Wheat,’ a tetraploid wheat TILLING population derived from, cv. Kronos (Uauy et al. 2009) was screened. We found 40 mutant M2 plants, with 28 of them leading to unique amino acid substitutions (10 homozygous and 18 heterozygous). Similar to our TILLING assay in barley, neither homozygous nor heterozygous lines with mutation outside of the AP2/ERF domain (26 lines) displayed a branched spike. Of the remaining two (one homozygous and one heterozygous) carrying mutations inside the AP2/ERF protein domain, plant T4-2447 (G61S) proved to confer mild spike branching (File S2, Table S7, and Figure S4). Furthermore, the same TILLING population was screened for mutations in the homeologous B genome copy of the gene (TtBH-B1) via which the TILLING plant T4-2432 was identified. This plant harbored a mutation giving rise to a premature stop codon at amino acid position 14 (Q14X, heterozygous form). Neither homozygous nor heterozygous progenies of this mutant plant showed any spike branching (Table S7), indicating that the TtBH-B1 copy does not actively contribute to branch formation in ‘Miracle-Wheat.’
COM2 is downstream of the spike architecture gene Six-rowed spike 4 (Vrs4); microarray analysis of com2.g reveals COM2 regulatory interactions
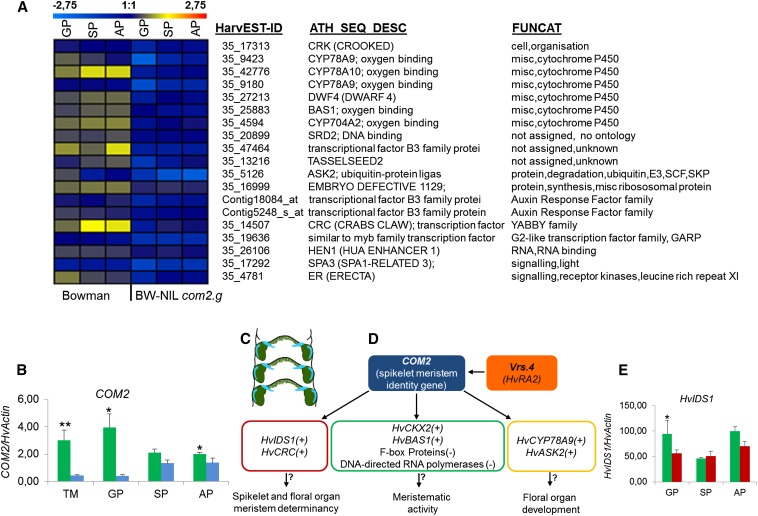
To identify potential downstream target genes of the putative barley transcription factor COM2, microarray analysis was performed in the barley spike-branching mutant BW-NIL(com2.g) and its respective wild type cv. Bowman (Figure 4A, Figure S5A, and File S1). Independent quantitative RT-PCR (qRT-PCR) analysis was performed to confirm the microarray data and to validate genes not present (Figure 4, B and E) on the array including the barley Vrs4 (HvRAMOSA2), which controls SM determinacy and row type (Koppolu et al. 2013). Loss-of-function vrs4 alleles promote lateral spikelet fertility as well as occasional branch formation, the latter trait resembling the com2 phenotype (Figure S6). We tested COM2 transcripts in the BW-NIL(vrs4.k) mutant and the corresponding wild type cv. Bowman by independent qRT-PCR at three spike developmental stages also used for the microarray experiment, and the triple mound stage. COM2 transcripts were significantly down-regulated in the vrs4.k mutant (Figure 4B, blue column) compared to the wild-type barley cv. Bowman (Figure 4B, green). The significant down-regulation was observed at early spike developmental stages, i.e., TM and GP, as well as in the late stage of AP (Figure 4B). This suggests that Vrs4, which has been reported to be highly expressed during early (TM to GP) barley spike development (Koppolu et al. 2013), may function upstream of COM2. Moreover, our observations in COM2 mRNA in situ hybridization indicated that COM2 and Vrs4 are expressed in overlapping spikelet primordia domains (Figure 4C), supporting a possible interaction.
Figure 4.
Transcriptome analysis of com2.g using microarray and independent qRT-PCR, as well as model of putative COM2 interactions. (A) Heat map of genes conjointly down-regulated in the BW-NIL(com2.g) mutant as compared to corresponding wild type cv. Bowman. For up-regulated genes in the mutant, see Figure S5B. (Scale bar above heat map indicates transcript level between wild type and mutant with blue indicating down-regulation and red indicating up-regulation). (B) COM2 expression in mutant BW 903 (vrs4.k) (blue) compared to corresponding wild type cv. Bowman (green). Mean ± SE of three biological replicates. (C) Schematic drawing of central and lateral SM at the triple mound stage. Six-rowed spike 4 (Vrs4; green) and COM2 (light blue) are expressed in overlapping domains of the lateral and central spikelets. (D) Model of putative wild-type COM2 interactions. (+) and (−) indicate up- or down-regulation of the wild-type allele, respectively, in comparison to the mutant BW-NIL(com2.g). (E) HvIDS1 expression in BW-NIL(com2.g) (red) as compared to corresponding wild type cv. Bowman (green). In both B and E, the mean ± SE of three biological replicates is shown. All expression values in both B and E were log10 transformed. Asterisks show the significance level calculated by Student’s t-test, (no asterisk corresponds to P > 0.05. Single, double, and triple asterisks stand for P ≤ 0.05, P ≤ 0.01 and P ≤ 0.001, respectively). Developmental stages include: TM, triple mound; GP, glume primordium; SP, stamen primordium; and AP, awn primordium. Genes including HvIDS1, HvCKX2, and COM2 were not present on the array.
Among the genes significantly down-regulated in com2.g were those engaged in hormonal metabolism [barley cytokinin oxidase/dehydrogenase (HvCKX2) and barley phyB activation tagged suppressor 1 protein (HvBAS1)], spikelet determinacy, and floral organ differentiation and development (Figure 4A and Figure S5B). The low transcript levels of the barley HvCKX2 (Figure S5B) may result in higher concentration of bioactive cytokinins in the com2.g inflorescence consistent with higher meristematic activity (Mok and Mok 2001; Zhu et al. 2013). The putative ortholog of maize INDETERMINATE SPIKELET 1 (IDS1), the barley HvIDS1 (an AP2-like gene), was also significantly down-regulated in the com2.g mutant (Figure 4E). In maize, the gene specifies a determinate SM fate and thereby limits the number of floral meristems (Chuck et al. 1998). This is in agreement with the loss of SM determinacy seen in com2 mutant inflorescences.
Other genes down-regulated were mostly involved in floral organ development and fertility (Figure 4A and Figure S5B). These include orthologs of the Arabidopsis genes cytochrome P450, CYP78A9, and CYP78A10, which control floral organ size and ovule integument development; CRABS CLAW (CRC), involved in floral meristem determinacy; and ARABIDOPSIS SKP1-LIKE2 (ASK2), which plays a role in embryogenesis and postembryonic development (Bowman and Smyth 1999; Liu et al. 2004; Sotelo-Silveira et al. 2013). Down-regulation of these genes is in accordance with the com2.g mutant phenotype, especially the low fertility associated in the more severe allele of mutant irregular spike 25.
In contrast to this, genes related to meristematic activity were up-regulated in com2.g mutant, including genes encoding F-box proteins (Figure S5A) that control degradation of cellular proteins (Jain et al. 2007). The increase in transcripts encoding for subunits of RNA polymersase I, II, and III (Figure S5A) hints at a connection between translational mechanisms and the high meristematic activity observed in com2.g (Figure 4D).
Discussion
COM2/TtBH-A1 confer a branchless spike in the tribe Triticeae
Among grasses, species from the tribe Triticeae have acquired a specific form of inflorescences in which spikelets, the actual building blocks of grass inflorescences, are directly attached to the main axis (or rachis). In contrast to other grass species like rice and maize, little is known about the genetic determinants that regulate inflorescence specification in Triticeae (Zhang and Yuan 2014). Genes responsible for row types in barley (Komatsuda et al. 2007; Ramsay et al. 2011; Koppolu et al. 2013) and for the free-threshing character in wheat (Simons et al. 2006) are among the few that have been characterized so far. Here, we report on a gene in tetraploid wheat (TtBH-A1) and barley (COM2) containing an AP2/ERF domain that represses inflorescence branch formation. Across grass species, TtBH-A1/COM2 shows 100% sequence conservation within the AP2/ERF domain and a highly conserved protein coding region. Nonfunctional forms of this protein always cause inflorescence branching, suggesting a consistent role in preventing formation of any ectopic branch-like meristems in grass inflorescences. In the maize mutant bd1 and rice mutant fzp/bfl1, the SMs acquire indeterminate branched meristem (BM) identity through the reiterated formation of axillary meristems that prevent the transition of spikelet to floral meristem identity (Chuck et al. 2002; Komatsu et al. 2003; Zhu et al. 2003). A similar pattern of direct conversion of SMs to branch-like meristems was observed in barley and tetraploid wheat. The branch-like meristem resembles IM-like meristems that produce secondary spike-like structures. Since SS formation in hexaploid bread wheat appears to be under the control of WFZP (Dobrovolskaya et al. 2015), the ortholog of TtBH, it is possible that mild homoeoalleles of wfzp-D and/or wfzp-A cause a SS-like phenotype due to lost SM determinacy, but fail to initiate spike branching as seen in tetraploid ‘Miracle-Wheat’ or ‘Compositum-Barley.’ This may explain our tetraploid wheat TILLING mutant, T4-2447 (G61S), in which we predominantly found additional spikelet formation but not spike branching.
Polyploidy and its association with increased grain number per spike
In all diploid grass species in which noncanonical branch formation is the result of a mutation in branch repressor genes like BD1, FZP/BFL1, or MOS1, no increase in grain number was reported. The latter is often due to associated inferior floret fertility. Although we have not quantified the impact of spike branching on grain number or grain size in barley, the fertility penalty was clearly visible and most extreme in the mutant irregular spike 25. Here, the majority of spikelets failed to set seeds, a phenomenon also observed in severe mutant alleles in rice FZP/BFL1 (Komatsu et al. 2003; Zhu et al. 2003). According to our microarray analysis, COM2 is also engaged in pathway(s) related to spikelet/floret fertility, in addition to branch repression, suggesting that similar factors play a role in diploid barley. Interestingly, such negative pleiotropic effects on fertility were not observed in polyploid wheats. The recessive single-gene inheritance of the bht locus rather suggests that the A genome copy of TtBH-A1 solely contributes to branch repression in tetraploid wheats, while the B genome copy appears inactive. This is in accordance with the very low transcript levels of WFZP-B in hexaploid wheat (Dobrovolskaya et al. 2015). In our study, we have not tested the expression pattern of the two homoeoalleles in tetraploid wheat. So, we do not (yet) know whether such transcript pattern is also maintained during later stages of floret development/fertility. It could well be that even low TtBH-B1 transcript levels may still be sufficient to trigger expression of downstream genes involved in floral fertility, or that TtBH-B1 transcripts are even elevated during later stages of floral development due to differential regulation. Future work has to resolve what role these different homoeoalleles of TtBH/WFZP play during the subsequent floral differentiation process for retaining fertility. This buffering effect of polyploidy on mutations might be the reason for the stable fertility in ‘Miracle-Wheat,’ resulting in improved grain number per spike and elevated sink capacity. It has to be realized, though, that such prospective sink capacity may depend on genetic background, phenology, and/or source capacity of the breeding line. So, the exploitation of the bht allele in combination with the genetic marker developed in this study may provide an interesting opportunity for introducing the spike-branching trait into modern varieties with conceivable implications for boosting wheat’s yield potential.
Branched spike: a domestication-related trait in wheat
Our resequencing analysis of the TtBH-A1 gene in wild-type and spike-branching tetraploid wheat accessions showed the presence of a single allele (i.e., bht) in all ‘Miracle-Wheat’ accessions. This is clear proof of a single selection event during the domestication process of tetraploid wheat, which most likely took place in cultivated emmer (AABB; T. dicoccum L.) wheat. Domestication of emmer wheat from its wild progenitor T. dicoccoides L. was an important step in the evolution of modern polyploid wheat varieties (Salamini et al. 2002). This process includes emergence of traits, such as hulled seeds and nonbrittle rachis, and started >10,000 years ago (Salamini et al. 2002). However, timescale and site, and when and where the mutation underlying ‘Miracle-Wheat’ initially occurred remain unclear. ‘Miracle-Wheat’ has been cultivated under various names in different parts of the world, especially before the emergence of modern-days breeding activities (Ball and Leighty 1916). Evidently, this ancient trait captivated farmers and present-day scientists alike simply because of its magnificent appearance and promise of wealth. Introducing this genetic resource to modern wheat breeding may be a worthwhile endeavor.
The COM2 genetic framework of interactions to putative targets
Our microarray analysis provided novel insights into the transcriptional regulation and interaction of COM2 in barley, suggesting that it may act downstream of the spike architecture gene Vrs4 (HvRA2) (Figure 4D). The formation of spike branching in vrs4.k, accompanied by a lowered COM2 expression and the presence of the Vrs4 cis-recognition motif 5′-GCGGCA-3′ in the 5′ UTR region (−44 bp to −39 bp of the start codon) of COM2 all point in this direction (Husbands et al. 2007; Koppolu et al. 2013). This putative interaction, however, is up to now unknown from maize (i.e., between ZmRA2 and ZmBD1; Chuck et al. 2002; Bortiri et al. 2006), suggesting that this pathway (Vrs4–COM2) appears to be tribe specific and related to inflorescence shape. In fact, a careful evaluation of ZmBD1 expression in ra2 mutants may provide a better understanding of branch repression pathways in different grass tribes. Additionally, HvCKX2 is among the genes commonly down-regulated in both vrs4.k (Koppolu et al. 2013) and com2.g mutants. Mutation in rice OsCKX2 and barley HvCKX2 has already been reported to increase primary and secondary branches in rice as well as higher number of grains in barley (Ashikari et al. 2005; Zalewski et al. 2012; Li et al. 2013)
The down-regulation of the putative barley ortholog of maize IDS1 (HvIDS1) remains intriguing. Although the role of HvIDS1 in barley is still unknown, the putative ortholog of IDS1 in wheat, the Q gene, confers the free-threshing character (Faris et al. 2003; Simons et al. 2006). Providing HvIDS1 has a similar function as IDS1 in maize, then lower expression of HvIDS1 leads to the loss of SM determinacy in com2 mutants. It thus seems that due to mutations in COM2 the SM loses its identity and converts back to an IM-like meristem. The consequence is a small-sized and indeterminate spike, visible as a lateral branch. Elucidating more of the underlying genetic regulatory pathways related to meristematic development and subsequently inflorescence architecture in grasses may provide valuable insights into the manipulation of yield-relevant traits in various crop plants.
Supplementary Material
Acknowledgments
We thank H. Bockelman (US Department of Agriculture–Agricultural Research Service) and M. Grau [Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) gene bank] for providing initial mutant germplasm; M. van Slageren (Royal Botanic Gardens, Kew, UK) for useful discussion on the Lobel reference; the International Barley Sequencing Consortium for prepublication access to sequence information of chromosome 2H; H. Ernst for taking photographs; and M. Pürschel, A. Marlow, E. Miatton, V. Talamé, J. Simmonds, R. Voss, and C. Weissleder for excellent technical support. A.B. was supported by the Scientific and Technological Research Council of Turkey 2214/A International Research Fellowship Programme as a visiting scholar in the John Innes Centre. This work was partly supported by grants from the IPK Gatersleben and the German Federal Ministry of Education and Research, GABI-FUTURE Start Young Investigator Program grant 0315071 to T.S.
Footnotes
Communicating editor: R. S. Poethig
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.176628/-/DC1.
Literature Cited
- Amagai Y., Martinek P., Watanabe N., Kuboyama T., 2014. Microsatellite mapping of genes for branched spike and soft glumes in Triticum monococcum L. Genet. Resour. Crop Evol. 61: 465–471. [Google Scholar]
- Ashikari M., Sakakibara H., Lin S., Yamamoto T., Takashi T., et al. , 2005. Cytokinin oxidase regulates rice grain production. Science 309: 741–745. [DOI] [PubMed] [Google Scholar]
- Ball, C. R., and C. E. Leighty, 1916 Alaska and stoner, or “miracle,” wheats: two varieties much misrepresented. Bulletin No. 357, US Department of Agriculture, Washington, DC. [Google Scholar]
- Boden S. A., Cavanagh C., Cullis B. R., Ramm K., Greenwood J., et al. , 2015. Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat. Nature Plants 1: 14016. [DOI] [PubMed] [Google Scholar]
- Bortiri E., Chuck G., Vollbrecht E., Rocheford T., Martienssen R., et al. , 2006. ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18: 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J. L., Smyth D. R., 1999. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development 126: 2387–2396. [DOI] [PubMed] [Google Scholar]
- Castiglioni P., Pozzi C., Heun M., Terzi V., Muller K. J., et al. , 1998. An AFLP-based procedure for the efficient mapping of mutations and DNA probes in barley. Genetics 149: 2039–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G., Meeley R. B., Hake S., 1998. The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev. 12: 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G., Muszynski M., Kellogg E., Hake S., Schmidt R. J., 2002. The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298: 1238–1241. [DOI] [PubMed] [Google Scholar]
- Derbyshire P., Byrne M. E., 2013. MORE SPIKELETS1 is required for spikelet fate in the inflorescence of Brachypodium. Plant Physiol. 161: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries J. N., Sybenga J., 1984. Chromosomal location of 17 monogenically inherited morphological markers in rye (Secale cereale L.) using the translocation tester set. J Plant Breeding 92: 117–139. [Google Scholar]
- Dobrovolskaya O., Pont C., Sibout R., Martinek P., Badaeva E., et al. , 2015. FRIZZY PANICLE drives supernumerary spikelets in bread wheat. Plant Physiol. 167: 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druka A., Franckowiak J., Lundqvist U., Bonar N., Alexander J., et al. , 2010. Exploiting induced variation to dissect quantitative traits in barley. Biochem. Soc. Trans. 38: 683–688. [DOI] [PubMed] [Google Scholar]
- Echeverry-Solarte, M., A. Kumar, S. Kianian, E. E. Mantovani, S. Simsek et al., 2014 Genome-wide genetic dissection of supernumerary spikelet and related traits in common wheat. Plant Genome DOI:10.3835/plantgenome2014.03.0013. [DOI] [PubMed]
- Faris J. D., Fellers J. P., Brooks S. A., Gill B. S., 2003. A bacterial artificial chromosome contig spanning the major domestication locus Q in wheat and identification of a candidate gene. Genetics 164: 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands A., Bell E. M., Shuai B., Smith H. M., Springer P. S., 2007. LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res. 35: 6663–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M., Nijhawan A., Arora R., Agarwal P., Ray S., et al. , 2007. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 143: 1467–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klindworth D. L., Klindworth M. M., Williams N. D., 1997. Telosomic mapping of four genetic markers in durum wheat. J. Hered. 88: 229–232. [Google Scholar]
- Komatsu M., Chujo A., Nagato Y., Shimamoto K., Kyozuka J., 2003. FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 130: 3841–3850. [DOI] [PubMed] [Google Scholar]
- Komatsuda T., Pourkheirandish M., He C. F., Azhaguvel P., Kanamori H., et al. , 2007. Six-rowed barley originated from a mutation in a homeodomain-leucine zipper I-class homeobox gene. Proc. Natl. Acad. Sci. USA 104: 1424–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppolu R., Anwar N., Sakuma S., Tagiri A., Lundqvist U., et al. , 2013. Six-rowed spike4 (Vrs4) controls spikelet determinacy and row-type in barley. Proc. Natl. Acad. Sci. USA 110: 13198–13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D. W., Paul M. J., 2014. Source/sink interactions underpin crop yield: the case for trehalose 6-phosphate/SnRK1 in improvement of wheat. Front. Plant Sci. 5: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhao B., Yuan D., Duan M., Qian Q., et al. , 2013. Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl. Acad. Sci. USA 110: 3167–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Ni W., Griffith M. E., Huang Z., Chang C., et al. , 2004. The ASK1 and ASK2 genes are essential for Arabidopsis early development. Plant Cell 16: 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Obel, M. de, 1591 Icones stirpium [tomus primus]. Available at: http://bibdigital.rjb.csic.es/ing/Libro.php?Libro=4360&Hojas. [Google Scholar]
- Lolas I. B., Himanen K., Gronlund J. T., Lynggaard C., Houben A., et al. , 2010. The transcript elongation factor FACT affects Arabidopsis vegetative and reproductive development and genetically interacts with HUB1/2. Plant J. 61: 686–697. [DOI] [PubMed] [Google Scholar]
- Mayer K. F. X., Martis M., Hedley P. E., Simkova H., Liu H., et al. , 2011. Unlocking the barley genome by chromosomal and comparative genomics. Plant Cell 23: 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles D. J., Slafer G. A., 2007. Sink limitations to yield in wheat: How could it be reduced? J. Agric. Sci. 145: 139. [Google Scholar]
- Mok D. W., Mok M. C., 2001. Cytokinin metabolism and action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 89–118. [DOI] [PubMed] [Google Scholar]
- Pennell A. L., Halloran G. M., 1983. Inheritance of supernumerary spikelets in wheat. Euphytica 32: 767–776. [Google Scholar]
- Ramsay L., Comadran J., Druka A., Marshall D. F., Thomas W. T. B., et al. , 2011. INTERMEDIUM-C, a modifier of lateral spikelet fertility in barley, is an ortholog of the maize domestication gene TEOSINTE BRANCHED 1. Nat. Genet. 43: 169–172. [DOI] [PubMed] [Google Scholar]
- Röder M. S., Korzun V., Wendehake K., Plaschke J., Tixier M. H., et al. , 1998. A microsatellite map of wheat. Genetics 149: 2007–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini L., Vecchietti A., Nicoloso L., Stein N., Franzago S., et al. , 2006. Candidate genes for barley mutants involved in plant architecture: an in silico approach. Theor. Appl. Genet. 112: 1073–1085. [DOI] [PubMed] [Google Scholar]
- Salamini F., Ozkan H., Brandolini A., Schafer-Pregl R., Martin W., 2002. Genetics and geography of wild cereal domestication in the near east. Nat. Rev. Genet. 3: 429–441. [DOI] [PubMed] [Google Scholar]
- Sharman B. C., 1944. Branched heads in wheat and wheat hybrids. Nature 153: 497–498. [Google Scholar]
- Simons K. J., Fellers J. P., Trick H. N., Zhang Z., Tai Y. S., et al. , 2006. Molecular characterization of the major wheat domestication gene Q. Genetics 172: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo-Silveira M., Cucinotta M., Chauvin A. L., Chavez Montes R. A., Colombo L., et al. , 2013. Cytochrome P450 CYP78A9 is involved in Arabidopsis reproductive development. Plant Physiol. 162: 779–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschermak E., 1914. Über die Vererbungsweise von Art- und Gattungsbastarden innerhalb der Getreidegruppe. [On the inheritance of species- and genus-hybrids among cereals.] Mitt. landw. Lehrkanzeln k. k. Hochschule für Bodenkultur Wien II. [Google Scholar]
- Uauy C., Paraiso F., Colasuonno P., Tran R. K., Tsai H., et al. , 2009. A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biol. 9: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski W., Orczyk W., Gasparis S., Nadolska-Orczyk A., 2012. HvCKX2 gene silencing by biolistic or Agrobacterium-mediated transformation in barley leads to different phenotypes. BMC Plant Biol. 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. B., Yuan Z., 2014. Molecular control of grass inflorescence development. Annu. Rev. Plant Biol. 65(65): 553. [DOI] [PubMed] [Google Scholar]
- Zhu J. Y., Sae-Seaw J., Wang Z. Y., 2013. Brassinosteroid signalling. Development 140: 1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q. H., Hoque M. S., Dennis E. S., Upadhyaya N. M., 2003. Ds tagging of BRANCHED FLORETLESS 1 (BFL1) that mediates the transition from spikelet to floret meristem in rice (Oryza sativa L). BMC Plant Biol. 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mutant plants from wheat and barley TILLING analysis are available upon request. File S3 contains the reference DNA sequence information of the TtBH and COM2 of some wheat and barley cultivars, respectively.